Abstract
Breast cancer is the most prevalent cancer in American women. Dietary factors are thought to have a strong influence on breast cancer incidence. This study utilized a meal-feeding protocol with female Sprague-Dawley rats to evaluate effects of two ratios of carbohydrate:protein on promotion and early progression of breast tissue carcinomas. Mammary tumors were induced by N-methyl-N-nitrosourea (MNU) at 52 d of age. Post-induction, animals were assigned to consume either a low protein high carbohydrate diet (LPHC; 15% and 60% of energy, respectively) or a high protein moderate carbohydrate diet (HPMC; 35% and 40% of energy, respectively) for 10 wk. Animals were fed 3 meals/day to mimic human absorption and metabolism patterns. The rate of palpable tumor incidence was reduced in HPMC relative to LPHC (12.9 ± 1.4%/wk vs. 18.2 ± 1.3%/wk). At 3 wk, post-prandial serum insulin was larger in the LPHC relative to HPMC (+136.4 ± 33.1 pmol/L vs. +38.1 ± 23.4 pmol/L), while at 10 wk there was a trend for post-prandial IGF-I to be increased in HPMC (P = 0.055). There were no differences in tumor latency, tumor surface area, or cumulative tumor mass between diet groups. The present study provides evidence that reducing the dietary carbohydrate:protein ratio attenuates the development of mammary tumors. These findings are consistent with reduced post-prandial insulin release potentially diminishing the proliferative environment required for breast cancer tumors to progress.
Background
Breast cancer is the most common cancer in American women, with over 40,000 annual deaths and nearly 200,000 estimated new cases in 2009 [1]. Given the high prevalence of breast cancer and associated physical, social, and psychological detriments [2], reducing cancer incidence through modifiable risk factors such as nutrition is a high public health priority. Estimates suggest that cancer incidence could be reduced by ~1/3 through modifications in diet, including changes in total energy intake and diet content of fat, protein or carbohydrate [3]. With increased attention on higher protein, low carbohydrate diets for weight management, secondary effects of these diets on tumor development are important to evaluate.
Some studies report that dietary protein, and particularly consumption of animal protein, is linked to increased risk of breast cancer [4,5]. However, recent large-scale evaluations have failed to identify intakes of meat, eggs, or dairy products as risk factors [6]. Conversely, epidemiological studies have reported protective effects of dietary protein on breast cancer incidence [7] and mortality [8]. A recent case-controlled study suggests that a dietary pattern including the highest relative levels of animal protein is inversely associated with breast cancer risk [9].
Evidence is now accumulating that dietary carbohydrates, glycemic load, and hyperinsulinemia are associated with increased cancer risk [10,11] and mortality [12]. Higher levels of circulating and post-prandial insulin have been reported to enhance cellular proliferation and tumor development [13] through upregulation of the PI3K/Akt signal pathway which is implicated in breast cancer promotion and tumorigenesis [14]. Furthermore, insulin stimulates the ovaries to produce androgens [15], which have been linked to increased breast cancer risk [16]. To our knowledge, no studies have attempted to compare the effects of dietary protein versus carbohydrates in an experimental model of breast cancer.
We have previously demonstrated that feeding rats diets with increased protein and reduced carbohydrates enhances muscle insulin signaling and glucose uptake, attenuates post-prandial hyperinsulinemia, and stimulates skeletal muscle protein synthesis [17,18]. Our experimental model uses a meal-feeding protocol with three discrete meals each day to mimic human eating patterns and allow for isolation of post-prandial meal responses. This study examined the effects of feeding diets differing in ratios of carbohydrate/protein, but within the Dietary Reference Intake (DRI) acceptable macronutrient distribution range, on tumor incidence, size, and development, in a chemically-induced model of breast cancer. We hypothesized that a diet with a reduced carbohydrate:protein ratio would have a favorable impact on breast cancer development by tempering post-prandial insulin response.
Methods
Experimental Model
Female 45 d old Sprague-Dawley rats (n = 74; Harlan-Teklad, Indianapolis, IN) with a mean body weight of 135 ± 4.8 g were housed individually in stainless steel wire-bottomed cages and maintained at 24°C with 12 h reverse light cycle (light: 1900-0700 h) and free access to water. Rats were trained to consume three meals each day using a modified AIN-93G diet [19] (60% of energy from carbohydrate, 15% protein, and 25% fat; LPHC) as the baseline control. The meal pattern consisted of a 3 g breakfast meal (20% of total energy) consumed between 0700-0720 h, followed by free access to food between 1300-1400 h (~40% total energy) and 1800-1900 h (~40% total energy). At 52 d of age, animals were randomly assigned to consume either the baseline low protein high carbohydrate diet (LPHC; n = 37) or a high protein moderate carbohydrate diet (40% carbohydrate, 35% protein, and 25% fat; HPMC; n = 37) (Table 1). Both diet treatments were isoenergetic. Tumors were induced in all animals at 52 d of age by a single intraperitoneal dose of N-methyl-N-nitrosourea (MNU) at 50 mg/kg body weight in 1 ml of 0.9% NaCl [20]. Rats were weighed and food intake measured each week. The animal protocol was approved by the University of Illinois Institutional Animal Care and Use Committee.
Table 1.
Diet Composition for Treatment Groups
| Diet Component | LPHC | HPMC |
|---|---|---|
| Corn Starch | 396.69 | 264.46 |
| Sucrose | 99.96 | 66.64 |
| Maltodextrin | 132.02 | 88.01 |
| Casein | 154.85 | 361.27 |
| Soybean Oil | 116.24 | 116.24 |
| t-Butylhydroquinone | 0.014 | 0.014 |
| Choline | 2.5 | 2.5 |
| AIN93G Minerals | 35 | 35 |
| AIN93G Vitamins | 10 | 10 |
| Cellulose | 50 | 50 |
| Cystine | 2.36 | 2.36 |
Grams per kg diet. LPHC and HPMC represent low protein and high protein diet groups, respectively.
Measurements
At 3 wk post-MNU treatment, rats were randomly selected from LPHC (n = 12) and HPMC (n = 12) groups and evaluated for metabolic changes associated with diet treatments prior to tumor appearance. Rats were euthanized either after overnight food deprivation (fasted) or 90 min following consumption of a 3 g breakfast meal. Trunk blood was collected in K3EDTA vacutainers (BD, Franklin Lakes, NJ), placed on ice, and subsequently centrifuged at 1500 × G at 4°C for 20 min. Plasma was decanted and stored at -80°C for further analysis. Soleus and gastrocnemius muscles of the left leg were rapidly excised and frozen in liquid nitrogen, while the soleus and gastrocnemius of the right leg were dissected and weighed. Likewise, the left retroperitoneal fat pad was excised and frozen and the right retroperitoneal fat pad dissected and weighed. Liver was excised, weighed, and frozen. The 3 wk time point was intended to capture metabolic differences that could affect tumor promotion, while avoiding confounding pathology associated with tumor development.
At 10 wk post-induction, rats from LPHC (n = 12) and HPMC (n = 12) were measured for body fat using dual-energy X-ray absorptiometery (DXA) (Hologic, Inc., Bedford, MA). To isolate diet effects on body composition, only rats without palpable tumors were scanned. Rats were sedated with medetomidine hydrochloride (Orion, Espoo, Finland) at 0.3 mg/kg body weight 10 min prior to scanning, and were revived with atipamezole (Orion, Espoo, Finland) at 0.15 mg/kg body weight following the scan. All rats (LPHC: n = 25; HPMC: n = 25) were euthanized in a similar manner 3-4 d after DXA scanning and blood, soleus and gastrocnemius muscles, adipose, and liver were collected and weighed as previously described. Mammary tumors were rapidly excised, weighed, rinsed in saline, and a portion was fixed for 24 hr in 10% neutral buffered formalin for histological analysis. Fixed tumors were stored in 75% ethanol, then subsequently dehydrated and imbedded in paraffin. Imbedded tumors were then sectioned at 2-4 μM and stained with hematoxylin and eosin. Tumor sections were graded histologically on the following semi-quantitative scale: 0 - low grade adenocarcinoma, 1 - high grade adenocarcinoma, 2 - mild mammary hyperplasia, 3 - marked mammary hyperplasia.
Carcinogenesis Parameters
Animals were monitored for mammary tumor appearance beginning at 5 wk post-induction through the remainder of the study and after animal euthanasia. Rats were palpated for mammary tumors 2 times/wk. When a tumor was detected, the date and tumor location were recorded. Tumor incidence and latency to tumor appearance were determined qualitatively by palpation. Tumor dimensions of length and width were measured orthogonally by caliper. Tumor surface area was calculated by the following formula: 4π × (length/2) × (width/2). Following euthanasia, all tumors were excised and weighed.
Metabolic Analyses
Plasma glucose was measured by glucose oxidase assay (Thermo, Waltham, MA). Plasma insulin was measured by commercial RIA kit (Millipore, Billerica, MA). Plasma IGF-I was measured by RIA after dissociation from IGFBP by Sephadex G-50 chromatography in 0.2 M formic acid. Eluant containing the free IGF was collected and lyophilized (Labconco Freeze Dry Systems, Kansas City, MO). IGF-I concentrations were measured using [125I]-IGF-I as a competitive radioligand and a polyclonal anti-human antibody (National Hormone and Pituitary Program, NIDDK, Torrance, CA). Bound radioactivity was measured using a gamma counter (Packard Instruments, Meriden, CT) and concentrations were determined relative to a standard curve prepared with recombinant human IGF-I [21].
Statistical Analysis
Data are expressed as mean ± SEM. Plasma glucose, insulin, and IGF-I were analyzed using 2-way ANOVA and between-diet differences were evaluated at each time point. Tumor incidence and time of appearance were evaluated using linear regression analysis. Histological classification of excised tumors was analyzed with a Mann-Whitney U test. All other carcinogenesis parameters, as well as body weight and food intake were analyzed by a Student's t-test. The level of significance was set at P < 0.05. All analyses were performed using SPSS Version 15.0 (Chicago, IL).
Results
Animal Weight Gain, Food Intake, and Body Composition
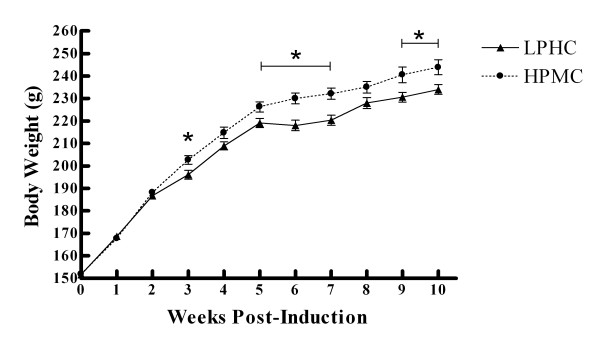
Body weights of HPMC and LPHC groups were not different at baseline (Figure 1). At 3 wk, there were no differences between groups in body weight or weights of gastrocnemius, soleus, or retroperitoneal fat pad (Table 2). Body weight was greater in the HPMC group at wks 3, 5-7, and 9-10 (P < 0.05) (Figure 1). There were no differences in food intake between groups at any time during the study. At 10 wk, there were no differences in gastrocnemius or soleus weights between diet groups (Table 2) or in % body fat measured by DXA. However, retroperitoneal fat and liver were heavier in the HPMC group.
Figure 1.
Body Weights; Values represent Means ± SEM. * indicates significant difference (P > 0.05).
Table 2.
Body Composition Measures
| LPHC | HPMC | P value | ||
|---|---|---|---|---|
| Gastrocnemius weight (g) | 3 wk | 1.32 ± 0.03 | 1.32 ± 0.05 | 0.939 |
| 10 wk | 1.54 ± 0.02 | 1.57 ± 0.03 | 0.398 | |
| Soleus weight (g) | 3 wk | 0.245 ± 0.006 | 0.237 ± 0.009 | 0.472 |
| 10 wk | 0.299 ± 0.007 | 0.309 ± 0.006 | 0.281 | |
| Adipose weight (g) | 3 wk | 0.374 ± 0.056 | 0.358 ± 0.055 | 0.844 |
| 10 wk | 0.443 ± 0.037 | 0.585 ± 0.047 | 0.021 | |
| Liver weight (g) | 3 wk | - | - | - |
| 10 wk | 5.60 ± 0.13 | 6.04 ± 0.11 | 0.012 | |
| % Body fat | 10 wk | 10.8 ± 0.6 | 11.2 ± 0.7 | 0.588 |
Values represent Mean ± SEM, n = 6 for each fasted and fed group.
Serum Glucose, Insulin, & IGF-I Concentrations
Serum glucose was not different between groups at 3 or 10 wk (Table 3) in either fasted or post-prandial blood samples. At 3 wk, fasting insulin tended to be higher in the HPMC group (P = 0.09), while the meal response of serum insulin was greater in the LPHC group compared to HPMC group (+136.4 ± 33.1 pmol/L vs. +38.1 ± 23.4 pmol/L, respectively; P = 0.035). At 10 wk, post-prandial serum insulin was elevated in both LPHC and HPMC groups compared to fasted animals. Serum IGF-I was not significantly modified by diet or meal-feeding at 3 wk. At 10 wk there was a trend for serum IGF-I to be increased post-prandially in the HPMC group (P = 0.055).
Table 3.
Plasma Metabolic Measurements
| Fasted | Fed | Time Effect P Value |
Time × Diet Effect P Value |
|||
|---|---|---|---|---|---|---|
| Glucose (mmol/L) | 3 wk | LPHC | 6.86 ± 0.50 | 6.74 ± 0.44 | 0.854 | 0.841 |
| HPMC | 7.16 ± 0.48 | 6.84 ± 0.47 | 0.642 | |||
| 10 wk | LPHC | 6.73 ± 0.18 | 6.54 ± 0.27 | 0.565 | 0.656 | |
| HPMC | 6.70 ± 0.27 | 6.74 ± 0.31 | 0.917 | |||
| Insulin (pmol/L) | 3 wk | LPHC | 77.4 ± 11.6 | 213.8 ± 33.1 | 0.003 | 0.075 |
| HPMC | 125.1 ± 19.8 | 163.2 ± 23.4 | 0.350 | |||
| 10 wk | LPHC | 86.2 ± 10.4 | 194.4 ± 29.7 | 0.002 | 0.520 | |
| HPMC | 77.5 ± 5.8 | 220.2 ± 37.6 | 0.003 | |||
| IGF-I (μg/L) | 3 wk | LPHC | 240 ± 24 | 272 ± 26 | 0.396 | 0.564 |
| HPMC | 274 ± 10 | 268 ± 32 | 0.917 | |||
| 10 wk | LPHC | 205 ± 30 | 211 ± 17 | 0.851 | 0.221 | |
| HPMC | 237 ± 17 | 299 ± 23 | 0.055 | |||
Values represent Mean ± SEM, n = 6 for each fasted and fed group
Carcinogenesis Parameters
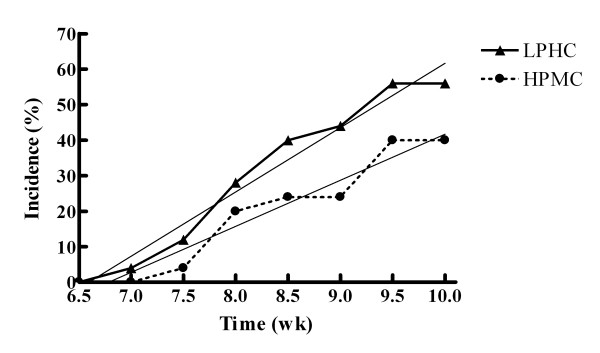
At 3 wk post-induction, no animals had palpable tumors and no tumors were found during dissection. Initial palpable tumors were detected at 51 d in the LPHC group and 55 d in the HPMC group. Surface area of palpable tumors was not different between treatments at any time point. The rate of tumor incidence was higher in the LPHC group with relative slopes of tumor incidence of 18.2 ± 1.3%/wk and 12.9 ± 1.4%/wk (P < 0.05) for the LPHC and HPMC groups, respectively (Figure 2). Other characteristics of tumor incidence, tumor latency (average time to first tumor development), and cumulative tumor mass at 10 wk, were not different between treatments (Table 4). All excised tumors were confirmed histologically to be mammary ductal carcinomas, and there were no differences between groups in the grade of the carcinomas.
Figure 2.
Tumor Incidence; Comparison of slopes of linear regression (P = 0.019); LPHC: y = 18.10x - 119.3, r2 = 0.968; HPMC: y = 12.95x - 87.86, r2 = 0.937.
Table 4.
Carcinogenesis Parameters
| LPHC | HPMC | P value | |
|---|---|---|---|
| Tumor Incidence at 10 wk | 0.56 | 0.4 | > 0.05 |
| Tumor Latency (wk) | 8.36 ± 0.21 | 8.60 ± 0.26 | > 0.05 |
| Cumulative Tumor Mass (g) | 2.22 ± 0.67 | 2.51 ± 1.40 | > 0.05 |
| Rate of Tumor Incidence (% per wk) | 18.2 ± 1.3 | 12.9 ± 1.4 | 0.019 |
Values represent Means ± SEM.
Discussion
The objective of this study was to identify potential effects of manipulating the dietary carbohydrate:protein ratio on the promotion of MNU-induced breast cancer tumors. A major finding of our study is that a HPMC diet with an reduced carbohydrate:protein ratio significantly reduced the rate of incidence of palpable mammary tumors. Although tumor latency was not different between groups, once palpable tumors began to appear, the rate of tumor appearance proceeded more rapidly in the LPHC group. This suggests that either reduced dietary carbohydrate or elevated dietary protein (or both), can attenuate the early development of mammary tumors. In agreement with our previous study [17], post-prandial insulin at 3 wk was elevated in the LPHC group and not in the HPMC group. Thus, animals in the LPHC group were likely exposed to higher, repeated elevations in serum insulin following each discrete meal during the period leading to the emergence of palpable mammary tumors. Post-prandial insulin at 10 wk was elevated in both groups, although host metabolism was likely confounded by tumor pathology, and this change does not necessarily diminish the early effect of post-prandial insulin on tumor promotion.
Nearly two decades ago, hyperinsulinemia was identified as a significant risk factor for breast cancer, independent of body composition or adiposity [22]. Cohort studies have found that women surgically treated for breast cancer with high fasted insulin levels had substantially increased risk for reoccurrence and death [23] and that the highest tertile of fasted insulin levels was associated with a 2-fold increase in post-menopausal breast cancer risk [24,25]. Most recently, the presence of metabolic syndrome was reported to be associated with breast cancer risk [26], and insulin resistance and post-prandial hyperinsulinemia are central features of metabolic syndrome [27], leading to suggestions that metabolic syndrome may serve as a prognostic factor for breast cancer [28]. With respect to dietary factors, a positive association between high glycemic index/load and breast cancer risk has been identified [29,30]. High total carbohydrate intake [31] and sucrose intake have been associated with unfavorable outcomes for breast cancer.
One of the mechanisms by which insulin has been proposed to increase breast cancer risk is via IGF-I. Elevated insulin can increase serum free IGF-I concentrations [32]. Overexpression of IGF-I in mammary epithelium in vivo leads to hyperplasia, spontaneous tumorogenesis, and enhanced susceptibility to chemical carcinogens [33], and circulating IGF-I appears to be important for the onset and subsequent development of mammary tumorigenesis in in vivo experiments [34,35]. In the present study, the elevated post-prandial insulin observed in the LPHC group was not associated with increased serum IGF-I concentrations.
Clinically, the effect of IGF-I on breast cancer remains equivocal. Elevated serum IGF-I concentrations appear to increase risk of pre-, but not post-menopausal breast cancer, while higher blood glucose levels were associated with increased risk for developing post-menopausal breast cancer [36]. However, a case control study identified a weak association for IGF-I with breast cancer in women under the age of fifty [37]. Most recently, the Women's Health Initiative study found a strong association between fasting insulin levels and breast cancer in post-menopausal women, but no association of free IGF-I [38].
IGF-I bioactivity is modulated by IGF binding proteins (IGFBP), which can block IGF-I from binding to its receptor. At least 75% of circulating IGF-I is bound to IGFBP-3 [39], though IGFBP-3 levels remain fairly constant within individuals [40]. IGFBP-1 and IGFBP-2 levels are inversely associated with insulin levels [41], which suggests that chronic hyperinsulinemia could depress binding protein interaction and thus increase free IGF-I. Serum IGF-I levels have also been shown to relate to protein intake [42]. In the present study at 10 wk, there was a trend for post-prandial IGF-I to be higher (p = 0.055) in the HPMC group. Despite the elevation of IGF-I in the HPMC group, the rate of tumor incidence was significantly lower.
Consistent with our findings, early animal experiments investigating the influence of dietary protein on breast cancer found that feeding a higher protein diet prior to administration of a carcinogen resulted in a reduction of tumor prevalence [43], suggesting a protective effect of protein during the initiation phase. Additionally, increased dietary protein fed after tumor induction had no independent effect on the promotion phase of carcinogenesis [44]. In contrast to the findings of the present study, Hawrylewicz et al. found higher dietary protein (33% vs. 15%) increased the number of tumors and tumor weight in MNU-induced rats, despite no difference in tumor incidence or latency [45]. However, this study differed substantially from the current study in that the rats were exposed in utero from dams fed the test diets and then consumed the same test diets ad libitum after weaning.
A further consideration is that elevated dietary protein was accomplished at the expense of dietary carbohydrate, and since tumors are obligate glucose consumers, HPMC may have been less energetically favorable to neoplastic tissues. Similarly, a ketogenic diet (80% medium chain triglycerides) fed to mice with implanted colon adenocarcinomas reduced tumor weight, while the inclusion of hydroxybutyrate in drinking water counteracted the stimulation of tumor growth by insulin infusion [46]. However, fasted glucose was not different between groups at either 3 or 10 wks, and in reflecting current dietary guidelines, the HPMC diet was not low enough in carbohydrate to induce ketogenesis.
A limitation of the current study is its relatively short length. At 10 wks, tumor incidence had only reached 40% in the HPMC group. Due to the labor-intensive nature of the meal-feeding protocol, this study was designed for 10 wk, with a goal of both groups exceeding 50% tumor incidence. However, within the short period of time, the study still achieved divergent rates of tumor appearance between diet groups.
Conclusion
In total, the present study provides evidence that reducing the dietary carbohydrate:protein ratio attenuates the progression of mammary tumors, and this finding is consistent with reduced post-prandial insulin release potentially diminishing the proliferative environment required for breast cancer tumors to progress. These results warrant additional investigation into the potentially protective effect that elevated dietary protein and reduced carbohydrate may have on breast cancer development.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
CM participated in the design of the study, coordinated the study, performed the statistical analysis, and drafted the manuscript. RV assisted in performing the study and helped to draft the manuscript. DL conceived of the study, participated in its design, and helped to draft the manuscript. SD helped in conception of the study and its design. KS helped in the conception of the study and its design. MW performed the histochemical analysis. SMD helped to draft the manuscript. All authors read and approved the final manuscript.
Contributor Information
Christopher J Moulton, Email: cmoulto2@illinois.edu.
Rudy J Valentine, Email: rvalenti@illinois.edu.
Donald K Layman, Email: dlayman@illinois.edu.
Suzanne Devkota, Email: devkota@uchicago.edu.
Keith W Singletary, Email: kws@illinois.edu.
Matthew A Wallig, Email: mawallig@illinois.edu.
Sharon M Donovan, Email: sdonovan@illinois.edu.
Acknowledgements
This work was supported by a grant from the Illinois Council on Food and Agricultural Research. The authors would like to acknowledge the assistance of Dr. Marcia Siegel with the IGF-I RIA.
References
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- Arndt V, Merx H, Stegmaier C, Ziegler H, Brenner H. Persistence of restrictions in quality of life from the first to the third year after diagnosis in women with breast cancer. J Clin Oncol. 2005;23:4945–53. doi: 10.1200/JCO.2005.03.475. [DOI] [PubMed] [Google Scholar]
- Cannon G. Translating science into improved health. Forum Nutr. 2003;56:186–7. [PubMed] [Google Scholar]
- Toniolo P, Riboli E, Shore RE, Pasternack BS. Consumption of meat, animal products, protein, and fat and risk of breast cancer: A prospective cohort study in new york. Epidemiology. 1994;5:391–7. doi: 10.1097/00001648-199407000-00003. [DOI] [PubMed] [Google Scholar]
- Prieto-Ramos F, Serra-Majem L, La Vecchia C, Ramon JM, Tresserras R, Salleras L. Mortality trends and past and current dietary factors of breast cancer in spain. Eur J Epidemiol. 1996;12:141–8. doi: 10.1007/BF00145499. [DOI] [PubMed] [Google Scholar]
- Pala V, Krogh V, Berrino F, Sieri S, Grioni S, Tjonneland A, Olsen A, Jakobsen MU, Overvad K. Meat, eggs, dairy products, and risk of breast cancer in the european prospective investigation into cancer and nutrition (EPIC) cohort. Am J Clin Nutr. 2009;90:602–12. doi: 10.3945/ajcn.2008.27173. [DOI] [PubMed] [Google Scholar]
- Goodwin PJ, Ennis M, Pritchard KI, Koo J, Trudeau ME, Hood N. Diet and breast cancer: Evidence that extremes in diet are associated with poor survival. J Clin Oncol. 2003;21:2500–7. doi: 10.1200/JCO.2003.06.121. [DOI] [PubMed] [Google Scholar]
- Holmes MD, Stampfer MJ, Colditz GA, Rosner B, Hunter DJ, Willett WC. Dietary factors and the survival of women with breast carcinoma. Cancer. 1999;86:826–35. doi: 10.1002/(SICI)1097-0142(19990901)86:5<826::AID-CNCR19>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Edefonti V, Decarli A, La Vecchia C, Bosetti C, Randi G, Franceschi S, Dal Maso L, Ferraroni M. Nutrient dietary patterns and the risk of breast and ovarian cancers. Int J Cancer. 2008;122:609–13. doi: 10.1002/ijc.23064. [DOI] [PubMed] [Google Scholar]
- Stoll BA. Upper abdominal obesity, insulin resistance and breast cancer risk. Int J Obes Relat Metab Disord. 2002;26:747–53. doi: 10.1038/sj.ijo.0801998. [DOI] [PubMed] [Google Scholar]
- Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: A review. Am J Clin Nutr. 2007;86:s836–42. doi: 10.1093/ajcn/86.3.836S. [DOI] [PubMed] [Google Scholar]
- Reaven GM. Banting lecture 1988. role of insulin resistance in human disease. Diabetes. 1988;37:1595–607. doi: 10.2337/diabetes.37.12.1595. [DOI] [PubMed] [Google Scholar]
- Borugian MJ, Sheps SB, Kim-Sing C, Van Patten C, Potter JD, Dunn B, Gallagher RP, Hislop TG. Insulin, macronutrient intake, and physical activity: Are potential indicators of insulin resistance associated with mortality from breast cancer? Cancer Epidemiol Biomarkers Prev. 2004;13:1163–72. [PubMed] [Google Scholar]
- Nicholson KM, Streuli CH, Anderson NG. Autocrine signalling through erbB receptors promotes constitutive activation of protein kinase B/Akt in breast cancer cell lines. Breast Cancer Res Treat. 2003;81:117–28. doi: 10.1023/A:1025765215765. [DOI] [PubMed] [Google Scholar]
- Poretsky L, Cataldo NA, Rosenwaks Z, Giudice LC. The insulin-related ovarian regulatory system in health and disease. Endocr Rev. 1999;20:535–82. doi: 10.1210/er.20.4.535. [DOI] [PubMed] [Google Scholar]
- Eliassen AH, Missmer SA, Tworoger SS, Spiegelman D, Barbieri RL, Dowsett M, Hankinson SE. Endogenous steroid hormone concentrations and risk of breast cancer among premenopausal women. J Natl Cancer Inst. 2006;98:1406–15. doi: 10.1093/jnci/djj376. [DOI] [PubMed] [Google Scholar]
- Baum JI, Layman DK, Freund GG, Rahn KA, Nakamura MT, Yudell BE. A reduced carbohydrate, increased protein diet stabilizes glycemic control and minimizes adipose tissue glucose disposal in rats. J Nutr. 2006;136:1855–61. doi: 10.1093/jn/136.7.1855. [DOI] [PubMed] [Google Scholar]
- Norton LE, Layman DK, Bunpo P, Anthony TG, Brana DV, Garlick PJ. The leucine content of a complete meal directs peak activation but not duration of skeletal muscle protein synthesis and mammalian target of rapamycin signaling in rats. J Nutr. 2009;139:1103–9. doi: 10.3945/jn.108.103853. [DOI] [PubMed] [Google Scholar]
- Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: Final report of the american institute of nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–51. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- Thompson HJ, McGinley JN, Rothhammer K, Singh M. Rapid induction of mammary intraductal proliferations, ductal carcinoma in situ and carcinomas by the injection of sexually immature female rats with 1-methyl-1-nitrosourea. Carcinogenesis. 1995;16:2407–11. doi: 10.1093/carcin/16.10.2407. [DOI] [PubMed] [Google Scholar]
- Monaco MH, Gronlund DE, Bleck GT, Hurley WL, Wheeler MB, Donovan SM. Mammary specific transgenic over-expression of insulin-like growth factor-I (IGF-I) increases pig milk IGF-I and IGF binding proteins, with no effect on milk composition or yield. Transgenic Res. 2005;14:761–73. doi: 10.1007/s11248-005-7219-8. [DOI] [PubMed] [Google Scholar]
- Bruning PF, Bonfrer JM, van Noord PA, Hart AA, de Jong-Bakker M, Nooijen WJ. Insulin resistance and breast-cancer risk. Int J Cancer. 1992;52:511–6. doi: 10.1002/ijc.2910520402. [DOI] [PubMed] [Google Scholar]
- Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Madarnas Y, Hartwick W, Hoffman B, Hood N. Fasting insulin and outcome in early-stage breast cancer: Results of a prospective cohort study. J Clin Oncol. 2002;20:42–51. doi: 10.1200/JCO.20.1.42. [DOI] [PubMed] [Google Scholar]
- Kabat GC, Kim M, Caan BJ, Chlebowski RT, Gunter MJ, Ho GY, Rodriguez BL, Shikany JM, Strickler HD, Vitolins MZ, Rohan TE. Repeated measures of serum glucose and insulin in relation to postmenopausal breast cancer. Int J Cancer. 2009;125:2704–10. doi: 10.1002/ijc.24609. [DOI] [PubMed] [Google Scholar]
- Kabat GC, Cross AJ, Park Y, Schatzkin A, Hollenbeck AR, Rohan TE, Sinha R. Meat intake and meat preparation in relation to risk of postmenopausal breast cancer in the NIH-AARP diet and health study. Int J Cancer. 2009;124:2430–5. doi: 10.1002/ijc.24203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnoli C, Berrino F, Abagnato CA, Muti P, Panico S, Crosignani P, Krogh V. Metabolic syndrome and postmenopausal breast cancer in the ORDET cohort: A nested case-control study. Nutr Metab Cardiovasc Dis. 2009. in press . [DOI] [PMC free article] [PubMed]
- Lann D, LeRoith D. Insulin resistance as the underlying cause for the metabolic syndrome. Med Clin North Am. 2007;91 doi: 10.1016/j.mcna.2007.06.012. 1063,77, viii. [DOI] [PubMed] [Google Scholar]
- Pasanisi P, Berrino F, De Petris M, Venturelli E, Mastroianni A, Panico S. Metabolic syndrome as a prognostic factor for breast cancer recurrences. Int J Cancer. 2006;119:236–8. doi: 10.1002/ijc.21812. [DOI] [PubMed] [Google Scholar]
- Augustin LS, Dal Maso L, La Vecchia C, Parpinel M, Negri E, Vaccarella S, Kendall CW, Jenkins DJ, Francesch S. Dietary glycemic index and glycemic load, and breast cancer risk: A case-control study. Ann Oncol. 2001;12:1533–8. doi: 10.1023/A:1013176129380. [DOI] [PubMed] [Google Scholar]
- Sieri S, Pala V, Brighenti F, Pellegrini N, Muti P, Micheli A, Evangelista A, Grioni S, Contiero P, Berrino F, Krogh V. Dietary glycemic index, glycemic load, and the risk of breast cancer in an italian prospective cohort study. Am J Clin Nutr. 2007;86:1160–6. doi: 10.1093/ajcn/86.4.1160. [DOI] [PubMed] [Google Scholar]
- Wen W, Shu XO, Li H, Yang G, Ji BT, Cai H, Gao YT, Zheng W. Dietary carbohydrates, fiber, and breast cancer risk in chinese women. Am J Clin Nutr. 2009;89:283–9. doi: 10.3945/ajcn.2008.26356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brismar K, Fernqvist-Forbes E, Wahren J, Hall K. Effect of insulin on the hepatic production of insulin-like growth factor-binding protein-1 (IGFBP-1), IGFBP-3, and IGF-I in insulin-dependent diabetes. J Clin Endocrinol Metab. 1994;79:872–8. doi: 10.1210/jc.79.3.872. [DOI] [PubMed] [Google Scholar]
- de Ostrovich KK, Lambertz I, Colby JK, Tian J, Rundhaug JE, Johnston D, Conti CJ, DiGiovanni J, Fuchs-Young R. Paracrine overexpression of insulin-like growth factor-1 enhances mammary tumorigenesis in vivo. Am J Pathol. 2008;173:824–34. doi: 10.2353/ajpath.2008.071005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Cui K, Miyoshi K, Hennighausen L, Green JE, Setser J, LeRoith D, Yakar S. Reduced circulating insulin-like growth factor I levels delay the onset of chemically and genetically induced mammary tumors. Cancer Res. 2003;63:4384–8. [PubMed] [Google Scholar]
- Lann D, LeRoith D. The role of endocrine insulin-like growth factor-I and insulin in breast cancer. J Mammary Gland Biol Neoplasia. 2008;13:371–9. doi: 10.1007/s10911-008-9100-x. [DOI] [PubMed] [Google Scholar]
- Krajcik RA, Borofsky ND, Massardo S, Orentreich N. Insulin-like growth factor I (IGF-I), IGF-binding proteins, and breast cancer. Cancer Epidemiol Biomarkers Prev. 2002;11:1566–73. [PubMed] [Google Scholar]
- Vatten LJ, Holly JM, Gunnell D, Tretli S. Nested case-control study of the association of circulating levels of serum insulin-like growth factor I and insulin-like growth factor binding protein 3 with breast cancer in young women in norway. Cancer Epidemiol Biomarkers Prev. 2008;17:2097–100. doi: 10.1158/1055-9965.EPI-08-0212. [DOI] [PubMed] [Google Scholar]
- Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Rohan TE, Manson JE, Li J, Ho GY, Xue X, Anderson GL, Kaplan RC, Harris TG, Howard BV, Wylie-Rosett J, Burk RD, Strickler HD. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2009;101:48–60. doi: 10.1093/jnci/djn415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: Biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- Juul A, Scheike T, Pedersen AT, Main KM, Andersson AM, Pedersen LM, Skakkebaek NE. Changes in serum concentrations of growth hormone, insulin, insulin-like growth factor and insulin-like growth factor-binding proteins 1 and 3 and urinary growth hormone excretion during the menstrual cycle. Hum Reprod. 1997;12:2123–8. doi: 10.1093/humrep/12.10.2123. [DOI] [PubMed] [Google Scholar]
- Ahmed RL, Thomas W, Schmitz KH. Interactions between insulin, body fat, and insulin-like growth factor axis proteins. Cancer Epidemiol Biomarkers Prev. 2007;16:593–7. doi: 10.1158/1055-9965.EPI-06-0775. [DOI] [PubMed] [Google Scholar]
- Norat T, Dossus L, Rinaldi S, Overvad K, Gronbaek H, Tjonneland A, Olsen A, Clavel-Chapelon F, Boutron-Ruault MC, Boeing H, Lahmann PH, Linseisen J, Nagel G, Trichopoulou A, Trichopoulos D, Kalapothaki V, Sieri S, Palli D, Panico S, Tumino R, Sacerdote C, Bueno-de-Mesquita HB, Peeters PH, van Gils CH, Agudo A, Amiano P, Ardanoz E, Martinez C, Quirós R, Tormo MJ. Diet, serum insulin-like growth factor-I and IGF-binding protein-3 in european women. Eur J Clin Nutr. 2007;61:91–8. doi: 10.1038/sj.ejcn.1602494. [DOI] [PubMed] [Google Scholar]
- Clinton SK, Alster JM, Imrey PB, Nandkumar S, Truex CR, Visek WJ. Effects of dietary protein, fat and energy intake during an initiation phase study of 7,12-dimethylbenz[a]anthracene-induced breast cancer in rats. J Nutr. 1986;116:2290–302. doi: 10.1093/jn/116.11.2290. [DOI] [PubMed] [Google Scholar]
- Clinton SK, Alster JM, Imrey PB, Simon J, Visek WJ. The combined effects of dietary protein and fat intake during the promotion phase of 7,12-dimethylbenz(a)anthracene-induced breast cancer in rats. J Nutr. 1988;118:1577–85. doi: 10.1093/jn/118.12.1577. [DOI] [PubMed] [Google Scholar]
- Hawrylewicz EJ, Huang HH, Liu JM. Dietary protein, enhancement of N-nitrosomethylurea-induced mammary carcinogenesis, and their effect on hormone regulation in rats. Cancer Res. 1986;46:4395–9. [PubMed] [Google Scholar]
- Beck SA, Tisdale MJ. Effect of insulin on weight loss and tumour growth in a cachexia model. Br J Cancer. 1989;59(5):677–81. doi: 10.1038/bjc.1989.140. [DOI] [PMC free article] [PubMed] [Google Scholar]