Abstract
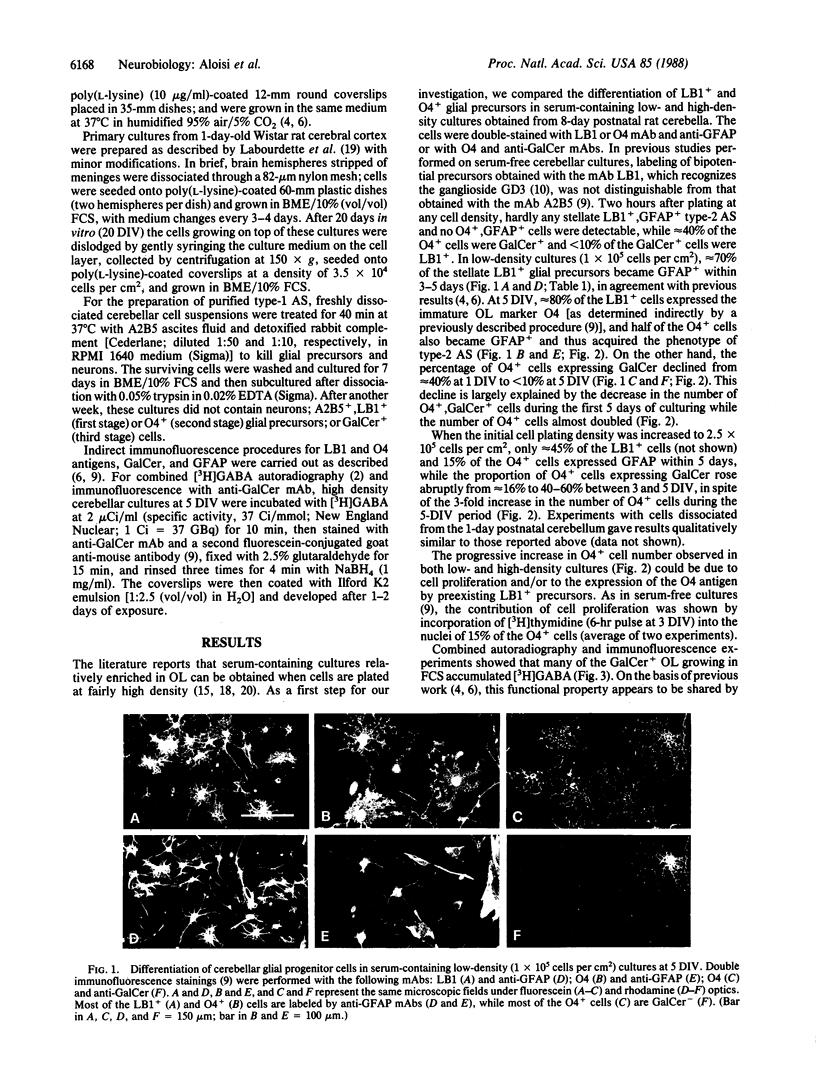
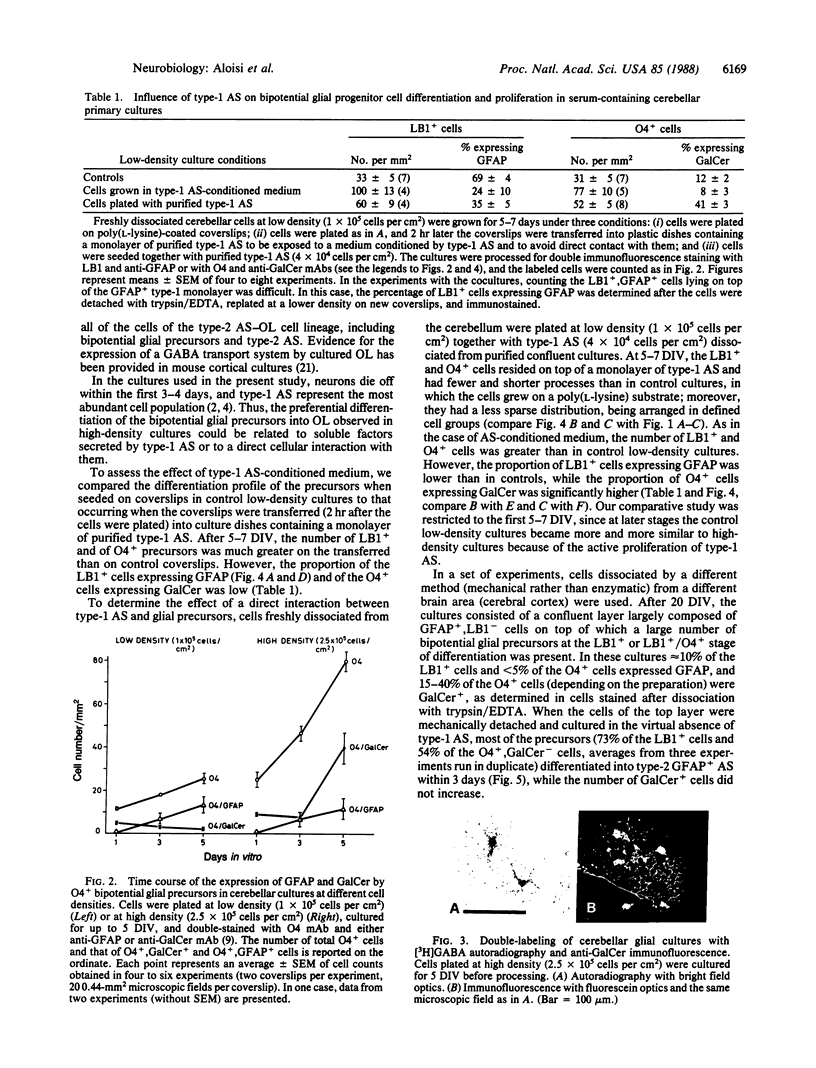
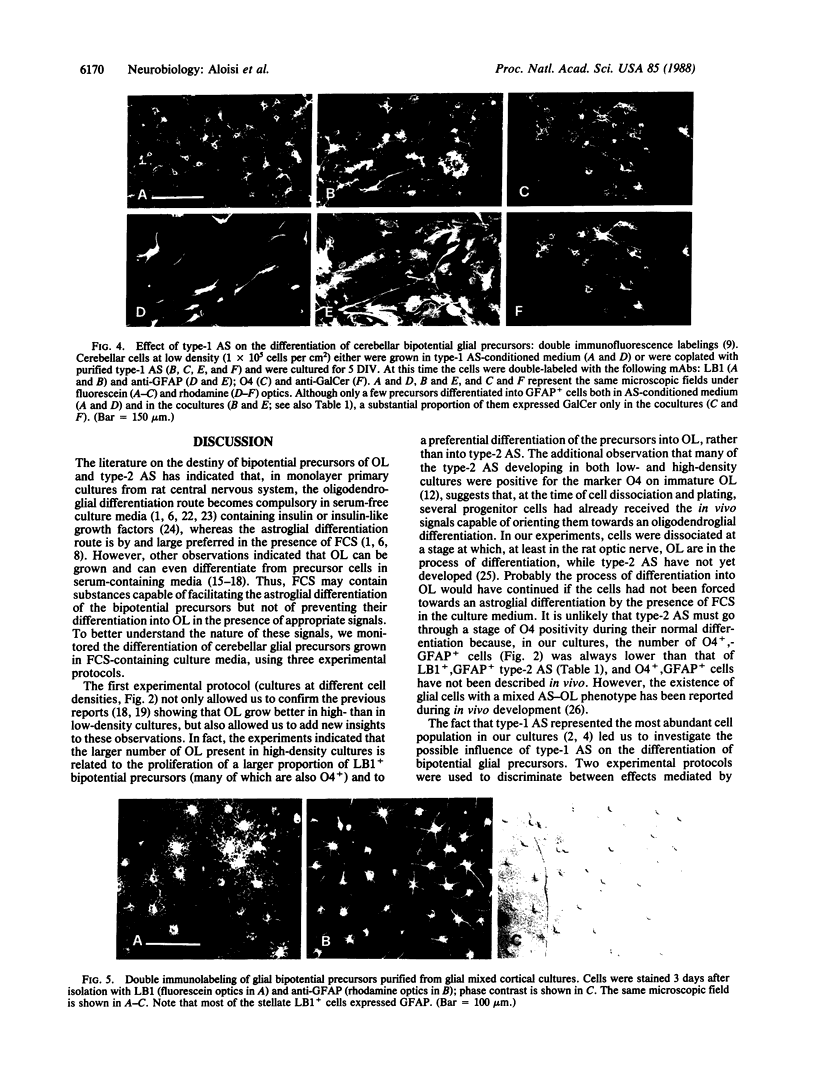
The differentiation of bipotential precursors of oligodendrocytes (OL) and type-2 astrocytes (AS) was followed in primary cultures from 8-day postnatal rat cerebellum by labeling the cells with the antibodies LB1 (which binds to the surface disialoganglioside GD3 present in glial precursors, type-2 AS, and immature OL), O4 (a marker of immature and mature OL binding to surface sulfatide), anti-galactocerebroside (GalCer, a marker of OL), and anti-glial fibrillary acidic protein (GFAP, a marker of AS). Two hours after plating, hardly any LB1+, GFAP+ cells were detectable, 40% of the O4+ cells were GalCer+, and none of the O4+ cells were GFAP+. Upon culturing cells plated at a density of 1 x 10(5) cells per cm2 in the presence of fetal calf serum, most of the LB1+ precursors differentiated into type-2 AS, even if most of them had already expressed the O4 antigen. Thus, in culture, most type-2 AS seem to derive from progenitor cells that were differentiating in vivo into OL. In higher density cultures (2.5 x 10(5) cells per cm2), however, many precursors differentiated into GalCer+ OL, rather than into AS. As a possible source of the signals responsible for the behavior of the glial precursors in high-density cultures, we focused our attention on type-1 AS, the most abundant cell type in the cultures. We found that, in low-density cultures maintained for 5-7 days in a medium conditioned by type-1 AS, the proliferation of the precursors was enhanced and their differentiation into OL or AS was prevented. In contrast, when cerebellar cells were coplated with type-1 AS dissociated from purified cultures, not only did the precursors proliferate more than in control cultures, but also a larger proportion of them differentiated into GalCer+ OL. In conclusion, type-1 AS appear to facilitate the differentiation of bipotential glial precursors into OL through direct cell-cell interactions. The influence of type-1 AS on the differentiation of the LB1+ and O4+ precursors is supported also by experiments with glial cortical cultures.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloisi F., Agresti C., Levi G. Glial conditioned media inhibit the proliferation of cultured rat cerebellar astrocytes. Neurochem Res. 1987 Feb;12(2):189–195. doi: 10.1007/BF00979536. [DOI] [PubMed] [Google Scholar]
- Barbarese E., Pfeiffer S. E. Developmental regulation of myelin basic protein in dispersed cultures. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1953–1957. doi: 10.1073/pnas.78.3.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bologa-Sandru L., Siegrist H. P., Z'Graggen A., Hofmann K., Wiesmann U., Dahl D., Herschkowitz N. Expression of antigenic markers during the development of oligodendrocytes in mouse brain cell cultures. Brain Res. 1981 Apr 6;210(1-2):217–229. doi: 10.1016/0006-8993(81)90895-7. [DOI] [PubMed] [Google Scholar]
- Choi B. H., Kim R. C. Expression of glial fibrillary acidic protein by immature oligodendroglia and its implications. J Neuroimmunol. 1985 Jun;8(4-6):215–235. doi: 10.1016/s0165-5728(85)80064-3. [DOI] [PubMed] [Google Scholar]
- Curtis R., Cohen J., Fok-Seang J., Hanley M. R., Gregson N. A., Reynolds R., Wilkin G. P. Development of macroglial cells in rat cerebellum. I. Use of antibodies to follow early in vivo development and migration of oligodendrocytes. J Neurocytol. 1988 Feb;17(1):43–54. doi: 10.1007/BF01735376. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant C., Raff M. C. Proliferating bipotential glial progenitor cells in adult rat optic nerve. Nature. 1986 Feb 6;319(6053):499–502. doi: 10.1038/319499a0. [DOI] [PubMed] [Google Scholar]
- Gallo V., Bertolotto A., Levi G. The proteoglycan chondroitin sulfate is present in a subpopulation of cultured astrocytes and in their precursors. Dev Biol. 1987 Sep;123(1):282–285. doi: 10.1016/0012-1606(87)90450-7. [DOI] [PubMed] [Google Scholar]
- Gallo V., Ciotti M. T., Aloisi F., Levi G. Developmental features of rat cerebellar neural cells cultured in a chemically defined medium. J Neurosci Res. 1986;15(3):289–301. doi: 10.1002/jnr.490150302. [DOI] [PubMed] [Google Scholar]
- Gallo V., Suergiu R., Levi G. Kainic acid stimulates GABA release from a subpopulation of cerebellar astrocytes. Eur J Pharmacol. 1986 Dec 16;132(2-3):319–322. doi: 10.1016/0014-2999(86)90624-2. [DOI] [PubMed] [Google Scholar]
- Goldman J. E., Geier S. S., Hirano M. Differentiation of astrocytes and oligodendrocytes from germinal matrix cells in primary culture. J Neurosci. 1986 Jan;6(1):52–60. doi: 10.1523/JNEUROSCI.06-01-00052.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone S. R., Levi G., Wilkin G. P., Schneider A., Ciotti M. T. Subpopulations of rat cerebellar astrocytes in primary culture: morphology, cell surface antigens and [3H]GABA transport. Brain Res. 1986 Jan;389(1-2):63–75. doi: 10.1016/0165-3806(86)90173-2. [DOI] [PubMed] [Google Scholar]
- Keilhauer G., Meier D. H., Kuhlmann-Krieg S., Nieke J., Schachner M. Astrocytes support incomplete differentiation of an oligodendrocyte precursor cell. EMBO J. 1985 Oct;4(10):2499–2504. doi: 10.1002/j.1460-2075.1985.tb03962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labourdette G., Roussel G., Ghandour M. S., Nussbaum J. L. Cultures from rat brain hemispheres enriched in oligodendrocyte-like cells. Brain Res. 1979 Dec 21;179(1):199–203. doi: 10.1016/0006-8993(79)90508-0. [DOI] [PubMed] [Google Scholar]
- Labourdette G., Roussel G., Nussbaum J. L. Oligodendroglia content of glial cell primary cultures, from newborn rat brain hemispheres, depends on the initial plating density. Neurosci Lett. 1980 Jun;18(2):203–209. doi: 10.1016/0304-3940(80)90327-4. [DOI] [PubMed] [Google Scholar]
- Levi G., Aloisi F., Wilkin G. P. Differentiation of cerebellar bipotential glial precursors into oligodendrocytes in primary culture: developmental profile of surface antigens and mitotic activity. J Neurosci Res. 1987;18(3):407–417. doi: 10.1002/jnr.490180305. [DOI] [PubMed] [Google Scholar]
- Levi G., Gallo V., Ciotti M. T. Bipotential precursors of putative fibrous astrocytes and oligodendrocytes in rat cerebellar cultures express distinct surface features and "neuron-like" gamma-aminobutyric acid transport. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1504–1508. doi: 10.1073/pnas.83.5.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy K. D., de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980 Jun;85(3):890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMorris F. A., Smith T. M., DeSalvo S., Furlanetto R. W. Insulin-like growth factor I/somatomedin C: a potent inducer of oligodendrocyte development. Proc Natl Acad Sci U S A. 1986 Feb;83(3):822–826. doi: 10.1073/pnas.83.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. H., Abney E. R., David S., Ffrench-Constant C., Lindsay R., Patel R., Stone J., Raff M. C. Is reactive gliosis a property of a distinct subpopulation of astrocytes? J Neurosci. 1986 Jan;6(1):22–29. doi: 10.1523/JNEUROSCI.06-01-00022.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. H., David S., Patel R., Abney E. R., Raff M. C. A quantitative immunohistochemical study of macroglial cell development in the rat optic nerve: in vivo evidence for two distinct astrocyte lineages. Dev Biol. 1985 Sep;111(1):35–41. doi: 10.1016/0012-1606(85)90432-4. [DOI] [PubMed] [Google Scholar]
- Noble M., Murray K. Purified astrocytes promote the in vitro division of a bipotential glial progenitor cell. EMBO J. 1984 Oct;3(10):2243–2247. doi: 10.1002/j.1460-2075.1984.tb02122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Abney E. R., Cohen J., Lindsay R., Noble M. Two types of astrocytes in cultures of developing rat white matter: differences in morphology, surface gangliosides, and growth characteristics. J Neurosci. 1983 Jun;3(6):1289–1300. doi: 10.1523/JNEUROSCI.03-06-01289.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Abney E. R., Fok-Seang J. Reconstitution of a developmental clock in vitro: a critical role for astrocytes in the timing of oligodendrocyte differentiation. Cell. 1985 Aug;42(1):61–69. doi: 10.1016/s0092-8674(85)80101-x. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Abney E. R., Miller R. H. Two glial cell lineages diverge prenatally in rat optic nerve. Dev Biol. 1984 Nov;106(1):53–60. doi: 10.1016/0012-1606(84)90060-5. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Miller R. H., Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983 Jun 2;303(5916):390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Mirsky R., Fields K. L., Lisak R. P., Dorfman S. H., Silberberg D. H., Gregson N. A., Leibowitz S., Kennedy M. C. Galactocerebroside is a specific cell-surface antigenic marker for oligodendrocytes in culture. Nature. 1978 Aug 24;274(5673):813–816. [PubMed] [Google Scholar]
- Reynolds R., Steffen C., Herschkowitz N. High-affinity uptake of gamma-[3H]aminobutyric acid by isolated mouse oligodendrocytes in culture. Neurochem Res. 1987 Oct;12(10):885–890. doi: 10.1007/BF00966310. [DOI] [PubMed] [Google Scholar]
- Saneto R. P., de Vellis J. Characterization of cultured rat oligodendrocytes proliferating in a serum-free, chemically defined medium. Proc Natl Acad Sci U S A. 1985 May;82(10):3509–3513. doi: 10.1073/pnas.82.10.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachner M. Cell type-specific surface antigens in the mammalian nervous system. J Neurochem. 1982 Jul;39(1):1–8. doi: 10.1111/j.1471-4159.1982.tb04694.x. [DOI] [PubMed] [Google Scholar]
- Wilkin G. P., Levi G., Johnstone S. R., Riddle P. N. Cerebellar astroglial cells in primary culture: expression of different morphological appearances and different ability to take up [3H]D-aspartate and [3H]GABA. Brain Res. 1983 Nov;312(2):265–277. doi: 10.1016/0165-3806(83)90143-8. [DOI] [PubMed] [Google Scholar]
- Williams B. P., Abney E. R., Raff M. C. Macroglial cell development in embryonic rat brain: studies using monoclonal antibodies, fluorescence activated cell sorting, and cell culture. Dev Biol. 1985 Nov;112(1):126–134. doi: 10.1016/0012-1606(85)90126-5. [DOI] [PubMed] [Google Scholar]






