Abstract
Context
Increased dietary intake of marine omega-3 fatty acids is associated with prolonged survival in patients with coronary heart disease. However, the mechanisms underlying this protective effect are poorly understood.
Objective
To investigate the association of omega-3 fatty acid blood levels with temporal changes in telomere length, an emerging marker of biological age.
Design, Setting, and Participants
Prospective cohort study of 608 ambulatory outpatients in California with stable coronary artery disease recruited from the Heart and Soul Study between September 2000 and December 2002 and followed up to January 2009 (median, 6.0 years; range, 5.0-8.1 years).
Main Outcome Measures
We measured leukocyte telomere length at baseline and again after 5 years of follow-up. Multivariable linear and logistic regression models were used to investigate the association of baseline levels of omega-3 fatty acids (docosahexaenoic acid [DHA] and eicosapentaenoic acid [EPA]) with subsequent change in telomere length.
Results
Individuals in the lowest quartile of DHA3EPA experienced the fastest rate of telomere shortening (0.13 telomere-to-single-copy gene ratio [T/S] units over 5 years; 95% confidence interval [CI], 0.09-0.17), whereas those in the highest quartile experienced the slowest rate of telomere shortening (0.05 T/S units over 5 years; 95% CI, 0.02-0.08; P<.001 for linear trend across quartiles). Levels of DHA+EPA were associated with less telomere shortening before (unadjusted β coefficient × 10−3=0.06; 95% CI, 0.02-0.10) and after (adjusted β coefficient × 10−3=0.05; 95% CI, 0.01-0.08) sequential adjustment for established risk factors and potential confounders. Each 1-SD increase in DHA+EPA levels was associated with a 32% reduction in the odds of telomere shortening (adjusted odds ratio, 0.68; 95% CI, 0.47-0.98).
Conclusion
Among this cohort of patients with coronary artery disease, there was an inverse relationship between baseline blood levels of marine omega-3 fatty acids and the rate of telomere shortening over 5 years.
Multiple epidemiologic studies, including several large randomized controlled trials, have demonstrated higher survival rates among individuals with high dietary intake of marine omega-3 fatty acids and established cardiovascular disease.1-4 On this basis, the Ameri-can Heart Association recommends increased oily fish intake and the use of omega-3 fatty acid supplements for the primary and secondary prevention of coronary heart disease.5 The mechanisms underlying this protective effect are poorly understood but are thought to include anti-inflammatory, antiplatelet, anti-hypertensive, antiarrhythmic, and triglyceride-lowering effects.6 There is ongoing interest in the identification of novel mechanisms of cardiovascular benefit from omega-3 fatty acids.
Telomeres are tandem repeat DNA sequences (TTAGGG)n that form a protective cap at the ends of eukaryotic chromosomes.7 During somatic cell division, DNA polymerase cannot fully replicate the 3′ end of linear DNA, resulting in an obligate and progressive loss of telomeric repeats. This process may eventually result in cellular senescence or apoptosis.8 These observations have led to telomere length emerging as a novel marker of biological age, which integrates the cumulative lifetime burden of genetic factors and environmental stressors independent of chronological age.9 Moreover, a robust association between short telomeres and cardiovascular morbidity and mortality has been documented in several populations.10-12
Little is known concerning the dynamic regulation of telomere length over time, although it has recently become apparent that telomeres may lengthen as well as shorten.13,14 Given the cardioprotective effects of omega-3 fatty acids, we sought to determine whether omega-3 fatty acid levels were associated with changes in leukocyte telomere length over 5 years in a prospective cohort study of outpatients with coronary artery disease.
METHODS
Participants
The Heart and Soul Study is a prospective cohort study investigating the influence of psychosocial factors on cardiovascular events in stable coronary artery disease. The enrollment process has been previously described.15 Eligible participants were recruited from outpatient clinics in the San Francisco Bay Area of California if they met at least 1 of the following inclusion criteria: (1) history of myocardial infarction; (2) angiographic evidence of at least 50% stenosis by area in at least 1 coronary artery; (3) evidence of exercise-induced ischemia on treadmill electrocardiogram or stress nuclear perfusion imaging; or (4) history of coronary revascularization. Individuals were excluded if they had a history of myocar-dial infarction in the past 6 months, deemed themselves unable to walk 1 block, or were planning to move out of the local area within 3 years.
The study protocol was approved by the University of California San Francisco Committee on Human Research, the Research and Development Committee at the San Francisco Veterans Administration (VA) Medical Center, the Medical Human Subjects Committee at Stanford University, the Human Subjects Committee at the VA Palo Alto Health Care System, and the Data Governance Board of the Community Health Network of San Francisco. All participants provided written informed consent.
Between September 2000 and December 2002, a total of 1024 participants enrolled in the study and completed a baseline examination including a venous blood draw. Five years later, all surviving participants were invited to return for a repeat examination. Of the 1024 original enrollees, 195 had died before the 5-year examination. Between September 2005 and December 2007, 667 (80%) of the eligible 829 participants completed the 5-year follow-up examination. Of 667 participants who completed the 5-year examination, 29 were excluded because they did not have baseline omega-3 measurements, 23 were excluded because they did not have baseline telomere length measurements, and 7 were excluded because they did not have 5-year telomere length measurements, leaving 608 participants for this analysis. Compared with the 221 participants who were alive at 5 years but not included in the analysis, these 608 participants had similar ages (P=.27), baseline omega-3 fatty acid levels (P=.14), and baseline telomere length (P=.24)
Marine Omega-3 Fatty Acid Assay
Levels of the marine omega-3 fatty acids docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) were measured in fasting whole blood. Fatty acid methyl esters generated by treatment with boron trifluoride-methanol were analyzed by capillary gas chromatography (GC2010 [Shimadzu Corp, Columbia, Maryland] equipped with a 100-m SP2560 column [Supelco; Belle-fonte, Pennsylvania]) and identified by comparison with a known standard (GLC-727; Nuchek Prep, Elysian, Minnesota). Blood levels of marine omega-3 fatty acids (EPA+DHA) are expressed as a percentage of total fatty acid methyl esters.16,17 The coefficient of variation for EPA+DHA was 5% to 6%.
Telomere Length Assay
Genomic DNA was isolated according to standard procedures from peripheral blood leukocytes collected at baseline and follow-up study visits and stored at −70°C. Purified DNA samples were diluted in 96-well microtiter source plates to a fixed concentration of 3 ng/μL. Relative mean telomere length was measured from DNA by a quantitative polymer-ase chain reaction (qPCR) assay that compares mean telomere repeat sequence copy number (T) to a reference single-copy gene copy number (S) in each sample as previously described and validated by comparison with Southern blot terminal restriction fragment analysis.18 Standard curves were derived from serially diluted reference DNA as previously described and validated.18-20 The T/S ratio was determined from the mean quantity of reference DNA found to match with each experimental sample for the copy number of the targeted template (the number of telomere repeats for T and the number of beta-globin gene copies for S).
The primers for the telomere qPCR were tel1b (5′-CGGTTT[GTTTGG] 5GTT-3′) and tel2b (5′-GGCTTG [CCTTAC]5CCT-3′), each used at a final concentration of 900 nM. Human beta-globin qPCR primers were hbg1 (5′-GCTTCTGACACAACTGT-GTTCACTAGC-3′), used at a final concentration of 300 nM, and hbg2 (5′-CACCAACTTCATCCACGTTCACC-3′), used at a final concentration of 700 nM. All PCRs were carried out on a Roche Lightcycler 480 real-time PCR machine (Roche Applied Science, India-napolis, Indiana).
The T/S ratio at baseline and follow-up for each participant was measured in duplicate. When the duplicate T/S value and the initial value varied by more than 7%, the sample was run for a third time, and the 2 closest values were used to calculate the mean. Approximately 15% of samples required assay in triplicate. Using this method, the interassay coefficient of variability for telomere length measurement was 3.7%. The intra-assay coefficient of variability was 2.5%.
Other Measurements
Baseline demographics, age, sex, cardiovascular comorbidities, income, education, and medical history were determined by questionnaire. Race/ethnicity has previously been shown to be associated with telomere length21 and was therefore ascertained for inclusion in multivariable adjustment models. Participants self-identified their race/ethnicity by questionnaire in the following categories: Hispanic, Asian, black, white, and other.
Exercise capacity was determined at peak exertion during a symptom-limited exercise treadmill test as previously described.22 Medication use was determined by having participants bring bottles to the study appointment, during which study personnel recorded all medications. Medication use was categorized dichotomously using Epocrates Rx (San Mateo, California). Information regarding omega-3 fatty acid supplements was not collected. Waist and hip circumferences were measured with a flexible plastic measure to the nearest 0.1 cm.
Fasting venous blood samples were obtained at the baseline visit to measure serum biomarkers. Fasting high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and C-reactive protein (CRP) were measured in a clinical laboratory setting. C-reactive protein was measured using the Roche Integra high-sensitivity assay (Roche, Indianapo-lis) or (because of a change in the laboratory) the Beckman Extended Range high-sensitivity CRP assay (Beck-man, Galway, Ireland). The R&D Systems (Minneapolis) Quantikine HS IL-6 Immunoassay was used to determine the concentration of interleukin 6 (IL-6). Blood samples were also obtained at the follow-up visit and stored as buffy coat, serum, and plasma.
All patients underwent complete resting 2-dimensional echocardiography and Doppler examination using an Acuson Sequoia ultrasound system (Siemens Medical Solutions, Mountain View, California) with a 3.5-MHz transducer. Left ventricular ejection fraction was calculated as (end diastolic volume – end systolic volume)/end diastolic volume.
Statistical Analyses
In the absence of established cutoffs, we categorized participants a priori by quartiles of omega-3 fatty acid levels for descriptive purposes. Continuous variables with a skewed distribution were natural logarithm transformed (DHA+EPA, HDL-C, LDL-C, triglycerides, CRP, and IL-6). Differences in means and proportions of baseline characteristics were compared using 1-way analysis of variance and the χ2 test, respectively. Based on a total of 608 participants (n=152 in each quartile), we had more than 80% power to detect an interquartile difference in the rate of telomere shortening of 0.07 T/S units over 5 years, assuming an SD of 0.2. The significance level was a 2-tailed α of .05. Analysis of covariance was used to calculate the mean change in telomere length in each quartile, adjusted for age and baseline telomere length.
We used generalized linear regression models to determine the relationship between the natural logarithm of omega-3 fatty acid levels and change in telomere length (T/S ratio) as a continuous variable. Mixed models were not required because the difference between baseline and follow-up telo-mere length for each participant collapsed into a single observation rather than repeated measures. Multivariable adjustment was made for potential demographic, clinical, and biochemical confounders based on published associations and biological plausibility.23-25 Adjustment was also made for known downstream mediators of omega-3 fatty acid effects, including blood pressure, triglyceride levels, and biomarkers of systemic inflammation. Grouped covariates were added sequentially in multivariable models to assess the incremental attenuation attributable to each adjustment step. Within each adjustment step, all covariates were added simultaneously. The final adjustment model also included baseline telomere length, which has previously been identified as the most powerful predictor of subsequent change in telomere length.13,26
Covariate selection was checked by visual inspection of directed acyclic graphs.27 Inclusion criteria for adjustment covariates were confounding in the directed acyclic graph, biological plausibility of the confounding relationship, and adequate ascertainment of the variable. Exclusion criteria for adjustment covariates were colliders in the directed acyclic graph, lack of biological plausibility, and inadequate ascertainment of the variable. No purely statistical criteria were used to select covariates.
The complete list of covariates included in the final adjusted model was age, sex, race/ethnicity, income level, education level, exercise capacity, prior myocardial infarction, history of type 2 diabetes mellitus, prior smoking, left ventricular ejection fraction, waist-hip ratio, systolic blood pressure, diastolic blood pressure, log triglycerides, log LDL-C, log HDL-C, log CRP, log IL-6, multivitamin use, statin use, β-blocker use, and baseline telomere length. No significant colinearity was present in the final adjustment models, with all pairwise correlation coefficients less than 0.7. The entry order of adjustment did not significantly alter the effect estimates. The assumption of linearity was checked by visual inspection of component plus residual plots. The normality assumption was checked by review of residual histograms and normal quantile-quantile plots.
We then used multivariable logistic regression models to explore the association between omega-3 fatty acid levels and telomere shortening (as a dichotomous variable, defined as >10% reduction in telomere length over 5 years). There is no established cutoff for defining telomere shortening. We chose a 10% cutoff a priori because it has reasonable face validity. We did not choose a lower cutoff because, given the inter-assay (3.7%) and intra-assay (2.5%) coefficients of variation, we could not be sure that this would indicate true shortening. The strategy for covariate selection and adjustment was identical to that described for the linear regression models. Model adequacy was confirmed using the Hosmer-Lemeshow goodness-of-fit test. The presence of outlying and influential points was explored by visual inspection of standardized Pearson residuals.
To explore potential effect modifiers, we tested for interaction between omega-3 fatty acid levels and age, baseline telomere length, sex, race/ethnicity, smoking, income, education, and type 2 diabetes in the final multivariable-adjusted model. Stratified analysis was also performed for subgroups of age, baseline telomere length, sex, race/ethnicity, smoking, income, education, and type 2 diabetes melli-tus. All subgroup analyses were pre-specified. Statistical analysis was performed using Intercooled Stata, version 10.0 (Stata Corp, College Station, Texas).
RESULTS
All measurements except for follow-up telomere length were made at the baseline visit. The distribution of baseline omega-3 fatty acid levels was right-skewed (mean, 4.3%; median, 3.7%). In quartile analysis, higher levels of baseline omega-3 fatty acids were significantly associated with older age, white race/ethnicity, higher income, higher education level, and higher HDL-C and were inversely associated with history of myocardial infarction, type 2 diabetes mellitus, current smoking, waist-hip ratio, triglyceride levels, CRP, and IL-6 (Table 1). There was no significant association between omega-3 fatty acid levels and baseline telomere length (unadjusted P=.06; adjusted for age, P=.20).
Table 1.
Baseline Characteristics of Study Population by Quartile of Marine Omega-3 Fatty Acid Levels
| Characteristics | Quartile of Marine Omega-3 Fatty Acid Level |
P Value (1-Way ANOVA or χ2) | |||
|---|---|---|---|---|---|
| 1 (n = 152) | 2 (n = 152) | 3 (n = 152) | 4 (n = 152) | ||
| Marine omega-3 fatty acid level, mean (range), % | 2.3 (1.1-2.8) | 3.3 (2.9-3.7) | 4.3 (3.8-5.1) | 7.3 (5.2-18.4) | |
| Age, mean (SD), y | 64 (11) | 66 (9) | 67 (10) | 67 (10) | .005 |
| Male, No. (%) | 128 (84) | 123 (81) | 120 (79) | 128 (84) | .55 |
| White, No. (%) | 84 (55) | 84 (55) | 96 (63) | 98 (64) | <.001 |
| Income ≥$50 000, No. (%) | 19 (13) | 25 (16) | 34 (23) | 52 (35) | .001 |
| Education beyond high school, No. (%) | 106 (69) | 96 (63) | 112 (74) | 130 (86) | <.001 |
| Exercise capacity, mean (SD), METs | 7.5 (2.9) | 7.3 (3.0) | 8.0 (3.1) | 9.1 (3.6) | .06 |
| Prior myocardial infarction, No. (%) | 94 (62) | 82 (55) | 69 (45) | 72 (47) | .02 |
| Prior congestive heart failure, No. (%) | 21 (13) | 23 (15) | 21 (14) | 25 (17) | .90 |
| Prior stroke, No. (%) | 22 (14) | 21 (14) | 13 (9) | 19 (13) | .40 |
| Type 2 diabetes, No. (%) | 47 (31) | 45 (30) | 27 (18) | 27 (18) | .004 |
| Smoking, No. (%) | |||||
| Current | 41 (27) | 31 (20) | 20 (13) | 9 (6) | <.001 |
| Past | 68 (45) | 78 (51) | 87 (57) | 74 (49) | .17 |
| Waist-hip ratio, mean (SD) | 0.96 (0.08) | 0.96 (0.08) | 0.95 (0.08) | 0.94 (0.07) | .04 |
| Blood pressure, mean(SD), mm Hg | |||||
| Systolic | 134 (20) | 134 (21) | 134 (21) | 129 (19) | .13 |
| Diastolic | 76 (11) | 75 (12) | 74 (11) | 74 (11) | .33 |
| Left ventricular ejection fraction, mean (SD), % | 62 (9) | 62 (8) | 63 (8) | 63 (10) | .22 |
| Statin use, No. (%) | 95 (63) | 113 (74) | 102 (67) | 113 (74) | .06 |
| β-Blocker use, No. (%) | 100 (66) | 89 (59) | 85 (56) | 77 (51) | .06 |
| ACE inhibitor/ARB use, No. (%) | 74 (49) | 83 (55) | 73 (48) | 79 (52) | .60 |
| Aspirin use, No. (%) | 118 (78) | 124 (82) | 119 (78) | 114 (75) | .58 |
| Vitamin use, No. (%) | 25 (16) | 33 (22) | 38 (25) | 32 (21) | .33 |
| HDL-C, mean (SD), mg/dL | 45 (14) | 44 (13) | 47 (15) | 48 (14) | .03a |
| LDL-C, mean (SD), mg/dL | 111 (39) | 102 (29) | 103 (33) | 100 (32) | .04a |
| Triglycerides, mean (SD), mg/dL | 208 (239) | 137 (88) | 121 (79) | 99 (50) | <.001a |
| C-reactive protein, mean (SD), mg/L | 4.7 (6.5) | 4.3 (5.6) | 3.6 (6.5) | 3.0 (7.9) | <.001a |
| Interleukin 6, mean (SD), mg/dL | 3.6 (2.7) | 3.0 (2.2) | 2.8 (2.1) | 2.5 (2.1) | <.001a |
| Baseline telomere length, mean (SD), T/S units | 0.96 (0.25) | 0.93 (0.22) | 0.89 (0.19) | 0.91 (0.20) | .06 |
| Year 5 telomere length, mean (SD), T/S units | 0.83 (0.15) | 0.83 (0.13) | 0.82 (0.15) | 0.86 (0.15) | .16 |
Abbreviations: ACE, angiotensin-converting enzyme; ANOVA, analysis of variance; ARB, angiotensin II receptor blocker; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MET, metabolic equivalent task; T/S, telomere-to-single-copy gene ratio.
SI conversions: To convert HDL-C and LDL-C to mmol/L, multiply by 0.0259; to convert triglycerides to mmol/L, multiply by 0.0113.
Significance test by 1-way ANOVA after natural log transformation.
Baseline omega-3 fatty acid levels were positively correlated with 5-year change in telomere length (r =0.13; P=.001). The relationships between quartile of baseline omega-3 fatty acid levels and mean absolute and relative changes in telomere length, adjusted for age and baseline telomere length, are shown in the Figure. Individuals in the lowest quartile experienced a decrease in telomere length of 0.13 T/S units (95% confidence interval [CI], 0.09-0.17), whereas those in the highest quartile experienced a decrease of 0.05 T/S units (95% CI, 0.02-0.08; P<.001 for trend using linear contrasts).
Figure. Absolute and Relative Mean Changes in Telomere Length Over 5 Years by Quartile of Omega-3 Fatty Acid Level, Adjusted for Age and Baseline Telomere Length.
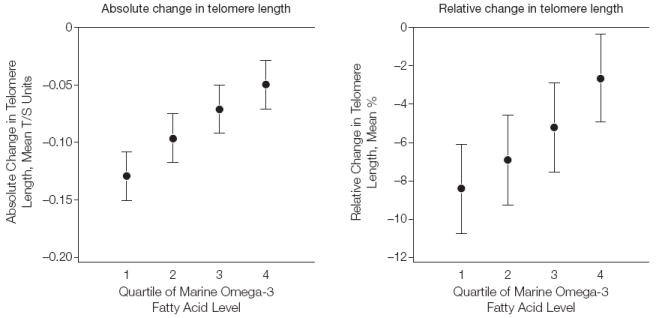
Error bars indicate 95% confidence intervals. T/S indicates telomere-to-single-copy gene ratio. P<.001 for linear trend for both absolute and relative change. See Table 1 for definitions of quartiles.
In the unadjusted linear regression model, higher baseline log omega-3 fatty acid levels were associated with increase in absolute telomere length (unadjusted β coefficient=0.06; 95% CI 0.02-0.10). After sequential adjustment for demographics, comorbidi-ties, blood pressure, serum lipids, inflammatory biomarkers, medications, and baseline telomere length (Table 2), the association of baseline log omega-3 fatty acid levels with lengthening of telomeres over time persisted (adjusted β coefficient=0.05; 95% CI, 0.01-0.08). The greatest attenuation in the β coefficient occurred after adjustment for baseline telomere length.
Table 2.
Association of Log Omega-3 Fatty Acid Levels (as a Continuous Variable) With Absolute Increase in Leukocyte Telomere Length Over 5 Years
| Modela | β Coefficient ×10−3 (95% Confidence Interval) | P Value | Model R2 |
|---|---|---|---|
| Unadjusted | 0.06 (0.02-0.10) | .001 | 0.02 |
| Model 1 | 0.08 (0.04-0.13) | <.001 | 0.08 |
| Model 2 | 0.09 (0.04-0.14) | <.001 | 0.13 |
| Model 3 | 0.09 (0.04-0.14) | <.001 | 0.13 |
| Model 4 | 0.09 (0.04-0.14) | <.001 | 0.14 |
| Model 5 | 0.05 (0.01-0.08) | .008 | 0.68 |
All covariates were modeled continuously except the following categorical variables: sex (male, female); ethnicity (Hispanic, Asian, black, white, other); income (<$10 000, $10 000-$19 999, $20 000-$29 999, $30 000-$39 999, $40 000-$50 000, ≥$50 000); education (no formal schooling, 5th grade or less, 6th-8th grade, 9th-11th grade, high school graduate, some college/vocational school, college degree, graduate/professional degree); prior myo-cardial infarction; type 2 diabetes; current smoking status; vitamin use; statin use; β-blocker use. In the final adjusted model (model 5), the linear regression equation is change in telomere length=0.101+0.05(log omega-3 level)−0.003(age)−0.06(male)−0.03(Asian)−0.02(black)−0.03(white)−0.03(other)−0.006(income $10 000-$19 999) −0.008(income $20 000-$29 999)−0.001(income $30 000-$39 999)−0.005(income $40 000-$49 999)−0.003 (income ≥$50 000)+0.04(≤5th grade)+0.12(6th-8th grade)+0.09(9th-11th grade)+0.08(high school graduate) +0.12(some college)+0.09(college degree)+0.11(graduate degree)+0.001(exercise capacity)−0.005(prior myocardial infarction)−0.02(type 2 diabetes)−0.007(smoking)−0.001(left ventricular ejection fraction)−0.12(waist-hip ratio)−0.0003(systolic blood pressure)+0.001(diastolic blood pressure)+0.005(log triglycerides)−0.03(log low-density lipoprotein cholesterol)−0.005(log high-density lipoprotein cholesterol)+0.003(log C-reactive protein) −0.007(log interleukin 6)−0.02(multivitamin use)+0.0009(statin use)+0.02(β-blocker use)−0.77(baseline telomere length)+0.009(baseline telomere length×log omega-3 level). Model 1 was adjusted for age, sex, race/ethnicity, income, education, and exercise capacity. Model 2=model 1 + prior myocardial infarction, type 2 diabetes, smoking, left ventricular ejection fraction, waist-hip ratio, systolic and diastolic blood pressure, log triglycerides, log low-density lipoprotein cholesterol, and log high-density lipoprotein cholesterol. Model 3=model 2 + log C-reactive protein and log interleukin 6. Model 4=model 3 + use of vitamins, statins, and β-blockers. Model 5=model 4 + baseline telomere length and baseline telomere length × log omega-3 level.
During the median 6-year (range, 5.0-8.1 years) follow-up period, 276 participants (45%) exhibited greater than 10% reduction in telomere length. Each 1-SD increase in baseline log omega-3 fatty acid levels was associated with a 19% decrease in the odds of telomere shortening (unadjusted odds ratio, 0.81; 95% CI, 0.69-0.95). After sequential adjustment for demographics, comorbidities, blood pressure, serum lipids, inflammatory biomarkers, medications, and baseline telomere length (Table 3), each 1-SD increase in baseline log omega-3 fatty acid levels was associated with a 32% decrease in the odds of telomere shortening (adjusted odds ratio, 0.68; 95% CI, 0.47-0.98).
Table 3.
Association of Log Omega-3 Fatty Acid Levels (as a Continuous Variable per 1-SD Increase) With Leukocyte Telomere Shortening, Defined as >10% Decrease in Telomere Length Over 5 Years
| Modela | Odds Ratio per 1-SD Increase (95% Confidence Interval) | P Value |
|---|---|---|
| Unadjusted | 0.81 (0.69-0.95) | .01 |
| Model 1 | 0.72 (0.60-0.88) | .001 |
| Model 2 | 0.68 (0.54-0.85) | .001 |
| Model 3 | 0.68 (0.54-0.86) | .001 |
| Model 4 | 0.68 (0.54-0.86) | .001 |
| Model 5 | 0.68 (0.47-0.98) | .04 |
All covariates were modeled continuously except the following categorical variables: sex (male, female); ethnicity (Hispanic, Asian, black, white, other); income (<$10 000, $10 000-19 999, $20 000-29 999, $30 000-39 999, $40 000-50 000, ≥$50 000); education (no formal schooling, 5th grade or less, 6th-8th grade, 9th-11th grade, high school graduate, some college/vocational school, college degree, graduate/professional degree); prior myo-cardial infarction; type 2 diabetes; current smoking status; vitamin use; statin use; β-blocker use. In the final adjusted model (model 5), the linear regression equation is log odds of telomere shortening=−1.06−0.87(log omega-3 level)+0.05(age)+0.87(male)+0.68(Asian)+0.91 (black)+0.57(white)+0.58(other)−0.3(income $10 000-$19 999)−0.008(income $20 000-$29 999)+0.56 (income $30 000-$39 999)−0.44(income $40 000-$49 999)−0.14(income ≥$50 000)−16(≤5th grade)−18 (6th-8th grade)−18(9th-11th grade)−18(high school graduate)−18(some college)−18(college degree)−18 (graduate degree)+0.07(exercise capacity)+0.39(prior myocardial infarction)+0.24(type 2 diabetes)+0.09 (smoking)+0.01(left ventricular ejection fraction)+4.0 (waist-hip ratio)+0.01(systolic blood pressure)−0.03 (diastolic blood pressure)−0.05(log triglycerides)+0.54 (log low-density lipoprotein cholesterol)−0.11(log high-density lipoprotein cholesterol)−0.005(log C-reactive protein)+0.09(log interleukin 6)+0.64(multivitamin use) −0.21(statin use)−0.35(β-blocker use)+10(baseline telomere length)+0.0002(baseline telomere length×log omega-3 level). Model 1 was adjusted for age, sex, race/ethnicity, income, education, and exercise capacity. Model 2=model 1 + prior myocardial infarction, type 2 diabetes, smoking, left ventricular ejection fraction, waist-hip ratio, systolic and diastolic blood pressure, log triglycerides, log low-density lipoprotein cholesterol, and log high-density lipoprotein cholesterol. Model 3=model 2 + log C-reactive protein and log interleukin 6. Model 4=model 3 + use of vitamins, statins, and β-blockers. Model 5=model 4 + baseline telomere length and baseline telomere length × log omega-3 level.
In multivariable models, we found no evidence that the effect of baseline omega-3 fatty acid levels on telomeric aging was modified by age, sex, race/ethnicity, smoking, income, education, or type 2 diabetes (all P>.10). However, the effect of baseline omega-3 fatty acids on telomeric aging was stronger in participants with longer baseline telomere length (P=.04 for interaction).
COMMENT
Leukocyte telomere length is an emerging marker of biological age that independently predicts morbidity and mortality in patients with cardiovascular diseases.28 In this longitudinal study, we observed that baseline levels of marine omega-3 fatty acids were associated with decelerated telomere attrition over 5 years. The association was linear and persisted after adjustment for potential confounders. These findings raise the possibility that omega-3 fatty acids may protect against cellular aging in patients with coronary heart disease.
Several studies have reported cross-sectional associations between longer telomeres and nutritional supplements, including multivitamins, vitamin C, vitamin D, vitamin E, and folic acid.29-33 The lack of longitudinal data on telomere trajectory limits the degree to which causal inferences can be made from these studies.34 Residual confounding due to measured and unmeasured factors may also have contributed to the observed associations between health supplements and telomere length.35 Statins mitigate the excess cardiovascular risk conferred by short telomeres and inhibit telomere shortening in vitro.36,37 However, in large epidemiological studies, there was no association between statin use and telomere length.38
Marine omega-3 fatty acid supplementation has been associated with improved survival after myocardial infarction in observational studies and randomizeinicaltrials.1 Althoughthe mechanisms involved remain incompletely understood, there is increasing evidence that omega-3 fatty acids exert direct effects on aging and age-related diseases.39 Omega-3 fatty acid supplementation and blood levels are both associated with attenuation of age-associated increases in pulse-wave velocity, a key marker of vascular stiffness.40,41 They are also associated with slowing of age-related cognitive decline and reduced incidence of age-related macular degeneration.42,43 In rodents, dietary enrichment with omega-3 fatty acids prolongs life span by approximately one-third.44 The present findings identify deceleration of telomere attrition as a potentially novel pathway for the antiaging effects of marine omega-3 fatty acids.
We observed that sequential adjustment for known downstream mediators of omega-3 fatty acids, including beneficial effects on blood pressure, lipids, and markers of systemic inflammation, did not attenuate the inverse association between omega-3 levels and telomere attrition rate, suggesting that 1 or more alternative mechanisms may be involved or that single measurements of these mediators may not sufficiently capture their long-term effects.
One possible explanation for the association of omega-3 fatty acids with decelerated telomere attrition may lie in the paradigm of oxidative stress, a powerful driver of telomere shortening and organismal aging.45 Reactive oxygen species specifically target the GGG triplets in telomeric DNA and result in increased telomere attrition during mitosis.46 Marine omega-3 fatty acids are incorporated into membrane phospholipids, as is arachidonic acid.47 Although EPA and DHA are more readily oxidized ex vivo than arachidonic acid, supplementation with omega-3 fatty acids has been associated with lower levels of F2-isoprostanes, an established standard for measurement of systemic oxidative stress, and with higher levels of the antioxidant enzymes catalase and superoxide dismutase.44,48 In vitro studies have identified a direct stimulatory effect of omega-3–derived J3-isoprostanes on Nrf2, a master transcriptional regulator of the cellular antioxidant response.49
A second potential mechanism for the association of slowing of omega-3 fatty acid levels with decelerated telomere attrition is increased activity of the enzyme telomerase.50 Until recently, expression of telomerase was thought to be limited to germ cells, stem cells, and cancer cells.51 However, low-level telomerase activity has now been demonstrated in peripheral blood mono-nuclear cells.52 The adoption of comprehensive lifestyle changes, which included daily supplementation with 3 g of omega-3 fish oil, was associated with a significant increase in telomerase activity in normal adult human leukocytes.53 In contrast, in cultured colorectal adenocarcinoma cells, EPA and DHA suppressed telomerase activity and reduced telomerase levels.54 There are no studies to date exploring the biological effect of omega-3 fatty acids on telomerase in noncancerous tissues. Taken together, these observations lead us to speculate that omega-3 fatty acids might exert bidirectional effects on telomerase depending on cellular context: in healthy tissues, they may enhance telomerase activity while suppressing it in cancerous cells. Such properties would be highly desirable in the development of treatments targeting telomeric aging.
Although we found an inverse association between baseline omega-3 fatty acid levels and subsequent rate of telomere shortening over time, we found no significant association between omega-3 fatty acid levels and telomere length at baseline. As to why omega-3 fatty acid levels were not associated with longer baseline telomere length, it should be emphasized that the level of omega-3 fatty acids is only one of many influences on telomere length in this heterogeneous sample. Numerous other factors, including systemic inflammation, obesity, oxidative stress, and lack of physical activity, are believed to cumulatively determine telomere length throughout adult life. Our paired-sample longitudinal study was specifically designed to isolate the effect of omega-3 fatty acid levels on subsequent within-person changes in telomere length. Further studies are needed to understand the interindividual variability in the complex integration of bidirectional influences on telomere length.
Among the strengths of the present study is the detailed characterization of demographic, clinical, and biochemical covariates. In addition, the longitudinal study design improves on prior cross-sectional studies. However, several limitations should be considered in the interpretation of our results. First, the association reported in this study is observational, and therefore no definitive conclusions can be made regarding causality. Although we adjusted for multiple carefully measured potential confounding variables, the possibility of residual confounding by measured or unmeasured covariates cannot be excluded. For example, the absence of detailed dietary information may have contributed to residual confounding by other nutritional factors associated with telomere length, such as red meat intake.55 To definitively address the question of whether omega-3 fatty acids inhibit cellular aging, a double-blind, randomized, placebo-controlled trial would be necessary. Second, our measurements were restricted to telomere length in leukocytes and do not necessarily reflect telomere trajectory in other cell compartments such as myocardium, endothelium, or the atherosclerotic plaque. Third, due to long-term storage of blood samples, we could not measure biomarkers of oxidative stress or telomerase activity, which might elucidate the mechanisms of deceleration of telomeric aging by omega-3 fatty acids. Fourth, our study sample comprised mainly male outpatients with coronary artery disease, a population that might be particularly responsive to the effects of omega-3 fatty acids. The findings may not be generalizable to other patient populations, and further studies are required to validate our findings in other demographic groups.
In summary, among patients with stable coronary artery disease, there was an inverse relationship between baseline blood levels of marine omega-3 fatty acids and the rate of telomere shortening over 5 years.
Acknowledgments
Funding/Support: Dr Farzaneh-Far is supported by an American Heart Association Fellow-to-Faculty Transition Award (grant 0875014N). The Heart and Soul Study was supported by the Department of Veterans Affairs (Epidemiology Merit Review Program), the National Heart, Lung, and Blood Institute (grant R01 HL079235), the Robert Wood Johnson Foundation (Generalist Physician Faculty Scholars Program), the American Federation for Aging Research (Paul Beeson Faculty Scholars in Aging Research Program), the Ischemia Research and Education Foundation, and the Nancy Kirwan Heart Research Fund. Dr Lin is supported by the Bernard and Barbro Foundation.
Role of the Sponsor: The funding organizations had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Dr Farzaneh-Far had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Farzaneh-Far, Epel, Whooley.
Acquisition of data: Farzaneh-Far, Lin, Epel, Harris, Whooley.
Analysis and interpretation of data: Farzaneh-Far, Lin, Epel, Harris, Blackburn, Whooley.
Drafting of the manuscript: Farzaneh-Far.
Critical revision of the manuscript for important intellectual content: Lin, Epel, Harris, Blackburn, Whooley.
Statistical analysis: Farzaneh-Far, Lin.
Obtained funding: Farzaneh-Far, Whooley.
Administrative, technical, or material support: Lin, Epel, Harris, Blackburn, Whooley.
Study supervision: Farzaneh-Far, Whooley.
Financial Disclosures: Dr Harris reports that he is an advisor to, a speaker for, and has received research grants from companies with interests in omega-3 fatty acids, including GlaxoSmithKline and Monsanto. In addition, he has recently founded a company (OmegaQuant Analytics) to offer blood omega-3 fatty acid testing. No other disclosures were reported.
References
- 1.Lee JH, O’Keefe JH, Lavie CJ, Marchioli R, Harris WS. Omega-3 fatty acids for cardioprotection. Mayo Clin Proc. 2008;83(3):324–332. doi: 10.4065/83.3.324. [DOI] [PubMed] [Google Scholar]
- 2.Marchioli R, Barzi F, Bomba E, et al. GISSI-Prevenzione Investigators. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)–Prevenzione. Circulation. 2002;105(16):1897–1903. doi: 10.1161/01.cir.0000014682.14181.f2. [DOI] [PubMed] [Google Scholar]
- 3.Burr ML, Fehily AM, Gilbert JF, et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART) Lancet. 1989;2(8666):757–761. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- 4.Albert CM, Campos H, Stampfer MJ, et al. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med. 2002;346(15):1113–1118. doi: 10.1056/NEJMoa012918. [DOI] [PubMed] [Google Scholar]
- 5.Kris-Etherton PM, Harris WS, Appel LJ. American Heart Association Nutrition Committee. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106(21):2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 6.Harris WS, Miller M, Tighe AP, Davidson MH, Schaefer EJ. Omega-3 fatty acids and coronary heart disease risk: clinical and mechanistic perspectives. Atherosclerosis. 2008;197(1):12–24. doi: 10.1016/j.atherosclerosis.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106(6):661–673. doi: 10.1016/s0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- 8.Blackburn EH. Structure and function of telomeres. Nature. 1991;350(6319):569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- 9.Olovnikov AM. Telomeres, telomerase, and aging: origin of the theory. Exp Gerontol. 1996;31(4):443–448. doi: 10.1016/0531-5565(96)00005-8. [DOI] [PubMed] [Google Scholar]
- 10.Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361(9355):393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 11.Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ. White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol. 2003;23(5):842–846. doi: 10.1161/01.ATV.0000067426.96344.32. [DOI] [PubMed] [Google Scholar]
- 12.Farzaneh-Far R, Cawthon RM, Na B, Browner WS, Schiller NB, Whooley MA. Prognostic value of leuko-cyte telomere length in patients with stable coronary artery disease: data from the Heart and Soul Study. Arterioscler Thromb Vasc Biol. 2008;28(7):1379–1384. doi: 10.1161/ATVBAHA.108.167049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aviv A, Chen W, Gardner JP, et al. Leukocyte telo-mere dynamics: longitudinal findings among young adults in the Bogalusa Heart Study. Am J Epidemiol. 2009;169(3):323–329. doi: 10.1093/aje/kwn338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epel ES, Merkin SS, Cawthon R, et al. The rate of leukocyte telomere shortening predicts mortality from cardiovascular disease in elderly men. Aging. 2008;1(1):81–88. doi: 10.18632/aging.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whooley MA, de Jonge P, Vittinghoff E, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300(20):2379–2388. doi: 10.1001/jama.2008.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris WS, Von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med. 2004;39(1):212–220. doi: 10.1016/j.ypmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 17.Harris WS. The omega-3 index as a risk factor for coronary heart disease. Am J Clin Nutr. 2008;87(6):1997S–2002S. doi: 10.1093/ajcn/87.6.1997S. [DOI] [PubMed] [Google Scholar]
- 18.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gil ME, Coetzer TL. Real-time quantitative PCR of telomere length. Mol Biotechnol. 2004;27(2):169–172. doi: 10.1385/MB:27:2:169. [DOI] [PubMed] [Google Scholar]
- 20.Lin J, Epel E, Cheon J, et al. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. J Immunol Methods. doi: 10.1016/j.jim.2009.09.012. [published online ahead of print October 21 2009] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunt SC, Chen W, Gardner JP, et al. Leukocyte telomeres are longer in African Americans than in whites: the National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study. Aging Cell. 2008;7(4):451–458. doi: 10.1111/j.1474-9726.2008.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA. 2003;290(2):215–221. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Njajou OT, Hsueh WC, Blackburn EH, et al. Association between telomere length, specific causes of death, and years of healthy life in health, aging, and body composition: a population-based cohort study. J Gerontol A Biol Sci Med Sci. 2009;64(8):860–864. doi: 10.1093/gerona/glp061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valdes AM, Andrew T, Gardner JP, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366(9486):662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 25.Bekaert S, De Meyer T, Rietzschel ER, et al. Askle-pios Investigators. Telomere length and cardiovascular risk factors in a middle-aged population free of overt cardiovascular disease. Aging Cell. 2007;6(5):639–647. doi: 10.1111/j.1474-9726.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 26.Nordfjäll K, Svenson U, Norrback KF, Adolfsson R, Lenner P, Roos G. The individual blood cell telo-mere attrition rate is telomere length dependent. PLoS Genet. 2009;5(2):e1000375. doi: 10.1371/journal.pgen.1000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol. 2008;8:70. doi: 10.1186/1471-2288-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuster JJ, Andres V. Telomere biology and cardiovascular disease. Circ Res. 2006;99(11):1167–1180. doi: 10.1161/01.RES.0000251281.00845.18. [DOI] [PubMed] [Google Scholar]
- 29.Richards JB, Valdes AM, Gardner JP, et al. Higher serum vitamin D concentrations are associated with longer leukocyte telomere length in women. Am J Clin Nutr. 2007;86(5):1420–1425. doi: 10.1093/ajcn/86.5.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu Q, Parks CG, DeRoo LA, Cawthon RM, Sandler DP, Chen H. Multivitamin use and telomere length in women. Am J Clin Nutr. 2009;89(6):1857–1863. doi: 10.3945/ajcn.2008.26986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furumoto K, Inoue E, Nagao N, Hiyama E, Miwa N. Age-dependent telomere shortening is slowed down by enrichment of intracellular vitamin C via suppression of oxidative stress. Life Sci. 1998;63(11):935–948. doi: 10.1016/s0024-3205(98)00351-8. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka Y, Moritoh Y, Miwa N. Age-dependent telomere-shortening is repressed by phosphorylated alpha-tocopherol together with cellular longevity and intracellular oxidative-stress reduction in human brain microvascular endotheliocytes. J Cell Biochem. 2007;102(3):689–703. doi: 10.1002/jcb.21322. [DOI] [PubMed] [Google Scholar]
- 33.Paul L, Cattaneo M, D’Angelo A, et al. Telomere length in peripheral blood mononuclear cells is associated with folate status in men. J Nutr. 2009;139(7):1273–1278. doi: 10.3945/jn.109.104984. [DOI] [PubMed] [Google Scholar]
- 34.Aviv A. The epidemiology of human telomeres: faults and promises. J Gerontol A Biol Sci Med Sci. 2008;63(9):979–983. doi: 10.1093/gerona/63.9.979. [DOI] [PubMed] [Google Scholar]
- 35.Aviv A. Leukocyte telomere length: the telo-mere tale continues. Am J Clin Nutr. 2009;89(6):1721–1722. doi: 10.3945/ajcn.2009.27807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brouilette SW, Moore JS, McMahon AD, et al. West of Scotland Coronary Prevention Study Group. Telo-mere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007;369(9556):107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 37.Satoh M, Minami Y, Takahashi Y, Tabuchi T, Itoh T, Nakamura M. Effect of intensive lipid-lowering therapy on telomere erosion in endothelial progenitor cells obtained from patients with coronary artery disease. Clin Sci (Lond) 2009;116(11):827–835. doi: 10.1042/CS20080404. [DOI] [PubMed] [Google Scholar]
- 38.Spyridopoulos I, Haendeler J, Urbich C, et al. Stat-ins enhance migratory capacity by upregulation of the telomere repeat-binding factor TRF2 in endothelial progenitor cells. Circulation. 2004;110(19):3136–3142. doi: 10.1161/01.CIR.0000142866.50300.EB. [DOI] [PubMed] [Google Scholar]
- 39.Visioli F, Hagen TM. Nutritional strategies for healthy cardiovascular aging: focus on micronutrients. Pharmacol Res. 2007;55(3):199–206. doi: 10.1016/j.phrs.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 40.Tomiyama H, Takazawa K, Osa S, et al. Do eicosa-pentaenoic acid supplements attenuate age-related increases in arterial stiffness in patients with dyslipid-emia? a preliminary study. Hypertens Res. 2005;28(8):651–655. doi: 10.1291/hypres.28.651. [DOI] [PubMed] [Google Scholar]
- 41.Hamazaki K, Terashima Y, Itomura M, et al. The relationship between n-3 long-chain polyunsatu-rated fatty acids and pulse wave velocity in diabetic and non-diabetic patients under long-term hemodi-alysis: a horizontal study. Clin Nephrol. 2009;71(5):508–513. doi: 10.5414/cnp71508. [DOI] [PubMed] [Google Scholar]
- 42.Albanese E, Dangour AD, Uauy R, et al. Dietary fish and meat intake and dementia in Latin America, China, and India: a 10/66 Dementia Research Group population-based study. Am J Clin Nutr. 2009;90(2):392–400. doi: 10.3945/ajcn.2009.27580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tan JS, Wang JJ, Flood V, Mitchell P. Dietary fatty acids and the 10-year incidence of age-related macular degeneration: the Blue Mountains Eye Study. Arch Ophthalmol. 2009;127(5):656–665. doi: 10.1001/archophthalmol.2009.76. [DOI] [PubMed] [Google Scholar]
- 44.Jolly CA, Muthukumar A, Avula CP, Troyer D, Fernandes G. Life span is prolonged in food-restricted autoimmune-prone (NZB × NZW)F(1) mice fed a diet enriched with (n-3) fatty acids. J Nutr. 2001;131(10):2753–2760. doi: 10.1093/jn/131.10.2753. [DOI] [PubMed] [Google Scholar]
- 45.Milne GL, Yin H, Morrow JD. Human biochemistry of the isoprostane pathway. J Biol Chem. 2008;283(23):15533–15537. doi: 10.1074/jbc.R700047200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Epel ES. Psychological and metabolic stress: a recipe for accelerated cellular aging? Hormones (Athens) 2009;8(1):7–22. doi: 10.14310/horm.2002.1217. [DOI] [PubMed] [Google Scholar]
- 47.Pepe S. Dietary polyunsaturated fatty acids and age-related membrane changes in the heart. Ann N Y Acad Sci. 2007;1114:381–388. doi: 10.1196/annals.1396.046. [DOI] [PubMed] [Google Scholar]
- 48.Romieu I, Garcia-Esteban R, Sunyer J, et al. The effect of supplementation with omega-3 polyunsat-urated fatty acids on markers of oxidative stress in elderly exposed to PM(2.5) Environ Health Perspect. 2008;116(9):1237–1242. doi: 10.1289/ehp.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao L, Wang J, Sekhar KR, et al. Novel n-3 fatty acid oxidation products activate Nrf2 by destabilizing the association between Keap1 and Cullin3. J Biol Chem. 2007;282(4):2529–2537. doi: 10.1074/jbc.M607622200. [DOI] [PubMed] [Google Scholar]
- 50.Chan SR, Blackburn EH. Telomeres and telomerase. Philos Trans R Soc Lond B Biol Sci. 2004;359(1441):109–121. doi: 10.1098/rstb.2003.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong JM, Collins K. Telomere maintenance and disease. Lancet. 2003;362(9388):983–988. doi: 10.1016/S0140-6736(03)14369-3. [DOI] [PubMed] [Google Scholar]
- 52.Weng NP, Levine BL, June CH, Hodes RJ. Regulated expression of telomerase activity in human T lymphocyte development and activation. J Exp Med. 1996;183(6):2471–2479. doi: 10.1084/jem.183.6.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9(11):1048–1057. doi: 10.1016/S1470-2045(08)70234-1. [DOI] [PubMed] [Google Scholar]
- 54.Eitsuka T, Nakagawa K, Suzuki T, Miyazawa T. Polyunsaturated fatty acids inhibit telomerase activity in DLD-1 human colorectal adenocarcinoma cells: a dual mechanism approach. Biochim Biophys Acta. 2005;1737(1):1–10. doi: 10.1016/j.bbalip.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 55.Nettleton JA, Diez-Roux A, Jenny NS, Fitzpatrick AL, Jacobs DR., Jr Dietary patterns, food groups, and telomere length in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2008;88(5):1405–1412. doi: 10.3945/ajcn.2008.26429. [DOI] [PMC free article] [PubMed] [Google Scholar]


