Abstract
While most programmed cell death (PCD) in animal development is reliant upon the caspase-dependent apoptotic pathway and subsequent cleavage of caspase substrates, we found that PCD in Drosophila larval midgut occurs normally in the absence of the main components of the apoptotic machinery. However, when some of the components of the autophagic machinery were disrupted, midgut destruction was severely delayed. These studies demonstrate that Drosophila midgut PCD is executed by a novel mechanism where caspases are apparently dispensable, but that requires autophagy.
Keywords: apoptosis, caspases, programmed cell death, metamorphosis, decay
Most of the developmentally programmed cell death (PCD) that has been studied in metazoans has an obligate requirement for the canonical apoptotic machinery involving caspase activation. In Drosophila, activation of the initiator caspase Dronc requires association with the adaptor protein Ark (Apaf-1 related killer). Dronc then activates downstream effector caspases Dcp-1 and Drice, which in turn cleave cellular substrates to bring about the execution of apoptosis. The importance of this canonical pathway is evident from the studies involving Drosophila loss-of-function mutants of dronc, ark, drice and dcp-1. dronc, ark and combined drice dcp-1 double mutants result in lethality with inhibition of most developmental PCD. However, this canonical pathway is dispensable for some developmental PCD such as extra embryonic amniose-rosa cells and larval midgut. Interestingly the larval midgut, which undergoes PCD in the absence of dronc or ark, maintains high levels of caspase activity. This suggests that midgut cell death requires a caspase(s) other than Dronc, Drice and Dcp-1, which is activated by a Dronc-independent mechanism.
In our recent study we set out to determine how larval midgut undergoes PCD in the absence of the canonical cell death pathway. Our initial studies demonstrate that ark dronc double mutants and dcp-1 dronc drice triple mutants undergo midgut destruction normally. We then tested mutants of strica, a long prodomain caspase that has been suggested to function redundantly with Dronc during oogenesis. Surprisingly, strica alone or strica dronc drice triple mutants undergo normal midgut PCD.
We detected high levels of caspase activity in the multiple caspase mutant midguts. This led us to conclude that another effector caspase(s) must be active in the midgut. Quantification of transcript levels of effector caspases during midgut death reveals that drice and decay levels are elevated. As we had shown that drice mutants do not affect midgut cell death we focused on the role of decay. Knockdown of decay in the midgut results in a dramatic reduction of capase activity, which is completely abolished by co-expression of the baculovirus caspase inhibitor p35. Surprisingly, when caspase activity is abolished, midgut histolysis occurs normally. These data indicate that while Decay contributes most of the caspase activity in dying midguts, caspases are not required for midgut degradation.
In addition to caspases, complete removal of fly salivary glands requires autophagy. Therefore, we examined whether autophagy plays a role in midgut PCD. Examination of autophagy using the pGFP-Atg8a marker shows a dramatic increase in autophagic puncta in dying midguts, which are absent from earlier stage midguts. Consistent with this observation we find that several Atg genes are transcriptionally upregulated at the time of midgut cell death. Furthermore, inhibition of autophagy using mutants and dsRNA-mediated knockdown (RNAi) of Atg1, Atg2 and Atg18 severely delays midgut degradation as observed by the persistence of the larval midgut in pupal sections at 12 hours after puparium formation. These data indicate an important role for autophagy in midgut destruction. Curiously, in autophagy-deficient midguts, high levels of caspase activity are present. However, the combined inhibition of both autophagy and caspase activity had no greater effect on midgut degradation than inhibition of autophagy alone.
Although the clear conclusions from these studies are that midgut cell death can occur independently of caspases, whereas inhibition of autophagy severely delays this process, a number of issues remain unresolved. For instance, it remains unknown whether high levels of caspase activity play a role in midgut PCD. We find that dying midguts become TUNEL-positive suggesting that caspases contribute to the cell death phenotype even though they are not essential for the execution of cell death. It is also well known that ecdysone upregulates several caspases (dronc, drice, strica) and ark, along with several Atg genes. The role of this transcriptional control during midgut PCD is not known, but it does imply that the canonical apoptosis and autophagy pathways may collaborate in midgut destruction. Alternatively, this may suggest independent pathways with the apoptotic machinery playing a novel non-cell death role (Fig. 1). Another possibility is that this may be due to redundancy between these pathways with autophagy being important for midgut destruction only when caspases are absent. However, this would seem unlikely, as inhibition of autophagy alone is sufficient to inhibit midgut destruction. One implication of our study is that autophagy may be acting as either a mechanism of cell death by depletion of specific survival factors, or that intracellular digestion by autophagy eventually leads to midgut removal or death caused by depletion of critical resources. The Atg mutant midgut phenotypes seem to show a partial contraction of gastric caeca, and this suggests that other catabolic pathways may be involved. It is still unclear if premature activation of autophagy is sufficient to induce larval midgut cell death. Perhaps the autophagic machinery provides a means to activate lysosomal proteases that are eventually necessary for midgut destruction. We also do not know the morphological characteristics of midgut cells dying in the absence of caspases, and whether they differ from those of wild-type animals. Future studies will focus on the molecular mechanism of midgut destruction, and whether autophagy is acting as a direct cell death regulator in this tissue.
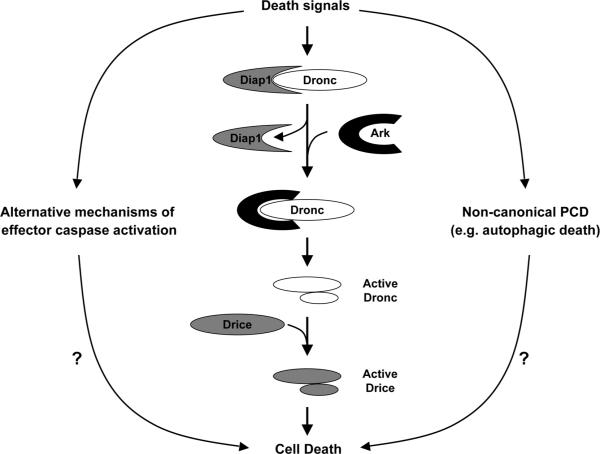
Figure 1.
Most developmental cell death in Drosophila is mediated by the canonical caspase activation pathway involving the initiator caspase Dronc and the effector caspase Drice. Drice function can be compensated by a similar caspase Dcp-1 (not shown here) in drice mutant animals. The Drosophila inhibitor of apoptosis protein (Diap1) prevents Dronc activation, whereas Ark, an adaptor that forms a Dronc-activation scaffold often called `apoptosome,' promotes Dronc activation. As discussed in this `punctum,' in Drosophila larval midgut, the Dronc-Ark-Drice/Dcp-1 pathway is dispensable for PCD. It appears that in the absence of this pathway, a noncanonical cell death mechanism involving autophagy mediates PCD, although how autophagy mediates midgut PCD is currently unknown. In the midgut, activation of the caspase Decay can occur without the Dronc-Ark-Drice/Dcp-1 pathway, suggesting an alternative mechanism(s) of caspase activation. However, the role of Decay in midgut cell death, if any, is unclear, as its inhibition/depletion has no effect on midgut PCD.