Abstract
Besides the importance of the renin-angiotensin system (RAS) in the circulation and other organs, the local RAS in the kidney has attracted a great attention in research in last decades. The renal RAS plays an important role in the body fluid homeostasis and long-term cardiovascular regulation. All major components and key enzymes for the establishment of a local RAS as well as two important angiotensin II (Ang II) receptor subtypes, AT1 and AT2 receptors, have been confirmed in the kidney. In addition to renal contribution to the systemic RAS, the intrarenal RAS plays a critical role in the regulation of renal function as well as in the development of kidney disease. Notably, kidney AT1 receptors located at different cells and compartments inside the kidney are important for normal renal physiological functions and abnormal pathophysiological processes. This mini-review focuses on: 1) the local renal RAS and its receptors, particularly the AT1 receptor and its mechanisms in physiological and pathophysiological processes, and 2) the chemistry of the selective AT1 receptor blocker, losartan, and the potential mechanisms for its actions in the renal RAS-mediated disease.
Keywords: AT1 blocker, renal RAS, renal remodeling, losartan
1. INTRODUCTION
The renin-angiotensin system (RAS) in normal and abnormal patterns has attracted significant interests in studies on renal functions under both physiological and pathological conditions [1]. In addition to the importance of the RAS and its functions in the circulation and other organs, the local RAS in the kidney has attracted a great attention in research in last decades [2].
The renal RAS plays an important role in body fluid homeostasis. Important evidence is that renin, a key enzyme secreted from the juxtaglomerular apparatus cells, is in the control of cleaving the substrate of RAS, angiotensinogen, to produce decapeptide angiotensin (Ang) I. Although renin is the major contribution from the kidney to the systemic RAS, other key components such as angiotensinogen, angiotensin converting enzyme (ACE), and Ang II have also been discovered in the kidney, independent of the circulating RAS. Therefore, in addition to its contribution to the systemic RAS, a local RAS has been demonstrated in the kidney [3].
Ang II type 1 (AT1) receptors have been demonstrated in the kidney [4], where they play critical roles in the renal function, long-term regulation of blood pressure, and disease development [5]. The role of Ang II type 2 (AT2) receptors remains controversial, although recent studies showed various functions of renal AT2 receptors in natriuresis, intrarenal vascular dilation, and the suppression of renin synthesis in the kidney [6]. This review focuses on: 1) the local renal RAS and its receptors, particularly AT1 receptors and their mechanisms in physiological and pathological status, and 2) the chemistry of the selective AT1 receptor blocker losartan and its potential mechanisms in the renal RAS-mediated disease.
2. THE CHEMICAL STRUCTURE OF LOSARTAN AND ITS PHARMACOLOGIC EFFECTS
Although a large array of emerging new variants of losartan (e.g. irbesartan, candesartan, and olmesartan) have been applied in the management of various cardiovascular disorders [7, 8], losartan is the first clinically-used angiotensin receptor blocker and is the prototype in elucidating possible roles of angiotensin receptors. As such, it remains an important agent in basic and clinical research.
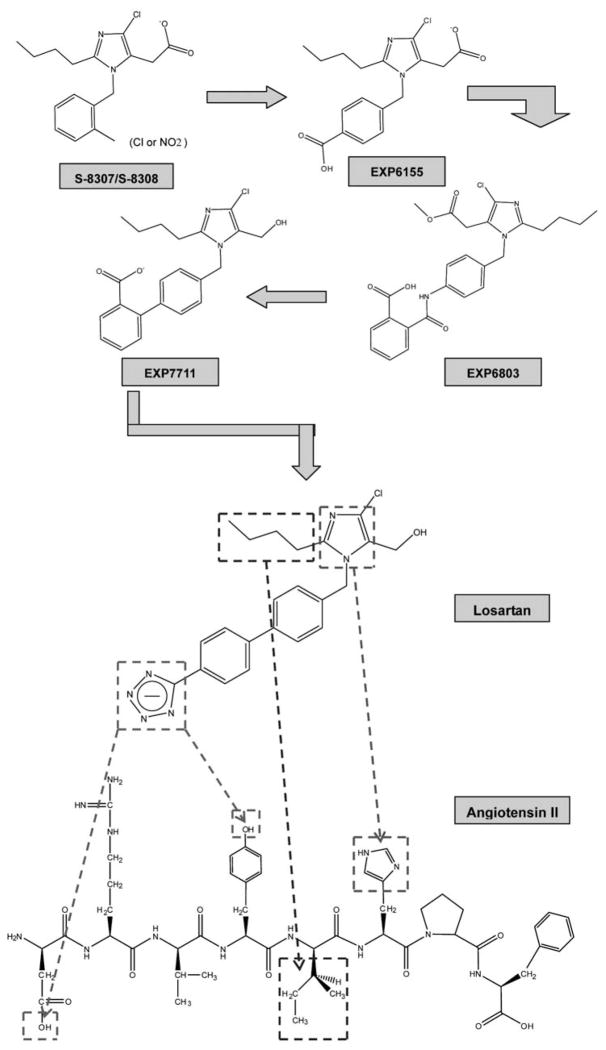
Losartan, also known as DuP 753, is a selective non-peptide antagonist blocking the AT1 receptors. It was first discovered in 1992 [9]. Its chemical structure is 2-Butyl-4-chloro-1-[(2′-(1H-etrazol-5-yl) (1, 1′-biphenyl-4-yl) methyl]-1H-imidazole-5- methanol with the molecular formula of C22H22N6O and molecular weight of 461.01 (Fig. 1). The chemical part of the imidazole-5-acetic acid derivatives is for the drug effect on cardiovascular systems. The compounds of losartan, like S-8307 and S-8308, are specific for AT1 receptor binding (Fig. 1). However, their anti-hypertensive effects are fairly weak [10]. Compared to the leading compounds with Ang II, acetic acid group, imidazole ring, and n-butyl side chain of S-8307/ S-8308 might mimic the C-terminal carboxylate group, histidine imidazole ring, and isoleucine n-butyl side chain, respectively. The additional benzyl group of S-8307 / S-8308 is derived from the resembling group of tyrosine residue in Ang II. EXP6155 is modified from the leading compounds by adding a para-COOH group in the benzyl group in order to strictly mimic anion charges in Ang II N-terminus. As EXP6803 and EXP7711, both magnitude of AT1 receptor binding affinity and hydrophobicity are significantly increased resulting from additional phenyl ring and replaced amide bond with a carbon-carbon bond. Furthermore, to replace ortho-COOH group by para-tetrazole ring can further increase intrinsic activity by an order of magnitude, and subsequent lipophilic specificity contributing to a more metabolic stability of losartan, while the oral bioavailability is well maintained [11].
Fig. (1). Chemistry comparison between losartan and Ang II.
The leading compound (S-8307/S-8308) is modified in its benzyl side chain for more hydrophobicity and oral bioavailability. Addition of the tetrazole group is mimic the anion charges in the N-terminal of Ang II. The lower part of figure indicates several parts in losartan, which are closly mimic those in Ang II: the imidazole ring and the n-butyl side chain in losartan is mimicing the side chains of His6 and Ile5 in the COOH-terminal of Ang II; the anion charged tetrazole ring in the para-position of losartan is well fitting that in Asp1 and Tyr4 of the Ang II NH2-terminal residues.
Losartan is a competitive antagonist selectively binding to AT1 receptors with a dissociation constant of approximate 0.1 nM [12]. In human liver, losartan undergoes both oxidative and conjugative metabolism, leading to formation of hydroxylated and carboxylic acid derivatives and glucuronide metabolite with modification in the tetrazole moiety, respectively [13]. However, in rodents, the oxidative pathway dominates metabolism of losartan in the liver [13]. EXP3174 with comparable potency and binding selectivity to that of losartan is a carboxylic acid metabolite in the oxidative pathway, which is the reason why an inhibition of Ang II-induced precurssor responses by losartan in vivo exhibits a biphasic property [14, 15]. Previous studies suggested that EXP3174 was a noncompetitive antagonist, whose insurmountable inhibition may be due to salt bridge interaction between Lys199 in AT1 receptors with a free carboxylate on imidazole ring in EXP3174 [16]. Stronger effects elicited by several other variants (e.g. candesartan) should also be due to the insurmountable binding ability with AT1 receptors [17]. Besides treatment of hypertension, losartan may also delay and regress progression of diabetic nephropathy, and may be able to prevent renal disease progression in patients with type 2 diabetes, hypertension, and other variety arrays of diseases (e.g. glomerulonephritis, nephritic syndromes, etc) [18]. Moreover, evidences prove that long-term use of high-dose and even supramaximal-dose of losartan or other variants (e.g. candesartan) may further alleviate the persisted and/or progressive state of chronic renal diseases independent of their hemodynamic effects [19–21].
3. INTRARENAL RAS
It has been demonstrated that the concentration of intrarenal Ang II is much higher (about 1000-fold) than that in the circulation [22], not only suggesting an existence of the independent local RAS in the kidney, but also indicating an important role (paracrine/autocrine regulation) of the local RAS in renal homeostasis.
All key components of RAS have been demonstrated in the kidney. Both mRNA and protein of angiotensinogen have been shown in proximal tubule cells [23]. As the blood-urinary barrier is not readily permeable to the circulating angiotensinogen [24, 25], the main origin of intraluminal angiotensinogen is synthesized and secreted from proximal tubular cells [26]. Juxtaglomerular apparatus cells are not the only source of intrarenal renin, cells of glomeruli, proximal and distal tubules, and connecting tubules also express renin mRNA and protein [27–29]. Furthermore, the distal portion of afferent arteriole extending and towards the interlobular artery has been shown to express renin under certain conditions [30]. In various animal models, ACE has been found on endothelial cells of the intrarenal vasculature, as well as on proximal tubular cells [31]. The brush border of proximal tubular segments is the predominant site for ACE in the human kidney. However, very little ACE expression is observed in endothelial cells of the renal microvasculature [32]. With the similar pattern of expression in both human and rodents [33–35], ACE is detected in cells in almost all nephron segments [except the thick ascending limb of the Loop of Henle], glomerular visceral and parietal epithelium, as well as smooth muscle cells and the endothelium of the intrarenal vasculature. Collectrin, another novel mammalian homolog of ACE [36, 37], has also been found in the apical membrane and in the cytoplasm of collecting duct epithelial cells [38].
Luminal angiotensinogen is synthesized and secreted from proximal tubular cells and is subsequently cleaved to Ang I by renin via autocrine/paracrine mechanisms. The ACE activity throughout the lumen, except the distal tubule [39], can readily convert Ang I into Ang II. Distribution of Ang II in the kidney shows fairly heterogeneity. Levels of Ang II in the medulla are higher than those in the cortex [40]. Ang II was detected in the tubular fluid, as well as in the interstitium in both the medulla and cortex [41]. However, the latter compartment seems to be more effective in production of intrarenal Ang II [42, 43]. Receptor-mediated endocytosis has been suggested to be the intracellular pathway for Ang II formation [22, 24] and the accumulation of Ang II in endosomes and/or the intermicrovillar clefts in tubular cells has been observed [45, 46]. Substantial bodies of evidence have further indicated that ACE2, the main enzyme for production of Ang [1–7], also exists and plays a protective role in the kidney [47].
AT1 receptor mRNA has been detected in almost all parts of nephron segments, including juxtaglomerular apparatus, glomeruli, macula densa, and intrarenal arterial vasculature [48]. In rodents, AT1b subtype transcript is restricted in the glomeruli. In contrast, mRNA of AT1a subtype is widely distributed along nephron segments (e.g. proximal tubules, thick ascending limb of the loop of Henle, distal tubules, macula densa cells, and collecting ducts), intrarenal microvasculature, and mesangial cells. However, its expression is not less abundant than that of AT1b mRNA in the glomeruli [49]. The intrarenal distribution of AT1 receptor protein is consistent with that of AT1 mRNA [50, 51]. In addition to AT1 receptors, AT2 receptor mRNA and protein are also consistently distributed in the glomerular epithelium, proximal tubules, collecting ducts, and other parts of the intrarenal vasculature [51, 52].
4. INTRARENAL RAS ACTIVATION AND LOSARTAN
The intrarenal RAS can be activated in a great variety of diseases, including hypertension, diabetes, glomerulonephritis, etc [53–56]. Inappropriate activation of the intrarenal RAS can cause functional (e.g. hemodynamic and/or transport functions) and/or structural (e.g. glomerular and/or interstitial injury) disturbances that are of great importance in the development of renal and cardiovascular disorders.
Under a number of conditions, mRNA of angiotensinogen in proximal tubular cells is elevated by the mechanism of Ang II-induced and AT1 receptor internalization-mediated pathways [57, 58], as well as by α2 and/or β1 receptor activation resulting from neurotransmitter releasing from the sympathetic nerve terminals in the proximal tubules [59, 60]. The synthesis and secretion of renin from juxtaglomerular apparatus cells, as well as from other nephric segments (e.g. proximal tubule cells), can be augmented as a result of AT1 receptor activation and the overload of sympathetic outflow [61, 62]. Previous studies have shown that the secondary intrarenal renin expression and secretion (i.e. proximal tubules, connecting tubules, and collecting ducts) are elevated by the activation of AT1 receptors occurred in cardiovascular diseases (e.g. hypertension, diabetes) [27, 28, 63, 64]. In addition, ACE was released to the lumen from proximal tubule apical membranes [65, 66] and was abnormally neoexpressed throughout the nephron (especially in the glomerular tuft) [67]. The enzyme can be upregulated in the proximal tubular epithelium and fibroblast/myofibroblast cells in the interstitium by over-stimulation of AT1 or α2 and/or β1 receptors [68–71].
The elevation of the RAS substrates and key enzymes, described above, contributes to the maintenance and increase of Ang II in the tubular lumen, although the circulating Ang II may be decreased in certain cardiovascular diseases (e.g. hypertension) [72]. Ang II levels in the renal interstitium were indeed increased in the cardiovascular diseases and diabetes [73, 74]. In addition, in the diseased kidney, Ang II in the intracellular compartment was significantly elevated [75]. Losartan lowers intrarenal Ang II via its direct binding to AT1 receptors [76]. As discussed above, the upregulation of transcripts of angiotensinogen, renin, and ACE are mediated by AT1 receptor activation, as well as by an elevation of intracellular Ang II [75]. Taken together, the benefits of losartan in attenuation of intrarenal Ang II production are likely mediated by the “key point break-down” effect in blocking the activated positive feedback loop (detailed in Fig. 2).
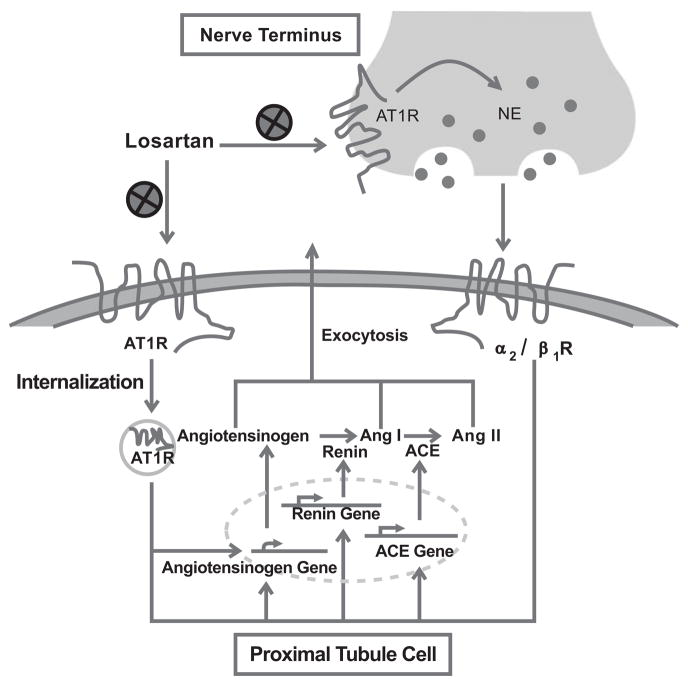
Fig. (2). Losartan and RAS-mediated activation.
This figure shows that increased intrarenal Ang II (either unltrafiltered from the glomerulus or the renal local tissue) activates AT1 receptors, in the proximal tubular cell followed by expression of angiotensinogen, renin, and ACE. As a result, angiotensinogen and Ang I are catalyzed in situ, and to be released to the extracellular space (as well as the tubular space). Losartan can directly abrogate AT1 receptors. Moreover, the proximal tubule is innervated by renal sympathetic nerve termini, whose subsequent catecholamine in turn stimulates α2/β1 receptors. AT1 receptors are located at the nerve terminals. Losartan is able to attenuate activation of intrarenal RAS by inhibiting renal sympathetic outflow.
5. LOSARTAN AND MEDICAL PROBLEMS OF THE KIDNEY
5.1. Losartan and Renal Hemodynamics
5.1.1. Losartan and Glomerular Filtration Rate
Although plasma Ang II concentrations decreased in some diseases, consistently elevated intrarenal Ang II can constrict both afferent and efferent arterioles, which has been recently demonstrated using an in vivo method of intravital tapered-tip lens-probe video-microscopy system [77]. The GFR is reduced because of the activation of AT1 receptors and the decrease of renal blood flow.
As a major structural and functional component of the filtration barrier, the mesangial-capillary unit is consisting of extracellular matrix of mesangium coupling glomerular/intraglomerular mesangial cells with capillaries [78] and is crucial in regulating local capillary blood flow, glomerular hydrostatic pressure, ultrafiltration surface area, and the GFR [79]. Increased levels of Ang II can lead to contractions of mesangial cells via AT1 receptors [80, 81] and a decrease of the GFR.
Although losartan can inhibit the effects of elevated intrarenal Ang II-induced reduction of the GFR, its effect on the overall GFR is variable given the influence of gross renal hemodynamics and autoregulation. When the blood pressure remains within the renal autoregulatory range, the GFR is generally increased by losartan [82]. However, if a reduction of the blood pressure is beyond the renal autoregulatory range or when there is an impairment of renal autoregulation function [83], the GFR may be increased [84], unchanged [85], or decreased [86] by losartan. The effects of losartan on the GFR become more difficult to determine in patients with established injury glomeruli losing its ability in maintaining the GFR, and with more severe renal disease, in which the afferent and/or efferent arterioles become less responsive to Ang II [87].
5.1.2. Losartan and Renal Autoregulation
Renal autoregulation in maintaining relatively constant renal blood flow (RBF) and the GFR regardless of liability of perfusion pressure is the most critical characteristic of the renal circulation [88]. Although losartan shows little influence on the autoregulation of the renal circulation under normal condition [90], it has been shown to ameliorate the impaired autoregulation induced by long-term abnormal activation of intrarenal RAS [89].
Intrarenal Ang II plays a fundamental role in the regulation of the sensitivity of the tubuloglomerular feedback mechanism [91], which serves as a secondary mechanism involved in renal autoregulation to provide a balance between reabsorption capabilities of renal tubules and filter load by regulating the GFR [92, 93]. Increased intrarenal Ang II can cause a left-shift and an increase slope of tubuloglomerular feedback function [94], which may be mediated by its direct effects on arteriolar smooth muscle cells and mesangial cells, as well as by the regulation and modification of the Na+/H+ exchanger and Na+/2Cl−/K+ cotransporter in macula densa cells [95–97]. Losartan can increase the distal nephron volume and sodium delivery by blocking the Ang II-mediated shift in the tubuloglomerular feedback [98].
In addition, the myogenic response, a primary mechanism involved in renal autoregulation, is the most effective and quick response in the kidney. Several lines of evidence have shown that chronically elevated Ang II blunts autoregulatory responsiveness of the afferent arterioles [99], which is prevented by losartan [100]. Recently, a so called “third element” involved in renal autoregulation has been described [100]. Nonetheless, whether any local renal RAS component plays a role in this mechanism is uncertain and requires further studies.
5.2. Losartan and Renal Tubular Functions
In physiological circumstances, intrarenal Ang II is regarded as one of the most powerful sodium-retaining hormones in the body, through either direct (AT1/AT2 receptor mediated) or indirect (secondary activation of effective molecules, e.g. aldosterone, vasopressin, etc) actions on water and electrolytes transport functions of renal tubules [101]. As mentioned above, in various cardiovascular diseases and diabetes, the renal local RAS can be aberrantly activated. Losartan has not only been shown to lower intrarenal Ang II concentrations, but it also normalizes renal excretion functions [102, 103]. The access of losartan and its metabolite EXP3174 to the glomerular filter suggests that the drug can directly regulate intraluminal Ang II-mediated regulation of water and electrolytes transport.
5.2.1. Losartan and Proximal Tubules
The regulation of sodium and water is the most important function of intraluminal Ang II in the proximal tubule (PT), which is partly independent of the systemic Ang II in the circulation [104]. The increased intraluminal Ang II can stimulate the apical Na+/H+ exchanger (NHE3) [105, 106], the basolateral Na+/K+ ATPase [107], and the basolateral electrogenic Na+/3HCO3− cotransporter (NBCe1-A) [108], H+-ATPase (V-type H+ Pumps), and NHE3 trafficking [109–112], via AT1 receptors in proximal tubule cells. Interestingly, unlike the situation in the distal tubules (discussed below), the nuclear events concerning the pumps and transporters seem to be little altered by the continuous stimulation of AT1 receptors (at least for NHE3 and Na+/K+ ATPase) [111]. In vitro perfusion studies indicated that such effects can be elicited by applying Ang II in either the apical or basolateral surface. As a result, losartan can reduce the reabsorption of sodium and, subsequently, increase the luminal fluid volume [113] by reducing intraluminal Ang II (Fig. 3). In addition, losartan normalizes the elevated proximal tubular acidification and bicarbonate reabsorption induced by the AT1 receptor activation [114, 115] (Fig. 3).
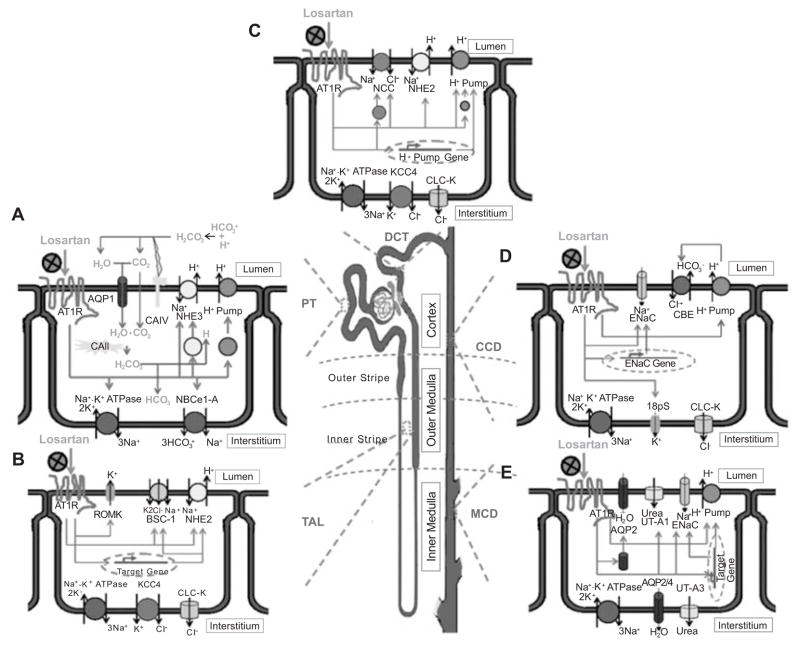
Fig. (3). Losartan and renal tubule functions.
A. In the proximal tubules (PT), increased intraluminal Ang II can, via AT1 receptors, directly stimulate type 3 Na+-H+ cotransporter (NHE3) and H+ pump (V-type H+-ATPase) in the apical membrane, and Na+-K+ ATPase and type 1-A of the eletrogenic Na+-3HCO3− cotransporter (NBCe1-A) in the basolateral membrane, as well as the protein trafficking of NHE3 and vacuolar H+ pump toward the apical membrane. As a result, Na+ reabsorption and H+ secretion will be increased. Elevated H+ in the lumen would then to form carbonic acid together with the luminal HCO3−. Then, membrane-bond type IV of the carbonic anhydrase (CAIV) is able to catalyze H2CO3 into water and carbon dioxide. Subsequently, almost all H2O and majority of CO2 would pass through the gas/water channel aquaporin 1 (AQP1) into the cytoplasm, while very limited CO2 diffuses into the cytoplasm. Soluble type II carbonic anhydrase (CAII) can condense H2O and CO2 to re-create H2CO3 in the cytoplasm. Finally, the HCO3− and H+ from H2CO3, are transported to the interstitium and lumen by H+ pump and NBCe1-A, respectively. Thus, in the proximal tubules, losartan can lower sodium and bicarbonate together with water absorption and lessen tubular acidification via AT1 receptors locating in either the apical and basolateral surface.
B. In the thick ascending limb of loop of Henle (TAL), Ang II can inhibit Na+-K+-2Cl− cotransporter (NKCC2) or bumetanide-sensitive channel-1 (BSC-1), and type 2 NHE (NHE2) in the apical membrane of the TAL. However, abnormal or persisted increased intrarenal Ang II can increase expression of BSC-1 and NHE2 and stimulate apical ROMK potassium channel on both apical and basolateral surface. The apical BSC-1 and ROMK can be interdependent to facilitate NaCl transepithelial transport. The Na+-K+ ATPase, type 4 K+-Cl− cotransporter (KCC4), and kidney specific chloride channel (CLC-K, K1 or K2 subtype), especially K2 subtype, whereas in distal convoluted tubules (DCT), in the basolateral membrane are coupled for transepithelial transport of Na+ and Cl−. The mechanism of bicarbonate reabsortion is similar to that in the PT. An increased NaCl and bicarbonate absorption can be effectively attenuated by losartan. As a result, losartan can further lower NaCl gradient in the medulla.
C. In the DCT, elevated intrarenal Ang II can stimulate NHE2, amiloride-sensitive Na+-Cl− cotransporter (NCC), and H+ pump, as well as increase of expression and trafficking of either NCC or H+ pump, which are undergoing the AT1 receptor pathway. The subsequent increased bicarbonate and NaCl transepithelial transport are similar to those indicated in A and B. Consequently, in the DCT, losartan may be in part abolish NaCl and HCO3− transepithelial transport together with inhibiting the tubular over-acidification by abnormal intrarenal Ang II in diseases.
D. In the cortical collecting ducts (CCD), activation of AT1 receptor can directly stimulate aldosterone-sensitive epithelial sodium channel (ENaC) and enhance expression of α-subunit of ENaC independent of aldosterone. In addition, AT1 receptor activation can stimulate basolateral 18pS potassium channel, which is related to sodium transepithelial transport. Recent studies indicated that activated AT1 receptors can enhance transepithelial transport of Cl− indirectly via its activation on apical H+ pump, which would in turn stimulate Cl− transport by AE2 (Cl−-HCO3− exchanger). Losartan can influence its natriuresis and diuresis in the CCD. E. In the medullary collecting ducts (MCD) [divided into outer and inner medullary collecting ducts (OMCD, IMCD)], Ang II can directly increase expression of apical ENaC and H+ pump. Thus, both sodium and bicarbonate transport can be elevated by increased intrarenal Ang II in diseases via the AT1 receptor pathway. Another intriguing phenomenon elicited by AT1 stimulation is that, activity of the apical UT-A1 (urea transporter-A subtype 1), and the trafficking of apical AQP2, can be both activated by Ang II. Consequently, losartan not only can further augment its inhibition of sodium and bicarbonate absorption, and tubular acidification, but also lessen urea gradient in the medulla in order to exquisitely regulate urinary volume and osmolality.
In addition to AT1 receptors, there are data showing that intraluminal Ang II can trigger AT2 receptor-mediated endocytosis of albumin [116]. However, whether this effect is detrimental or beneficial remain to be determined.
5.2.2. Losartan and Distal Tubules
Recent findings have indicated that intrarenal Ang II inhibits NaCl and bicarbonate reabsorption acutely in the medullary thick ascending limb (TAL) under physiological conditions [117,118,119]. However, in the TAL of the loop of Henle either in the medullary or cortical, the expression of Na+/H+ exchanger 3 (NHE3) and Na+-2Cl−-K+ cotransporter [NKCC2/BSC-1 (type 1 bumetanide-sensitive cotransporter)] is increased by chronically over-activation of AT1 receptors in the apical membrane [111,120]. In addition, previous studies have shown that Ang II activates 70pS K+ channels [renal outer medullary inward rectifier ATP-sensitive potassium channel (ROMK)] that are coupling to the Na+-K+-2Cl− cotransporter in the TAL [121]. Consequently, although the inhibition of the apical NHE3 and/or BSC-1 may be in the medullary [122], TAL cells can be stimulated by Ang II, and over-stimulated AT1 receptors lead to an enhancement of Na+ and HCO3− reabsorption in the TAL. Because the TAL segment is water impermeable, trans-epithelial NaCl absorption to the medulla is attenuated by the application of losartan followed by a decrease in countercurrent multiplication in the kidney [123]. Thus, losartan can ameliorate the disturbance of water and electrolytes and acid-base balance in this part of nephron segment.
Moreover, intrarenal Ang II via AT1 receptors can stimulate Na+/H+ exchanger 2 (NHE2) in both the distal convoluted tubule (DCT) and connecting tubule (CNT), and vacuolar H+-ATPase in the late distal segment, associated with synthesis and insertion of H+-ATPase into apical membranes, suggesting a possible action of losartan in natriuresis and diuresis in the distal tubular segments [124–127]. In addition, recent evidence has shown that trafficking of Na+ -Cl− cotransporter (NCC) in the DCT apical membrane is stimulated by the activation of AT1 receptors [128]. It is reasonable to think that the inhibition of AT1 receptors by losartan is beneficial to electrolytes excretion in the kidney.
5.2.3. Losartan and Collecting Ducts
In the isolated and perfused cortical collecting duct (CCD), intrarenal Ang II can directly stimulate epithelial sodium channel (ENaC) on the apical membrane [129], induce the expression of α-subunit of ENaC independent of aldosterone [130], and increase 18 pS potassium channel activity in the basolateral surface [131]. In addition, Ang II increases trans-epithelial chloride transport in intercalated cells of the CCD, which may be mediated by pendrin and H+-ATPase dependent pathways [132, 133]. The elevated intrarenal RAS activity exhibits a urinary concentrating ability in the inner medullary collecting ducts (IMCD) via vasopressin-stimulated urea permeability and the activation of urea transporter (UT-A1) mediated by AT1 receptors [134–136]. A recent study by Lee Yu-Jung et al. has indicated that the activation of AT1 receptors in IMCD cells stimulates aquaporin 2 (AQP2) in the plasma membrane and enhances urinary concentrating mechanisms in the MCD [137]. Taken together, anti-Ang II effects of losartan in the renal tubules are further augmented in the collecting duct [138]. Apart from that in the PT, the regulation of H+-ATPase in the MCD by Ang II seems to be a paradox. Ang II can inhibit the secretion of H+ in the OMCD [139], but a persistent activation of AT1 receptors may increase expression of the β1 subunit of H+-ATPase followed by the upregulation of H+-ATPase in the apical membrane of the MCD [140]. It has been demonstrated that the increased intrarenal Ang II can intensely augment tubular acidification and reabsorption of bicarbonate throughout the nephric segments, suggesting a possible effect of losartan in ameliorating the acid-base balance via its blockade of AT1 receptors.
Aldosterone, a sodium-retaining hormone mainly synthesized and secreted from zona glomerulosa cells of the adrenal cortex by AT1 receptor activation, can increase the number of epithelial sodium and potassium channels in the apical membrane and enhance the activity of Na+/K+-ATPase located in the basolateral membrane of principal cells in eliciting anti-dinatriuresis effects [141]. Moreover, arginine vasopressin (AVP) from the hypothalamus consequent to an abnormal activation of AT1 receptors in the circumventricular organs in the brain also plays a critical role in the electrolyte transport and urinary concentrating in the kidney [142]. Furthermore, the type 2 AVP receptors (V2 receptors) in the IMCD are up-regulated by Ang II [143]. Consequently, the amelioration of fluid and electrolysis disorders by losartan results in part from its effects on lowering plasma levels of aldosterone and AVP.
5.3. Losartan and Glomerular Permselectivity
The glomeruli selectively filter water, electrolytes, and low-molecular-weight products, while restricting albumin and large-molecular macromolecules to the Bowman’s capsule from plasma [144]. This function of the kidney, based on the glomerular permselectivity, is attributed to the unique morphology of the glomerular filtration barrier consisting of the endothelial glycocalyx, fenestrated endothelium, glomerular basement membrane (GBM), and slit diaphragm of podocyte located from capillary to Bowman’s space [145]. Proteinuria, a common complication in various arrays of cardiovascular and kidney diseases [56, 146], mainly resulted from the impairment of the ultrafiltration barrier, can be effectively protected by the application of losartan [56, 147, 148].
5.3.1. Losartan and GBM
Recent studies have indicated that the impairment of the GBM is the primary culprit in pathogenesis of proteinuria [149, 150], which may be caused by the disturbance of either charge-selectivity or size-selectivity that can both be improved by losartan [151, 152].
Heparin sulfate proteoglycans (HSPGs) are rich in negative charges and contribute to the charge-selective function of the GBM [153]. Previous studies have shown that elevated Ang II inhibits the production and secretion of HSPGs from podocytes [154] and mesangial cells [155] via the AT1-mediated pathway. However, the data regarding whether HSPGs in glomerular endothelial cells are affected are limited. In addition, a loss of heparin sulfate (HS) also results in an impairment of the charge-selective filter [153]. The activation of AT1 receptors induces the expression of heparanase, an endo-β(1, 4)-d-glucuronidase involved in the cleavage of HS [156] and mainly synthesized in podocytes of the glomeruli [157]. Hence, the amelioration of impairment of charge-selectivity in the GBM by losartan is, at least in part, due to the maintenance of HSPGs via the pathways described above. Furthermore, the preservation of HS by losartan may also result from a reduction of radical oxygen species (ROS) that can depolymerize HS [157, 158].
The size-selectivity of the GBM is mainly supported by collagens in the GBM. Several lines of evidence have shown that Ang II stimulates aberrant deposition of type I collagen and the α3 chain of type IV collagen from mesangial and podocyte cells, respectively [159,160]. By blocking AT1 receptors, losartan attenuates the Ang II-induced collagen synthesis and partly protects thickening of the GBM, beneficial to the size-selective filter of the GBM. However, it was noted that synthesis of the α3 chain of type IV collagen may be indirectly from the action of TGF-β1 and vascular endothelial growth factor (VEGF) induced by Ang II [160–162]. The finding that TGF-β1 induces metalloproteinase-9 (MMP-9) expressing on the cell surface of podocytes [163] is intriguing and suggests a role of MMP-9 in degrading type IV collagen and laminin in the GBM [164]. It is known that Ang II causes an elevation of TGF-β1. As a result, losartan lessens the GBM hydrolysis by the blockade of the Ang II-TGF-β1 axis.
5.3.2. Losartan and Slit Diaphragm
Since the identification of the molecular determinant of the Finnish type of congenital nephritic syndrome as nephrin, a type I integrated membrane protein in podocytes [165], the function of slit diaphragm in the permeability of the GBM has been well accepted of extreme importance [166]. Current therapeutic trials have indicated that losartan can ameliorate destruction of the podocyte and its slit in the diseased kidney [167, 168]. Recent studies demonstrated that the elevated Ang II reduced the expression of the nephrin and podocin [169] that played a role in the maintenance and regulation of the zipper-like arranged slit diaphragm [170]. The enhanced intrarenal Ang II induced podocyte apoptosis leading to podocyte detachment and the impairment of slit diaphragm via the AT1 receptor activation [171,172], which can be inhibited by losartan. In addition, by blocking AT1 receptors losartan can ameliorate the slit function through an alternate mechanism, in which excess of Ang II can bind to AT2 receptors to elicit the opposite effects on the slit diaphragm and podocytes [171]. Although losartan can also be employed to prevent the detachment of podocytes from the GBM associated with a decrease of α3β1 integrin, the detailed mechanism is lacking [168]. In addition to the nephrin and podocin, there are a number of other molecules, such as NEPH1/2, CD2AP, α-actin-4, etc, locating in the slit diaphragm and podocyte cytoplasm, which may also be important in the GBM permselectivity [170]. The relationship among Ang II, losartan, and those molecules in the regulation of the GBM permselectivity needs to be further elucidated.
5.4. Losartan and Renal Fibrosis
Besides hemodynamic, tubular transport, and glomerular filter regulatory functions, the role of Ang II in the kidney has been considered in cellular remodeling (e.g. hypertophy, proliferation, apoptosis, differentiation, and transition) and ECM reorganization (e.g. synthesis, degradation, etc), both of which are involved in pathophysiological events of either stromal or parenchymal components of the kidney [173–175]. The common feature of these pathological outcomes is “renal fibrosis”, which is manifested as glomerulosclerosis and/or interstitial fibrosis.
5.4.1. Losartan and Glomerulosclerosis
Glomerulosclerosis is one of the most key elements in the end-stage of renal diseases [176]. A large body of evidence has demonstrated the potential effects of losartan on protection and regression of glomerulosclerosis [177–179]. The main pathological manifestation of glomerulosclerosis is considered to be promotion of mesangial cell growth and ECM deposition. Furthermore, increasingly growing data suggest that podocyte injury is the initial stage and acts as a key role in the pathogenesis of glomerulosclerosis. It has been shown that Ang II plays a critical role in the progression of glomerular mesangial and podocte remodeling (see details below), however, whether endothelial and parietal epithelial cells take part in Ang II-mediated glomerulosclerosis are not clear at the present.
5.4.1.1. Losartan and Mesangium
Previous studies demonstrated that mesangial cells exclusively expressed AT1 receptors but not AT2 receptors [180]. Both in vivo and in vitro studies have shown that Ang II induces cellular hypertrophy, proliferation, and acute injury of mesangial cells, which are prevented by losartan [56, 181–183]. The ECM in mesangium, such as type I collagen, fibronectin, laminin, etc, also are promoted by elevated Ang II via AT1 receptor activation [184], which is blocked by losartan [185].
The degradation of ECM is another important aspect involved in tissue fibrosis. In addition, the enhancement of both mRNA and protein of plasminogen activator inhibitor-1 (PAI-1) and tissue inhibitors of matrix metalloproteases (TIMP) in mesangial cells by Ang II is effectively decreased by losartan [186, 187], which in turn provokes matalloproteinases and, thereby, ECM turnover [188].
It has been demonstrated that losartan can prevent in part pathogenic processes caused by Ang II involved in renal fibrosis. However, it should be noted that glomerular mesangial fibrosis is not directly mediated by AT1 receptors, but follows the downstream accumulation of TGF-β1 synthesis [184, 186, 189]. In addition, a novel fibrotic mediator, connective tissue growth factor (CTGF) induced by Ang II independent of TGF-β1 [190], also mediates renal fibrosis. Taken together, both the primary and secondary fibrotic factors could be in part blocked by losartan [191].
5.4.1.2. Losartan and Podocyte
The fact that podocytes involved in pathogenesis in glomerulosclerosis has attracted an attention for a long time [192]. Due to its terminal differentiation property, the podocyte lacks ability to proliferate [192] following a variety of injuries except certain conditions (e.g. HIV-induced nephrosis) [193].
Foot process (FP) effacement may be the initial step in the development of podocyte injury. Preliminary studies showed that an increased Ang II in the kidney elicited dysfunction of nephrin. The impairment of the GBM permeability is not a unique outcome, FP effacement of podocytes is another detrimental event induced by destruction of the nephrin. The dynamics of the cytoskeleton (especially actin) in the PF of podocytes are disorganized by the impact of deterioration of the nephrin [166] and this process may be linked by adaptor protein Nck [194]. Thus, early application of losartan in cardiovascular diseases may ameliorate the progression of FP effacement by inhibiting AT1 receptors on podocytes and attenuating insults of the nephrin induced by increased Ang II. In light of this, losartan should be considered worthy of controlling and improving podocyturia in patients [195].
Furthermore, an abnormally over-activated intrarenal RAS can elicit podocytopenia resulting from apoptosis and the detachment of podocytes mediated by elevated Ang II. Consequently, loss of podocytes may trigger a sequence of events in the development of glomerulosclerosis. The insufficient coverage induced by the loss of podocyte is likely to denude specific areas of the GBM followed by formation of microaneurysm in the glomerular capillary tuft resulting from the loss of effective tensile function supported by normal podocytes [196]. The bulging capillary loops would then adjoin to the Bowman’s capsule to form a local tuft adhesion, which is thought to be the initial committed task to the development of focal segmental glomerulosclerosis (FSGS) [56, 197]. The misdirected filtration resulting from FSGS further leads to the deterioration of podocytes lost and triggers abutting interstitial fibroblasts activation, which accelerates and worsens FSGS and finally leads to the progression of the loss of the nephron [198–200]. Losartan may be used to prevent and attenuate the apoptosis caused by Ang II to maintain the number and function of the podocyte. Notably, the activation of both AT1 and AT2 receptors can induce podocyte apoptosis [201]. This may be the reason why losartan can not massively abrogate the apoptosis effect by elevated intrarenal RAS, and the combination of ACEI and ARB should be more optimal in controlling glomerulosclerosis in clinic. In addition, the benefit to the detachment of podocytes by losartan is also attributed to the protection of podocytopenia.
Epithelial-to-mesenchymal transition (EMT), an abrupt event observed in the renal tubular cells related to pathogenesis of interstitial dysfunction (see details below), also occurred in the glomerular podocyte [163]. The dedifferentiation of the podocyte can be a fibroblast-like behavior that is expression of fibronectin, collagen I, MMP, etc, which may potentially trigger glomerulosclerosis. The EMT in podocytes has been shown to be promoted by TGF-β1 [163]. Therefore, it is likely that losartan can attenuate this event by blocking AT1 receptors and reducing the levels of TGF-β1.
5.4.2. Losartan and Interstitial Fibrosis
The interstitial fibrosis, another type of renal fibrosis, always accompanying with a great variety of cardiovascular and/or kidney diseases, has been frequently demonstrated in the deterioration of renal functions. Both in vitro and in vivo evidence have shown that Ang II causes proliferation of interstitial fibroblasts, and further increases the expression and secretion of ECM, including collagen, fibronectin, etc, via activation of AT1 receptors locating on interstitial fibroblast cells [202, 203]. Various studies have demonstrated that the growth-promoting effect on fibroblasts is directed by TGF-β1 via a paracrine/autocrine route subsequent to primary activation of AT1 receptors by elevated Ang II [204]. Furthermore, the secondary increased TGF-β1 may further induce fibroblasts transforming to the myofibroblasts [205], which in turn migrating and invading the periglomerular and/or tubulointerstitial compartments in correlating with the extent of interstitial scarring and functional outcome in clinical manifestation [206]. These may be the possible mechanisms underlying the protection and regression effects on renal interstitial fibrosis by losartan.
Besides the fibroblast cells, renal tubular cells may also be involved in the process of interstitial fibrosis. The cellular growth effect mediated by AT1 receptors on tubular cells closely resembles that on fibroblast cells [204]. Elevated TGF-β1, consequent to activation of AT1 receptors, can also cause hypertrophy and increased synthesis of ECM in tubular cells. However, instead of the transformation phenomenon in fibroblast cells, tubular cells have the ability to change into local interstitial fibroblast cells through a process called epithelial-to-mesenchymal transition (EMT), a reversible event of the mesenchymal-to-epithelial transition in renal embryogenesis [207]. Because of the loss of tubular cells and the formation/activation of more fibroblasts, these pathological processes may further lead to tubular atrophy and interstitial fibrosis in late stages of renal injury progression. Both Ang II and TGF-β1 promote this event [207]. Recent studies indicated that hepatocyte growth factor (HGF), an antagonist against tubular cell EMT, is suppressed by Ang II [208], suggesting that losartan can partly inhibit the pathogenesis via its indirect protection of the restoration of HGF.
5.5. Losartan and Inflammation in the Kidney
Inflammation is commonly occurred in progressive renal diseases subsequent to cardiovascular diseases, which is generally manifested as mononuclear cells infiltrating in the glomeruli and/or renal interstitium [209]. Losartan partly attenuates inflammatory processes consequent to the activation of intrarenal RAS [56, 210]. Several studies have indicated that Ang II is involved in inflammatory process in the kidney injury by induction of proinflammatory transcription factor NF-κB via either AT1 or AT2 receptors [211]. Subsequent NF-κB-induced transcription of various chemokines, for instance, monocyte chemoattractant protein-1 (MCP-1), RANTES, osteopontin, etc, leads to leukocyte infiltration in both the glomeruli and interstitium [212]. As expected, losartan can effectively attenuate inflammatory cell infiltration by reducing chemokines [213]. In addition, losartan can further block proliferation of leukocytes that may be directly stimulated by Ang II [211]. It should be noted that losartan may not be so efficient in abrogating leukocyte recruitment in the endothelium, because induction of RANTES in endothelial cells may be mediated by the activation of AT2 receptors [214]. Recent studies have shown that Toll-like receptor 4 (TLR4) on glomerular mesangial cells is upregulated, which is mediated by the activation of AT1 receptors [215]. As TLR4 binds to a variety of endogenous ligands, losartan can block this pathway to inhibit glomerular inflammation. Taking initial inflammatory events into account, Ang II can elicit an upregulation of adhesion molecules (e.g. vascular cellular adhesion molecule-1, intracellular adhesion molecule-1, integrins, etc) in circulating immune cells and the vascular endothelium, in order to trigger adhesion events [211], which can further snag ultrafiltration and perselectivity functions of the GBM. In light of this, the inhibition of inflammation by losartan can alternatively ameliorate glomerular filter functions.
Ang II-induced chemokines (e.g. MCP-1) and inflammatory cells can in turn induce a release of a wide range of growth factors, including Ang II, TGF-β1 from renal cells (either stromal or interstitial cells). Moreover, ECM production and/or deposition can also be further augmented [216]. Certainly, the positive feedback loop could be attenuated by an application of losartan. Because both AT1 and AT2 receptors are involved in the process, the treatment with a combination of ACEI and ARB should be the optimal regimen.
6. CONCLUDING REMARKS
All major components and key enzymes for an establishment of the local RAS in the kidney have been demonstrated. Importantly, the renal local RAS plays a critical role not only in renal hemodynamics and tubular functions together with long-term cardiovascular regulation, but also in renal pathological functional and structural remodeling, including disordered glomerular filter permselectivity, inflammation, and fibrosis. Notably, renal AT1 receptors extensively distributed in many cell types and tissue compartments inside the kidney are important for both normal renal physiological functions and abnormal pathophysiological processes. Although several mechanisms and pathways for actions of intrarenal Ang II are reviewed in this article, more studies are required for further understanding of these mechanisms. Nevertheless, information and data gained so far in the field strongly indicate that losartan is one of the most reasonable candidates that can be used in both medical studies and clinical trials for renal diseases initiated either primarily or secondary, acute or chronic. In addition, the chemical structure of losartan and its pharmacologic effects are briefly reviewed in this article, together with the collected data on use of this drug in renal diseases. The combined information should shed light on the investigation of losartan and its potential benefits, as well as mechanisms underlying prevention and treatment of cardiovascular and renal diseases.
Acknowledgments
This work was supported in part by NIH grants HL82779 (LZ) and HL83966 (LZ), HL600355, and by NSFC (No: 30871400) (ZX), Jiangsu NSF (BK2006703, 08KJB32001) (ZX, CM), Suzhou Grant (SZS0602, No.90134602, EE134704) (ZX, CM). We apologize to all authors whose work could not be cited due to space limitations.
References
- 1.Ram CVS. Angiotensin Receptor Blockers: Current Status and Future Prospects. Am J Med. 2008;121(8):656–663. doi: 10.1016/j.amjmed.2008.02.038. [DOI] [PubMed] [Google Scholar]
- 2.Billet S, Aguilar F, Baudry C, Clauser E. Role of angiotensin II AT1 receptor activation in cardiovascular diseases. Kidney Int. 2008;74(11):1379–1384. doi: 10.1038/ki.2008.358. [DOI] [PubMed] [Google Scholar]
- 3.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: From physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59(3):251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 4.Ruilope LM. Angiotensin receptor blockers: RAAS blockade and renoprotection. Curr Med Res Opin. 2008;24(5):1285–1293. doi: 10.1185/030079908x291921. [DOI] [PubMed] [Google Scholar]
- 5.Wolf G. Novel aspects of the renin-angiotensin-aldosterone-system. Front Biosci. 2008;13:4993–5005. doi: 10.2741/3058. [DOI] [PubMed] [Google Scholar]
- 6.Carey RM, Padia SH. Angiotensin AT2 receptors: control of renal sodium excretion and blood pressure. Trends Endocrinol Metab. 2008;19(3):84–87. doi: 10.1016/j.tem.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Macconi D, Remuzzi G. Candesartan and renal protection: more than blocking angiotensin type 1 receptor? Kidney Int. 2008;74(9):1112–1114. doi: 10.1038/ki.2008.420. [DOI] [PubMed] [Google Scholar]
- 8.Scott LJ, McCormack PL. Olmesartan medoxomil: a review of its use in the management of hypertension. Drugs. 2008;68(9):1239–1272. doi: 10.2165/00003495-200868090-00005. [DOI] [PubMed] [Google Scholar]
- 9.Duncia JV, Carini DJ, Chiu AT, Johnson AL, Price WA, Wong PC, Wexler RR, Timmermans PB. The discovery of DuP 753, a potent, orally active nonpeptide angiotensin II receptor antagonist. Med Res Rev. 1992;12(2):149–191. doi: 10.1002/med.2610120203. [DOI] [PubMed] [Google Scholar]
- 10.Furakawa Y, Kishimoto S, Nishikawa K. Hypotensive imidazole derivatives and hypotensive imidazole-5-acetic acid derivates. Patients issued to Takeda Chemical Industries Ltd; Osaka: 1982. [Google Scholar]
- 11.Timmermans PB, Carini DJ, Chiu AT, Duncia JV, Price WAJr, Wells GJ, Wong PC, Wexler RR, Johnson AL. Angiotensin II receptor antagonists. From discovery to antihypertensive drugs. Hypertension. 1991;18(5 Suppl):136–142. doi: 10.1161/01.hyp.18.5_suppl.iii136. [DOI] [PubMed] [Google Scholar]
- 12.Smith RD, Chiu AT, Wong PC, Herblin WF, Timmermans PBMWM. Pharmacology of Nonpeptide Angiotensin II Receptor Antagonists. Annu Rev Pharmacol Toxicol. 1992;32:135–165. doi: 10.1146/annurev.pa.32.040192.001031. [DOI] [PubMed] [Google Scholar]
- 13.Stearns RA, Miller RR, Doss GA, Chakravarty PK, Rosegay A, Gatto GJ, Chiu SH. The metabolism of DuP 753, a nonpeptide angiotensin II receptor antagonist, by rat, monkey, and human liver slices. Drug Metab Dispos. 1992;20:281–287. [PubMed] [Google Scholar]
- 14.Christ D, Kilkson T, Wong N, Lam G. Formation and disposition of BXP3174, a pharmacologically active metabolite of the novel angiotensin II receptor antagonist DuP 753. Third North American Meeting of the International Society for the Study of Xenobiotics; 1990. Abstract no.34. [Google Scholar]
- 15.Wong PC, Price WA, Chiu ET. In vivo pharmacology of DuP 753. Am J Hypertens. 1991;4:F288–F298. doi: 10.1093/ajh/4.4.288s. [DOI] [PubMed] [Google Scholar]
- 16.Fierens FLP, Vanderheyden PML, Gaborik Z, Minh TL, Backer JP, Hunyady L, Ijzerman A, Vauquelin G. Lys 199 mutation of the human angiotensin type 1 receptor differentially affects the binding of surmountable and insurmountable non-peptide antagonists. J Renin-Angiotensin-Aldosterone Syst. 2000;1:283–288. doi: 10.3317/jraas.2000.044. [DOI] [PubMed] [Google Scholar]
- 17.Feng YH, Zhou L, Qiu R, Zeng R. Single mutations at Asn295 and Leu305 in the cytoplasmic half of transmembrane -helix domain 7 of the AT1 receptor induce promiscuous agonist specificity for angiotensin II fragments: a pseudo-constitutive activity. Mol Pharmacol. 2005;68(2):347–355. doi: 10.1124/mol.105.011601. [DOI] [PubMed] [Google Scholar]
- 18.Galle J. Reduction of proteinuria with angiotensin receptor blockers. Nat Clin Pract Cardiovasc Med. 2008;5:S36–S43. doi: 10.1038/ncpcardio0806. [DOI] [PubMed] [Google Scholar]
- 19.Fujihara CK, Velho M, Malheiros DM, Zatz R. An extremely high dose of losartan affords superior renoprotection in the remnant model. Kidney Int. 2005;67(5):1913–1924. doi: 10.1111/j.1523-1755.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- 20.Yu C, Gong R, Rifai A, Tolbert EM, Dworkin LD. Long-term, high-dosage candesartan suppresses inflammation and injury in chronic kidney disease: nonhemodynamic renal protection. J Am Soc Nephrol. 2007;18(3):750–759. doi: 10.1681/ASN.2006070770. [DOI] [PubMed] [Google Scholar]
- 21.Burgess E, Muirhead N, Rene de Cotret P, Chiu A, Pichette V, Tobe S SMART (Supra Maximal Atacand Renal Trial) Investigators. Supramaximal dose of candesartan in proteinuric renal disease. J Am Soc Nephrol. 2009;20(4):893–900. doi: 10.1681/ASN.2008040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ingert C, Grima M, Coquard C, Barthelmebs M, Imbs JL. Contribution of angiotensin II internalization to intrarenal angiotensin II levels in rats. Am J Physiol. 2002;283:F1003–F1010. doi: 10.1152/ajprenal.00322.2001. [DOI] [PubMed] [Google Scholar]
- 23.Darby IA, Sernia C. In situ hybridization and immunohistochemistry of renal angiotensinogen in neonatal and adult rat kidneys. Cell Tissue Res. 1995;281:197–206. doi: 10.1007/BF00583388. [DOI] [PubMed] [Google Scholar]
- 24.Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, Zhang S, Cheng T, Inagami T, Ward K, Terreros DA, Lalouel JM. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999;34(6):F1265–F1274. doi: 10.1161/01.hyp.34.6.1265. [DOI] [PubMed] [Google Scholar]
- 25.Kobori H, Nishiyama A, Harrison-Bernard LM, Navar LG. Urinary Angiotensinogen as an Indicator of Intrarenal Angiotensin Status in Hypertension. Hypertension. 2003;41:F42–F49. doi: 10.1161/01.hyp.0000050102.90932.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobori H, Ozawa Y, Suzaki Y, Prieto-Carrasquero MC, Nishiyama A, Shoji T, Cohen EP, Navar LG. Young scholars award lecture: Intratubular angiotensinogen in hypertension and kidney diseases. Am J Hypertens. 2006;19:F541–F550. doi: 10.1016/j.amjhyper.2005.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moe OW, Ujiie K, Star RA, Miller RT, Widell J, Alpern RJ, Henrich WL. Renin expression in renal proximal tubule. J Clin Invest. 1993;91:F774–F779. doi: 10.1172/JCI116296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lantelme P, Rohrwasser A, Gociman B, Hillas E, Cheng T, Petty G, Thomas J, Xiao S, Ishigami T, Herrmann T, Terreros DA, Ward K, Lalouel JM. Effects of dietary sodium and genetic background on angiotensinogen and renin in mouse. Hypertension. 2002;39:F1007–F1014. doi: 10.1161/01.hyp.0000016177.20565.a0. [DOI] [PubMed] [Google Scholar]
- 29.Rohrwasser A, Ishigami T, Gociman B, Lantelme P, Morgan T, Cheng T, Hillas E, Zhang S, Ward K, Bloch-Faure M, Meneton P, Lalouel JM. Renin and kallikrein in connecting tubule of mouse. Kidney Int. 2003;64:2155–2162. doi: 10.1046/j.1523-1755.2003.00302.x. [DOI] [PubMed] [Google Scholar]
- 30.Gomez RA, Lynch KR, Chevalier RL, Everett AD, Johns DW, Wilfong N, Peach MJ, Carey RM. Renin and angiotensinogen gene expression and intrarenal renin distribution during ACE inhibition. Am J Physiol. 1988;254:F900–F906. doi: 10.1152/ajprenal.1988.254.6.F900. [DOI] [PubMed] [Google Scholar]
- 31.Alhenc-Gelas F, Baussant T, Hubert C, Soubrier F, Corvol P. The angiotensin converting enzyme in the kidney. J Hypertens. 1989;7(Suppl):S9–S14. doi: 10.1097/00004872-198909007-00003. [DOI] [PubMed] [Google Scholar]
- 32.Schulz WW, Hagler HK, Buja LM, Erdös EG. Ultrastructural localization of angiotensin I-converting enzyme (EC 3.4.15.1) and neutral metalloendopeptidase (EC 3.4.24.11) in the proximal tubule of the human kidney. Lab Invest. 1988;59:F789–F797. [PubMed] [Google Scholar]
- 33.Lely AT, Hamming I, van Goor H, Navis GJ. Renal ACE2 expression in human kidney disease. J Pathol. 2004;204:F587–F593. doi: 10.1002/path.1670. [DOI] [PubMed] [Google Scholar]
- 34.Gembardt F, Sterner-Kock A, Imboden H, Spalteholz M, Reibitz F, Schultheiss HP, Siems WE, Walther T. Organ-specific distribution of ACE2 mRNA and correlating peptidase activity in rodents. Peptides. 2005;26:F1270–F1277. doi: 10.1016/j.peptides.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye M, Wysocki J, William J, Soler MJ, Cokic I, Batlle D. Glomerular Localization and Expression of Angiotensin-Converting Enzyme 2 and Angiotensin-Converting Enzyme: Implications for Albuminuria in Diabetes. J Am Soc Nephrol. 2006;17:3067–3075. doi: 10.1681/ASN.2006050423. [DOI] [PubMed] [Google Scholar]
- 36.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A Human Homolog of Angiotensin-converting Enzyme. J Biol Chem. 2000;275:F33238–F33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 37.Rella M, Elliot JL, Revett TJ, Lanfear J, Phelan A, Jackson RM, Turner AJ, Hooper NM. Identification and characterisation of the angiotensin converting enzyme-3 (ACE3) gene: a novel mammalian homologue of ACE. BMC Genomics. 2007;8:F194. doi: 10.1186/1471-2164-8-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H, Wada J, Hida K, Tsuchiyama Y, Hiragushi K, Shikata K, Wang H, Lin S, Kanwar YS, Makino H. Transmembrane Glycoprotein, Is a Novel Homolog of ACE2 and Is Developmentally Regulated in Embryonic Kidneys. J Biol Chem. 2001;276:F17132–F17139. doi: 10.1074/jbc.M006723200. [DOI] [PubMed] [Google Scholar]
- 39.Casarini DE, Boim MA, Stella RC, Krieger-Azzolini MH, Krieger JE, Schor N. Angiotensin I-converting enzyme activity in tubular fluid along the rat nephron. Am J Physiol. 1997;272:F405–F409. doi: 10.1152/ajprenal.1997.272.3.F405. [DOI] [PubMed] [Google Scholar]
- 40.Navar LG, Imig JD, Zou L, Wang CT. Intrarenal production of angiotensin II. Semin Nephrol. 1997;17:F412–F422. [PubMed] [Google Scholar]
- 41.Siragy HM, Howell NL, Ragsdale NV, Carey RM. Renal Interstitial Fluid Angiotensin. Hypertension. 1995;25:F1021–F1024. doi: 10.1161/01.hyp.25.5.1021. [DOI] [PubMed] [Google Scholar]
- 42.Nishiyama A, Seth DM, Navar LG. Renal interstitial fluid angiotensin I and angiotensin II concentrations during local angiotensin-converting enzyme inhibition. J Am Soc Nephrol. 2002;13:F2207–F2212. doi: 10.1097/01.asn.0000026610.48842.cb. [DOI] [PubMed] [Google Scholar]
- 43.Cervenka L, Wang CT, Mitchell KD, Navar LG. Proximal Tubular Angiotensin II Levels and Renal Functional Responses to AT1 Receptor Blockade in Nonclipped Kidneys of Goldblatt Hypertensive Rats. Hypertension. 1999;33:F102–F107. doi: 10.1161/01.hyp.33.1.102. [DOI] [PubMed] [Google Scholar]
- 44.Zou LX, Imig JD, Hymel A, Navar LG. Renal Uptake of Circulating Angiotensin II in Val5-Angiotensin II Infused Rats Is Mediated by AT1 Receptor. Am J Hypertens. 1998;11:F570–F578. doi: 10.1016/s0895-7061(97)00410-x. [DOI] [PubMed] [Google Scholar]
- 45.Imig JD, Navar GL, Zou LX, O’Reilly KC, Allen PL, Kaysen JH, Hammond TG, Navar LG. Renal endosomes contain angiotensin peptides, converting enzyme, and AT1A receptors. Am J Physiol. 1999;277:F303–F311. doi: 10.1152/ajprenal.1999.277.2.F303. [DOI] [PubMed] [Google Scholar]
- 46.Zhuo JL, Imig JD, Hammond TG, Orengo S, Benes E, Navar LG. Ang II Accumulation in Rat Renal Endosomes During Ang II-Induced Hypertension: Role of AT1 Receptor. Hypertension. 2002;39:F116–F121. doi: 10.1161/hy0102.100780. [DOI] [PubMed] [Google Scholar]
- 47.Ingelfinger JR. Angiotensin-converting enzyme 2: implications for blood pressure and kidney disease. Curr Opin Nephrol Hypertens. 2009;18(1):79–84. doi: 10.1097/MNH.0b013e32831b70ad. [DOI] [PubMed] [Google Scholar]
- 48.Tufro-McReddie A, Harrison JK, Everett AD, Gomez RA. Ontogeny of type 1 angiotensin II receptor gene expression in the rat. J Clin Invest. 1993;91:F530–F537. doi: 10.1172/JCI116232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bouby N, Hus-Citharel A, Marchetti J, Bankir L, Corvol P, Llorens-Cortes C. Expression of type 1 angiotensin II receptor subtypes and angiotensin II-induced calcium mobilization along the rat nephron. J Am Soc Nephrol. 1997;8:F1658–F1667. doi: 10.1681/ASN.V8111658. [DOI] [PubMed] [Google Scholar]
- 50.Paxton WG, Runge M, Horaist C, Cohen C, Alexander RW, Bernstein KE. Immunohistochemical localization of rat angiotensin II AT1 receptor. Am J Physiol-Renal Physiol. 1993;264:F989–F995. doi: 10.1152/ajprenal.1993.264.6.F989. [DOI] [PubMed] [Google Scholar]
- 51.Miyata N, Park F, Li XF, Cowley AW. Distribution of angiotensin AT 1 and AT 2 receptor subtypes in the rat kidney. Jr Am J Physiol. 1999;277:F437–F446. doi: 10.1152/ajprenal.1999.277.3.F437. [DOI] [PubMed] [Google Scholar]
- 52.Ciuffo GM, Viswanathan M, Seltzer AM, Tsutsumi K, Saavedra JM. Glomerular angiotensin II receptor subtypes during development of rat kidney. Am J Physiol. 1993;265:F264–F271. doi: 10.1152/ajprenal.1993.265.2.F264. (Renal Fluid Electrolyte Physiol 34) [DOI] [PubMed] [Google Scholar]
- 53.Sadjadi J, Puttaparthi K, Welborn MB, 3rd, Rogers TE, Moe O, Clagett GP, Turnage RH, Levi M, Modrall JG. Upregulation of autocrine-paracrine renin-angiotensin systems in chronic renovascular hypertension. J Vasc Surg. 2002;36:F386–F392. doi: 10.1067/mva.2002.125016. [DOI] [PubMed] [Google Scholar]
- 54.Kennefick TM, Oyama TT, Thompson MM, Vora JP, Anderson S. Enhanced renal sensitivity to angiotensin actions in diabetes mellitus in the rat. Am J Physiol. 1996;271:F595–F602. doi: 10.1152/ajprenal.1996.271.3.F595. [DOI] [PubMed] [Google Scholar]
- 55.Ruiz-Ortega M, Gonzalez S, Seron D, Condom E, Bustos C, Largo R, Gonzalez E, Ortiz A, Egido J. ACE inhibition reduces proteinuria, glomerular lesions and extracellular matrix production in a normotensive rat model of immune complex nephritis. Kidney Int. 1995;48:1778–1791. doi: 10.1038/ki.1995.476. [DOI] [PubMed] [Google Scholar]
- 56.Mii A, Shimizu A, Masuda Y, Ishizaki M, Kawachi H, Iino Y, Katayama Y, Fukuda Y. Lab Invest. 2009;89(2):164. doi: 10.1038/labinvest.2008.128. [DOI] [PubMed] [Google Scholar]
- 57.Schunkert H, Ingelfinger JR, Hirsch AT, Tang SS, Litwin SE, Talsness CE, Dzau VJ. Evidence for tissue-specific activation of renal angiotensinogen mRNA expression in chronic stable experimental heart failure. J Clin Invest. 1992;90:F1523–F1529. doi: 10.1172/JCI116020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kobori H, Harrison-Bernard LM, Navar LG. Expression of Angiotensinogen mRNA and Protein in Angiotensin II-Dependent Hypertension. J Am Soc Nephrol. 2001;12:F431–F439. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang TT, Chen M, Lachance S, Delalandre A, Carrière S, Chan JSD. Isoproterenol and 8-bromocyclic adenosine monophosphate stimulate the expression of the angiotensinogen gene in opossum kidney cells. Kidney Int. 1994;46:F703–F710. doi: 10.1038/ki.1994.324. [DOI] [PubMed] [Google Scholar]
- 60.Wang TT, Lachance S, Delalandre A, Carrière S, Chan JSD. Alpha-adrenoceptors and angiotensinogen gene expression in opossum kidney cells. Kidney Int. 1995;48:F139–F145. doi: 10.1038/ki.1995.277. [DOI] [PubMed] [Google Scholar]
- 61.Bader M, Ganten D. Regulation of renin: new evidence from cultured cells and genetically modified mice. J Mol Med. 2000;78(3):F130–F139. doi: 10.1007/s001090000089. [DOI] [PubMed] [Google Scholar]
- 62.Paul M, Mehr AP, Kreutz R. Physiology of the renin-angotensin systems. Physiol Rev. 2006;86:F747–F803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 63.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, Navar LG. Enhancement of Collecting Duct Renin in Angiotensin II–Dependent Hypertensive Rats. Hypertension. 2004;44:F223–F229. doi: 10.1161/01.HYP.0000135678.20725.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prieto-Carrasquero MC, Kobori H, Ozawa Y, Gutierrez A, Seth D, Navar LG. AT1 receptor-mediated enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Am J T HYPERTENSIVE RATS Physiol Renal Physiol. 2005;289:F632–F637. doi: 10.1152/ajprenal.00462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kato I, Takada Y, Nishimura K, Hiwada K, Kokubu T. Increased urinary excretion of angiotensin converting enzyme in patients with renal diseases. J Clin Chem Clin Biochem. 1982;20:473–476. doi: 10.1515/cclm.1982.20.7.473. [DOI] [PubMed] [Google Scholar]
- 66.Pedraza-Chaverri J, Cruz C, del Socorro-Blancas M, Hernandez-Pando R, Ibarra-Rubio ME, Larriva-Sahd J, Tapia E. Renal Fail. 1995;17:377. doi: 10.3109/08860229509037603. [DOI] [PubMed] [Google Scholar]
- 67.Metzger R, Bohle RM, Pauls K, Eichner G, Alhenc-Gelas F, Danilov SM, Franke FE. Angiotensin-converting enzyme in non-neoplastic kidney diseases. Kidney Int. 1999;56:F1442–F1454. doi: 10.1046/j.1523-1755.1999.00660.x. [DOI] [PubMed] [Google Scholar]
- 68.Largo R, Gómez-Garre D, Soto K, Marron B, Blanco J, Gazapo RM, Plaza JJ, Eqido J. Angiotensin-converting enzyme is upregulated in the proximal tubules of rats with intense proteinuria. Hypertension. 1999;33:F732–F739. doi: 10.1161/01.hyp.33.2.732. [DOI] [PubMed] [Google Scholar]
- 69.Mezzano SA, Aros CA, Droguett A, Burgos ME, Ardiles LG, Flores CA, Carpio D, Vio CP, Ruiz-Ortega M, Egido J. Renal angiotensin II up-regulation and myofibroblast activation in human membranous nephropathy. Kidney Int Suppl. 2003a;64:S39–S45. doi: 10.1046/j.1523-1755.64.s86.8.x. [DOI] [PubMed] [Google Scholar]
- 70.Mezzano S, Droguett A, Burgos ME, Ardiles LG, Flores CA, Aros CA, Caorsi I, Vio CP, Ruiz-Ortega M, Egido J. Renin-angiotensin system activation and interstitial inflammation in human diabetic nephropathy. Kidney Int Suppl. 2003b;64:S64–S70. doi: 10.1046/j.1523-1755.64.s86.12.x. [DOI] [PubMed] [Google Scholar]
- 71.Vío CP, Jeanneret VA. Local induction of angiotensin-converting enzyme in the kidney as a mechanism of progressive renal diseases. Kidney Int Suppl. 2003;86:S57–S63. doi: 10.1046/j.1523-1755.64.s86.11.x. [DOI] [PubMed] [Google Scholar]
- 72.Kobori H, Ozawa Y, Suzaki Y, Nishiyama A. Enhanced Intra-renal Angiotensinogen Contributes to Early Renal Injury in Spontaneously Hypertensive Rats. J Am Soc Nephrol. 2005;16:2073–2080. doi: 10.1681/ASN.2004080676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nishiyama A, Seth DM, Navar LG. Angiotensin II type 1 receptor-mediated augmentation of renal interstitial fluid angiotensin II in angiotensin II-induced hypertension. J Hypertens. 2003;21:1897–1903. doi: 10.1097/00004872-200310000-00017. [DOI] [PubMed] [Google Scholar]
- 74.Ingert C, Grima M, Coquard C, Barthelmebsm M, Imbs JL. Effects of dietary salt changes on renal renin-angiotensin system in rats. Am J Physiol. 2002;283:F995–F1002. doi: 10.1152/ajprenal.00321.2001. [DOI] [PubMed] [Google Scholar]
- 75.Zhuo JL, Imig JD, Hammond TG, Orengo S, Benes E, Navar LG. Ang II Accumulation in Rat Renal Endosomes During Ang II-Induced Hypertension. Hypertension. 2002;39:F116–F121. doi: 10.1161/hy0102.100780. [DOI] [PubMed] [Google Scholar]
- 76.Zou LX, Imig JD, Hymel A, Navar LG. Renal Uptake of Circulating Angiotensin II in Val5-Angiotensin II Infused Rats Is Mediated by AT1 Receptor. Am J Hypertens. 1998;11:F570–F578. doi: 10.1016/s0895-7061(97)00410-x. [DOI] [PubMed] [Google Scholar]
- 77.Yamamoto T, Hayashi K, Matsuda H, Kubota E, Tanaka H, Ogasawara Y, Nakamoto H, Suzuki H, Saruta T, Kajiya F. In vivo visualization of angiotensin II- and tubuloglomerular feedback-mediated renal vasoconstriction. Kidney Int. 2001;60:F364–F369. doi: 10.1046/j.1523-1755.2001.00808.x. [DOI] [PubMed] [Google Scholar]
- 78.Inkyo-Hayasaka K, Sakai T, Kobayashi N, Shirato I, Tomino Y. Three-dimensional analysis of the whole mesangium in the rat. Kidney Int. 1996;50:F672–F683. doi: 10.1038/ki.1996.364. [DOI] [PubMed] [Google Scholar]
- 79.Kriz W, Elger M, Mundel P, Lemley KV. Structure-stabilizing forces in the glomerular tuft. J Am Soc Nephrol. 1995;5:F1731–F1739. doi: 10.1681/ASN.V5101731. [DOI] [PubMed] [Google Scholar]
- 80.Foidart J, Sraer J, Delarue F, Mahieu P, Ardaillou R. Evidence for mesangial glomerular receptors for angiotensin II linked to mesangial cell contractility. FEBS Lett. 1980;121:F333–F339. doi: 10.1016/0014-5793(80)80375-9. [DOI] [PubMed] [Google Scholar]
- 81.Madhun ZT, Ernsberger P, Ke FC, Zhou J, Hopfer U, Douglas JG. Signal transduction mediated by angiotensin II receptor subtypes expressed in rat renal mesangial cells. Regul Pept. 1993;44:F149–F157. doi: 10.1016/0167-0115(93)90238-4. [DOI] [PubMed] [Google Scholar]
- 82.Cervenka L, Navar LG. Renal responses of the nonclipped kidney of two-kidney/one-clip Goldblatt hypertensive rats to type 1 angiotensin II receptor blockade with candesartan. J Am Soc Nephrol. 1999;10(Suppl 11):S197–S201. [PubMed] [Google Scholar]
- 83.Bidani AK, Griffin KA. Long-term renal consequences of hypertension for normal and diseased kidneys. Curr Opin Nephrol Hypertens. 2002;11:F73–F80. doi: 10.1097/00041552-200201000-00011. [DOI] [PubMed] [Google Scholar]
- 84.Tamaki T, Nishiyama A, Yoshida H, He H, Fukui T, Yamamoto A, Aki Y, Kimura S, Iwao H, Miyatake A. Effects of EXP3174, a non-peptide angiotensin II receptor antagonist, on renal hemodynamics and renal function in dogs. Eur J Pharmacol. 1993;236:F15–F21. doi: 10.1016/0014-2999(93)90221-3. [DOI] [PubMed] [Google Scholar]
- 85.Omoro SA, Majid DSA, El Dahr SS, Navar LG. Roles of ANG II and bradykinin in the renal regional blood flow responses to ACE inhibition in sodium-depleted dogs. Am J Physiol. 2000;279:F289–F293. doi: 10.1152/ajprenal.2000.279.2.F289. [DOI] [PubMed] [Google Scholar]
- 86.Hall JE, Guyton AC, Smith MJ, Jr, Coleman TG. Chronic blockade of angiotensin II formation during sodium deprivation. Am J Physiol. 1979;237:F424–F432. doi: 10.1152/ajprenal.1979.237.6.F424. [DOI] [PubMed] [Google Scholar]
- 87.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: From physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59(3):F251–F287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 88.Navar LG. Renal autoregulation: perspectives from whole kidney and single nephron studies. Am J Physiol. 1978;234:F357–F370. doi: 10.1152/ajprenal.1978.234.5.F357. [DOI] [PubMed] [Google Scholar]
- 89.Inscho EW, Imig JD, Deichmann PC, Cook AK. Candesartan cilexetil protects against loss of autoregulatory efficiency in angiotensin II-infused rats. J Am Soc Nephrol. 1999;10(Suppl 11):S178–S183. [PubMed] [Google Scholar]
- 90.Persson P, Ehmke H, Kirchheim H. Influence of the renin—angiotensin system on the autoregulation of renal blood flow and glomerular filtration rate in conscious dogs. Acta Physiol Scand. 1988;134:F1–F7. doi: 10.1111/j.1748-1716.1988.tb08452.x. [DOI] [PubMed] [Google Scholar]
- 91.Navar LG, Inscho EW, Majid SA, Imig JD, Harrison-Bernard LM, Mitchell KD. Paracrine regulation of the renal microcirculation. Physiol Rev. 1996;76:F425–F536. doi: 10.1152/physrev.1996.76.2.425. [DOI] [PubMed] [Google Scholar]
- 92.Nishiyama A, Rahman M, Inscho EW. Role of Interstitial ATP and Adenosine in the Regulation of Renal Hemodynamics and Microvascular Function. Hypertens Res. 2004;27:F791–F804. doi: 10.1291/hypres.27.791. [DOI] [PubMed] [Google Scholar]
- 93.Loutzenhiser R, Griffin K, Williamson G, Bidani A. Renal autoregulation: new perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1153–R1167. doi: 10.1152/ajpregu.00402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Traynor T, Yang T, Huang YG, Krege JH, Briggs JP, Smithies O, Schnermann J. Tubuloglomerular feedback in ACE-deficient mice. Am J Physiol. 1999;276:F751–F757. doi: 10.1152/ajprenal.1999.276.5.F751. [DOI] [PubMed] [Google Scholar]
- 95.Khavandi K, Greenstein AS, Sonoyama K, Withers S, Price A, Malik RA, Heagerty AM. Myogenic tone and small artery remodelling: insight into diabetic nephropathy. Nephrology Dialysis Transplantation. 2008 doi: 10.1093/ndt/gfn583. abstract. [DOI] [PubMed] [Google Scholar]
- 96.Peti-Peterdi J, Bell PD. Regulation of macula densa Na:H exchange by angiotensin II. Kidney Int. 1998;54:F2021–F2028. doi: 10.1046/j.1523-1755.1998.00200.x. [DOI] [PubMed] [Google Scholar]
- 97.Kovács G, Peti-Peterdi J, Rosivall L, Bell PD. Angiotensin II directly stimulates macula densa Na-2Cl-K cotransport via apical AT1 receptors. Am J Physiol. 2002;282:F301–F306. doi: 10.1152/ajprenal.00129.2001. [DOI] [PubMed] [Google Scholar]
- 98.Braam B, Navar LG, Mitchell KD. Modulation of Tubuloglomerular Feedback by Angiotensin II Type 1 Receptors During the Development of Goldblatt Hypertension. Hypertension. 1995;25:F1232–F1237. doi: 10.1161/01.hyp.25.6.1232. [DOI] [PubMed] [Google Scholar]
- 99.Ichihara A, Inscho EW, Imig JD, Michel RE, Navar LG. Role of renal nerves in afferent arteriolar reactivity in angiotensin-induced hypertension. Hypertension. 1997;29:442–449. doi: 10.1161/01.hyp.29.1.442. [DOI] [PubMed] [Google Scholar]
- 100.Just A. Mechanisms of renal blood flow autoregulation: dynamics and contributions. Am J Physiol Regulatory Integrative Comp Physiol. 2007;292(1):R1–R17. doi: 10.1152/ajpregu.00332.2006. [DOI] [PubMed] [Google Scholar]
- 101.Navar LG, Prieto-Carrasquero MC, Kobori H. Handbook of Biologically Active Peptides. 2006 [Google Scholar]
- 102.Staahltoft D, Nielsen S, Janjua NR, Christensen S, Skott O, Marcussen N, Jonassen TE. Losartan treatment normalizes renal sodium and water handling in rats with mild congestive heart failure. Am J Physiol Renal Physiol. 2002;282:F307–F315. doi: 10.1152/ajprenal.00132.2001. [DOI] [PubMed] [Google Scholar]
- 103.Navar LG, Harrison-Bernard LM, Imig JD, Cervenka L, Mitchell KD. Renal responses to AT1 receptor blockade. Am J Hypertens. 2007;13:45S–54S. doi: 10.1016/s0895-7061(99)00248-4. [DOI] [PubMed] [Google Scholar]
- 104.Hashimoto S, Adams JW, Bernstein KE, Schnermann J. Micropuncture determination of nephron function in mice without tissue angiotensin-converting enzyme. Am J Physiol. 2005;288:F445–F452. doi: 10.1152/ajprenal.00297.2004. [DOI] [PubMed] [Google Scholar]
- 105.Saccomani G, Mitchell KD, Navar LG. Angiotensin II stimulation of Na+-H+ exchange in proximal tubule cells. Am J Physiol Renal Fluid Electrolyte Physiol. 1990;258:F1188–F1195. doi: 10.1152/ajprenal.1990.258.5.F1188. [DOI] [PubMed] [Google Scholar]
- 106.Liu FY, Cogan MG. Angiotensin II stimulation of hydrogen ion secretion in the rat early proximal tubule: modes of action, mechanism, and kinetics. J Clin Invest. 1988;82:601–607. doi: 10.1172/JCI113638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Garvin JL. Angiotensin stimulates bicarbonate transport and Na+/K+ ATPase in rat proximal straight tubules. J Am Soc Nephrol. 1991;1:1146–1152. doi: 10.1681/ASN.V1101146. [DOI] [PubMed] [Google Scholar]
- 108.Eiam-Ong S, Hilden SA, Johns CA, Madias NE. Stimulation of basolateral Na+-HCO3- cotransporter by angiotensin II in rabbit renal cortex. Am J Physiol. 1993;265:F195–F203. doi: 10.1152/ajprenal.1993.265.2.F195. [DOI] [PubMed] [Google Scholar]
- 109.Mitchell KD, Braam B, Navar LG. Hypertensinogenic mechanisms mediated by renal actions of renin-angiotensin system. Hypertension. 1992;19:I18–I27. doi: 10.1161/01.hyp.19.1_suppl.i18. [DOI] [PubMed] [Google Scholar]
- 110.Gluck SL, Lee BS, Wang SP, Underhill D, Nemoto J, Holliday LS. Plasma membrane V-ATPases in proton-transporting cells of the mammalian kidney and osteoclast. Acta Physiol Scand Suppl. 1998;643:203–212. [PubMed] [Google Scholar]
- 111.Kwon TH, Nielsen J, Kim YH, Knepper MA, Frokiaer J, Nielsen S. Regulation of sodium transporters in the thick ascending limb of rat kidney: response to angiotensin II. Am J Physiol Renal Physiol. 2003;285(1):F152–F165. doi: 10.1152/ajprenal.00307.2002. [DOI] [PubMed] [Google Scholar]
- 112.Leong PK, Devillez A, Sandberg MB, Yang LE, Yip DK, Klein JB, McDonough AA. Effects of ACE inhibition on proximal tubule sodium transport. Am J Physiol Renal Physiol. 2006;290:F854–F863. doi: 10.1152/ajprenal.00353.2005. [DOI] [PubMed] [Google Scholar]
- 113.Quan A, Baum M. Endogenous production of angiotensin II modulates rat proximal tubule transport. J Clin Invest. 1996;97(12):2878–2882. doi: 10.1172/JCI118745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Soleimani M, Grassl SM, Aronson PS. Stoichiometry of Na+-HCO3– cotransport in basolateral membrane vesicles isolated from rabbit renal cortex. J Clin Invest. 1987;79:1276–1280. doi: 10.1172/JCI112948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Boron WF. Acid-base transport by the renal proximal tubule. J Am Soc Nephrol. 2006;17:2368–2382. doi: 10.1681/ASN.2006060620. [DOI] [PubMed] [Google Scholar]
- 116.Pollock CA. Albumin transport and processing by the proximal tubule: physiology and pathophysiology. Curr Opin Nephrol Hypertens. 2007;16:359–364. doi: 10.1097/MNH.0b013e3281eb9059. [DOI] [PubMed] [Google Scholar]
- 117.Good DW. The thick ascending limb as a site of renal bicarbonate reabsorption. Semin Nephrol. 1993;13:225–235. [PubMed] [Google Scholar]
- 118.Good DW, George T, Wang DH. Angiotensin II inhibits HCO3– absorption via a cytochrome P-450-dependent pathway in MTAL. Am J Physiol Renal Physiol. 1999;276:F726–F736. doi: 10.1152/ajprenal.1999.276.5.F726. [DOI] [PubMed] [Google Scholar]
- 119.Lerolle N, Bourgeois S, Leviel F, Lebrun G, Paillard M, Houillier P. Angiotensin II inhibits NaCl absorption in the rat medullary thick ascending limb. Am J Physiol Renal Physiol. 2004;287:F404–F410. doi: 10.1152/ajprenal.00265.2003. [DOI] [PubMed] [Google Scholar]
- 120.Amemiya M, Loffing J, Lotscher M, Kaissling B, Alpern RJ, Moe OW. Expression of NHE-3 in the apical membrane of rat renal proximal tubule and thick ascending limb. Kidney Int. 1995;48:1206–1215. doi: 10.1038/ki.1995.404. [DOI] [PubMed] [Google Scholar]
- 121.Lu M, Zhu Y, Balazy M, Reddy KM, Falck JR, Wang W. Effect of angiotensin II on the apical K+ channel in the thick ascending limb of the rat kidney. J Gen Physiol. 1996;108:537–547. doi: 10.1085/jgp.108.6.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ecelbarger CA, Terris J, Hoyer JR, Nielsen S, Wade JB, Knepper MA. Localization and regulation of the rat renal Na+-K+-2Cl- cotransporter, BSC-1. Am J Physiol Renal Fluid Electrolyte Physiol. 1996;271:F619–F628. doi: 10.1152/ajprenal.1996.271.3.F619. [DOI] [PubMed] [Google Scholar]
- 123.Robert AF, Mark A. Mouse models and the urinary concentrating mechanism in the new millennium. Physiol Rev. 2007;87:1083–1112. doi: 10.1152/physrev.00053.2006. [DOI] [PubMed] [Google Scholar]
- 124.Wang T, Giebisch G. Effects of angiotensin II on electrolyte transport in the early and late distal tubule in rat kidney. Am J Physiol. 1996;40:F143–F149. doi: 10.1152/ajprenal.1996.271.1.F143. [DOI] [PubMed] [Google Scholar]
- 125.Barreto-Chaves ML, Mello-Aires M. Effect of luminal angiotensin II and ANP on early and late cortical distal tubule HCO3- reabsorption. Am J Physiol. 1996;271:F977–F984. doi: 10.1152/ajprenal.1996.271.5.F977. [DOI] [PubMed] [Google Scholar]
- 126.Wang T, Giebisch G. Effects of angiotensin II on electrolyte transport in the early and late distal tubule in rat kidney. Am J Physiol. 1996;271:F143–F149. doi: 10.1152/ajprenal.1996.271.1.F143. [DOI] [PubMed] [Google Scholar]
- 127.Levine DZ, Iacovitti M, Luck B, Hincke MT, Burns KD, Fryer JN. Surviving rat distal tubule bicarbonate reabsorption: effects of chronic AT1 blockade. Am J Physiol. 2000;278:F476–F483. doi: 10.1152/ajprenal.2000.278.3.F476. [DOI] [PubMed] [Google Scholar]
- 128.Sandberg MB, Riquier ADM, Pihakaski-Maunsbach K, McDonough AA, Maunsbach AB. ANG II provokes acute trafficking of distal tubule Na+-Cl– cotransporter to apical membrane. Am J Physiol Renal Physiol. 2007;293:F662–F669. doi: 10.1152/ajprenal.00064.2007. [DOI] [PubMed] [Google Scholar]
- 129.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT1 receptors. J Am Soc Nephrol. 2002;13:1131–1135. doi: 10.1097/01.asn.0000013292.78621.fd. [DOI] [PubMed] [Google Scholar]
- 130.Brooks HL, Allred AJ, Beutler KT, Coffman TM, Knepper MA. Targeted proteomic profiling of renal Na+ transporter and channel abundances in angiotensin II type 1a receptor knockout mice. Hypertension. 2002;39:470–473. doi: 10.1161/hy02t2.102959. [DOI] [PubMed] [Google Scholar]
- 131.Wei Y, Wang WH. Angiotensin II stimulates basolateral K channels in rat cortical collecting ducts. Am J Physiol Renal Physiol. 2003;284:F175–F181. doi: 10.1152/ajprenal.00211.2002. [DOI] [PubMed] [Google Scholar]
- 132.Pech V, Kim YH, Weinstein AM, Everett LA, Pham TD, Wall SM. Angiotensin II increases chloride absorption in the cortical collecting duct in mice through a pendrin-dependent mechanism. Am J Physiol Renal Physiol. 2007;292:F914–F920. doi: 10.1152/ajprenal.00361.2006. [DOI] [PubMed] [Google Scholar]
- 133.Pech V, Zheng W, Pham TD, Verlander JW, Wall SM. Angiotensin II Activates H+-ATPase in Type A Intercalated Cells. J Am Soc Nephrol. 2008;19(1):84–91. doi: 10.1681/ASN.2007030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Oliverio MI, Delnomdedieu M, Best CF, Li P, Morris M, Callahan MF, Johnson GA, Smithies O, Coffman TM. Abnormal water metabolism in mice lacking the type 1A receptor for ANG II. Am J Physiol. 2000;278:F75–F82. doi: 10.1152/ajprenal.2000.278.1.F75. [DOI] [PubMed] [Google Scholar]
- 135.Kato A, Klein JD, Zhang C, Sands JM. Angiotensin II increases vasopressin-stimulated facilitated urea permeability in rat terminal IMCDs. Am J Physiol. 2000;279:F835–F840. doi: 10.1152/ajprenal.2000.279.5.F835. [DOI] [PubMed] [Google Scholar]
- 136.Sands JM. Molecular mechanisms of urea transport. J Membr Biol. 2003;191(3):149–163. doi: 10.1007/s00232-002-1053-1. [DOI] [PubMed] [Google Scholar]
- 137.Lee YJ, Song IK, Jang KJ, Nielsen J, Frøkiær J, Nielsen S, Kwon TH. Increased AQP2 targeting in primary cultured IMCD cells in response to angiotensin II through AT1 receptor. Am J Physiol Renal Physiol. 2007;292:F340–F350. doi: 10.1152/ajprenal.00090.2006. [DOI] [PubMed] [Google Scholar]
- 138.Blount MA, Sands JM, Kent KJ, Smith TD, Price SR, Klein JD. Candesartan augments compensatory changes in medullary transport proteins in the diabetic rat kidney. Am J Physiol Renal Physiol. 2008;294(6):F1448–F1452. doi: 10.1152/ajprenal.00600.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wall SM, Fischer MP, Glapion DM, Calzada MDL. ANG II reduces net acid secretion in rat outer medullary collecting duct. Am J Physiol Renal Physiol. 2003;285:F930–F937. doi: 10.1152/ajprenal.00400.2002. [DOI] [PubMed] [Google Scholar]
- 140.Valles P, Wysocki J, Salabat MR, Cokic I, Ye M, LaPointe MS, Batlle D. Angiotensin II increases H+-ATPase B1 subunit expression in medullary collecting ducts. Hypertension. 2005;45:818–823. doi: 10.1161/01.HYP.0000154787.42718.a4. [DOI] [PubMed] [Google Scholar]
- 141.Navar LG, Inscho EW, Majid SA, Imig JD, Harrison-Bernard LM, Mitchell KD. Paracrine regulation of the renal microcirculation. Physiol Rev. 1996;76:425–536. doi: 10.1152/physrev.1996.76.2.425. [DOI] [PubMed] [Google Scholar]
- 142.Robert AF, Knepper MA. Mouse models and the urinary concentrating mechanism in the new millennium. Physiol Rev. 2007;87:1083–1112. doi: 10.1152/physrev.00053.2006. [DOI] [PubMed] [Google Scholar]
- 143.Wong NL, Tsui JK. Angiotensin II upregulates the expression of vasopressin V2 mRNA in the inner medullary collecting duct of the rat. Metabolism. 2003;52:290–295. doi: 10.1053/meta.2003.50047. [DOI] [PubMed] [Google Scholar]
- 144.Kanwar YS, Liu ZZ, Kashihara N, Wallner EI. Current status of the structural and functional basis of glomerular filtration and proteinuria. Semin Nephrol. 1991;11:390–413. [PubMed] [Google Scholar]
- 145.Haraldsson B, Sörensson J. Why do we not all have proteinuria? An update of our current understanding of the glomerular barrier. News Physiol Sci. 2004;19:7–10. doi: 10.1152/nips.01461.2003. [DOI] [PubMed] [Google Scholar]
- 146.Farbom P, Wahlstrand B, Almgren P, Skrtic S, Lanke J, Weiss L, Kjeldsen S, Hedner T, Melander O. Interaction between renal function and microalbuminuria for cardiovascular risk in hypertension: The nordic diltiazem study. Hypertension. 2008;52:115–122. doi: 10.1161/HYPERTENSIONAHA.107.109264. [DOI] [PubMed] [Google Scholar]
- 147.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. Effect of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 148.Khosla N, Bakris G. Lessons learned from recent hypertension trials about kidney disease. Clin J Am Soc Nephrol. 2006;1:229–235. doi: 10.2215/CJN.00840805. [DOI] [PubMed] [Google Scholar]
- 149.Isogai S, Mogami K, Shiina N, Yoshino G. Nephron. 1999;83:53. doi: 10.1159/000045473. [DOI] [PubMed] [Google Scholar]
- 150.Jarad G, Cunningham J, Shaw AS, Mine JH. Proteinuria precedes podocyte abnormalities inLamb2−/− mice, implicating the glomerular basement membrane as an albumin barrier. J Clin Invest. 2006;116(8):2272–2279. doi: 10.1172/JCI28414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yavuz DG, Ersoz O, Kucukkaya B, Budak Y, Ahiskali R, Ekiciol G, Emerk K, Akalin S. The effect of losartan and captopril on glomerular basement membrane anionic charge in a diabetic rat model. J Hypert. 1999;17(8):1217–1223. doi: 10.1097/00004872-199917080-00023. [DOI] [PubMed] [Google Scholar]
- 152.Deyneli O, Yavuz D, Velioglu A, Cacina H, Aksoy N, Haklar G, Taga Y, Akalm S. Effects of ACE Inhibition and Angiotensin II Receptor Blockade on Glomerular Basement Membrane Protein Excretion and Charge Selectivity in Type 2 Diabetic Patients. J Renin Angiotensin Aldosterone Syst. 2006;7(2):98–103. doi: 10.3317/jraas.2006.016. [DOI] [PubMed] [Google Scholar]
- 153.Raats CJ, Van Den Born J, Berden JH. Glomerular heparan sulfate alterations: Mechanisms and relevance for proteinuria. Kidney Int. 2000;57:385–400. doi: 10.1046/j.1523-1755.2000.00858.x. [DOI] [PubMed] [Google Scholar]
- 154.Brinkkoetter PT, Holtgrefe S, van der Woude FJ, Yard BA. Angiotensin II type 1-receptor mediated changes in heparan sulfate proteoglycans in human SV40 transformed podocytes. J Am Soc Nephrol. 2004;15:33–40. doi: 10.1097/01.asn.0000102476.50041.44. [DOI] [PubMed] [Google Scholar]
- 155.Van Det NF, Tamsma JT, van den Born J, Verhagen NAM, van den Heuvel LPWJ, Lowik WGM, Berden JHM, Bruijn JA, Daha MR, van der Woude FJ. Differential effects of angiotensin II and transforming growth factor beta on the production of heparan sulfate proteoglycan by mesangial cells in vitro. J Am Soc Nephrol. 1996;7:1015–1023. doi: 10.1681/ASN.V771015. [DOI] [PubMed] [Google Scholar]
- 156.Parish CR, Freeman C, Hulett MD. Heparanase: A key enzyme involved in cell invasion. Biochim Biophys Acta. 2001;1471:M99–M108. doi: 10.1016/s0304-419x(01)00017-8. [DOI] [PubMed] [Google Scholar]
- 157.Kramer A, van den Hoven M, Rops A, Wijnhoven T, van der Heuvel L, Lensen J, van Kuppevelt T, van Goor H, van der Vlag J, Navis G, Berden JH. Induction of glomerular heparanase expression in rats with Adriamycin nephropathy is regulated by reactive oxygen species and the rennin-angiotensin system. J Am Soc Nephrol. 2006;17:2513–2520. doi: 10.1681/ASN.2006020184. [DOI] [PubMed] [Google Scholar]
- 158.Raats CJ, Bakker MA, van den Born J, Berden JH. Hydroxyl radicals depolymerize glomerular heparan sulfate in vitro and in experimental nephrotic syndrome. J Biol Chem. 1997;272:26734–26741. doi: 10.1074/jbc.272.42.26734. [DOI] [PubMed] [Google Scholar]
- 159.Wolf G, Haberstroh U, Neilson EG. Angiotensin II stimulates the proliferation and biosynthesis of type I collagen in cultured murine mesangial cells. Am J Pathol. 1992;140:95–107. [PMC free article] [PubMed] [Google Scholar]
- 160.Chen S, Lee JS, Iglesias-de la Cruz MC, Kasama Y, Izquierdo-Lahuerta A, Wolf G, Ziyadeh FN. Angiotensin II stimulates alpha3(IV) collagen production in mouse podocytes via TGF-beta and VEGF signaling: Implications for diabetic nephropathy. Nephrol Dial Transplant. 2005;20:1320–1328. doi: 10.1093/ndt/gfh837. [DOI] [PubMed] [Google Scholar]
- 161.Rizkalla B, Forbes JM, Cooper ME, Cao Z. Increased renal vascular endothelial growth factor and angiopoietins by angiotensin II infusion is mediated by both AT1 and AT2 receptors. J Am Soc Nephrol. 2003;14:3061–3071. doi: 10.1097/01.asn.0000099374.58607.c9. [DOI] [PubMed] [Google Scholar]
- 162.Daniel C. Blocking of angiotensin II is more than blocking of transforming growth factor-β. Kidney Int. 2008;74:551–553. doi: 10.1038/ki.2008.290. [DOI] [PubMed] [Google Scholar]
- 163.Li YJ, Kang YS, Dai CS, Kiss LP, Wen XY, Liu YH. Epithelial-to-mesenchymal transition is a potential pathway leading to podocyte dysfunction and proteinuria. Am J Pathol. 2008;172(2):299–308. doi: 10.2353/ajpath.2008.070057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lenz O, Elliot SJ, Stetler-Stevenson WG. Matrix metalloproteinases in renal development and disease. J Am Soc Nephrol. 2000;11:574–581. doi: 10.1681/ASN.V113574. [DOI] [PubMed] [Google Scholar]
- 165.Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K. Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- 166.Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83:253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- 167.Mifsud SA, Allen TJ, Bertram JF, Hulthen UL, Kelly DJ, Cooper ME, Wilkinson-Berka JL, Gilbert RE. Podocyte foot process broadening in experimental diabetic nephropathy: amelioration with renin-angiotensin blockade. Diabetologia. 2001;44:878–882. doi: 10.1007/s001250100561. [DOI] [PubMed] [Google Scholar]
- 168.Marshall SM. The podocyte: a potential therapeutic target in diabetic nephropathy? Curr Pharm Des. 2007;13(26):2713–2720. doi: 10.2174/138161207781662957. [DOI] [PubMed] [Google Scholar]
- 169.Davis BJ, Cao Z, de Gasparo M, Kawachi H, Cooper ME, Allen TJ. Disparate effects of angiotensin II antagonists and calcium channel blockers on albuminuria in experimental diabetes and hypertension: potential role of nephrin. J Hypertens. 2003;21:209–216. doi: 10.1097/00004872-200301000-00031. [DOI] [PubMed] [Google Scholar]
- 170.Haraldsson B, Nyström J, Deen WM. Properties of the Glomerular Barrier and Mechanisms of Proteinuria. Physiol Rev. 2008;88:451–487. doi: 10.1152/physrev.00055.2006. [DOI] [PubMed] [Google Scholar]
- 171.Suzuki K, Han GD, Miyauchi N, Hashimoto T, Nakatsue T, Fujioka Y, Koike H, Shimizu F, Kawachi H. Angiotensin II type 1 and type 2 receptors play opposite roles in regulating the barrier function of kidney glomerular capillary wall. Am J Pathol. 2007;170:1841–1853. doi: 10.2353/ajpath.2007.060484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jia J, Ding G, Zhu J, Chen C, Liang W, Franki N, Singhal PC. Angiotensin II infusion induces nephrin expression changes and podocyte apoptosis. Am J Nephrol. 2008;28(3):500–507. doi: 10.1159/000113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wolf G, Neilson EG. Angiotensin II as a renal growth factor. J Am Soc Nephrol. 1993;3:1531–1540. doi: 10.1681/ASN.V391531. [DOI] [PubMed] [Google Scholar]
- 174.Egido J. Vasoactive hormones and renal sclerosis. Kidney Int. 1996;49:578–597. doi: 10.1038/ki.1996.82. [DOI] [PubMed] [Google Scholar]
- 175.Ruiz-Ortega M, Egido J. Angiotensin II modulates cell growth-related events and synthesis of matrix proteins in renal interstitial fibroblasts. Kidney Int. 1997;52:1497–1510. doi: 10.1038/ki.1997.480. [DOI] [PubMed] [Google Scholar]
- 176.Klahr S, Schreiner G, Ichikawa I. The progression of renal disease. N Engl J Med. 1988;318:1651–1666. doi: 10.1056/NEJM198806233182505. [DOI] [PubMed] [Google Scholar]
- 177.Mihailovic-Stanojevic N, Jovovic D, Miloradovic Z, Grujic-Milanovic J, Jerkic M, Markovic-Lipkovski J. Reduced progression of adriamycin nephropathy in spontaneously hypertensive rats treated by losartan. Nephrol Dial Transplant. 2009;24(4):1142–1150. doi: 10.1093/ndt/gfn596. [DOI] [PubMed] [Google Scholar]
- 178.Piecha G, Koleganova N, Gross ML, Geldyyev A, Adamczak M, Ritz E. Regression of glomerulosclerosis in subtotally nephrectomized rats: effects of monotherapy with losartan, spironolactone, and their combination. Am J Physiol Renal Physiol. 2008;295(1):137–144. doi: 10.1152/ajprenal.00065.2008. [DOI] [PubMed] [Google Scholar]
- 179.Teles F, Machado FG, Ventura BH, Malheiros DM, Fujihara CK, Silva LF, Zatz R. Regression of glomerular injury by losartan in experimental diabetic nephropathy. Kidney Int. 2009;75(1):72–79. doi: 10.1038/ki.2008.528. [DOI] [PubMed] [Google Scholar]
- 180.Matsubara H. Pathophysiological role of angiotensin II type 2 receptor in cardiovascular and renal diseases. Circ Res. 1998;83:F1182–F1191. doi: 10.1161/01.res.83.12.1182. [DOI] [PubMed] [Google Scholar]
- 181.Gomez-Garre D, Ruiz-Ortega M, Ortego M, Largo R, Lopez-Armada MJ, Plaza JJ, Gonzalez E, Egido J. Effects and interactions of endothelin-1 and angiotensin II on matrix protein expression and synthesis and mesangial cell growth. Hypertension. 1996;27:885–892. doi: 10.1161/01.hyp.27.4.885. [DOI] [PubMed] [Google Scholar]
- 182.Wolf G, Ziyadeh FN. The role of angiotensin II in diabetic nephropathy: emphasis on nonhemodynamic mechanisms. Am J Kidney Dis. 1997;29:153–163. doi: 10.1016/s0272-6386(97)90023-8. [DOI] [PubMed] [Google Scholar]
- 183.Peters H, Noble NA. Angiotensin II and L-arginine in tissue fibrosis: More than blood pressure. Kidney Int. 1997;51:1481–1486. doi: 10.1038/ki.1997.203. [DOI] [PubMed] [Google Scholar]
- 184.Kagami S, Border WA, Miller DE, Nobl NA. Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor- expression in rat glomerular mesangial cells. J Clin Invest. 1994;93:2431–2437. doi: 10.1172/JCI117251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shihab FS, Bennett WM, Tanner AM, Andoh TF. Angiotensin II blockade decreases TGF-beta1 and matrix proteins in cyclosporine nephropathy. Kidney Int. 1997;52(3):660–673. doi: 10.1038/ki.1997.380. [DOI] [PubMed] [Google Scholar]
- 186.Kagami S, Kuhara T, Okada K, Kuroda Y, Border WA, Noble NA. Dual effects of angiotensin II on the plasminogen/plasmin system in rat mesangial cells. Kidney Int. 1997;51:664–671. doi: 10.1038/ki.1997.96. [DOI] [PubMed] [Google Scholar]
- 187.Ding HL, Xu MT, Guo Y, Chen L, Zhang SL, Li F, Fu ZZ. Effect of losartan on the mRNA expressions of MT3-MMP and TIMP-2 in diabetic kidneys. Rev Diabet Stud. 2005;2(4):216–220. doi: 10.1900/RDS.2005.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Abrahamsen CT, Pullen MA, Schnackenberg CG, Grygielko ET, Edwards RM, Laping NJ, Brooks DP. Effects of angiotensins II and IV on blood pressure, renal function, and PAI-1 expression in the heart and kidney of the rat. Pharmacology. 2002;66:26–30. doi: 10.1159/000063252. [DOI] [PubMed] [Google Scholar]
- 189.Border WA, Noble NA. Interactions of transforming growth factor-β and angiotensin II in renal fibrosis. Hypertension. 1998;31:181–188. doi: 10.1161/01.hyp.31.1.181. [DOI] [PubMed] [Google Scholar]
- 190.Rodriguez-Vita J, Sabchez-Lopez E, Esteban V, Ruperez M, Egido J, Ruiz-Ortega M. Angiotensin II activates the smad pathway in vascular smooth muscle cells by a transforming growth factor-beta-independent mechanism. Circulation. 2005;111:2509–2517. doi: 10.1161/01.CIR.0000165133.84978.E2. [DOI] [PubMed] [Google Scholar]
- 191.Zhu S, Liu Y, Wang L, Meng QH. Transforming growth factor-{beta}1 is associated with kidney damage in patients with essential hypertension: renoprotective effect of ACE inhibitor and/or angiotensin II receptor blocker. Nephrol Dial Transplant. 2008;23(9):2841–2846. doi: 10.1093/ndt/gfn159. [DOI] [PubMed] [Google Scholar]
- 192.Kriz W, Gretz N, Lemley KV. Progression of glomerular diseases: Is the podocyte the culprit? Kidney Int. 1998;54:687–697. doi: 10.1046/j.1523-1755.1998.00044.x. [DOI] [PubMed] [Google Scholar]
- 193.D’Agati VD. The spectrum of focal segmental glomerulosclerosis: new insights. Curr Opin Nephrol Hypertens. 2008;17(3):271–281. doi: 10.1097/MNH.0b013e3282f94a96. [DOI] [PubMed] [Google Scholar]
- 194.Jones N, Blasutig IM, Eremina V, Ruston JM, Bladt F, Li H, Huang H, Larose L, Li SS, Takano T, Quaggin SE, Pawson T. Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature. 2006;440:818–823. doi: 10.1038/nature04662. [DOI] [PubMed] [Google Scholar]
- 195.Skoberne A, Konieczny A, Schiffer M. Glomerular epithelial cells in the urine: what has to be done to make them worthwhile? Am J Physiol Renal Physiol. 2009;296(2):F230–F241. doi: 10.1152/ajprenal.90507.2008. [DOI] [PubMed] [Google Scholar]
- 196.Kriz W, Hackenthal E, Nobiling R, Sakai T, Elger M. A role for podocytes to counteract capilary wall distention. Kidney Int. 1994;45:F369–F376. doi: 10.1038/ki.1994.47. [DOI] [PubMed] [Google Scholar]
- 197.Schartz MM, Lewis EJ. Focal segmental glomerulosclerosis: The cellular lesion. Kidney Int. 1985;28:968–974. doi: 10.1038/ki.1985.225. [DOI] [PubMed] [Google Scholar]
- 198.Kriz W, Hosser H, Hahnel B, Gretz N, Provoost AP. From segmental glomerulosclerosis to total nephron degeneration and interstitial fibrosis: a histopathological study in rat models and human glomerulopathies. Nephrol Dial Transplant. 1998;13:2781–2798. doi: 10.1093/ndt/13.11.2781. [DOI] [PubMed] [Google Scholar]
- 199.Kriz W, Hartmann I, Hosser H, Hahnel B, Kranzlin B, Provoost A, Gretz N. Tracer studies in the rat demonstrate misdirected filtration and peritubular filtrate spreading in nephrons with segmental glomerulosclerosis. J Am Soc Nephrol. 2001;12:496–506. doi: 10.1681/ASN.V123496. [DOI] [PubMed] [Google Scholar]
- 200.Kretzler MJ. Role of Podocytes in Focal Sclerosis: Defining the Point of No Return. Am Soc Nephrol. 2005;16(10):2830–2832. doi: 10.1681/ASN.2005080841. [DOI] [PubMed] [Google Scholar]
- 201.Ding G, Reddy K, Kapasi AA, Franki N, Gibbons N, Kasinath BS. Angiotensin II induces apoptosis in rat glomerular epithelial cells. Am J Renal Physiol. 2002;283:173–180. doi: 10.1152/ajprenal.00240.2001. [DOI] [PubMed] [Google Scholar]
- 202.Ruiz-Ortega M, Lorenzo O, Suzuki Y, Ruperez M, Egido J. Proinflammatory actions of angiotensin II. Curr Opin Nephrol Hypertens. 2001;10:321–329. doi: 10.1097/00041552-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 203.Johnson RJ, Alpers CE, Yoshimura A, Lombardi D, Pritzl P, Floege J, Schwartz SM. Renal injury from angiotensin II-mediated hypertension. Hypertension. 1992;19:464–474. doi: 10.1161/01.hyp.19.5.464. [DOI] [PubMed] [Google Scholar]
- 204.Wolf G. Link between angiotensin II and TGF-beta in the kidney. Miner Electrolyte Metab. 1998;24:174–180. doi: 10.1159/000057367. [DOI] [PubMed] [Google Scholar]
- 205.Desmouliére A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mezzano S, Droguett MA, Burgos ME, Ardiles LG, Aros CA, Caorsi I, Egido J. Overexpression of chemokines, fibrogenic cytokines, and myofibroblasts in human membranous nephropathy. Kidney Int. 2000;57:147–158. doi: 10.1046/j.1523-1755.2000.00830.x. [DOI] [PubMed] [Google Scholar]
- 207.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Matsumoto K, Morishita R, Tomita N, Moriguchi A, Komai N, Aoki M, Matsumoto K, Nakamura T, Higaki J, Ogihara T. Improvement of endothelial dysfunction by angiotensin II blockade accompanied by induction of vascular hepatocyte growth factor system in diabetic spontaneously hypertensive rats. Heart Vessels. 2003;18:18–25. doi: 10.1007/s003800300003. [DOI] [PubMed] [Google Scholar]
- 209.Cattell V. Macrophages in acute glomerular inflammation. Kidney Int. 1994;45:945–952. doi: 10.1038/ki.1994.128. [DOI] [PubMed] [Google Scholar]
- 210.Morrissey JJ, Klahr S. Differential effects of ACE and AT1 receptor inhibition on chemoattractant and adhesion molecule synthesis. Am J Physiol. 1998;274:580–586. doi: 10.1152/ajprenal.1998.274.3.F580. [DOI] [PubMed] [Google Scholar]
- 211.Ruiz-Ortega M, Esteban V, Ruperez M, Sanchez-Lopez E, Rodriguez-Vita J, Carvajal G, Egido J. Renal and vascular hypertension-induced inflammation: role of angiotensin II. Curr Opin Nephrol Hypertens. 2006;15:159–166. doi: 10.1097/01.mnh.0000203190.34643.d4. [DOI] [PubMed] [Google Scholar]
- 212.Ruiz-Ortega M, Lorenzo O, Ruperez M, Blanco J, Egido J. Systemic infusion of angiotensin II into normal rats activates nuclear factor-kappaB and AP-1 in the kidney: role of AT(1) and AT(2) receptors. Am J Pathol. 2001;158:1743–1756. doi: 10.1016/s0002-9440(10)64130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ruiz-Ortega M, Lorenzo O, Rupérez M, Esteban V, Suzuki Y, Mezzano S, Plaza JJ, Egido J. Role of the renin-angiotensin system in vascular diseases: expanding the field. Hypertension. 2001;38(6):1382–1387. doi: 10.1161/hy1201.100589. [DOI] [PubMed] [Google Scholar]
- 214.Wolf G, Ziyadeh FN, Thaiss F, Tomaszewski J, Caron RJ, Wenzel U, Zahner G, Helmchen U, Stahl RA. Angiotensin II stimulates expression of the chemokine RANTES in rat glomerular endothelial cells. Role of the angiotensin type 2 receptor. J Clin Invest. 1997;100:1047–1058. doi: 10.1172/JCI119615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wolf G, Bondeva T, Bohlender J, Roger T, Thaiss F, Wenzel U. Angiotensin II upregulates toll-like receptor 4 on mesangial cells. J Am Soc Nephrol. 2006;17:1585–1593. doi: 10.1681/ASN.2005070699. [DOI] [PubMed] [Google Scholar]
- 216.Mezzano SA, Ruiz-Ortega M, Egido J. Angiotensin II and renal fibrosis. Hypertension. 2001;38:635–638. doi: 10.1161/hy09t1.094234. [DOI] [PubMed] [Google Scholar]