Abstract
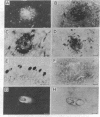
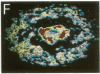
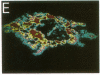
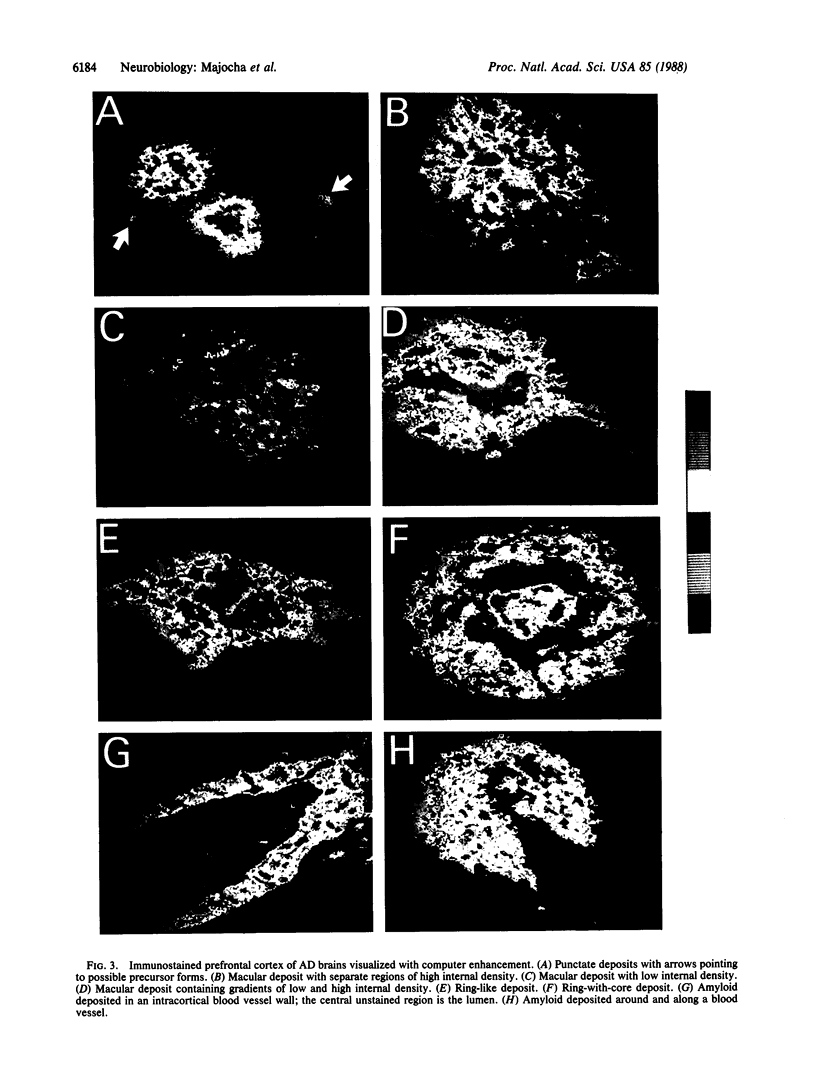
Monoclonal antibodies to the A4 amyloid polypeptide were used in immunocytochemical staining of the Alzheimer disease prefrontal cortex. Analysis of the resulting staining patterns allowed us to evaluate the amounts and distribution of amyloid-protein deposits exclusive of other senile-plaque components. Previously unappreciated infra-structural details of amyloid in the Alzheimer disease brain became accessible through computer-enhanced imaging procedures. Four discrete morphologic classes of amyloid deposits were observed and classified as punctate, macular, ring, and ring-with-core configurations. Computer imaging indicated that all four classes of immunostained deposits contain internal gradients of density. The classes were nonuniformly distributed with regard to size and location within cortical laminae. Our results support two separate but complementary hypotheses concerning the molecular neuropathology of Alzheimer disease in the prefrontal cortex. (i) Irrespective of cortical layer or morphology, density-gradient analyses suggest that amyloid deposits are elaborated through molecular and cellular events that may involve diffusion or coalescence of the A4 polypeptide. (ii) The distribution and morphology of prefrontal cortical amyloid deposits may be dependent upon underlying laminar-specific structures of the neocortex.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahmanyar S., Higgins G. A., Goldgaber D., Lewis D. A., Morrison J. H., Wilson M. C., Shankar S. K., Gajdusek D. C. Localization of amyloid beta protein messenger RNA in brains from patients with Alzheimer's disease. Science. 1987 Jul 3;237(4810):77–80. doi: 10.1126/science.3299701. [DOI] [PubMed] [Google Scholar]
- Benes F. M., Davidson J., Bird E. D. Quantitative cytoarchitectural studies of the cerebral cortex of schizophrenics. Arch Gen Psychiatry. 1986 Jan;43(1):31–35. doi: 10.1001/archpsyc.1986.01800010033004. [DOI] [PubMed] [Google Scholar]
- Benes F. M., Matthysse S. W., Davidson J., Bird E. D. The spatial distribution of neurons and glia in human cortex based on the poisson distribution. Anal Quant Cytol Histol. 1987 Dec;9(6):531–534. [PubMed] [Google Scholar]
- Brown B. A., Majocha R. E., Staton D. M., Marotta C. A. Axonal polypeptides cross-reactive with antibodies to neurofilament proteins. J Neurochem. 1983 Feb;40(2):299–308. doi: 10.1111/j.1471-4159.1983.tb11283.x. [DOI] [PubMed] [Google Scholar]
- Duyckaerts C., Hauw J. J., Bastenaire F., Piette F., Poulain C., Rainsard V., Javoy-Agid F., Berthaux P. Laminar distribution of neocortical senile plaques in senile dementia of the Alzheimer type. Acta Neuropathol. 1986;70(3-4):249–256. doi: 10.1007/BF00686079. [DOI] [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Gonatas N. K., Anderson W., Evangelista I. The contribution of altered synapses in the senile plaque: an electron microscopic study in Alzheimer's dementia. J Neuropathol Exp Neurol. 1967 Jan;26(1):25–39. doi: 10.1097/00005072-196701000-00003. [DOI] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Majocha R. E., Marotta C. A., Benes F. M. Immunostaining of neurofilament protein in human postmortem cortex: a sensitive and specific approach to the pattern analysis of human cortical cytoarchitecture. Can J Biochem Cell Biol. 1985 Jun;63(6):577–584. doi: 10.1139/o85-076. [DOI] [PubMed] [Google Scholar]
- Majocha R. E., Marotta C. A. Molecular and genetic investigations on Alzheimer brain amyloid. J Geriatr Psychiatry Neurol. 1988 Apr-Jun;1(2):65–70. doi: 10.1177/089198878800100202. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. C., Esiri M. M., Hiorns R. W., Wilcock G. K., Powell T. P. Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer disease. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4531–4534. doi: 10.1073/pnas.82.13.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst A., Basler V., Bron B., Ulrich J. Neuritic plaques in senile dementia of Alzheimer type: a Golgi analysis in the hippocampal region. Brain Res. 1983 Jun 6;268(2):249–254. doi: 10.1016/0006-8993(83)90490-0. [DOI] [PubMed] [Google Scholar]
- Rogers J., Morrison J. H. Quantitative morphology and regional and laminar distributions of senile plaques in Alzheimer's disease. J Neurosci. 1985 Oct;5(10):2801–2808. doi: 10.1523/JNEUROSCI.05-10-02801.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERRY R. D., GONATAS N. K., WEISS M. ULTRASTRUCTURAL STUDIES IN ALZHEIMER'S PRESENILE DEMENTIA. Am J Pathol. 1964 Feb;44:269–297. [PMC free article] [PubMed] [Google Scholar]
- Wong C. W., Quaranta V., Glenner G. G. Neuritic plaques and cerebrovascular amyloid in Alzheimer disease are antigenically related. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8729–8732. doi: 10.1073/pnas.82.24.8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zain S. B., Salim M., Chou W. G., Sajdel-Sulkowska E. M., Majocha R. E., Marotta C. A. Molecular cloning of amyloid cDNA derived from mRNA of the Alzheimer disease brain: coding and noncoding regions of the fetal precursor mRNA are expressed in the cortex. Proc Natl Acad Sci U S A. 1988 Feb;85(3):929–933. doi: 10.1073/pnas.85.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]