Abstract
PURPOSE
Retinal neovascularization (RNV) is a primary cause of blindness and involves the dysfunction of retinal capillaries. Recent studies have emphasized the beneficial effects of inhibitors of HMG-CoA reductase (statins) in preventing vascular dysfunction. In the present study, the authors characterized the therapeutic effects of statins on RNV.
METHODS
Statin treatment (10 mg/kg/d fluvastatin) was tested in a mouse model of oxygen-induced retinopathy. Morphometric analysis was conducted to determine the extent of capillary growth. Pimonidazole hydrochloride was used to assess retinal ischemia. Western blot and immunohistochemical analyses were used to assess protein expression levels and immunolocalization. Lipid peroxidation and superoxide radical formation were determined to assess oxidative changes.
RESULTS
Fluvastatin treatment significantly reduced the area of the capillary-free zone (P < 0.01), decreased the formation of neovascular tufts (P < 0.01), and ameliorated retinal ischemia. These morphologic and functional changes were associated with statin effects in preventing the upregulation of VEGF, HIF-1α, phosphorylated STAT3, and vascular expression of the inflammatory mediator ICAM-1 (P < 0.01). Superoxide production and lipid peroxidation in the ischemic retina were also reduced by statin treatment (P < 0.01).
CONCLUSIONS
These data suggest the beneficial effects of statin treatment in preventing retinal neovascularization. These beneficial effects appear to result from the anti-oxidant and anti-inflammatory properties of statins.
Retinal neovascularization (RNV), the inappropriate growth of retinal capillaries that occurs as a complication of pre-maturity, diabetes mellitus, or retinal vein occlusion, is a major cause of blindness worldwide. Therapies for RNV consist of the ablation of functional retinal tissue with laser photocoagulation, cryotherapy, and intravitreal injection of inhibitors of vascular endothelial growth factor (VEGF) and its receptors. Indeed, the overexpression of VEGF and its receptor VEGFR2 is considered a hallmark of RNV,1,2 and it has been associated with increased oxidative stress and the upregulation of inflammatory pathways.3 VEGF is a potent endothelial cell agonist that exerts dramatic effects on the retinal microvascular endothelium, and the inhibition of its action through antibodies, aptamers, and specific tyrosine kinase inhibitors has been shown to be beneficial in halting retinal vessel proliferation and leakage.3 However, recent studies have shown that the inhibition of VEGF expression and its activity in retina needs careful titration because of the physiological function of VEGF in promoting survival of the different retinal cell layers.4,5 Therefore, the quest for a more appropriate therapy able to “normalize” VEGF expression and retinal vessel function rather than to obliterate it remains open.
Recently, attention has been focused on the use of the lipid-lowering drugs, statins, to prevent cardiovascular disease.6 This class of drugs consists of inhibitors of HMG-CoA reductase, a rate-limiting enzyme involved in the biosynthesis of mevalonate and cholesterol.7 The vasculoprotective effect of statins has been extensively documented, and numerous studies have suggested that the protective action of statins on the microvasculature is often independent of their cholesterol-lowering effects and involves their anti-oxidant and anti-inflammatory properties.8–10
Indeed, the statin-mediated blockade of mevalonate synthesis also affects the synthesis of isoprenoids, which are required for protein isoprenylation. This an important mechanism involved in the activation of the signaling pathway, leading to oxidative and proinflammatory events.7 For example, isoprenylation is required for the activation of small G-proteins including Rac-1, a known regulator of redox signaling, through the induction of the superoxide-generator NAD(P)H oxidase.11
We have previously documented that selective inhibition of NAD(P)H oxidase prevents VEGF overexpression and RNV in a mouse model of retinopathy of prematurity.12 Our group and others13,14 have also shown that treatment with simvastatin (another statin) efficiently prevents hyperglycemia-induced retinal vascular permeability and leukostasis. In addition, our study demonstrated that statin effects in preventing diabetes-induced retinal vascular dysfunction are associated with the blockade of VEGF expression and NAD(P)H oxidase activation and with the inhibition of the transcription factor signal transducer and activator of transcription 3 (STAT3).14 The latter is an important regulator of angiogenic responses because of its ability to modulate VEGF expression and activity.15–17 STAT3 also represents a convergence point for the cellular activity of several cytokines and proinflammatory mediators, such as IL-6 and TNFα.18 Based on this evidence, we conducted studies in a model of oxygen-induced retinopathy to determine whether treatment with statins could be beneficial in preventing RNV, thus supporting their use in the treatment of advanced stages of ischemic retinopathies, among them proliferative diabetic retinopathy and retinopathy of prematurity.
MATERIALS AND METHODS
Materials
Fluvastatin was obtained from Sigma-Aldrich (St. Louis, MO) and was suspended in sterile saline and injected in a final volume of 50 µL. Anti-VEGF was from Oncogene (San Diego, CA), and anti-HIF-1α and anti-ICAM-1 were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti–phospho-Tyr-705-STAT3 was from Cell Signaling Technology (Beverly, CA), and anti-STAT3 was from Becton Dickinson Transduction Laboratories (San Diego, CA).
Animal Model of Retinal Neovascularization and Treatment Protocols
All experiments were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and our institutional guidelines.
Retinal neovascularization was induced with the use of a mouse model of oxygen-induced retinopathy according to the protocol of Smith et al.19 and as previously described.20,21 In this model, mice are exposed to 75% oxygen tension from postnatal day (P) 7 to P12 and then transferred again to room air. Elevated oxygen tension during the hyperoxic stage, from P7 to P12, inhibits the development of the retinal vasculature. When the mice are brought to room air, at P12, the retina becomes ischemic, VEGF expression is upregulated, and intravitreal neovascularization takes place (P17), whereas the central part of the retina remains poorly vascularized. At P17, which is the peak of the neovascularization stage, the animals were euthanatized. Eight sets of six pups each were used for each treatment group. We established three treatment groups, as follows: normal age-matched control mice (P17), mice subjected to oxygen-induced retinopathy and injected (P12–P17) with PBS (OIR+PBS), and mice subjected to oxygen-induced retinopathy and injected (P12–P17) with 10 mg/kg/d fluvastatin (OIR+F). Drug and vehicle were administered daily by intraperitoneal injection, and all animals were killed at P17. At the end of the treatment time in each mouse, the eyeballs, isolated retinas, or both were excised and processed for imaging or molecular analysis. Statin treatment did not affect the growth rate of the neonatal mice, as assessed by monitoring of body weight at the beginning and end of treatment, from P12 to P17 (data not shown).
Morphometric Analysis
Retinal capillary density and distribution were assessed in flatmount preparations labeled with biotinylated isolectin B4 (Griffonia simplicifolia) and Texas Red–conjugated avidin D.12,20,21 Images were captured with a confocal microscope (LSM510; Carl Zeiss, Thornwood, NY) in digital format (Spot System; Diagnostic Instruments, Sterling Heights, MI). Areas of vaso-obliteration and capillary tuft formation were measured in masked fashion (IP Laboratory Spectrum Scientific Image System; Signal Analytics, Vienna, VA).
Assessment of Retinal Ischemia
Retinal ischemia was assessed with the use of pimonidazole hydrochloride (Hypoxyprobe-1 [H-1]; Chemicon, Temecula, CA) according to the manufacturer’s instructions. Briefly, treated and control mice were injected intraperitoneally with 50 mg/kg H-1 1 hour before kill to allow stabilization of the probe in the hypoxic retinal tissue. Detection of H-1–positive (hypoxic) areas was achieved by immunohistochemical analysis of frozen retinal sections after fixation with 4% paraformaldehyde using a primary antibody against the H-1 probe (provided in the kit) and FITC-conjugated secondary antibodies. Images were captured and subjected to morphometric analysis to compare retinal fluorescence levels in the treatment groups.
Protein Analysis
Western blot analysis was conducted as described12,15,16 to determine levels of VEGF, phosphorylated/activated STAT3 (PYSTAT3), and HIF-1α. For the determination of VEGF protein levels, retinal extracts were subjected to heparin binding-affinity columns according to the protocol of Ferrara and Henzel.22
Assessment of ROS Production and Oxidative Stress
Reactive oxygen species (ROS) production was measured by fluorescence staining of retinal frozen sections with 2,7-dichlorodihydro-fluorescein, as described.12 Briefly, pools of three retinas per group were homogenized in ice-cold lysis buffer (50 mM phosphate buffer, pH 7.0, 1 mM EGTA, 150 mM sucrose, and protease inhibitors). Preincubation of the tissue homogenates with a mixture of 100 IU/mL superoxide dismutase (SOD; Sigma Aldrich, St. Louis, MO) and 1000 IU/mL catalase (Sigma Aldrich) was used as a negative control to assess the specificity of the reaction.
Oxidative stress in the retinal tissue extracts was assessed by measuring lipid peroxide levels according to a previously described protocol.12 Standard curves were established using tetraethoxypropane as standard. Lipid peroxide levels are expressed as microgram malondialdehyde (MDA) per milligram total tissue proteins.
Immunohistochemistry
To determine intercellular adhesion molecule (ICAM)-1 immunolocalization, we performed immunohistochemical analysis on retinal frozen sections fixed with 4% paraformaldehyde, as previously described.12 Western blot analysis was also used for better quantification of ICAM-1 protein levels.
Statistical Analysis
One-way ANOVA and post hoc comparison were performed to determine the statistical significance of the observed differences among the treatment groups. Data differences were considered statistically significant when P < 0.05
RESULTS
Fluvastastin Effects on Retinal Vessel Growth and Distribution
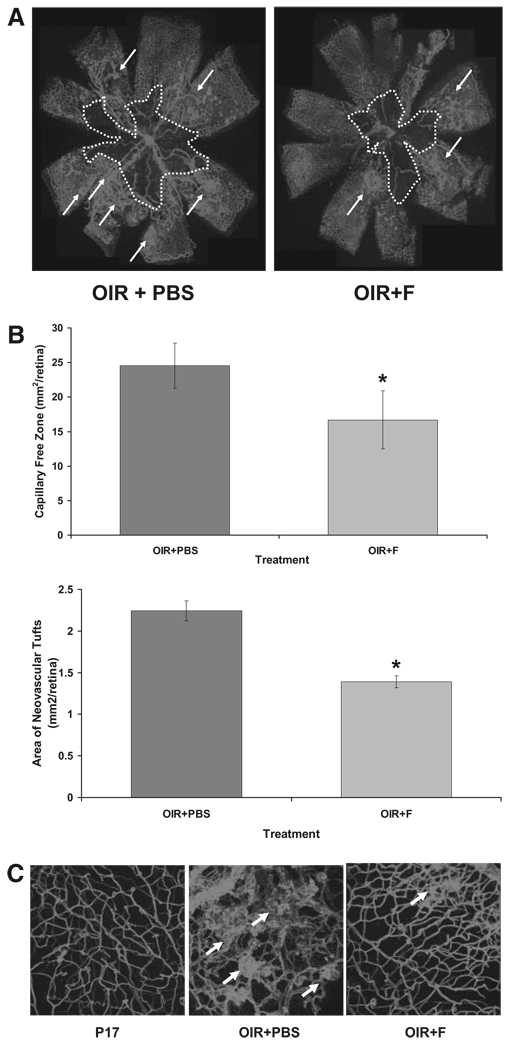
Morphometric analysis of retinal flatmounts stained with Texas Red–isolectin B4 was conducted to assess vessel growth and distribution in the ischemic retinas (Fig. 1A). Quantification of the observed changes revealed that fluvastatin treatment significantly decreased the number of neovascular tufts and reduced the capillary-free area (Fig. 1B, bar histograms).
FIGURE 1.
(A) Flatmounted mouse retinas stained with isolectin B4 to identify areas of neovascularization (arrows) and capillary-free zones (dotted white line). (B) Histograms representing the results of morphometric analysis of retinal flatmounts measuring the capillary-free areas (upper bars) and neovascular tufts (lower bars). OIR+PBS, sham ischemic mouse retina at postnatal day 17; OIR+F, ischemic mouse retina at postnatal day 17 injected with 10 mg/kg/d fluvastatin (P12–P17). *P < 0.01 vs. OIR+PBS; x = mean ± SE; n = 11. (C) Three-dimensional images (z-stacks, 20× magnification) of retinal flatmounts stained with isolectin B4 (Texas Red demonstrating retinal capillary morphology in the different treatment groups).
Microimages taken at higher magnification further confirmed the observed changes in retinal vessel distribution followed to fluvastatin treatment (OIR+F in Fig. 1C) and compared with ischemic retinas (OIR+PBS in Fig. 1C).
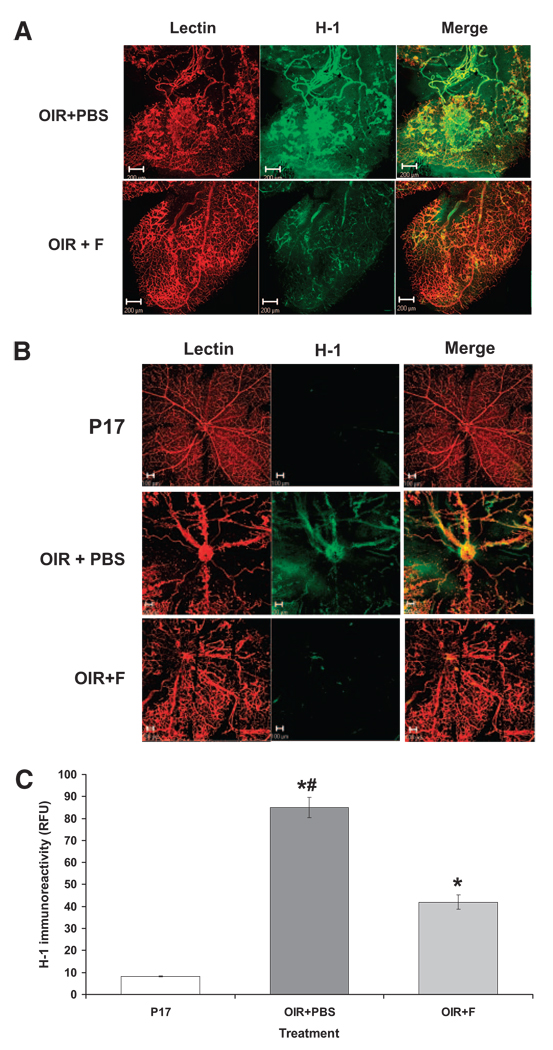
Fluvastatin Effects on Retinal Ischemia
Oxygen-induced retinal neovascularization appears to be driven by tissue ischemia because of vessel drop-out in the central retina during hyperoxia. Our data indicating that fluvastatin prevented hypoxia-induced retinal vessel growth and decreased vessel drop-out suggested that fluvastatin may improve retinal ischemia. Therefore, we tested the extent of retinal ischemia by using the hypoxic probe H-1. Immunolocalization of the hypoxic probe H-1, shown in Figure 2, reveals that treatment with fluvastatin (OIR+F) significantly reduces the immunoreactivity for H-1 in the ischemic retina (OIR+PBS) in the midperiphery (Fig. 2A) and the central (Fig. 2B) areas. These data are also confirmed by morphometric analysis measuring ratios of H-1–specific fluorescent areas compared with total retinal surface, as shown in Figure 2C.
FIGURE 2.
Assessment of retinal ischemia measured by identification of positive areas (FITC) for H-1 and counterstained with isolectin B4 (Texas Red) to identify (A) retinal midperiphery and (B) central retina. (C) Morphometric analysis measuring H-1–specific immunofluorescence in the whole retina. *P < 0.01 vs. P17; #P < 0.01 vs. OIR+PBS; x = mean ± SE; n = 8.
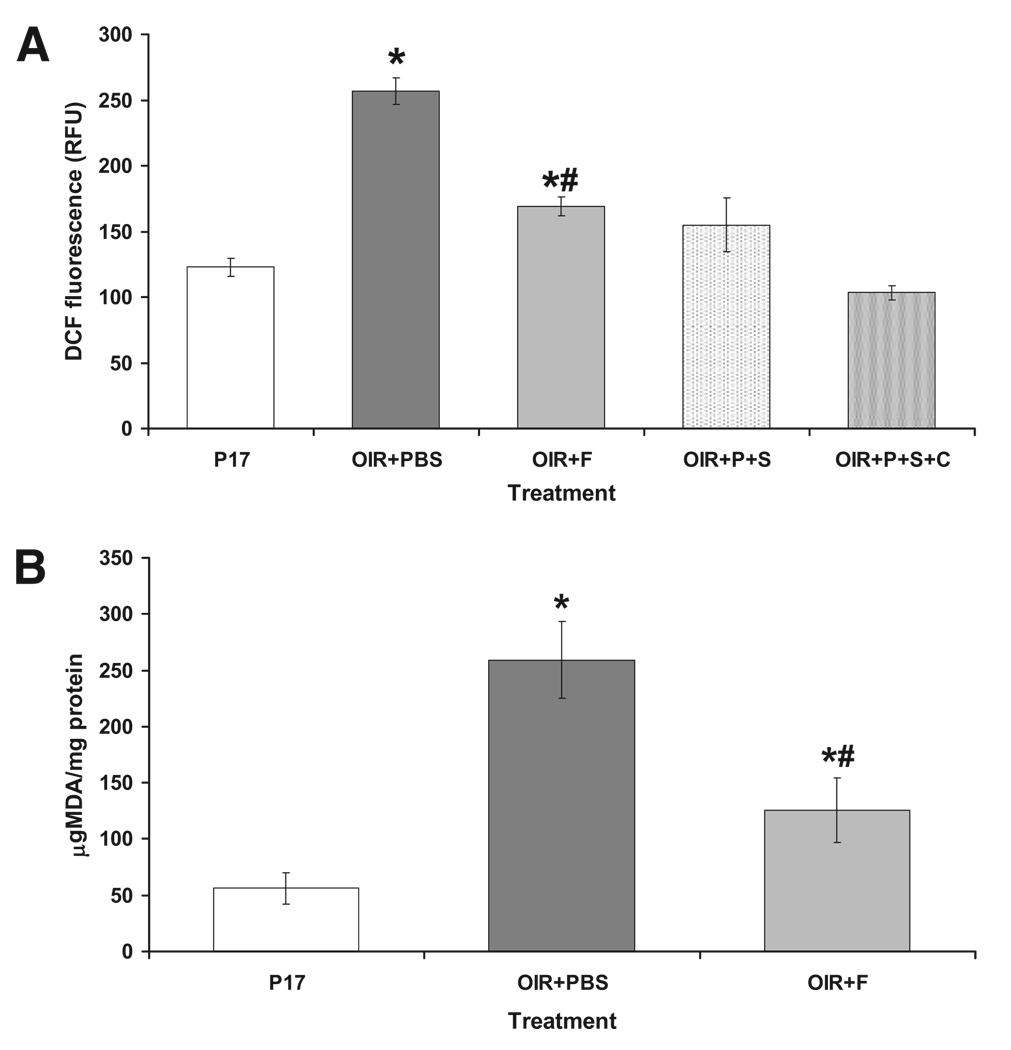
Fluvastatin Effects on Retinal Oxidative Stress
Increased production of ROS and oxidative stress has been shown to be involved in the induction of pathologic neovascularization. To assess ROS production in the murine retinas after the different treatments, we determined the formation of superoxide anion (O2−•), hydroxyl radical (OH−•), and peroxynitrite (ONOO−•) in retinal tissue homogenates with the fluorescence probe 2,7-dychlorofluorescein (DCF). As shown in Figure 3A, DCF fluorescence is increased 2.5-fold in ischemic retinas (OIR+PBS) compared with normal age-matched control retinas (P17; P < 0.01; n = 8). Treatment with fluvastatin (OIR+F) significantly decreased ischemia-induced production of ROS (Fig 3A).
FIGURE 3.
(A) Measurements of DCF-specific fluorescence to assess ROS production in murine retinas subjected to different treatments. DCF was incubated with retinal homogenates. To control the specificity of the reaction of 100 µL OIR+PBS, homogenates were also preincubated with 100 µL SOD (OIR+P+S) alone or with a mixture of 100 IU SOD and 1000 IU catalase (OIR+P+S+C). (B) Measurement of lipid peroxide levels to assess fluvastatin effects on oxidative stress in the ischemic retina. Lipid peroxides are measured as microgram MDA normalized by total protein. *P < 0.01 vs. P17; #P < 0.01 vs. OIR+PBS; x = mean ± SE; n = 8.
We further determined the effects of fluvastatin treatment on oxidative stress in the ischemic retinas by assessing the formation of lipid peroxides. As shown in Figure 3B, the formation of lipid peroxides, measured as microgram MDA per milligram total protein, was increased threefold in the ischemic retina (P < 0.01 vs. control P17; n = 8); treatment with fluvastatin (OIR+F), however, blocked this effect.
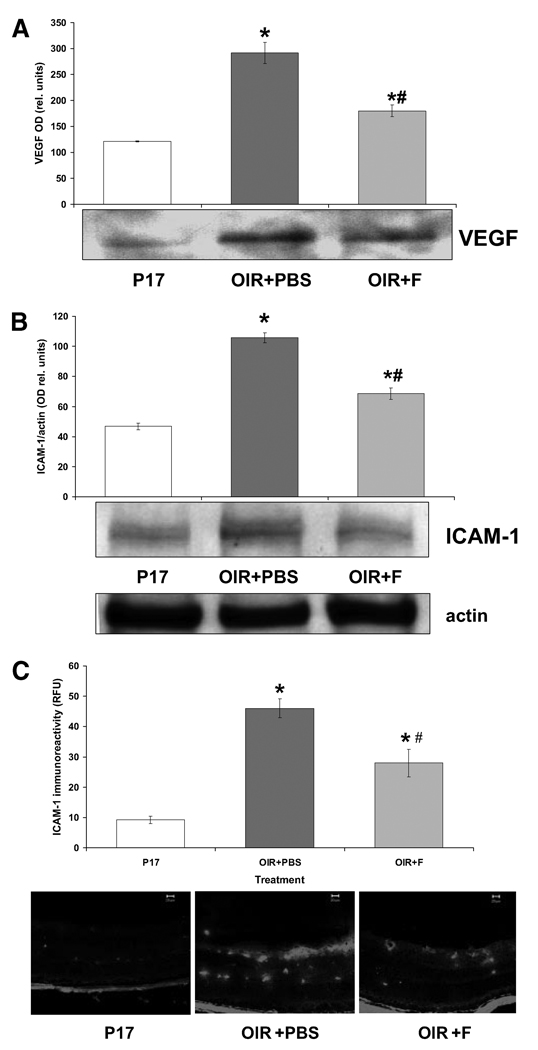
Fluvastatin Effects on Expression of VEGF and ICAM-1
Increased VEGF expression has been shown to be a primary factor in inducing and sustaining retinal neovascularization. Because of the fluvastatin effects on retinal vessel growth and distribution in the OIR mice, we sought to assess the expression of VEGF in the murine retinas after the different treatment protocols. As shown in Figure 4A, VEGF expression, measured by Western blot analysis, was significantly increased (P < 0.01; n = 5) in the retinas of the OIR mice (OIR+PBS) injected with saline compared with the normal age-matched control retinas (P17). Treatment with fluvastatin significantly reduced ischemia-induced VEGF expression (Fig. 4A; OIR+F) in the murine retinas.
FIGURE 4.
Measurement of VEGF and ICAM-1 expression by Western blot and immunohistochemical analyses. (A) Immunoblots showing VEGF-specific immunoreactivity and quantified as measurements of optical density (histogram). *P < 0.01 vs. P17; #P < 0.01 vs. OIR+PBS; x = mean ± SE; n = 5. (B) Immunoblot showing ICAM-1–specific immunoreactivity and measured as optical density units relative to actin. *P < 0.01 vs. P17; #P < 0.01 vs. OIR+PBS; x = mean ± SE; n = 5. (C) Immunohistochemical analysis demonstrating ICAM-1–specific immunofluorescence (FITC) and quantified by morphometric analysis (bar histograms). *P < 0.01 vs. P17; #P < 0.01 vs. OIR_PBS; x = mean ± SE; n = 3.
It has been shown that VEGF effects on retinal neovascularization involve inflammatory mechanisms such as the up-regulation of ICAM-1. Therefore, we assessed the expression of ICAM-1 in our experimental groups by immunohistochemical and morphometric analyses. As shown in Figure 4B, ICAM-1 expression, as measured by Western blot analysis, was significantly increased in the retinas of OIR mice (OIR+PBS) compared with age-matched controls (P17), whereas treatment with fluvastatin (OIR+F) prevented this effect. In addition, immunohistochemical analysis demonstrated ICAM-1 localization in vascular and perivascular areas (Fig. 4). Morphometric analysis confirmed the Western results showing an increase in ICAM-1 expression levels in the ischemic retina (OIR+PBS), which was significantly attenuated by the statin treatment (Fig. 4C, histogram).
Fluvastatin Effects on Transcriptional Activators
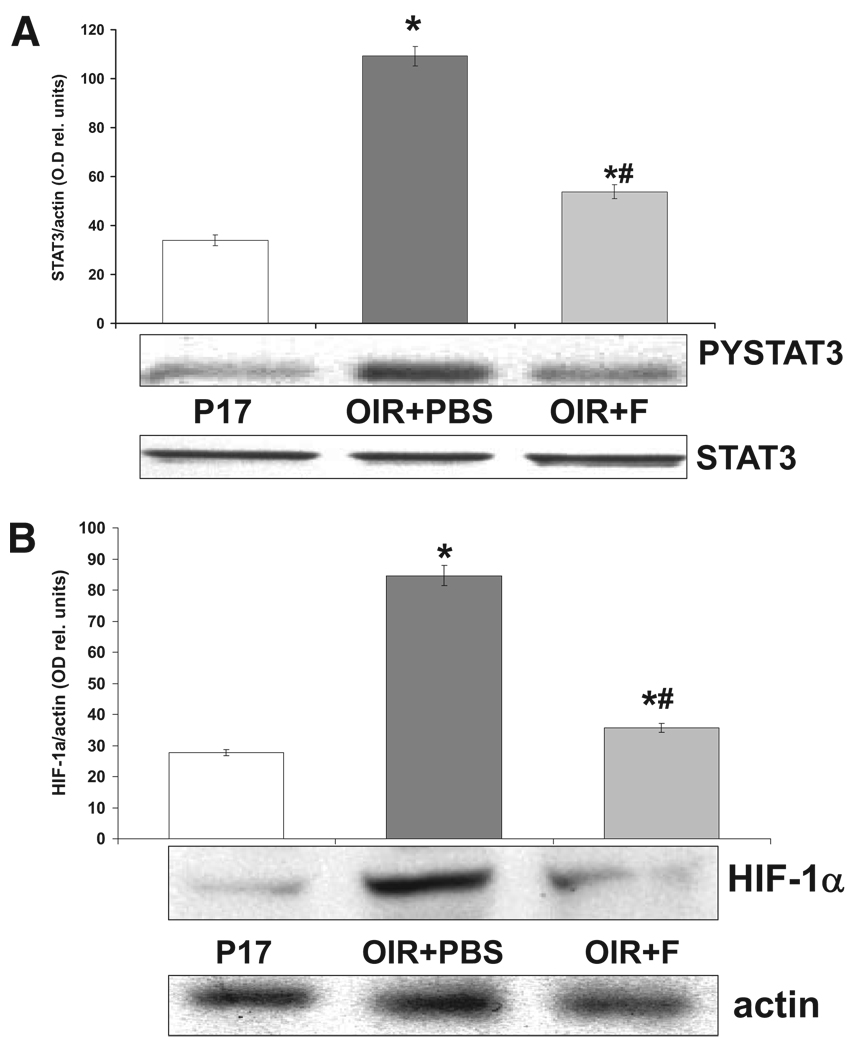
The functional and morphologic changes we observed were associated with changes in the expression levels of VEGF and ICAM-1, thus suggesting that treatment with statins could influence the activation of transcriptional regulators involved in the expression of these factors. Therefore, we determined the effects of statin treatment on the activation pattern of the transcription factors STAT3 and HIF-1, known modulators of VEGF and ICAM-1 expression and activity. As shown in Figure 5A, immunoreactivity for phospho-(Tyr705)-STAT3, the activated form of STAT3 (PYSTAT3 in Fig. 5), measured by Western blot analysis, was significantly increased in the ischemic retina (OIR+PBS) in comparison with retinas of age-matched controls (P17), but treatment with fluvastatin prevented this effect (OIR+F). In addition, expression levels of the inducible subunit of the HIF-1 transcription factor, HIF-1α, was increased in the ischemic retina (OIR+PBS) compared with age-matched controls, shown in Figure 5B, and treatment with fluvastatin (OIR+F) significantly reduced this effect.
FIGURE 5.
Fluvastatin effects on activation of STAT3 and HIF-1. (A) Western blot analysis showing immunoreactivity specific for the activated form of STAT3 phospho-Tyr705-STAT3 (PYSTAT3) and measured as optical density values normalized compared with total STAT3 protein (histogram). *P < 0.01 vs. P17; #P < 0.01 vs. OIR+PBS; x = mean ± SE; n = 5. (B) Western blot analysis showing immunoreactivity specific for HIF-1α, the inducible subunit of transcription factor HIF-1. Data are expressed as values of optical density normalized compared with actin (histogram). *P < 0.01 vs. P17; #P < 0.01 vs. OIR+PBS; x = mean ± SE; n = 5.
CONCLUSIONS
Retinal neovascularization is a major cause of morbidity in potentially blinding diseases such as ischemic retinopathies, including proliferative diabetic retinopathy, retinal vein occlusion, and retinopathy of prematurity. Treatments for retinal neovascularization are limited and involve invasive procedures; thus, the need for better therapies is an important area for research emphasis.
In this study we assessed the effects of the drug fluvastatin in preventing retinal neovascularization in a model of oxygen-induced retinopathy. Our results show that treatment with fluvastatin prevented neovascularization, normalized retinal vessel distribution, and decreased retinal ischemia. Furthermore, these morphologic and functional changes were associated with modifications of molecular signaling events such as inactivation of the transcriptional regulators STAT3 and HIF-1 and diminished the expression of VEGF and ICAM-1 in the ischemic retina.
Clinical evidence has repeatedly suggested the use of statins for the management of ischemic retinopathies, particularly diabetic retinopathy3,23 and age-related macular degeneration.24 This hypothesis has been extrapolated from studies demonstrating that statins effectively prevent cardiovascular abnormalities associated with, and consequent to, metabolic conditions such as diabetes and hypercholesterolemia.25 In addition, studies conducted on patients with diabetes have shown that statin treatment may prevent the formation of hard exudates because of their lipid-lowering properties.25,26 Ultimately, the lack of clinical trials designed ad hoc makes it difficult to determine whether treatment with statins is beneficial for ocular diseases involving dysfunction of the retinal vasculature. Thus, the results of our study provide critical molecular evidence supporting the bulk of clinical observations suggesting the use of statins for the treatment of ischemic retinopathies.
Moreover, our data showing that fluvastatin prevents lipid peroxidation and overexpression of VEGF and ICAM-1 in the ischemic retina suggest that their beneficial effects are correlated with statin anti-oxidant and anti-inflammatory activities. Indeed, increased formation of reactive oxygen species and inflammation cause retinal microvessel dysfunction and promote vascular permeability, followed by pathologic neovascularization.3 These data are in agreement with previous studies by our group and others13,14 that have shown, in the streptozotocin-induced diabetic rat model, that treatments with simvastatin (another HMG-CoA reductase inhibitor) significantly prevent diabetes-induced vascular permeability and leukostasis and decrease hyperglycemia-induced VEGF and ICAM-1 protein expression. These effects were also correlated with decreased oxidative stress markers and diminished activity of the superoxide anion generator NAD(P)H oxidase.14 We have previously shown that the activity of this oxidase is upregulated in the ischemic retina, and its selective inhibition prevents retinal neovascularization.12 It is tempting to speculate that the data obtained in the present study may involve statin inhibitory effects on NAD(P)H oxidase activity by preventing prenylation/activation of the small G-protein Rac-1.27 Nevertheless, we cannot rule out the involvement of the cholesterol-lowering ability of these drugs. Indeed, the formation of cholesterol-containing lipid rafts is important for the modulation of the intracellular signaling pathway potentially involving Rac-1, NAD(P)H oxidase, and STAT3.28,29 Of particular interest, it is our observation that fluvastatin treatment, while blocking pathologic neovascularization, favors physiological revascularization of the central retina. Previous work has shown that pathologic neovascularization in the ischemic retina is driven by the upregulation of inflammatory events and that blockade of ICAM-1 expression prevents it.30–34 Thus, our data suggest that the anti-inflammatory effects of fluvastatin in blocking ICAM-1 expression are likely to be involved in its ability to preserve normal vascularization while blocking the formation of pathologic neovascular tufts.
Furthermore, our present studies show that fluvastatin effects in decreasing VEGF and ICAM-1 expression in the ischemic retina are associated with the inhibition of STAT3 and of HIF-1 activation. These transcription factors are known inducers of VEGF gene expression and angiogenesis.35–37 In addition, STAT3 is a key mediator of cellular responses to inflammatory stimuli18 and a downstream effector of VEGF activity in endothelial cells.15,16 We have previously shown that VEGF-dependent STAT3 activation in retinal endothelial cells is mediated by VEGF receptor 2 (VEGFR2).15 Other investigators have shown that VEGFR2 activation in endothelial cells is caused by redox-dependent events involving NAD(P)H oxidase activity,38,39 further underscoring the role of this oxidase in the regulation of VEGF-induced cellular responses and implicating it in the effects of statins we observed in our studies.
Finally, several studies have demonstrated proangiogenic and antiangiogenic effects of statins treatment. These dual effects of statins have been explained by a dose-dependent mechanism, with high doses of statins inhibitory and low doses of statins stimulatory for angiogenic events.40–42 However, clinical studies have shown that this concept may be controversial and that the same doses of statins could favor coronary vessel growth while reducing cancer-induced angiogenesis.6 It is clear that more ad hoc clinical trials should be conducted to respond to these controversies. In our experiments, we used a relatively high dose of fluvastatin, theoretically favoring the antiangiogenic properties of this drug. They did not, however, prevent normal revascularization, further suggesting the interesting concept of a homeostatic regulatory effect of statins on retinal vessel growth. In summary, the results of these studies provide biochemical and morphologic evidence supporting a possible therapeutic role for statins in the treatment of ischemic retinopathy during the proliferative stage.
Acknowledgments
The authors thank Tahira Lemtalsi, Jun Yao Liu, and Telina Franklin for their excellent technical assistance.
Supported by Fight for Sight, Juvenile Diabetes Research Foundation Grant 1–2005-1086, and National Institutes of Health/National Eye Institute Grants EY04618 and EY11766.
Footnotes
Disclosure: M. Bartoli, None; M. Al-Shabrawey, None; M. Labazi, None; M.A. Behzadian, None; M. Istanboli, None; A.B. El-Remessy, None; R.W. Caldwell, None; D.M. Marcus, None; R.B. Caldwell, None
References
- 1.Caldwell RB, Bartoli M, Behzadian MA, et al. Vascular endothelial growth factor and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Diabetes Metab Res Rev. 2003;19(6):442–455. doi: 10.1002/dmrr.415. [DOI] [PubMed] [Google Scholar]
- 2.Shama N, Inachulev T. Role of vascular endothelial growth factor in ocular angiogenesis. Ophthalmol Clin North Am. 2006;19(3):335–344. doi: 10.1016/j.ohc.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Caldwell RB, Bartoli M, Behzadian MA, et al. Vascular endothelial growth factor and diabetic retinopathy: role of oxidative stress. Curr Drug Targets. 2005;6(4):511–524. doi: 10.2174/1389450054021981. [DOI] [PubMed] [Google Scholar]
- 4.Kilic U, Kilic E, Jarve A, et al. Human vascular endothelial growth factor protects axotomized retinal ganglion cells in vivo by activating ERK-1/2 and Akt pathways. J Neurosci. 2006;26(48):12439–12446. doi: 10.1523/JNEUROSCI.0434-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishijima K, Ng YS, Zhong L, et al. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol. 2007;171(1):53–67. doi: 10.2353/ajpath.2007.061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paraskevas KI, Tzovaras AA, Briana DD, Mikhaidis DP. Emerging indications for statins: a pluripotent family of agents with several potential applications. Curr Pharm Des. 2007;13(35):3622–3636. doi: 10.2174/138161207782794194. [DOI] [PubMed] [Google Scholar]
- 7.Bonetti PO, Lerman LO, Napoli C, Lerman A. Statin effects beyond lipid lowering-are they clinical relevant? Eur Heart J. 2003;24(3):225–248. doi: 10.1016/s0195-668x(02)00419-0. [DOI] [PubMed] [Google Scholar]
- 8.Bellosta S, Ferri N, Arnaboldi L, Bernini F, Paoletti R, Corsini A. Pleiotropic effects of statins in atherosclerosis and diabetes. Diabetes Care. 2000;23 suppl 2:B72–B78. [PubMed] [Google Scholar]
- 9.Bellosta S, Ferri N, Bernini F, Paoletti R, Corsini A. Non-lipid-related effects of statins. Ann Med. 2000;32(3):164–176. doi: 10.3109/07853890008998823. [DOI] [PubMed] [Google Scholar]
- 10.Danesh FR, Kanwar YS. Modulatory effects of HMG-CoA reductase inhibitors in diabetic microangiopathy. FASEB J. 2004;18(7):805–815. doi: 10.1096/fj.03-0839rev. [DOI] [PubMed] [Google Scholar]
- 11.Takayama T, Wada A, Tsutamoto T, et al. Contribution of vascular NAD(P)H oxidase to endothelial dysfunction in heart failure and the therapeutic effects of HMG-CoA reductase inhibitor. Circ J. 2004;68(11):1067–1075. doi: 10.1253/circj.68.1067. [DOI] [PubMed] [Google Scholar]
- 12.Al-Shabrawey M, Bartoli M, El-Remessy AB, et al. Inhibition of NAD(P)H oxidase activity blocks vascular endothelial growth factor overexpression and neovascularization during ischemic retinopathy. Am J Pathol. 2005;167(2):599–607. doi: 10.1016/S0002-9440(10)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyara S, Kiryu J, Yamashiro Y, et al. Simvastatin inhibits leukocyte accumulation and vascular permeability in the retinas of rats with streptozotocin-induced diabetes. Am J Pathol. 2004;164(5):1697–1706. doi: 10.1016/S0002-9440(10)63728-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Shabrawey M, Bartoli M, El-Remessy A, et al. Role of NAD(P)H oxidase and STAT3 in statin-mediated protection against diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008;49(7):3231–3238. doi: 10.1167/iovs.08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartoli M, Gu X, Tsai NT, et al. Vascular endothelial growth factor activates STAT proteins in aortic endothelial cells. J Biol Chem. 2000;275(43):33189–33192. doi: 10.1074/jbc.C000318200. [DOI] [PubMed] [Google Scholar]
- 16.Bartoli M, Platt D, Lemtalsi T, et al. VEGF differentially activates STAT3 in microvascular endothelial cells. FASEB J. 2003;17(11):1562–1564. doi: 10.1096/fj.02-1084fje. [DOI] [PubMed] [Google Scholar]
- 17.Platt DH, Bartoli M, El-Remessy AB, et al. Peroxynitrite increases VEGF expression in vascular endothelial cells via STAT3. Free Radic Biol Med. 2005;39(10):1353–1361. doi: 10.1016/j.freeradbiomed.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimura A. Signal transduction of inflammatory cytokines and tumor development. Cancer Sci. 2006;97(6):439–447. doi: 10.1111/j.1349-7006.2006.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith LE, Wesolowski E, McLellan A, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35(1):101–111. [PubMed] [Google Scholar]
- 20.Gu X, El-Remessy AB, Brooks SE, Al-Shabrawey M, Tsai NT, Caldwell RB. Hyperoxia induces retinal vascular endothelial cell apoptosis through formation of peroxynitrite. Am J Physiol Cell Physiol. 2003;285(3):C546–C554. doi: 10.1152/ajpcell.00424.2002. [DOI] [PubMed] [Google Scholar]
- 21.Gu X, Samuel S, Al-Shabrawey M, et al. Effects of sustained hyperoxiaon revascularization in experimental retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2002;43(2):496–502. [PubMed] [Google Scholar]
- 22.Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparins-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989;161(2):851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- 23.Cusik M, Chew Ey, Chan CC, Kruth HS, Murphy RP, Ferris FL., 3rd Histopathology and regression of retinal hard exudates in diabetic retinopathy after reduction of elevated serum lipid levels. Ophthalmology. 2003;110(11):2126–2133. doi: 10.1016/j.ophtha.2003.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Coccheri S. Approaches for prevention to cardiovascular complications and events in diabetes mellitus. Drugs. 2007;67(7):997–1026. doi: 10.2165/00003495-200767070-00005. [DOI] [PubMed] [Google Scholar]
- 25.Guymer RH, Chiu AW, Lim L, Baird PN. HMG-CoA reductase inhibitors (statins) do they have a role in age-related macular degeneration? Surv Ophthalmol. 2005;50(2):194–206. doi: 10.1016/j.survophthal.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Gupta A, Gupta V, Thapar S, Bhansali A. Lipid-lowering drug atorvastatin as an adjunct in the management of diabetic macular edema. Am J Ophthalmol. 2004;137(4):675–682. doi: 10.1016/j.ajo.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 27.Lahera V, Goicoechea M, de Vinuesa SG, et al. Endothelial dysfunction, oxidative stress and inflammation in atherosclerosis: beneficial effects of statins. Curr Med Chem. 2007;14(2):243–248. doi: 10.2174/092986707779313381. [DOI] [PubMed] [Google Scholar]
- 28.Sehgal PB, Guo GG, Shah M, Kumar V, Patel K. Cytokine signaling: STATs in plasma membrane rafts. J Biol Chem. 2002;277(14):12067–12074. doi: 10.1074/jbc.M200018200. [DOI] [PubMed] [Google Scholar]
- 29.Zuo L, Ushio-Fukai M, Hilenski LL, Alexander RW. Microtubules regulate angiotensin II type 1 receptor and Rac-1 localization in caveolae/lipid rafts: role in redox signaling. Arterioscler Thromb Vasc Biol. 2004;24(7):1223–1228. doi: 10.1161/01.ATV.0000132400.25045.2a. [DOI] [PubMed] [Google Scholar]
- 30.Ishida S, Yamashiro K, Usui T, et al. Leukocytes mediate retinal vascular remodeling during development and vaso-obliteration in disease. Nat Med. 2003;9(6):781–788. doi: 10.1038/nm877. [DOI] [PubMed] [Google Scholar]
- 31.Miyamoto K, Khosrof S, Bursell SE, et al. Vascular endothelial growth factor (VEGF)-induced retinal vascular permeability is mediated by intercellular adhesion molecule-1 (ICAM-1) Am J Pathol. 2000;156(5):1733–1739. doi: 10.1016/S0002-9440(10)65044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu M, Perez VL, Ma N, et al. VEGF increases retinal vascular ICAM-1 expression in vivo. Invest Ophthalmol Vis Sci. 1999;40(8):1808–1812. [PubMed] [Google Scholar]
- 33.Miyamoto K, Khosrof S, Bursell SE, et al. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci U S A. 1999;96(19):10836–10841. doi: 10.1073/pnas.96.19.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakurai E, Taguchi H, Anand A, et al. Targeted disruption of the CD18 or ICAM-1 gene inhibits choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44(6):2743–2749. doi: 10.1167/iovs.02-1246. [DOI] [PubMed] [Google Scholar]
- 35.Arjamaa O, Nikinmaa M. Oxygen-dependent diseases in the retinal the role of hypoxia-inducible factors. Exp Eye Res. 2006;83(3):473–483. doi: 10.1016/j.exer.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 36.Hilfiker-Kleiner D, Limbourg A, Drexler H. STAT3-mediated activation of myocardial capillary growth. Trends Cardiovasc Med. 2005;15(4):152–157. doi: 10.1016/j.tcm.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Chen Z, Han ZC. STAT3: a critical transcription activator in angiogenesis. Med Res Rev. 2008;28(2):185–200. doi: 10.1002/med.20101. [DOI] [PubMed] [Google Scholar]
- 38.Ushio-Fukai M, Tang Y, Fukai T, et al. Novel role of gp91(phox)-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res. 2002;91(12):1160–1167. doi: 10.1161/01.res.0000046227.65158.f8. [DOI] [PubMed] [Google Scholar]
- 39.Ushio-Fukai M. VEGF signaling through NAD(P)H oxidase-derived ROS. Antioxid Redox Signal. 2007;9(6):731–739. doi: 10.1089/ars.2007.1556. [DOI] [PubMed] [Google Scholar]
- 40.Urbich C, Dembach E, Ziher AM, Dimmeler S. Double-edge role of statins in angiogenesis signaling. Circ Res. 2002;90(6):737–744. doi: 10.1161/01.res.0000014081.30867.f8. [DOI] [PubMed] [Google Scholar]
- 41.Vincent L, Soria C, Mirshahi F, et al. Cerivastatin, an inhibitor of 3-hydroxy-3-methylglutaryl coenzyme a reductase, inhibits endothelial cell proliferation induced by angiogenic factors in vitro and angiogenesis in in vivo models. Arterioscler Thromb Vasc Biol. 2002;22(4):623–629. doi: 10.1161/01.atv.0000012283.15789.67. [DOI] [PubMed] [Google Scholar]
- 42.Weis M, Heeschen C, Glassford AJ, Cooke JP. Statins have biphasic effects on angiogenesis. Circulation. 2002;105(6):739–745. doi: 10.1161/hc0602.103393. [DOI] [PubMed] [Google Scholar]