Abstract
PURPOSE
Inhibitors of 3-hydroxy-3-methylglutaryl CoA reductase (statins) reduce signs of diabetic retinopathy in diabetic patients and animals. Indirect clinical evidence supports the actions of statins in improving cardiovascular function, but the mechanisms of their protective actions in the retina are not understood. Prior studies have implicated oxidative stress and NADPH oxidase–mediated activation of signal transducer and activator of transcription 3 (STAT3) in diabetes-induced increases in expression of vascular endothelial growth factor (VEGF) and intercellular adhesion molecule (ICAM)-1 and breakdown of the blood–retinal barrier (BRB). Because statins are known to be potent antioxidants, the hypothesis for the current study was that the protective effects of statins in preventing diabetic retinopathy involve blockade of diabetes-induced activation of NADPH oxidase and STAT3.
METHODS
The hypothesis was tested by experiments in which rats with streptozotocin (STZ)-induced diabetes and retinal endothelial cells maintained in high-glucose medium were treated with simvastatin. Blood–retinal barrier (BRB) function was assayed by determining extravasation of albumin. Oxidative stress was assayed by measuring lipid peroxidation, protein nitration of tyrosine, dihydroethidine oxidation, and chemiluminescence. Immunoprobe techniques were used to determine the levels of NADPH oxidase subunit expression and STAT3 activation.
RESULTS
These studies showed that simvastatin blocks diabetes or high-glucose–induced increases in VEGF and ICAM-1 and preserves the BRB by a process involving blockade of diabetes/high-glucose–induced activation of STAT3 and NADPH oxidase. Statin treatment also prevents diabetes-induced increases in expression of the NADPH oxidase catalytic and subunit NOX2.
CONCLUSIONS
These results suggest that simvastatin protects against the early signs of diabetic retinopathy by preventing NADPH oxidase-mediated activation of STAT3.
Diabetic retinopathy is the leading cause of acquired blindness among working-age adults in developed countries worldwide. Vision loss in this disease correlates strongly with increased vascular permeability.1 The retinal vascular leakage results in retinal edema that can lead to decreased visual acuity when it involves the macula. The overexpression of vascular endothelial cell growth factor (VEGF) plays a key role in this process (for review, see Ref. 2).
The increased formation of reactive oxygen species (ROS) is thought to be a key event in the pathogenesis of diabetic retinopathy, probably through increasing VEGF expression (Liu J, et al. IOVS 2007;48:ARVO E-Abstract 4984).3,4 We have shown that treatments that reduce ROS formation also prevent the early signs of diabetic retinopathy in a streptozotocin (STZ) diabetic rat model.4,5 A major source of ROS in vascular cells is activity of NADPH oxidase. Vascular endothelial cells express all the components of phagocytic NADPH oxidase, including the catalytic subunit NOX2 (formerly known as gp91phox), p22phox, p47phox, p67phox, and Rac1.6 NADPH oxidase has been shown to be critically involved in the VEGF-induced angiogenesis7 and in the neovascularization that occurs in response to hindlimb ischemia.8 We have recently demonstrated that ROS derived from NADPH oxidase are important for hypoxia-induced VEGF expression and angiogenesis in a model of ischemic retinopathy. 9 Moreover, we have data showing that inhibiting NADPH oxidase or deleting NOX2 blocks diabetes-induced increases in leukocyte adhesion to the vessel wall and prevents breakdown of the blood–retinal barrier (BRB).10
Signal transducer and activator of transcription 3 (STAT3) is critically involved in a wide variety of biological processes, including inflammation and angiogenesis. The role of STAT3 in angiogenesis has been shown to involve its action in increasing VEGF expression.11–15 Recent studies have identified STAT3 as a direct transcriptional activator of the VEGF gene.12,13 Furthermore, STAT3 is required for PI3K-Akt-mediated expression of HIF-1, a key regulator of VEGF expression.16 Our group has recorded data that show that diabetes or high glucose treatment causes sustained activation of STAT3 by a mechanism involving activation of NADPH oxidase (Liu J, et al. IOVS 2007;48:ARVO E-Abstract 4984). Moreover, inhibiting STAT3 in the retina by transduction with dominant negative STAT3 blocks the actions of diabetes that causes overexpression of VEGF and breakdown of the BRB.
Treatment with statins, inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme of cholesterol synthesis in the liver, has been shown to reduce the cardiovascular complications of diabetes. Clinical studies of diabetic patients have shown promising efficacy of statins in reducing signs of diabetic retinopathy.17 The mechanisms of these protective actions are not understood. However, studies in patients and experimental animal models have shown that in addition to lowering plasma lipid levels, statins are also highly effective in reducing the formation of ROS, limiting vascular inflammation and improving vascular endothelial cell function.18 In the present study, we investigated the impact of statin treatment on diabetes-induced upregulation of VEGF and ICAM-1 and vascular permeability in relation to activation of STAT3 and NADPH oxidase. We demonstrated in this study that treatment with statin blocks the effects of diabetes or high glucose in causing increases in VEGF and ICAM-1 expression and preserves the BRB in the diabetic rat retina by a process that involves blockade of NADPH oxidase-mediated activation of STAT3.
MATERIALS AND METHODS
Simvastatin (Calbiochem, La Jolla, CA) was prepared by opening the lactone ring and activating it. Briefly, native simvastatin (42 mg) was dissolved in 95% ethanol (1 mL). NaOH (0.1 N, 0.15 mL) was added and heated (50°C, 2 hours), followed by neutralization with HCl to a pH of ~7.2. Distilled water was added to bring the volume to 3 mL, and the mixture was freeze dried. The open form of simvastatin was dissolved in distilled water to a concentration of 100 mM, and aliquots were stored at 80°C until use for both the in vivo and in vitro studies.
Bovine serum albumin (BSA)-Alexa-Fluor 488 conjugate was from Invitrogen-Molecular Probes (Eugene, OR). Antibodies against phospho-Tyr705-STAT3 were from Cell Signaling Technology (Beverly, MA), and antibodies against STAT3 were from BD-Transduction Laboratories (San Diego, CA). Anti-VEGF was from Oncogene (San Diego, CA), anti-ICAM-1 from Santa Cruz Biotechnology (Santa Cruz, CA), anti-NOX-2 and anti-p47phox from Upstate Biochemicals (Charlottesville, VA), and anti-nitrotyrosine antibody and 3-nitrotyrosine were from Cayman Chemical Company (Ann Arbor, MI).
Treatment of Animals
All procedures with animals were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the institutional animal care and use committee (Animal Welfare Assurance no. A3307–01). Male Sprague-Dawley rats, weighing 250 to 300 g at the beginning of the study, were used. Diabetes was induced by intravenous injection of streptozotocin (STZ, 50 mg/kg, dissolved in 0.1 M sodium citrate buffer [pH 4.5]). Two groups of diabetic rats received either simvastatin (5 mg/kg/d, SC) or no treatment. Based on body surface area, this dose is 27.8 mg/m2/d, which falls within the dose range used in humans (i.e., 20–80 mg/d or 10.8–43.2 mg/m2/d). Age-matched control rats received only the vehicle. Rats were considered diabetic if their blood glucose was greater than 350 mg/dL. After 4 weeks, the animals were killed, and their blood was collected for analysis of blood glucose, cholesterol, and triglyceride levels. The results of these blood chemistry analyses, which have been reported by us previously,19 showed that simvastatin treatment of the diabetic animals significantly lowered the total cholesterol levels ([asterisks refer to *P < 0.05 versus control, **P < 0.05 versus diabetic] control = 78 mg/dL, diabetic 121 mg/dL*, diabetic + statin = 81 mg/dL**) and triglyceride levels (control = 53 mg/dL, diabetic = 391 mg/dL*, diabetic+statin = 135 mg/dL**). Blood glucose levels were not significantly altered by statin treatment (control = 106 mg/dL, diabetic = 506 mg/dL*, diabetic + statin = 486 mg/dL*). These results are consistent with those of previous studies showing that statin treatment blocks diabetes-induced increases in cholesterol and triglycerides in the STZ diabetic rat model.20,21
Retinas from different subgroups of animals were prepared for biochemical and morphologic analysis. For the biochemical studies, the animals were killed by decapitation, and the retinas were removed, snap frozen in liquid nitrogen, and stored at −80°C. Other groups were prepared for analysis of BRB function, immunofluorescence analysis, or dihydroethidine imaging, as explained later.
Tissue Culture
Bovine retinal endothelial cells (BRECs) were prepared as described previously.22 The cells were used between passages 6 and 9. Eighty-five percent confluent cultures were switched to serum-free medium containing normal d-glucose levels (5.5 mM, NG) or high glucose (25 mM, HG). The cells were maintained in these culture conditions in the presence or absence of simvastatin (10 µM), and expression of VEGF and ICAM-1 and activation of STAT3 were determined.
Measurement of BRB Function
Integrity of the BRB was measured as described previously.4,23 Briefly, rats received tail vein injections of BSA-Alexa-Fluor 488 conjugate (100 mg/kg). After 30 minutes, the animals were killed, and plasma samples were collected for analysis of Alexa-Fluor 488 concentration, and the eyes were rapidly removed, embedded in OCT, and frozen in liquid nitrogen. Serial sections (10 µm) collected at 60-µm intervals were imaged with a fluorescence microscope fitted with a spot camera. Images were collected from 10 retinal areas (200 µm2) in each section. The average retinal fluorescence intensity in each animal was calculated and normalized to plasma fluorescence intensity.
Measurement of ROS Formation
Retinal lipid peroxide concentration was determined by the method of Ohkawa et al.24 This method measures thiobarbituric acid reactivity by assaying malonaldehyde formation during acid hydrolysis of lipid peroxides, as described elsewhere.4
ROS formation was further evaluated by measuring protein nitration of tyrosine as a biomarker for peroxynitrite.4 Peroxynitrite is formed by the reaction of superoxide with nitric oxide and can be assayed by determining immunoreactivity for nitrotyrosine.
Superoxide production in retinal tissue sections was assayed by digital imaging microfluorometry of the oxidation of dihydroethidine (DHE) into ethidium bromide, which binds to DNA in the nucleus and fluoresces red.25 Serial cryosections from fresh-frozen retinas of control or diabetic rats treated with or without simvastatin were first incubated in NADPH (15 minutes, 100 µM) followed by incubation with DHE (20 minutes, 37°C). Images were obtained with a laser scanning confocal microscope. To demonstrate specificity of the DHE reaction for superoxide anion formation we preincubated some sections from nontreated diabetic rats with PEG-SOD (400 U) for 20 minutes, followed by DHE reaction.
NADPH oxidase activity in cultured BREC was measured using lucigenin chemiluminescence as described by Sorescu et al.26 The cells were maintained in 5.5 (normal) or 25 mM (high) d-glucose for 2 days before the assay. Specificity of the reaction for NADPH oxidase was demonstrated by the absence of reaction when NADH was used as substrate and by inhibition of the reaction with apocynin (10 mM).
Western Blot Analysis
Western blot analysis was performed as described before.10,27 For measurement of VEGF, protein samples were processed according to the heparin affinity protocol of Ferrara and Henzel.28 Briefly, protein samples (100 µg) were adjusted to a volume of 1 mL using 10 µM Tris (pH 7.4) and 100 µM NaCl and incubated overnight with 50 mL of equilibrated heparin-agarose beads (Sigma-Aldrich, St. Louis, MO). Samples were boiled in sodium dodecyl sulfate-sample buffer (100°C, 10 minutes) to elute the proteins that were then electrophoresed, transferred to nitrocellulose membranes, and probed with anti-VEGF antibody (Oncogene). Equal protein loading was verified by actin labeling of protein blots prepared from the same samples.
Statistical Analysis
Values are presented as the mean ± SEM with n values provided. Statistical significance of differences among the analyzed groups was determined by performing one way ANOVA and post hoc comparison. P < 0.05 was considered statistically significant.
RESULTS
Effect of Simvastatin on BRB Function and Expression of VEGF and ICAM-1
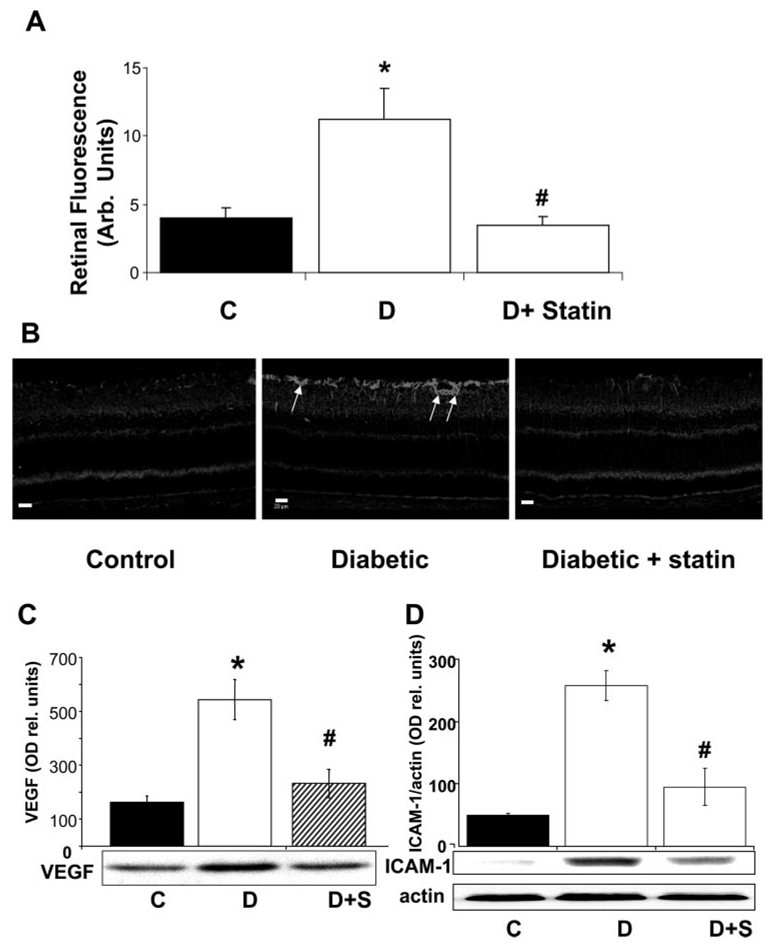
Our studies in STZ diabetic rats showed that retinal vascular permeability as indicated by extravasation of FITC-albumin was increased by ~2.7-fold in the diabetic retinas compared with the control rats (Fig. 1A). Treatment of the diabetic animals with simvastatin (5 mg/kg/d, SC) blocked this effect.
FIGURE 1.
Analysis of the effect of simvastatin (5 mg/kg/d, SC) on diabetes- induced increases in retinal vascular permeability and upregulation of VEGF and ICAM-1 protein in the STZ-induced diabetic rat retina. (A) Rats were injected intravenously with Alexa-Fluor 488-BSA (100 mg/kg), and permeability was determined by morphometric analysis of fluorescence intensity in serial sections. Diabetic retinas had a threefold increase in fluorescence compared with the control (C). Treatment of diabetic rats with simvastatin blocked the permeability increase. (B) Representative images showing the distribution of VEGF immunostaining. VEGF immunoreactivity is concentrated in glial cell processes within the inner retina (arrows). (C) Western blot analysis of VEGF protein showed a significant increase in VEGF expression in the diabetic retina that was blocked by simvastatin. (D) Western blot analysis of ICAM-1 protein showed a five- to sixfold increase in ICAM-1 expression in the diabetic retina that was blocked by simvastatin. Data represent the mean ± SEM of six in each group. *P < 0.01 vs. control; #P < 0.01 vs. diabetic. C, control; D, diabetic; S, simvastatin.
Immunolocalization studies showed increases in VEGF immunoreactivity within the inner retina of the diabetic rats (Fig. 1B), accompanied by increases in ICAM-1 immunoreactivity within the retinal vessels (data not shown). Western blot analysis followed by densitometric analysis showed that levels of VEGF and ICAM-1 protein in the diabetic retinas were increased by ~3.5- and 5-fold, respectively (Figs. 1C, 1D). Treatment of the diabetic animals with simvastatin (5 mg/kg/d, SC) blocked each of these effects.
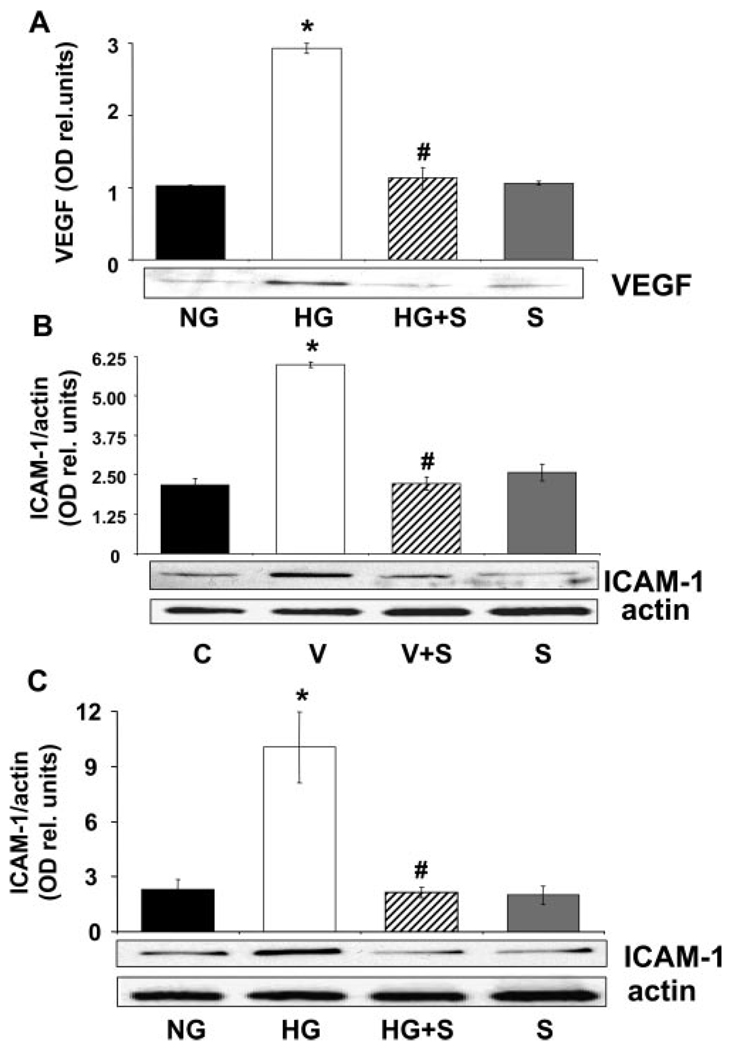
The retina is a complex tissue made up of a variety of cells, including neurons, glia, and vascular cells. Our group has shown in earlier work that vascular endothelial cells are important as a source of VEGF and are a target of VEGF in the diabetic retina (Liu J, et al. IOVS 2007;48:ARVO E-Abstract 4984).27 VEGF is also known to increase endothelial cell expression of ICAM-1.29 Therefore, we determined the effects of statin treatment on high-glucose– mediated increases in expression of VEGF and ICAM-1 and on VEGF-induced increases in ICAM-1 expression in cultured bovine retinal endothelial cells (BRECs). The results of these experiments showed that high-glucose treatment (25 mM d-glucose, 72 hours) caused prominent increases in VEGF and ICAM-1 protein levels and that VEGF treatment (25 ng/mL, 24 hours) caused increases in ICAM-1 comparable with those induced by high glucose. Each of these effects was completely blocked by simvastatin (10 µM, Fig. 2). These results indicate that the protective effects of simvastatin in blocking breakdown of the BRB in diabetes/high-glucose conditions are associated with normalization of VEGF and ICAM-1 expression levels in retinal endothelial cells.
FIGURE 2.
Measurement of VEGF and ICAM-1 protein levels in BRECs treated with high glucose (25 mM d-glucose, 3 days) or VEGF (20 ng/mL, 24 hours) with or without simvastatin (10 µM) versus control medium (5.5 mM d-glucose). Western blot analyses showed that the high glucose-induced increase in VEGF (A) and ICAM-1 (B) expression was blocked by simvastatin treatment. (C) Western blot analysis shows that the VEGF-induced increase in ICAM-1 expression was blocked by simvastatin treatment. Data represent the mean ± SEM of three to four experiments. *P < 0.01 vs. control, #P < 0.01 vs. high glucose. C, control; HG, high glucose; S, simvastatin; V, VEGF.
Effect of Simvastatin on Diabetes-Induced Activation of STAT3
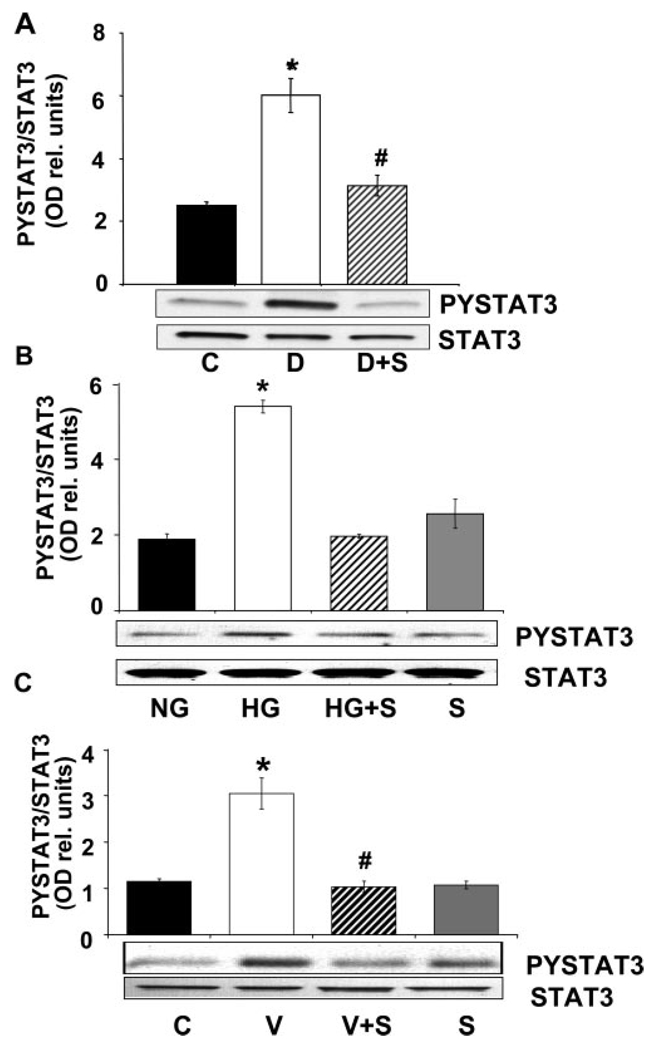
We have shown before that diabetes or high glucose–induced increases in ICAM-1 and VEGF expression (Liu J, et al. IOVS 2007;48:ARVO E-Abstract 4984) and peroxynitrite-induced increases in VEGF expression30 involve the activation of STAT3. To test whether the actions of statin treatment in normalizing VEGF and ICAM-1 expression in the diabetic retina or in high-glucose– treated BRECs involves blockade of STAT3 activation, we determined the effects of simvastatin on STAT3 phosphorylation of tyrosine 705 by Western blot. These experiments showed a significant increase in phosphorylation of STAT3 in retinal tissues of diabetic animals (Fig. 3A) and in cultured BRECs treated with high glucose or VEGF (Figs. 3B, 3C). These effects of diabetes, high glucose, and VEGF in causing activation of STAT3 were completely blocked by simvastatin treatment (5 mg/kg/d, SC).
FIGURE 3.
Analysis of STAT3 activation in the diabetic retina and high glucose-treated BRECs. (A) Western blot analysis of STAT3 activation as shown by phosphorylation of tyrosine 705 demonstrates that diabetes caused significant activation of STAT3 compared with the control. This effect was blocked by simvastatin (5 mg/kg/d, SC). Data represent the mean ± SEM for six animals. (B) Western blot analysis showing that high glucose treatment of BRECs (25 mM d-glucose, 72 hours) increased STAT3 activation above the control levels (5.5 mM d-glucose) and that simvastatin (10 µM) blocked the effect. (C) Western blot analysis showing that VEGF treatment of BRECs (20 ng/mL, 24 hours) increased STAT3 activation above the control levels and that simvastatin (10 µM) blocked the effect. Data represent the mean ± SEM of three experiments. *P < 0.01 vs. control, #P < 0.01 vs. high glucose. C, control; HG, high glucose, V, VEGF; S, simvastatin.
Effect of Simvastatin on Oxidative Stress and NOX2 Expression
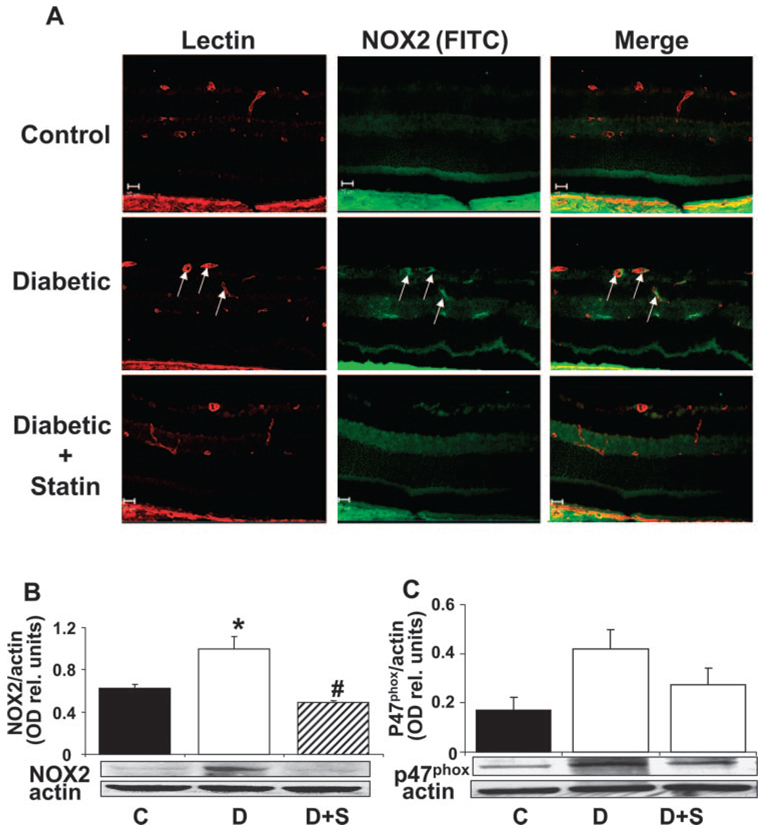
Our previous studies have shown that diabetes- and high glucose– induced activation of STAT3 is mediated by NADPH-derived ROS (Liu J, et al. IOVS 2007;48:ARVO E-Abstract 4984). We also have shown that NOX2/NADPH oxidase activity is upregulated by ischemia/hypoxia and that this process is critically involved in the induction of VEGF expression and retinal neovascularization in the mouse model of ischemic retinopathy. 9 To test whether the protective actions of simvastatin in blocking diabetes and high glucose–induced activation of STAT3, induction of VEGF and ICAM-1 expression, and breakdown of the BRB involves an effect on NOX2/NADPH oxidase, we next determined statin’s effects on NOX2/NADPH oxidase in the diabetic retina. Double label immunolocalization studies showed that NOX2 was increased within the diabetic retina and that the protein was localized to the retinal vessels (Fig. 4A). Western blot analysis demonstrated significant increases in levels of NOX2 protein in the diabetic retina (Fig. 4B). Furthermore, expression of p47phox, a cytosolic, regulatory subunit of NADPH oxidase, also appeared to increase in the diabetic retina (Fig. 4C). However, the data were more variable, and the group differences were not statistically significant. Simvastatin treatment (5 mg/kg/d, SC) blocked these effects.
FIGURE 4.
Analysis of NOX2 (gp91phox) and p47phox in control, diabetic, and simvastatin-treated diabetic (5 mg/kg/d, SC) retinas. (A) Retinal sections from control, diabetic, and statin-treated diabetic retinas were reacted with GSI lectin to label the vessels (red) and gp91phox antibody to label NOX2 (green). Merged images show low levels of NOX2 in the normal retina and marked increases in the diabetic retina, especially within the retinal vessels (yellow). (B) Western blot analysis showed a significant increase in NOX2 protein expression in the diabetic retinas compared with the control, which was blocked by statin treatment. (C) Western blot analysis shows increased levels of p47phox protein in the diabetic retina which was inhibited by statin treatment. Data represent the mean ± SEM of six animals/group. *P < 0.01 vs. control, #P < 0.01 vs. diabetic. C, control; D, diabetic; S, simvastatin.
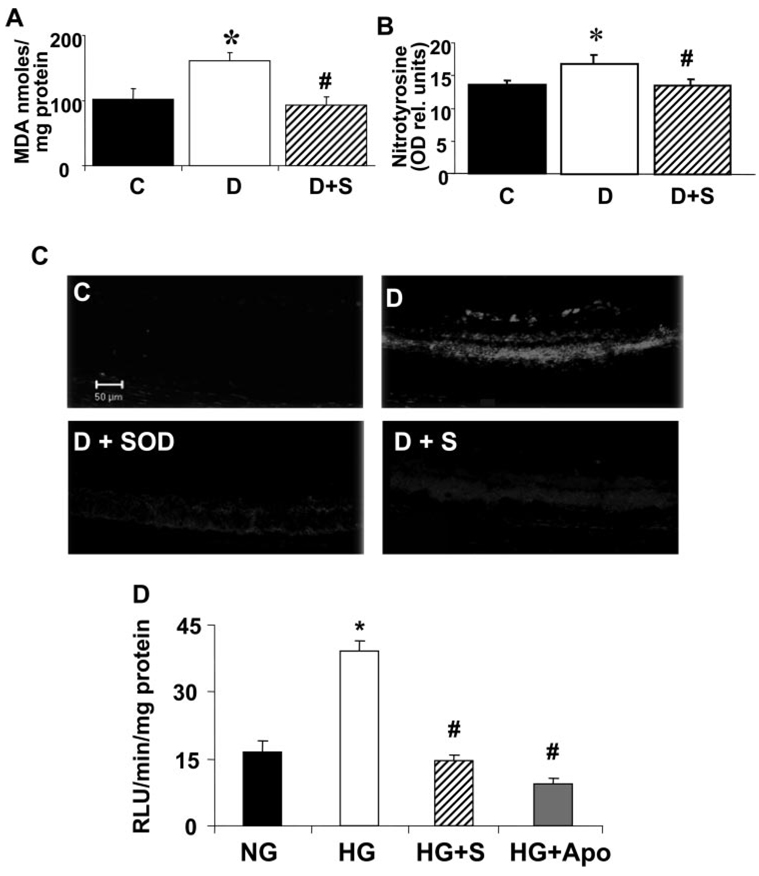
The increases in NOX2 expression were associated with significant increases in oxidative stress, as shown by measurement of lipid peroxidation and nitrotyrosine formation in retinal tissue extracts (Figs. 5A, 5B). Simvastatin treatment (5 mg/kg/d, SC) blocked both effects. Analysis of superoxide anion formation using DHE imaging showed a marked increase in conversion of DHE to ethidium bromide in the diabetic retinas (Fig. 5C). The reaction was markedly decreased in retinas from diabetic animals treated with simvastatin and was completely blocked when the retinal slices were pretreated with SOD, indicating the specificity of the reaction for superoxide.
FIGURE 5.
Analysis of ROS formation in the diabetic retina and high glucose-treated BRECs. (A) Levels of lipid peroxides in control, diabetic, and simvastatin-treated (5 mg/kg/d, SC) diabetic retinas as determined by the amount of thiobarbituric acid reactivity with malondialdehyde shows a significant increase in formation of lipid peroxides in the diabetic retina that was blocked by the statin treatment. Data represent the mean ± SEM of six animals/group. *P < 0.01 vs. untreated control, #P < 0.01 vs. diabetic. (B) Analysis of tyrosine nitration in control, diabetic, and simvastatin-treated (5 mg/kg/d) diabetic retinas shows a significant increase in peroxynitrite formation in the diabetic retina that was blocked by the statin treatment. Data represent the mean ± SEM of six animals/group. *P < 0.01 vs. untreated control, #P < 0.01 vs. diabetic. (C) Superoxide generation in the diabetic retina as determined by imaging of the oxidative fluorescent dye DHE in retinal sections in vitro shows a marked increase in the diabetic retinas that was blocked by the statin treatment. The DHE reaction was reduced in retinal sections treated with PEG-SOD, demonstrating specificity of the reaction for superoxide. (D) Analysis of superoxide generation in BRECs treated with high glucose (25 mM d-glucose, 5 days) using chemiluminescence showed a significant increase compared with control cultures treated with normal glucose media (5.5 mM d-glucose). The high-glucose effect was blocked by simvastatin (10 µM) or apocynin (10 mM). Blockade of the chemiluminescence reaction with apocynin demonstrated specificity of the reaction for NADPH oxidase. Data represent the mean ± SEM of four samples/group. *P < 0.01 vs. control, #P < 0.01 vs. high glucose. C, control; D, diabetic; S, simvastatin; NG, normal glucose; HG, high glucose; APO, apocynin.
To confirm the role of the endothelium in ROS formation, we used chemiluminescence techniques to measure superoxide formation in cultured BRECs treated with high glucose in the presence or absence of simvastatin, apocynin, or SOD. These experiments confirmed the requirement for NADPH oxidase activity in high glucose–induced production of ROS (Fig. 5D). The high-glucose treatment caused a significant increase in chemiluminescence compared with the control cells. The high-glucose effect was blocked in the cells treated with simvastatin. Control studies using the NADPH oxidase inhibitor apocynin showed a marked decrease in the chemiluminescence signal, demonstrating specificity of the reaction for NADPH oxidase activity.
DISCUSSION
In summary, our data show that simvastatin treatment blocked diabetes-induced increases in retinal levels of VEGF and ICAM-1 protein and prevented the associated breakdown of the BRB. These protective actions involved abrogation of diabetes-induced ROS formation, blockade of STAT3 activation, and normalization of NOX2 and p47phox expression. Moreover, statin treatment also blocked high-glucose– induced activation of both STAT3 and NADPH oxidase and normalized VEGF and ICAM-1 protein expression in retinal microvascular endothelial cells. Taken together, these results suggest that statins prevent the early signs of diabetic retinopathy by blocking NADPH oxidase–mediated activation of STAT3 in the vascular endothelium. Protective actions of simvastatin in blocking breakdown of the BRB and blood– brain barriers in diabetic rats have been described previously.31,32 However, to our knowledge, this is the first study to suggest a mechanism for the protective actions of statins in preventing diabetic retinopathy.
Increases in expression of NOX2 and activity of NADPH oxidase have been implicated in hypoxia-induced increases in VEGF expression9 and VEGF-induced angiogenesis.7 NADPH oxidase-derived ROS have also been shown to mediate permeability barrier dysfunction induced by TNF-α33 and angiotensin II34,35 treatment of cultured endothelial cells. Moreover, inhibiting NADPH oxidase with apocynin blocked vascular permeability increases in a lung model of ischemia–reperfusion injury.36 In the current study, increases in activity of NADPH oxidase and in expression of its catalytic subunit NOX2 and regulatory subunit p47phox accompanied diabetes-induced increases in vascular permeability in the retina, and statin treatment blocked these events.
Immunolabeling techniques showed that NOX2 was localized mainly to the retinal vessels. Furthermore our analyses of ROS formation as shown by assays of lipid peroxidation, peroxynitrite-mediated tyrosine nitration, and DHE imaging of superoxide formation confirmed significant increases in oxidative stress in the diabetic retina. Simvastatin treatment completely blocked the diabetes-induced increases in retinal vascular NOX2 expression and normalized the redox state, as shown by each of the assays.
Chemiluminescence studies of cultured retinal endothelial cells provided further support for the role of NADPH oxidase as a source of oxidative stress in the retinal vascular endothelium. The data showed that high-glucose exposure caused a significant increase in superoxide formation and that statin treatment completely blocked this effect. Furthermore, our finding that the NADPH oxidase inhibitor apocynin and superoxide dismutase were equally effective in blocking superoxide formation in the high-glucose–treated retinal endothelial cells, and that both agents reduced the signal to a low level suggests that NADPH oxidase is a major source of superoxide formation in the high glucose-treated cells.
Our studies in a mouse model of ischemic retinopathy have linked activation of NADPH oxidase with VEGF overexpression and retinal neovascularization.9 Although the mechanism involved in this process is not entirely clear, our studies in diabetic rats suggest that diabetes and high glucose–induced increases in VEGF and ICAM-1 expression, leukostasis, and breakdown of the BRB are linked to NADPH oxidase–dependent activation of STAT3 (Liu J, et al. IOVS 2007;48:ARVO E-Abstract 4984). In the present study, we showed that diabetes and high glucose induce activation of STAT3, as indicated by the fact that phosphorylation on Tyr705 was blocked by simvastatin treatment. Phosphorylation on Tyr705 is essential for STAT3 dimerization and nuclear translocation. STAT3 also has a conserved Ser727 residue, which is also a target for phosphorylation.37 Studies in several cell types indicate that a combination of tyrosine and serine phosphorylation is necessary for full activation of STAT3. The identity of the protein kinase responsible for STAT3 Ser727 phosphorylation in endothelial cells is unknown.
STAT3 has been shown to play a role in angiogenesis by inducing the expression of VEGF11–13,30 and HIF-1, a key regulator of the VEGF gene.16 STAT3 activation is also involved in inducing the expression of the gelatinase enzyme matrix metalloproteinase-2,38 which is important for angiogenic function.
These observations support the hypothesis that simvastatin blocks STZ diabetes-induced increases in ICAM-1 and VEGF expression and breakdown of the BRB by inhibiting NADPH oxidase activity and preventing the activation of STAT3. Because we saw effects of simvastatin in blocking STAT3 activation in high-glucose–treated retinal endothelial cells very similar to those that we saw in the diabetic retina, we believe that effects on the retinal vascular endothelium play a prominent role in the protective actions of simvastatin against diabetic retinopathy.
Clinical studies have suggested that statins prevent or reduce signs of diabetic retinopathy, as well as decreasing many of diabetes’ cardiovascular complications.17 The beneficial effects of statins on the vasculature have been attributed to their antioxidant and anti-inflammatory properties, as well as to their cholesterol- lowering ability.18,39 Studies in animals and tissue culture models have suggested that the former effects involve the inhibition of the isoprenylation/activation of small the G proteins Rac1 and RhoA.40,41 However, the question of whether this mechanism plays any role in the protective actions of statins in patients is controversial.18 Moreover, our results indicate that the protective actions of statins against diabetic retinopathy in rats are associated with blockade of diabetes-induced increases in plasma cholesterol. Given the localization of NADPH oxidase within cholesterol-rich lipid rafts and the requirement of enzyme assembly for activity, it is likely that the cholesterol-lowering action of statins contributes to their effects in levels of NADPH oxidase and STAT3 activation.
In summary, a better understanding of the mechanisms underlying the protective actions of statins in the retina can serve to guide development of new therapies for those patients who are unable to take statins due to muscle, liver, and neuronal toxicity associated with the dose needed for effect. Severe myopathy can cause acute renal failure in diabetic patients42 and statin-induced neuropathy is increasingly described.43 Targeting the NADPH oxidase–mediated activation of STAT3 may be helpful in avoiding statin-mediated toxicity.
Acknowledgments
Supported by the Juvenile Diabetes Research Foundation (JDRF) (MA); Fight For Sight (MA); JDRF (MB); AHA (ABE); National Heart, Lung, and Blood Institute Grant R01 HL70215 (RWC); National Eye Institute Grants R01 EY04618 and R01 EY11766, and Veterans Administration CDA and MRA (RBC).
Footnotes
Disclosure: M. Al-Shabrawey, None; M. Bartoli, None; A.B. El-Remessy, None; G. Ma, None; S. Matragoon, None; T. Lemtalsi, None; R.W. Caldwell, None; R.B. Caldwell, None
References
- 1.Antonetti DA, Lieth E, Barber AJ, Gardner TW. Molecular mechanisms of vascular permeability in diabetic retinopathy. Semin Ophthalmol. 1999;14:240–248. doi: 10.3109/08820539909069543. [DOI] [PubMed] [Google Scholar]
- 2.Caldwell RB, Bartoli M, Behzadian MA, et al. Vascular endothelial growth factor and diabetic retinopathy: role of oxidative stress. Curr Drug Targets. 2005;6:511–524. doi: 10.2174/1389450054021981. [DOI] [PubMed] [Google Scholar]
- 3.Obrosova IG, Minchenko AG, Marinescu V, et al. Antioxidants attenuate early up regulation of retinal vascular endothelial growth factor in streptozotocin-diabetic rats. Diabetologia. 2001;44:1102–1110. doi: 10.1007/s001250100631. [DOI] [PubMed] [Google Scholar]
- 4.El-Remessy AB, Behzadian MA, Abou-Mohamed G, Franklin T, Cald-well RW, Caldwell RB. Experimental diabetes causes breakdown of the blood-retina barrier by a mechanism involving tyrosine nitration and increases in expression of vascular endothelial growth factor and urokinase plasminogen activator receptor. Am J Pathol. 2003;162:1995–2004. doi: 10.1016/S0002-9440(10)64332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Remessy AB, Al-Shabrawey M, Khalifa Y, Tsai NT, Caldwell RB, Liou GI. Neuroprotective and blood-retinal barrier-preserving effects of cannabidiol in experimental diabetes. Am J Pathol. 2006;168:235–244. doi: 10.2353/ajpath.2006.050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ushio-Fukai M, Alexander RW. Reactive oxygen species as mediators of angiogenesis signaling: role of NAD(P)H oxidase. Mol Cell Biochem. 2004;264:85–97. doi: 10.1023/b:mcbi.0000044378.09409.b5. [DOI] [PubMed] [Google Scholar]
- 7.Ushio-Fukai M, Tang Y, Fukai T, et al. Novel role of gp91(phox)-containing NAD(P)H oxidase in vascular endothelial growth factorinduced signaling and angiogenesis. Circul Res. 2002;91:1160–1167. doi: 10.1161/01.res.0000046227.65158.f8. [DOI] [PubMed] [Google Scholar]
- 8.Tojo T, Ushio-Fukai M, Yamaoka-Tojo M, Ikeda S, Patrushev N, Alexander RW. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation. 2005;111:2347–2355. doi: 10.1161/01.CIR.0000164261.62586.14. [DOI] [PubMed] [Google Scholar]
- 9.Al-Shabrawey M, Bartoli M, El-Remessy AB, et al. Inhibition of NAD(P)H oxidase activity blocks vascular endothelial growth factor overexpression and neovascularization during ischemic retinopathy. Am J Pathol. 2005;167:599–607. doi: 10.1016/S0002-9440(10)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Shabrawey M, Sanders T, Rojas M, et al. Role of NADPH oxidase in vascular inflammation associated with diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008 March 31; doi: 10.1167/iovs.08-1755. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Funamoto M, Fujio Y, Kunisada K, et al. Signal transducer and activator of transcription 3 is required for glycoprotein 130-mediated induction of vascular endothelial growth factor in cardiac myocytes. J Biol Chem. 2000;275:10561–10566. doi: 10.1074/jbc.275.14.10561. [DOI] [PubMed] [Google Scholar]
- 12.Niu G, Wright KL, Huang M, et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 13.Wei D, Le X, Zheng L, et al. Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene. 2003;22:319–329. doi: 10.1038/sj.onc.1206122. [DOI] [PubMed] [Google Scholar]
- 14.Wei LH, Kuo ML, Chen CA, et al. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene. 2003;22:1517–1527. doi: 10.1038/sj.onc.1206226. [DOI] [PubMed] [Google Scholar]
- 15.Niu G, Bowman T, Huang M, et al. Roles of activated Src and Stat3 signaling in melanoma tumor cell growth. Oncogene. 2002;21:7001–7010. doi: 10.1038/sj.onc.1205859. [DOI] [PubMed] [Google Scholar]
- 16.Xu Q, Briggs J, Park S, et al. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene. 2005;24:5552–5560. doi: 10.1038/sj.onc.1208719. [DOI] [PubMed] [Google Scholar]
- 17.Yamagishi S, Nakamura K, Matsui T, Sato T, Takeuchi M. Potential utility of statins, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors in diabetic retinopathy. Med Hypotheses. 2006;66:1019–1021. doi: 10.1016/j.mehy.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 18.Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tawfik HE, El-Remessy AB, Matragoon S, Ma G, Caldwell RB, Caldwell RW. Simvastatin improves diabetes-induced coronary endothelial dysfunction. J Pharmacol Exp Therapeut. 2006;319:386–395. doi: 10.1124/jpet.106.106823. [DOI] [PubMed] [Google Scholar]
- 20.Mahfouz MM, Kummerow FA. Atorvastatin reduces the plasma lipids and oxidative stress but did not reverse the inhibition of prostacyclin generation by aortas in streptozotocin diabetic rats. Prostaglandins Other Lipid Mediat. 2005;76:59–73. doi: 10.1016/j.prostaglandins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Ceylan A, Karasu C, Aktan F, Ozansoy G. Simvastatin treatment restores vasoconstriction and the inhibitory effect of LPC on endothelial relaxation via affecting oxidizing metabolism in diabetic rats. Diabetes Nutr Metabol. 2004;17:203–210. [PubMed] [Google Scholar]
- 22.Behzadian MA, Wang XL, Jiang B, Caldwell RB. Angiostatic role of astrocytes: suppression of vascular endothelial cell growth by TGF-beta and other inhibitory factor(s) Glia. 1995;15:480–490. doi: 10.1002/glia.440150411. [DOI] [PubMed] [Google Scholar]
- 23.Antonetti DA, Barber AJ, Khin S, Lieth E, Tarbell JM, Gardner TW Penn State Retina Research Group. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells. Diabetes. 1998;47:1953–1959. doi: 10.2337/diabetes.47.12.1953. [DOI] [PubMed] [Google Scholar]
- 24.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 25.Miller FJ, Jr, Gutterman DD, Rios CD, Heistad DD, Davidson BL. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circul Res. 1998;82:1298–1305. doi: 10.1161/01.res.82.12.1298. [DOI] [PubMed] [Google Scholar]
- 26.Sorescu D, Somers MJ, Lassegue B, Grant S, Harrison DG, Griendling KK. Electron spin resonance characterization of the NAD(P)H oxidase in vascular smooth muscle cells. Free Radic Biol Med. 2001;30:603–612. doi: 10.1016/s0891-5849(00)00507-4. [DOI] [PubMed] [Google Scholar]
- 27.Bartoli M, Platt D, Lemtalsi T, et al. VEGF differentially activates STAT3 in microvascular endothelial cells. FASEB J. 2003;17:1562–1564. doi: 10.1096/fj.02-1084fje. [DOI] [PubMed] [Google Scholar]
- 28.Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989;161:851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- 29.Radisavljevic Z, Avraham H, Avraham S. Vascular endothelial growth factor up-regulates ICAM-1 expression via the phosphatidylinositol 3 OH-kinase/AKT/Nitric oxide pathway and modulates migration of brain microvascular endothelial cells. J Biol Chem. 2000;275:20770–20774. doi: 10.1074/jbc.M002448200. [DOI] [PubMed] [Google Scholar]
- 30.Platt DH, Bartoli M, El-Remessy AB, et al. Peroxynitrite increases VEGF expression in vascular endothelial cells via STAT3. Free Radic Biol Med. 2005;39:1353–1361. doi: 10.1016/j.freeradbiomed.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 31.Miyahara S, Kiryu J, Yamashiro K, et al. Simvastatin inhibits leukocyte accumulation and vascular permeability in the retinas of rats with streptozotocin-induced diabetes. Am J Pathol. 2004;164:1697–1706. doi: 10.1016/S0002-9440(10)63728-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mooradian AD, Haas MJ, Batejko O, Hovsepyan M, Feman SS. Statins ameliorate endothelial barrier permeability changes in the cerebral tissue of streptozotocin-induced diabetic rats. Diabetes. 2005;54:2977–2982. doi: 10.2337/diabetes.54.10.2977. [DOI] [PubMed] [Google Scholar]
- 33.Gertzberg N, Neumann P, Rizzo V, Johnson A. NAD(P)H oxidase mediates the endothelial barrier dysfunction induced by TNF-alpha. Am J Physiol Lung Cell Mol Physiol. 2004;286:L37–L48. doi: 10.1152/ajplung.00116.2003. [DOI] [PubMed] [Google Scholar]
- 34.Yamagishi S, Nakamura K, Ueda S, Kato S, Imaizumi T. Pigment epithelium-derived factor (PEDF) blocks angiotensin II signaling in endothelial cells via suppression of NADPH oxidase: a novel antioxidative mechanism of PEDF. Cell Tissue Res. 2005;320:437–445. doi: 10.1007/s00441-005-1094-8. [DOI] [PubMed] [Google Scholar]
- 35.Ying CJ, Xu JW, Ikeda K, Takahashi K, Nara Y, Yamori Y. Tea polyphenols regulate nicotinamide adenine dinucleotide phosphate oxidase subunit expression and ameliorate angiotensin II-induced hyperpermeability in endothelial cells. Hypertens Res. 2003;26:823–828. doi: 10.1291/hypres.26.823. [DOI] [PubMed] [Google Scholar]
- 36.Dodd OJ, Pearse DB. Effect of the NADPH oxidase inhibitor apocynin on ischemia-reperfusion lung injury. Am J Physiol Heart Circ Physiol. 2000;279:H303–H312. doi: 10.1152/ajpheart.2000.279.1.H303. [DOI] [PubMed] [Google Scholar]
- 37.Decker T, Kovarik P. Serine phosphorylation of STATs. Oncogene. 2000;19:2628–2637. doi: 10.1038/sj.onc.1203481. [DOI] [PubMed] [Google Scholar]
- 38.Xie TX, Wei D, Liu M, et al. Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene. 2004;23:3550–3560. doi: 10.1038/sj.onc.1207383. [DOI] [PubMed] [Google Scholar]
- 39.Sowers JR. Effects of statins on the vasculature: implications for aggressive lipid management in the cardiovascular metabolic syndrome. Am J Cardiol. 2003;91:14B–22B. doi: 10.1016/s0002-9149(02)03269-1. [DOI] [PubMed] [Google Scholar]
- 40.Delbosc S, Morena M, Djouad F, Ledoucen C, Descomps B, Cristol JP. Statins, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, are able to reduce superoxide anion production by NADPH oxidase in THP-1-derived monocytes. J Cardiovasc Pharmacol. 2002;40:611–617. doi: 10.1097/00005344-200210000-00015. [DOI] [PubMed] [Google Scholar]
- 41.Wassmann S, Laufs U, Baumer AT, et al. Inhibition of geranylgeranylation reduces angiotensin II-mediated free radical production in vascular smooth muscle cells: involvement of angiotensin AT1 receptor expression and Rac1 GTPase. Mol Pharmacol. 2001;59:646–654. doi: 10.1124/mol.59.3.646. [DOI] [PubMed] [Google Scholar]
- 42.Grundy SM. The issue of statin safety: where do we stand? Circulation. 2005;111:3016–3019. doi: 10.1161/CIRCULATIONAHA.105.557652. [DOI] [PubMed] [Google Scholar]
- 43.Vaughan TB, Bell DS. Statin neuropathy masquerading as diabetic autoimmune polyneuropathy. Diabetes Care. 2005;28:2082. doi: 10.2337/diacare.28.8.2082. [DOI] [PubMed] [Google Scholar]