Abstract
We have previously shown that T cells are required for the full development of angiotensin II-induced hypertension. However, the specific subsets of T cells that are important in this process are unknown. Th17 cells represent a novel subset that produces the proinflammatory cytokine interleukin 17 (IL17). We found that angiotensin II infusion increased IL17 production from T cells and IL17 protein in the aortic media. To determine the effect of IL17 on blood pressure and vascular function, we studied IL17−/− mice. The initial hypertensive response to angiotensin II infusion was similar in IL17−/− and C57BL/6J mice. However, hypertension was not sustained in IL17−/− mice, reaching levels 30 mmHg lower than in wild type mice by 4 weeks of angiotensin II infusion. Vessels from IL17−/− mice displayed preserved vascular function, decreased superoxide production, and reduced aortic T cell infiltration in response to angiotensin II. Gene array analysis on cultured human aortic smooth muscle cells revealed that IL17, in conjunction with tumor necrosis factor α, modulated expression of over 30 genes, including a number of inflammatory cytokines/chemokines. Examination of IL17 in diabetic humans showed that serum levels of this cytokine were significantly increased in those with hypertension compared to normotensive subjects. We conclude that IL17 is critical for the maintenance of angiotensin II-induced hypertension and vascular dysfunction and might be a therapeutic target for this widespread disease.
Keywords: Angiotensin II, inflammation, hypertension, interleukins, blood vessels
Introduction
There is emerging evidence that both innate and adaptive immune responses contribute to vascular dysfunction and hypertension1. Early studies by Svendsen2 and Bataillard et al3 suggested that the thymus was important in experimental hypertension in rodents. Pharmacological and pathological immune suppression, such as occurs with HIV, is associated with decreased blood pressure in animals and humans4–6. In addition, T lymphocytes infiltrate the kidney in hypertensive animals and efforts to reduce this decrease blood pressure7. Recently we provided further evidence that T cells are essential for the development of hypertension. Mice lacking T and B cells (RAG1−/− mice) had blunted hypertension in response to angiotensin II and little or none of the associated vascular dysfunction. Adoptive transfer of T, but not B, cells restored both the hypertensive response and vascular abnormalities in RAG1−/− mice. angiotensin II-induced hypertension was associated with increased numbers of T cells infiltrating the adventitia and periadvential fat of vessels8.
The specific subsets of T cells that are important in hypertension are not known. Traditionally, T helper cells are thought to differentiate into distinct subsets designated Th1 and Th2 with different functions and patterns of cytokine secretion9. Th1 cells primarily express interleukin (IL)-2, interferon (IFN)-γ, and TNF-β and mediate host defense against intracellular pathogens such as viruses and some bacteria. Th2 cells primarily express IL-4, IL-5, IL-6, and IL-13 and respond to extracellular stimuli such as allergens and helminths (reviewed in Abbas et al10). An imbalance in Th1/Th2 subsets is implicated in resistance and susceptibility to infection10, the pathogenesis of autoimmune diseases including diabetes11, the development of atherosclerosis12 and hypertensive kidney disease13. In 2005, a novel T helper subset that produces the unique cytokine, IL17, designated Th17 cells, was described14. There are 6 known members of the IL17 family designated IL17A-F of which IL17A and IL17F are produced by Th17 cells15. IL17A is the most widely studied and has been implicated in the pathogenesis of many autoimmune and inflammatory diseases such as rheumatoid arthritis (RA), psoriasis, multiple sclerosis, asthma, inflammatory bowel disease, and periodontal disease (reviewed in Tesmer et al15). Recent evidence also supports a role for IL17 in cardiovascular disease 16–18.
Importantly, IL17 synergizes with other cytokines, such as tumor necrosis factor α (TNFα), to modulate inflammatory responses19. Given our results that TNFα plays a critical role in angiotensin II-induced hypertension8, we hypothesized that IL17 might also contribute to the hypertensive phenotype. To test this hypothesis, we studied the production of IL17 by T cells in response to angiotensin II, the effect of angiotensin II on hypertension and vascular function in mice deficient in IL17, and the effects of IL17 on vascular smooth muscle cells via gene array. We also examined serum levels of IL17 in humans with and without hypertension. Our results suggest that Th17 cells and IL17 could serve as a therapeutic target for the treatment of hypertension and particularly the vascular dysfunction associated with hypertension.
Materials and Methods
Animal and Cell Culture Studies
C57BL/6J mice were obtained from Jackson Laboratories. IL17−/− mice were generated as described in Nakae et al20 and back-crossed to the C57BL/6J background. Blood pressure was measured by tail cuff or telemetry as previously described21, 22. Immunofluorescence was performed with a rat-anti-mouse IL-17 antibody (a kind gift from Dr. Jerry Y. Niederkorn - Department of Ophthalmology, University of Texas Southwestern Medical Center, Dallas, Texas). Isometric tension studies of aortic rings, vascular superoxide production, and T cell infiltration by FACS were performed as previously described8. Illumina whole genome beadchip microarray analysis was performed on 3 independent RNA samples from human aortic smooth muscle cells (HASMs) treated with TNFα (20ng/ml), IL17 (100ng/ml), TNFα (20ng/ml) plus IL17 (100ng/ml), or no treatment. The Institutional Animal Care and Use Committee at Emory University approved all animal protocols.
Patient population
IL-17A was measured in serum from 112 patients with type 2 diabetes and concomitant other risk factors for atherosclerosis. The Jagiellonian University Ethics Committee in Cracow, Poland approved collection of specimens. Clinical characteristics of the population are shown in supplemental Table 1 at http://hyper.ahajournals.org. IL17 levels were determined by quantitative immunoassay.
See online supplement at http://hyper.ahajournals.org for additional/expanded Materials and Methods section.
Results
Angiotensin II increased IL17 production from T cells
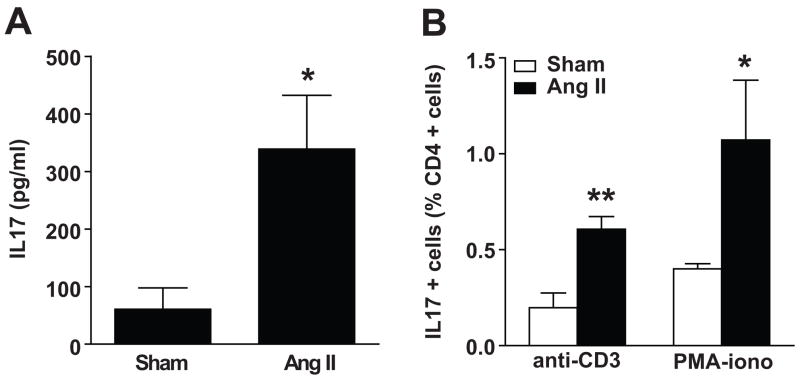
To investigate whether IL17 could play an important role in hypertension, we first determined if chronic infusion of angiotensin II increased production of IL17 from circulating T cells. Angiotensin II infusion for two weeks increased T cell production of IL17 by greater than 5-fold as determined by ELISA (Figure 1A). In keeping with this, intracellular staining showed that the number of circulating Th17 cells was increased by angiotensin II. At baseline, the number of CD4+ cells producing IL17 (Th17 cells) in response to CD3 or PMA/ionomycin was low (0.2% and 0.4%, respectively). This was increased 2–3 fold in mice with 2 weeks of angiotensin II-induced hypertension (Figure 1B).
Figure 1. Effect of angiotensin II on IL17 production from T cells.
C57BL/6J mice were treated for 14 d with vehicle (sham) or angiotensin II. T lymphocytes were then isolated, cultured, and stimulated with anti-CD3 antibodies or PMA-ionomycin. (A) ELISA for IL17 released into the media (n=6–8 per group). (B) Intracellular FACS staining for IL17 expressed as percentage of CD4+ cells (n=5–6 per group). Data are expressed as mean±SEM. *p<0.05 vs sham, **p<0.01 vs sham.
IL17 is required for the maintenance of angiotensin II-induced hypertension
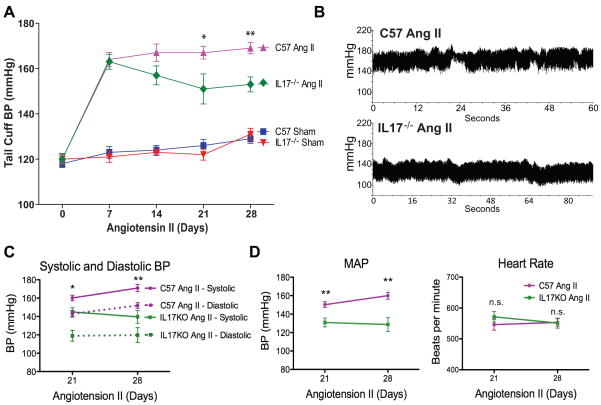
We then determined the effect of IL17 on blood pressure using both the noninvasive tail cuff method as well as invasive monitoring with radio telemetry catheters. Interestingly, the initial hypertensive response to angiotensin II was similar in the IL17−/− mice and C57BL/6J controls. By week 3, however, the C57BL/6J mice remained hypertensive while the blood pressure in IL17−/− mice started to decline (Figure 2A). By 4 weeks of angiotensin II infusion, the systolic pressure by telemetry was 30 mmHg lower in IL17−/− mice compared to wild type mice (Figures 2B and 2C). Diastolic pressures and mean arterial pressures were similarly decreased in IL-17−/− mice by three weeks of angiotensin II infusion, while heart rate was similar between the two groups (Figure 2C–D).
Figure 2. Effect of IL17 on angiotensin II-induced hypertension.
(A) Non-invasive blood pressure measurements obtained via the tail cuff method (n=8–9 per group). (B) Sample traces of telemetry recordings from freely moving mice after 4 weeks of angiotensin II infusion obtained via indwelling carotid artery catheters. Systolic and diastolic blood pressure (C), mean arterial pressure (MAP) and heart rate (D) from telemetry recordings (n=4–6 per group). Data are expressed as mean±SEM. *p<0.05 vs IL17−/− angiotensin II, **p<0.01 vs IL17−/− angiotensin II, n.s. = not significant.
Angiotensin II increased vascular levels of IL17 protein
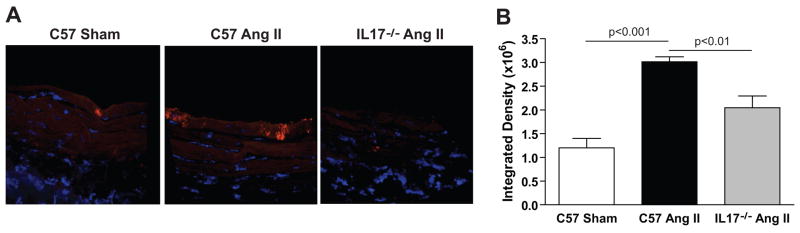
We performed immunofluorescence analysis for IL17 in sections from the thoracic aorta of C57BL/6J or IL17−/− mice treated with vehicle or angiotensin II for 4 weeks. Angiotensin II infusion markedly increased the levels of IL17 in the thoracic aorta with specific staining confined to the innermost medial layer of the vessel (Figure 3A). There was also some staining seen in the IL17−/− mice, perhaps due to cross-reactivity with isoforms other than IL17A, but quantification of intensity revealed that this was significantly lower than that seen in the C57BL/6J mice treated with angiotensin II (Figure 3B). The increase in IL17 protein in the aorta is not due to increased IL17 receptor expression, as this was similar between sham and angiotensin II-treated aortas by real-time RT-PCR (data not shown).
Figure 3. Effect of angiotensin II on IL17 protein in vessels.
Immunoflourescence for IL17 in sections from the thoracic aorta of C57BL/6J or IL17−/− mice treated with vehicle or angiotensin II for 4 weeks. (A) Representative confocal images taken at 60x/oil. (B) Integrated density quantification made from 20x Axiocam images (n=3–4 per group). Data are expressed as mean±SEM. p-values are shown on graph.
IL17 contributes to angiotensin II-induced vascular dysfunction
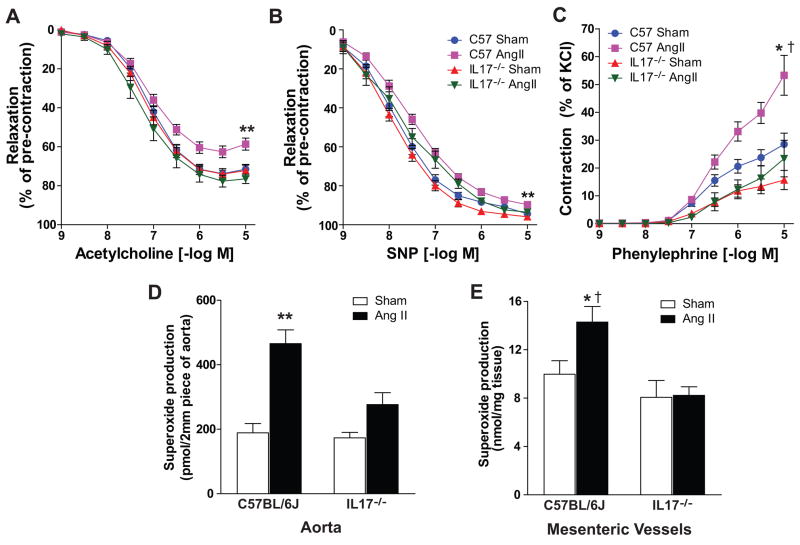
As previously reported, angiotensin II-induced hypertension was associated with blunted endothelium-dependent vasodilatation to acetylcholine in C57BL/6J mice with only mild impairment of endothelium–independent vasodilatation to sodium nitroprusside (Figures 4A and 4B). In contrast, endothelium-dependent vasodilatation was preserved in IL-17−/− mice (Figure 4A). Also, in keeping with prior studies, angiotensin II-induced hypertension markedly augmented contraction to phenylephrine from C57BL/6J mice23. This effect was dramatically blunted in IL17−/− mice (Figure 4C). These perturbations of vascular tone are thought to be at least partly mediated by alterations in superoxide production24. Indeed we found that angiotensin II more than doubled superoxide production in C57BL/6J aortas, and this effect was abrogated in mice deficient in IL17 (Figure 4D). To extend these results to resistance vessels, we confirmed that IL17 deficiency abrogated the increase in superoxide production in mesenteric vessels in response to angiotensin II (Figure 4E). Taken together, these results indicate that IL17 plays a critical role in altering vascular reactivity and increasing superoxide production in angiotensin II-induced hypertension.
Figure 4. Effect of IL17 on vascular function and superoxide production.
Aortic rings were isolated from C57BL/6J and IL17−/− mice exposed to 4 weeks of vehicle or angiotensin II. (A) Endothelium-dependent relaxation to increasing doses of acetylcholine (n=7–13 per group). (B) Endothelium-independent relaxation to increasing doses of sodium nitroprusside (n=7–13 per group). (C) Contraction to increasing doses of phenylephrine (n=5–9 per group). (D) Aortic superoxide production as measured by dihydroethidium/HPLC analysis (n=6–7 per group). (E) Superoxide production in mesenteric vessels as measured by dihydroethidium/HPLC analysis (n=4–5 per group). Data are expressed as mean±SEM. **p<0.001 vs remaining 3 groups. *p<0.05 vs C57BL/6J Sham, †p<0.01 vs IL17−/− Sham and IL17−/− angiotensin II.
IL17 modulated expression of adhesion molecules and inflammatory cytokines/chemokines in cultured human aortic smooth muscle cells
The above experiments suggest that IL17 could promote a pro-inflammatory milieu in the vessel wall. To investigate this further, we performed gene array analysis on cultured human aortic smooth muscle cells (HASMs) treated with and without IL17. Surprisingly, IL17 alone had little effect on gene expression in these cells. Only one gene, stanniocalcin-1, was modestly increased by IL17 treatment. Because IL17 has been shown to act synergistically with other potent cytokines such as TNFα and since we previously showed that TNFα is important in angiotensin II-induced hypertension8, we also analyzed the effect of IL17 in combination with TNFα on global gene expression in HASMs. Thirty-two genes were altered in response to IL17 plus TNFα compared to TNFα alone with 6 genes, including VCAM-1, being downregulated by IL17 and 26 genes, including a number of inflammatory cytokines/chemokines, being upregulated by IL17 (see supplemental Figure 1 and supplemental Tables 2–3 at http://hyper.ahajournals.org). Among the genes upregulated by IL17 were chemokine (C-C motif) ligand 8 (CCL8), colony stimulating factor 3 (granulocyte) (CSF3), chemokine (C-C motif) ligand 7 (CCL7), and chemokine (C-X-C motif) ligand 2 (CXCL2).
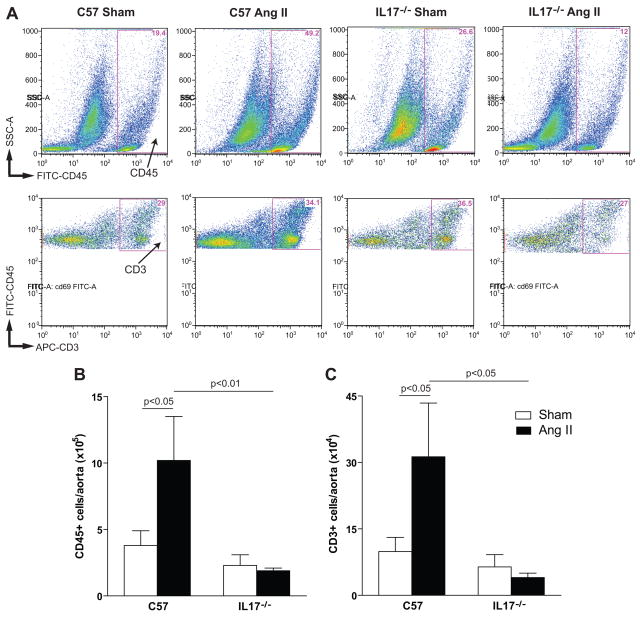
IL17−/− mice have reduced aortic T cell infiltration
We have previously shown that angiotensin II increases T cell infiltration into the vascular adventitia and periadventitial fat8. IL17 is known to promote chemotaxis of inflammatory cells in conditions such as rheumatoid arthritis25 and uveitis26. Moreover, our gene array analysis indicated that IL17 upregulates a number of cytokines/chemokines which could increase T cell infiltration. To test this hypothesis, we performed FACS analysis on collagenase-digested aortas from C57BL/6J and IL17−/− mice treated with 4 weeks of angiotensin II. As previously described, the number of vascular leukocytes (CD45 positive cells) and T cells (CD3 positive cells) increased by chronic angiotensin II infusion in C57BL/6J mice (Figure 5). Strikingly, this inflammatory response was abolished in IL17−/− mice, indicating that IL17 is essential for vascular T cell homing (Figure 5).
Figure 5. Effect of IL17 on aortic T cell infiltration.
(A) Examples of flow cytometric analysis of collagenase-digested aortic single cell suspensions from C57BL/6J and IL17−/− mice exposed to 4 weeks of vehicle or angiotensin II. Fluorescent staining was performed to detect CD45 (total leukocytes) and CD3 (T cells). Absolute numbers of total leukocytes (CD45+ cells, panel B) and total T cells (CD3+ cells, panel C) in whole aortas from vehicle-infused/sham treatment (open bars) and angiotensin II-infused (solid bars) mice (n=7–9 per group). p-values are shown on graph.
IL17 levels are increased in human hypertension
To determine if IL17 is important in human hypertension, we analyzed serum IL17 levels in type 2 diabetic patients with and without hypertension as well as other cardiovascular risk factors. A population with type II diabetes was studied since these subjects are prone to develop vascular dysfunction and hypertension, and effects of concomitant risk factors on vascular phenotypes are more pronounced. One hundred and twelve patients were analyzed (94 with hypertension or on antihypertensive medication and 18 normotensive individuals). The average IL17 level in the 18 normotensive individuals was 2.2+1.4 pg/ml and in the 94 hypertensive individuals was 7.1+1.6 pg/ml (Table 1). Multivariate analysis taking into account major risk factors for atherosclerosis and the presence of coronary artery disease (CAD) revealed that hypertension was most strongly independently associated with increased serum IL17 levels (p<0.005), while smoking and male sex correlated with decreased IL17 levels (p<0.04 and p<0.02, respectively) (Table 1).
Table 1.
Serum IL17 levels in diabetic patients with concomitant risk factors for coronary disease:
| Serum IL-17 level (pg/ml; mean ± SEM) | |||||||
|---|---|---|---|---|---|---|---|
| Factors | n with Factor | n no Factor | With Factor | No Factor | Type III Sum of Squares | F | p |
| Hypercholesterolemia | 74 | 38 | 8.1 ±2.3 | 4.9± 2.1 | 54 | 0.3 | 0.6 |
| Hypertension | 94 | 18 | 7.1 ± 1.6 | 2.2 ±1.4 | 1628 | 8.7 | 0.005 |
| Smoking | 22 | 90 | 5.2± 2.1 | 7.1± 1.6 | 1289 | 3.4 | 0.04 |
| Male sex | 51 | 61 | 5.2± 1.5 | 8.5± 2.4 | 1201 | 6.4 | 0.02 |
| Obesity | 103 | 9 | 6.8 ±1.3 | 5.5 ±2.1 | 228 | 1.2 | 0.3 |
| CAD | 73 | 39 | 5.7 ±2.3 | 6.6 ±1.8 | 39 | 0.2 | 0.6 |
CAD = coronary artery disease (angiographically)
Discussion
The present study adds to our prior knowledge regarding the inflammatory process in hypertension, as it identifies IL17, and by extension the Th17 subset of T cells, as essential in this disease. We showed that the hypertensive state caused by angiotensin II is associated with increased Th17 cells and increased T cell production of IL17. In vivo, angiotensin II increased IL17 protein deposition in the aortic wall. The hypertensive response induced by chronic angiotensin II infusion was decreased in IL17−/− mice compared to C57BL/6J controls. Gene array analysis revealed that in combination with TNFα, IL17 modulated inflammatory gene expression in cultured human aortic smooth muscle cells. Blood vessels from IL17−/− mice displayed preserved vascular function, decreased superoxide production, and decreased aortic T cell infiltration. Analysis of hypertensive and normotensive humans revealed a strong association between serum IL17 levels and hypertension. Taken together, these data suggest that IL17 promotes a vascular inflammatory response and is a critical mediator of angiotensin II-induced hypertension and vascular dysfunction.
We previously showed that T cells are essential for the full development of angiotensin II-induced hypertension, but the question remained as to which subsets of T cells and which cytokines were important in this process. This is the first report showing that Th17 cells, a novel subset of T helper cells distinct from Th1 and Th2, and interleukin 17 plays a critical role in angiotensin II-induced hypertension and vascular dysfunction. Although IL17 is classically produced by CD4+ T cells (Th17 cells), other sources could include γδTcells27, a subset of invariant natural killer T cells (iNKT17)28, CD8+ T cells (Tc17)29, and dendritic cells under special circumstances30. We therefore cannot exclude a contribution of non-CD4+ cells to IL17 production in hypertension.
It is important to note that the blood pressure in IL17 deficient mice after 4 weeks of angiotensin II infusion, although significantly decreased compared to control mice, is still higher than baseline. Thus, loss of IL17 is not sufficient to abolish all of the blood pressure effects of angiotensin II. It is likely that other T cell subsets/cytokines and/or T cell independent effects of angiotensin II, such as salt and water retention, are responsible for the IL17 independent elevations in blood pressure. Indeed, we and others have shown that TNFα plays a critical role in angiotensin II-induced hypertension8, 31, and deficiency of IL6, which functions both upstream and downstream of IL1715, has been shown to inhibit angiotensin II-induced hypertension32.
To investigate the global effect of IL17 on vascular gene expression and to understand how IL17 could affect the local inflammatory milieu, we performed microarray analysis on cultured human aortic smooth muscle cells. Remarkably, IL17 alone had little effect on gene expression with only one gene, stanniocalcin-1 (STC1), slightly elevated by IL17 treatment. Since IL17 has been shown to act synergistically with cytokines such as TNFα, and since we previously showed that TNFα is important in angiotensin II-induced hypertension, we performed additional studies examining the combination of IL17 and TNFα on global gene expression in HASMs. We found that this combination altered the expression of over 30 genes compared to TNFα alone. The decrease in VCAM-1 discovered in our gene array analysis was confirmed by real-time PCR and Western Blot analysis (see Supplemental Figure 2 at http://hyper.ahajournals.org). This effect on VCAM-1 is consistent with another report that IL17 inhibited TNFα induced VCAM-1 expression in fibroblasts33. More importantly, IL17 increased expression of a number of inflammatory cytokines/chemokines such as CCL8, CSF3, CXCL2, and CCL7. Chemokine (C-C motif) ligand 8 (CCL8), also known as monocyte chemotactic protein 2 (MCP-2), binds to cell surface receptors such as CCR1 and CCR5 expressed on leukocytes34. Such changes in cytokines/chemokines could provide an explanation for the decreased leukocyte infiltration observed in IL17−/− mice. Interestingly, another MCP family member, MCP-1/CCL2 has also been implicated in hypertension35, and CC chemokines including CCL2 and CCL8 are upregulated in adipose tissue of obese patients and associated with systemic inflammation36.
It is noteworthy that many IL17-related diseases in humans, such as rheumatoid arthritis, psoriasis, and periodontal disease are also associated with high blood pressure37–39. There is growing evidence that IL17 contributes to atherosclerotic vascular disease as well. Recently, it has been found that patients with acute myocardial infarction and unstable angina have an increase in peripheral Th17 cells and IL17 levels16. In keeping with this, IL17 upregulates C-reactive protein, a marker of inflammation and cardiovascular disease, in cultured hepatocytes and coronary artery smooth muscle cells17. Eid et al.18 found that coronary artery infiltrating T cells produced IL17, IFN-γ, or both, and that IL17 and IFN-γ acted synergistically to induce proinflammatory responses in vascular smooth muscle cells. Of note, HMG CoA reductase inhibitors, which are widely used for treatment of coronary disease, inhibit IL17 gene expression and secretion from human T cells40. It is possible that some of the cholesterol-independent beneficial effects of these agents, including their ability to lower blood pressure41, are related to their effect on IL17.
To extend our results to human hypertension, we examined serum levels of IL17 in normotensive and hypertensive individuals. IL17 was undetectable in a large proportion of patients, consistent with the observations of others18. The frequency of IL17 detection was similar (27–28%) in both groups but when the levels were compared, hypertensive individuals had a greater than 3-fold increase in serum IL17. This might actually be an underestimation since 88% of hypertensive patients were on inhibitors of the angiotensin 1 converting enzymes, which can suppress Th17 cells42.
Perspective
This is the first report demonstrating an important role for a specific subset of T cells in angiotensin II-induced hypertension and vascular dysfunction. Our results indicate that by targeting a small subset of T cells, it might be possible to control blood pressure and preserve vascular function without inducing global immunosuppression. Thus, IL17 and Th17 cells represent novel potential therapeutic targets for this widespread disease.
Supplementary Material
Acknowledgments
Sources of Funding
This work was funded by NIH Grants P01HL58000, R01HL39006, P01HL58000, and a Department of Veterans Affairs merit grant.
Footnotes
Conflict of Interest/Disclosure
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harrison DG, Guzik TJ, Goronzy J, Weyand C. Is hypertension an immunologic disease? Curr Cardiol Rep. 2008;10:464–469. doi: 10.1007/s11886-008-0073-6. [DOI] [PubMed] [Google Scholar]
- 2.Svendsen UG. Evidence for an initial, thymus independent and a chronic, thymus dependent phase of DOCA and salt hypertension in mice. Acta Pathol Microbiol Scand [A] 1976;84:523–528. doi: 10.1111/j.1699-0463.1976.tb00150.x. [DOI] [PubMed] [Google Scholar]
- 3.Bataillard A, Freiche JC, Vincent M, Sassard J, Touraine JL. Antihypertensive effect of neonatal thymectomy in the genetically hypertensive LH rat. Thymus. 1986;8:321–330. [PubMed] [Google Scholar]
- 4.Herrera J, Ferrebuz A, MacGregor EG, Rodriguez-Iturbe B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol. 2006;17:S218–225. doi: 10.1681/ASN.2006080918. [DOI] [PubMed] [Google Scholar]
- 5.Tian N, Gu JW, Jordan S, Rose RA, Hughson MD, Manning RD., Jr Immune suppression prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol. 2007;292:H1018–1025. doi: 10.1152/ajpheart.00487.2006. [DOI] [PubMed] [Google Scholar]
- 6.Seaberg EC, Munoz A, Lu M, Detels R, Margolick JB, Riddler SA, Williams CM, Phair JP. Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS. 2005;19:953–960. doi: 10.1097/01.aids.0000171410.76607.f8. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Iturbe B, Quiroz Y, Nava M, Bonet L, Chavez M, Herrera-Acosta J, Johnson RJ, Pons HA. Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. Am J Physiol Renal Physiol. 2002;282:F191–201. doi: 10.1152/ajprenal.0197.2001. [DOI] [PubMed] [Google Scholar]
- 8.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 10.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 11.Lafaille JJ. The role of helper T cell subsets in autoimmune diseases. Cytokine Growth Factor Rev. 1998;9:139–151. doi: 10.1016/s1359-6101(98)00009-4. [DOI] [PubMed] [Google Scholar]
- 12.Mazzolai L, Duchosal MA, Korber M, Bouzourene K, Aubert JF, Hao H, Vallet V, Brunner HR, Nussberger J, Gabbiani G, Hayoz D. Endogenous angiotensin II induces atherosclerotic plaque vulnerability and elicits a Th1 response in ApoE−/− mice. Hypertension. 2004;44:277–282. doi: 10.1161/01.HYP.0000140269.55873.7b. [DOI] [PubMed] [Google Scholar]
- 13.Shao J, Nangaku M, Miyata T, Inagi R, Yamada K, Kurokawa K, Fujita T. Imbalance of T-cell subsets in angiotensin II-infused hypertensive rats with kidney injury. Hypertension. 2003;42:31–38. doi: 10.1161/01.HYP.0000075082.06183.4E. [DOI] [PubMed] [Google Scholar]
- 14.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 15.Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. 2008;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng X, Yu X, Ding YJ, Fu QQ, Xie JJ, Tang TT, Yao R, Chen Y, Liao YH. The Th17/Treg imbalance in patients with acute coronary syndrome. Clin Immunol. 2008;127:89–97. doi: 10.1016/j.clim.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Patel DN, King CA, Bailey SR, Holt JW, Venkatachalam K, Agrawal A, Valente AJ, Chandrasekar B. Interleukin-17 stimulates C-reactive protein expression in hepatocytes and smooth muscle cells via p38 MAPK and ERK1/2-dependent NF-kappaB and C/EBPbeta activation. J Biol Chem. 2007;282:27229–27238. doi: 10.1074/jbc.M703250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eid RE, Rao DA, Zhou J, Lo SF, Ranjbaran H, Gallo A, Sokol SI, Pfau S, Pober JS, Tellides G. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation. 2009;119:1424–1432. doi: 10.1161/CIRCULATIONAHA.108.827618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruddy MJ, Wong GC, Liu XK, Yamamoto H, Kasayama S, Kirkwood KL, Gaffen SL. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J Biol Chem. 2004;279:2559–2567. doi: 10.1074/jbc.M308809200. [DOI] [PubMed] [Google Scholar]
- 20.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 21.Widder JD, Guzik TJ, Mueller CF, Clempus RE, Schmidt HH, Dikalov SI, Griendling KK, Jones DP, Harrison DG. Role of the multidrug resistance protein-1 in hypertension and vascular dysfunction caused by angiotensin II. Arterioscler Thromb Vasc Biol. 2007;27:762–768. doi: 10.1161/01.ATV.0000259298.11129.a2. [DOI] [PubMed] [Google Scholar]
- 22.Davis ME, Cai H, McCann L, Fukai T, Harrison DG. Role of c-Src in regulation of endothelial nitric oxide synthase expression during exercise training. Am J Physiol Heart Circ Physiol. 2003;284:H1449–1453. doi: 10.1152/ajpheart.00918.2002. [DOI] [PubMed] [Google Scholar]
- 23.Ryan MJ, Didion SP, Mathur S, Faraci FM, Sigmund CD. Angiotensin II-induced vascular dysfunction is mediated by the AT1A receptor in mice. Hypertension. 2004;43:1074–1079. doi: 10.1161/01.HYP.0000123074.89717.3d. [DOI] [PubMed] [Google Scholar]
- 24.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shahrara S, Pickens SR, Dorfleutner A, Pope RM. IL-17 induces monocyte migration in rheumatoid arthritis. J Immunol. 2009;182:3884–3891. doi: 10.4049/jimmunol.0802246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Z, Zhong W, Spencer D, Chen H, Lu H, Kawaguchi T, Rosenbaum JT. Interleukin-17 causes neutrophil mediated inflammation in ovalbumin-induced uveitis in DO11.10 mice. Cytokine. 2009;46:79–91. doi: 10.1016/j.cyto.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roark CL, Simonian PL, Fontenot AP, Born WK, O’Brien RL. gammadelta T cells: an important source of IL-17. Curr Opin Immunol. 2008;20:353–357. doi: 10.1016/j.coi.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michel ML, Keller AC, Paget C, Fujio M, Trottein F, Savage PB, Wong CH, Schneider E, Dy M, Leite-de-Moraes MC. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204:995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamada H, Garcia-Hernandez Mde L, Reome JB, Misra SK, Strutt TM, McKinstry KK, Cooper AM, Swain SL, Dutton RW. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J Immunol. 2009;182:3469–3481. doi: 10.4049/jimmunol.0801814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coury F, Annels N, Rivollier A, Olsson S, Santoro A, Speziani C, Azocar O, Flacher M, Djebali S, Tebib J, Brytting M, Egeler RM, Rabourdin-Combe C, Henter JI, Arico M, Delprat C. Langerhans cell histiocytosis reveals a new IL-17A-dependent pathway of dendritic cell fusion. Nat Med. 2008;14:81–87. doi: 10.1038/nm1694. [DOI] [PubMed] [Google Scholar]
- 31.Sriramula S, Haque M, Majid DS, Francis J. Involvement of tumor necrosis factor-alpha in angiotensin II-mediated effects on salt appetite, hypertension, and cardiac hypertrophy. Hypertension. 2008;51:1345–1351. doi: 10.1161/HYPERTENSIONAHA.107.102152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coles B, Fielding CA, Rose-John S, Scheller J, Jones SA, O’Donnell VB. Classic interleukin-6 receptor signaling and interleukin-6 trans-signaling differentially control angiotensin II-dependent hypertension, cardiac signal transducer and activator of transcription-3 activation, and vascular hypertrophy in vivo. Am J Pathol. 2007;171:315–325. doi: 10.2353/ajpath.2007.061078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schnyder B, Schnyder-Candrian S, Pansky A, Schmitz ML, Heim M, Ryffel B, Moser R. IL-17 reduces TNF-induced Rantes and VCAM-1 expression. Cytokine. 2005;31:191–202. doi: 10.1016/j.cyto.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 34.Gong W, Howard OM, Turpin JA, Grimm MC, Ueda H, Gray PW, Raport CJ, Oppenheim JJ, Wang JM. Monocyte chemotactic protein-2 activates CCR5 and blocks CD4/CCR5-mediated HIV-1 entry/replication. J Biol Chem. 1998;273:4289–4292. doi: 10.1074/jbc.273.8.4289. [DOI] [PubMed] [Google Scholar]
- 35.Jemaa R, Ben Ali S, Kallel A, Omar S, Feki M, Elasmi M, Haj-Taieb S, Sanhaji H, Kaabachi N. Association between the −2518G/A polymorphism in the monocyte chemoattractant protein-1 (MCP-1) gene and hypertension in Tunisian patients. Clin Biochem. 2009;42:34–37. doi: 10.1016/j.clinbiochem.2008.09.118. [DOI] [PubMed] [Google Scholar]
- 36.Huber J, Kiefer FW, Zeyda M, Ludvik B, Silberhumer GR, Prager G, Zlabinger GJ, Stulnig TM. CC chemokine and CC chemokine receptor profiles in visceral and subcutaneous adipose tissue are altered in human obesity. J Clin Endocrinol Metab. 2008;93:3215–3221. doi: 10.1210/jc.2007-2630. [DOI] [PubMed] [Google Scholar]
- 37.Panoulas VF, Douglas KM, Milionis HJ, Stavropoulos-Kalinglou A, Nightingale P, Kita MD, Tselios AL, Metsios GS, Elisaf MS, Kitas GD. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford) 2007;46:1477–1482. doi: 10.1093/rheumatology/kem169. [DOI] [PubMed] [Google Scholar]
- 38.Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol. 2006;55:829–835. doi: 10.1016/j.jaad.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 39.Holmlund A, Holm G, Lind L. Severity of periodontal disease and number of remaining teeth are related to the prevalence of myocardial infarction and hypertension in a study based on 4,254 subjects. J Periodontol. 2006;77:1173–1178. doi: 10.1902/jop.2006.050233. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Jin J, Peng X, Ramgolam VS, Markovic-Plese S. Simvastatin inhibits IL-17 secretion by targeting multiple IL-17-regulatory cytokines and by inhibiting the expression of IL-17 transcription factor RORC in CD4+ lymphocytes. J Immunol. 2008;180:6988–6996. doi: 10.4049/jimmunol.180.10.6988. [DOI] [PubMed] [Google Scholar]
- 41.Bautista LE. Blood pressure-lowering effects of statins: who benefits? J Hypertens. 2009;27:1478–1484. doi: 10.1097/HJH.0b013e32832b1e78. [DOI] [PubMed] [Google Scholar]
- 42.Platten M, Youssef S, Hur EM, Ho PP, Han MH, Lanz TV, Phillips LK, Goldstein MJ, Bhat R, Raine CS, Sobel RA, Steinman L. Blocking angiotensin-converting enzyme induces potent regulatory T cells and modulates TH1- and TH17-mediated autoimmunity. Proc Natl Acad Sci U S A. 2009;106:14948–14953. doi: 10.1073/pnas.0903958106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.