Abstract
Among hemodialysis patients the assessment of dry-weight remains a matter of clinical judgment because tests to assess dry-weight have not been validated. The objective of this study was to evaluate and validate relative plasma volume monitoring as a marker of dry-weight. We performed relative plasma volume monitoring using the Critline monitor at baseline and 8 weeks in 150 patients participating in the dry-weight reduction in hypertensive hemodialysis patients (DRIP) trial. The intervention group of 100 patients had dry-weight probed whereas 50 patients served as time controls. Relative plasma volume slopes were defined as flat when they were less than the median (1.33%/hour) at the baseline visit. Among predominantly (87%) African-American hemodialysis patients, we found that flat relative plasma volume slopes suggest a volume overloaded state because of the following reasons: 1) probing dry-weight in these patients leads to steeper slopes; 2) those with flatter slopes at baseline had greater weight loss; 3) both baseline relative plasma volume slopes and the intensity of weight loss were found to be important for subsequent change in relative plasma volume slopes; and most importantly 4) relative plasma volume slopes predicted the subsequent reduction in interdialytic ambulatory systolic BP. Those with the flattest slopes had the greatest decline in BP upon probing dry-weight. Both baseline relative plasma volume slopes and the change in relative plasma volume slopes were important for subsequent changes in ambulatory systolic BP. We conclude that relative plasma volume slope monitoring is a valid method to assess dry-weight among hypertensive hemodialysis patients.
Keywords: hypertension, hemodialysis, dry-weight, plasma volume, diagnostic test
Introduction
Although the adequacy of solute clearance in patients on chronic hemodialysis is routinely measured as part of dialysis quality assessment, volume status has no validated marker 1. Even after 50 years of dialysis, the assessment of volume remains a matter of clinical judgment 2; unfortunately the clinical examination performs poorly to assess volume 3. Whereas probing for dry weight can lead to intradialytic hypotension and uncomfortable symptoms, inadequate volume removal can lead to chronic volume overload with hypertension and left ventricular hypertrophy, which may evoke cardiovascular events and increase mortality 4, 5.
There has been a long-standing interest in developing volume markers 6. In general, four major types of objective measures of volume status have been investigated 7. These measures include the following: biochemical markers (eg. N-terminal pro-B-natriuretic peptide8), imaging markers (eg. inferior vena cava diameter9), bioimpedance analysis 10, and relative plasma volume (RPV) monitoring 11. Among these RPV monitoring is relatively easy, commercially available, and inexpensive to perform12. To monitor RPV a device is attached to the hemodialysis blood tubing that continuously and accurately measures the hematocrit by optical absorbance 13. Assuming no change in the red cell mass during hemodialysis and uniform mixing of red cells within the vasculature, the percent increase in hematocrit during ultrafiltration estimates the percent decrease in blood volume 13.
Most studies of RPV monitoring have revolved around efforts to predict and thus prevent intradialytic hypotension and symptoms 13. Using this monitor we have earlier reported RPV monitoring correlates with vena cava echography as well as with symptoms of and interventions for intradialytic hypotension 14. A few studies have examined the ability of RPV monitor to assess volume status, using the slope of the RPV decrease over the hemodialysis session and correlating that with other objective measures of volume status 11, 15, 16. All these studies have been small. Kooman et al in a recent review concluded the following 17: “Blood volume monitoring as a tool to assess dry weight needs further validation and standardization. Summarizing technological tools may certainly aid the clinician in the assessment of fluid state, but should always be interpreted in the clinical context of the patient. Controlled studies are needed to definitively establish the role of technological tools in detecting dry weight.”
The purpose of this study was to evaluate among hypertensive hemodialysis patients the diagnostic ability of RPV slope monitoring to assess dry weight. Accordingly, we sought the relationship of probing dry weight on RPV slopes. We then evaluated the relationship of baseline RPV slopes and change in RPV slopes on their ability to predict interdialytic ambulatory BP.
Methods
This is a pre-specified analysis of patients participating in the previously published dry-weight reduction in hypertensive hemodialysis patients (DRIP) trial 18. Briefly, we recruited patients 18 years of age or older on long-term hemodialysis for at least 3 months, who had hypertension defined as mean interdialytic ambulatory BP of 135/85 mm Hg or more. After a six hemodialysis run-in phase, during which baseline data were collected, patients were randomized in 1:2 proportion into control group vs. ultrafiltration trial group for 8 weeks. Pre and post BP and weights were averaged over these 6 treatment run-in phase. During this 24 dialysis treatment phase, patients were seen at each dialysis visit and had dry-weight probed as assessed by symptoms and signs related to hypovolemia7, 19. The ultrafiltration group underwent an additional weight loss of 0.1kg/10 kg body-weight per dialysis without increasing the time or frequency of dialysis. This additional weight loss was combined with the ultrafiltration volume required to remove interdialytic weight gain to achieve the desired reduction in dry-weight. If ultrafiltration was not tolerated based on symptoms and signs such as muscle cramps, need for excessive saline or symptomatic hypotension, the additional prescribed weight loss was reduced by 50%. If ultrafiltration was still not tolerated, the additional weight loss was further reduced by 50% until even 0.2 kg incremental weight loss per dialysis was not tolerated. At this point, the patient was said to be at his or her dry weight. Thus, by this protocol, each patient had to experience symptoms of volume depletion to be at dry-weight. The control group had regular physician visits but no additional reduction in dry-weight. No changes in antihypertensive medication were permitted during the trial.
Blood Pressure Monitoring
Ambulatory BP monitoring was performed after the mid-week hemodialysis session for 44 hours at baseline, 4 weeks and 8 weeks. Blood pressures were recorded every 20 minutes during the day (6 AM to 10 PM) and every 30 minutes during the night (10 PM to 6 AM) using a Spacelab 90207 ABP monitor (SpaceLabs Medical Inc, Redmond, WA, USA) in the non-access arm. Recordings began immediately after hemodialysis and terminated immediately before the subsequent dialysis. Accuracy of ambulatory BP recordings was confirmed against auscultated blood pressure at baseline. Hourly means were calculated. These means were then averaged over the entire course of recording to provide systolic and diastolic interdialytic ambulatory blood pressures. The mean interdialytic ambulatory BP served as the reference standard.
Relative Plasma Volume Monitoring
Of the 150 participants, 145 patients underwent successful intradialytic relative plasma volume monitoring which was performed once during the two week period at baseline before any intervention and in the last week of the 8 week trial. Relative plasma volume monitoring was performed with Crit-Line® III-TQA which is a clinically available device that incorporates photo-optical technology to non-invasively measure absolute hematocrit (Hemametrics, Salt Lake City, UT) 20. Hematocrit is measured every 20 seconds throughout the duration of hemodialysis. Measurements made by the machine have been validated against hematocrits measured by centrifugation 12. We exported the machine stored time and hematocrit data to a relational database for further analysis.
The study protocol was approved by the Institutional Review Boards (IRB) and the VA Research and Development Committee and all patients provided written informed consent. The trial was registered at ClinicalTrials.gov (NCT00067665).
Statistical Methods
Relative Plasma Volume
The change in plasma volume with ultrafiltration dialysis approximates a first order elimination kinetics. Relative plasma volume (RPV) change was calculated as follows: First, we calculated the fraction of blood free of hematocrit using the formula 100-hematocrit%. Next, we took the natural log of this fraction as the dependent variable. An advantage of log transformation is that the coefficients on the time variables reflect approximately the percent change in RPV. Independent variables were the following: 1) time elapsed since the beginning of dialysis; 2) indicator variables for group (ultrafiltration and control), visits (baseline and 8 weeks), and their interaction; and 3) interactions of these indicator variables with time elapsed. A mixed model was used to allow for repeated measurements within individuals. Details of the analyses are shown in supplemental methods (please see http://hyper.ahajournals.org)
All analyses were conducted using Stata 10.1 (Stata Corp., College Station, TX). The P values reported are two-sided and taken to be significant at <0.05.
Results
Table 1 shows the baseline clinical characteristics of the study population by quartiles of RPV slopes. Most of these characteristics were well matched. By play of chance, steeper RPV slopes at baseline were more often seen in those randomized to the ultrafiltration group. This disadvantaged the ultrafiltration group, since we subsequently show that BP response was dependent on quartiles of RPV slopes. Those with steeper slopes had greater ultrafiltration volume which is not surprising given the physiology that underlies RPV monitoring.
Table 1.
Clinical characteristics of the study Population
| Clinical characteristic | Quartile of relative plasma volume slope | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | total | p | |
| n | 37 (26%) | 36 (25%) | 36 (25%) | 36 (25%) | 145 (100%) |
|
| RPV Slope %/hr (range) | (−5.6, −2.4) | (−2.4, −1.3) | (−1.3, −.7) | (−.7, 0.3) | (−5.6, 0.3) | |
| Study Group | 0.03 | |||||
| Control | 13 (35%) | 5 (14%) | 15 (42%) | 16 (44%) | 49 (34%) | |
| Ultrafiltration | 24 (65%) | 31 (86%) | 21 (58%) | 20 (56%) | 96 (66%) | |
| Age (years) | 52.0 ± 15.0 | 53.6 ± 13.1 |
54.9 ± 11.2 |
56.5 ± 10.4 |
54.2 ± 12.6 | 0.5 |
| Ultrafiltration Volume (L) | 3.6 ± 1.3 | 3.4 ± 1.4 | 2.9 ± 1.3 | 1.7 ± 0.7 | 2.9 ± 1.4 | <0.00 1 |
| Baseline Post-dialysis Weight (kg) | 75.9 ± 16.7 | 87.3 ± 23.0 |
81.0 ± 13.2 |
78.6 ± 17.4 |
80.6 ± 18.1 | 0.08 |
| Change in Weight From Baseline | 0.3 ± 1.7 | −0.7 ± 2.6 | −0.4 ± 1.7 | −1.5 ± 3.2 | −0.5 ± 2.4 | 0.03 |
| Race | 0.3 | |||||
| White | 5 (14%) | 7 (19%) | 2 (6%) | 1 (3%) | 15 (10%) | |
| Black | 31 (84%) | 29 (81%) | 31 (86%) | 35 (97%) | 126 (87%) | |
| Asian | 1 (3%) | 0 (0%) | 1 (3%) | 0 (0%) | 2 (1%) | |
| American Indian/Alaska Native | 0 (0%) | 0 (0%) | 1 (3%) | 0 (0%) | 1 (1%) | |
| Other/Not Known | 0 (0%) | 0 (0%) | 1 (3%) | 0 (0%) | 1 (1%) | |
| Pre-dialysis seated BP (mmHg) | 154.6 ± 14.2/ 84.4 ± 10.9 |
163.3 ± 19.0 / 87.1 ± 12.1 |
160.6 ± 16.6 / 88.4 ± 10.7 |
158.8 ± 13.2 / 86.1 ± 10.6 |
159.3 ± 16.0 / 86.5 ± 11.1 |
0.13 / 0.5 |
| Body Mass Index (kg/m2) | 26.0 ± 4.9 | 27.9 ± 7.2 | 27.4 ± 5.3 | 27.2 ± 6.6 | 27.1 ± 6.0 | 0.6 |
| Years of Dialysis | 4.0 ± 6.1 | 4.2 ± 5.6 | 4.3 ± 5.6 | 3.7 ± 2.7 | 4.0 ± 5.1 | 1 |
| Etiology of end-stage renal disease | 0.11 | |||||
| Diabetes Mellitus | 12 (32%) | 18 (50%) | 16 (44%) | 11 (31%) | 57 (39%) | |
| Hypertension | 16 (43%) | 12 (33%) | 17 (47%) | 23 (64%) | 68 (47%) | |
| Glomerulonephritis | 2 (5%) | 1 (3%) | 3 (8%) | 0 (0%) | 6 (4%) | |
| Polycystic Kidney Disease | 2 (5%) | 1 (3%) | 0 (0%) | 0 (0%) | 3 (2%) | |
| Other | 5 (14%) | 4 (11%) | 0 (0%) | 2 (6%) | 11 (8%) | |
| History of | ||||||
| Congestive Heart Failure | 5 (14%) | 6 (17%) | 5 (14%) | 5 (14%) | 21 (14%) | 1 |
| Myocardial Infarction | 2 (5%) | 7 (19%) | 4 (11%) | 5 (14%) | 18 (12%) | 0.3 |
| Stroke | 6 (16%) | 4 (11%) | 4 (11%) | 0 (0%) | 14 (10%) | 0.12 |
| Urea Reduction Ratio (%) | 74.9 ± 7.4 | 74.3 ± 6.3 | 73.6 ± 6.1 | 72.9 ± 6.7 | 73.9 ± 6.6 | 0.6 |
| Albumin (g/dL) | 3.8 ± 0.4 | 3.7 ± 0.5 | 3.7 ± 0.4 | 3.6 ± 0.4 | 3.7 ± 0.4 | 0.3 |
| Hemoglobin (g/dL) | 12.3 ± 1.3 | 12.1 ± 1.3 | 12.3 ± 1.0 | 11.8 ± 1.2 | 12.1 ± 1.2 | 0.4 |
| Presence of Edema | 4 (11%) | 8 (22%) | 8 (22%) | 6 (17%) | 26 (18%) | 0.6 |
| Number receiving antihypertensive drugs | 30 (81%) | 29 (81%) | 32 (89%) | 31 (86%) | 122 (84%) | 0.7 |
| Number of antihypertensives in users | 2.1 ± 1.6 | 2.1 ± 1.6 | 2.2 ± 1.6 | 2.6 ± 1.6 | 2.2 ± 1.6 | 0.6 |
| Nature of antihypertensive agent | ||||||
| Dihydropyridine calcium channel blockers |
16 (43%) | 18 (50%) | 15 (42%) | 19 (53%) | 68 (47%) | 0.8 |
| Beta-blockers | 25 (68%) | 25 (69%) | 26 (72%) | 25 (69%) | 101 (70%) | 1 |
| ACE Inhibitors | 18 (49%) | 18 (50%) | 18 (50%) | 21 (58%) | 75 (52%) | 0.8 |
| Angiotension Receptor Blockers | 3 (8%) | 6 (17%) | 9 (25%) | 5 (14%) | 23 (16%) | 0.3 |
| Interdialytic ambulatory BP (mmHg) | 143.5 ± 10.4 / 83.7 ± 11.2 |
144.3 ± 10.2 / 81.4 ± 9.9 |
149.1 ± 10.5 / 85.2 ± 8.7 |
147.3 ± 9.5 / 82.7 ± 11.2 |
146.0 ± 10.3 / 83.3 ± 10.3 |
0.08 / 0.4 |
± indicates standard deviation. Parentheses have percent of patients.
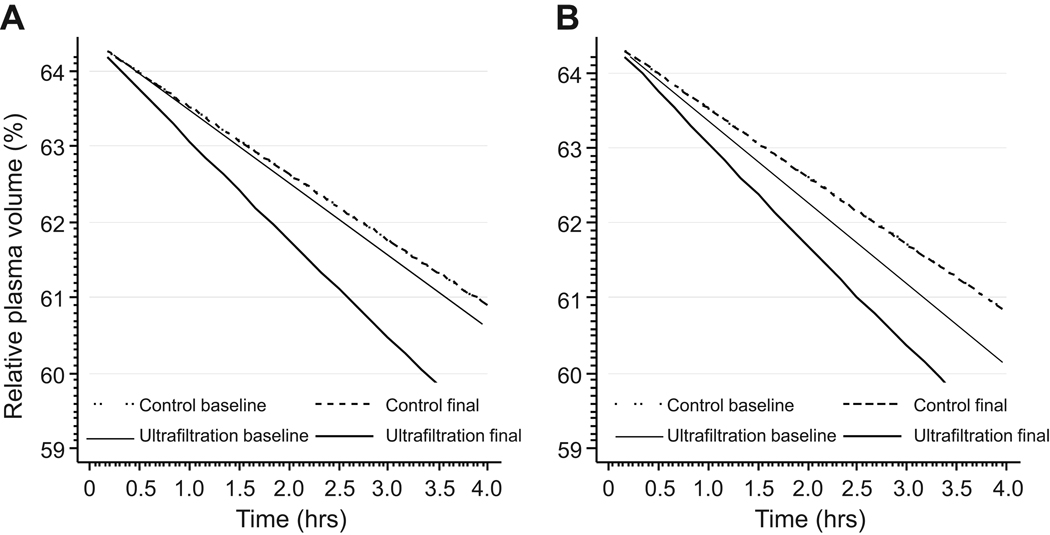
Unadjusted modeled changes in RPV between control and ultrafiltration groups are shown in Figure 1A. The intercepts of RPV slopes were not significantly different from each other and therefore were removed from the model. The resulting model was parsimonious and its model fit was similar to the parent model. In the control group, there was no change in RPV slope from baseline to final visit. In fact the RPV slopes were so similar that the RPV curves in controls from baseline to final were superimposed. The ultrafiltration group at baseline had RPV slope similar to controls and experienced a significant steepening of slope over 8 weeks.
Figure 1.
Panel A: Modeled changes in relative plasma volume (RPV) among the two groups of patients: control and ultrafiltration. The broken lines represent the control group and the solid lines ultrafiltration group. In the control group or ultrafiltration groups, RPV intercepts were similar at baseline and unchanged at final visit. The control group at baseline had an RPV slope of 1.40 %/hr. There was no change in RPV slope (1.40%/hr) at final visit. Accordingly, the two lines are superimposed on each other. The ultrafiltration group at baseline had an RPV slope of 1.53%/hr (NS from baseline control). The RPV slope significantly (p<0.001) steepened to 2.09%/hr at final visit. Panel B: Modeled RPV slopes adjusted for post-dialysis weight changes from baseline to end of study. Similar changes as unadjusted model were seen suggesting that changes in RPV slope persist even after accounting for changes in body weight.
The ultrafiltration group had additional volume reduction therapy which as expected led to reduction in post-dialysis weight from baseline to 8 weeks. If post-dialysis weight change was the sole cause of changes in RPV slopes then adjustment in the ultrafiltration group for post-dialysis weight change would abolish the RPV change. Figure 1B illustrates that if weight changes were accounted for it did not alter the magnitude or significance of change in RPV slopes.
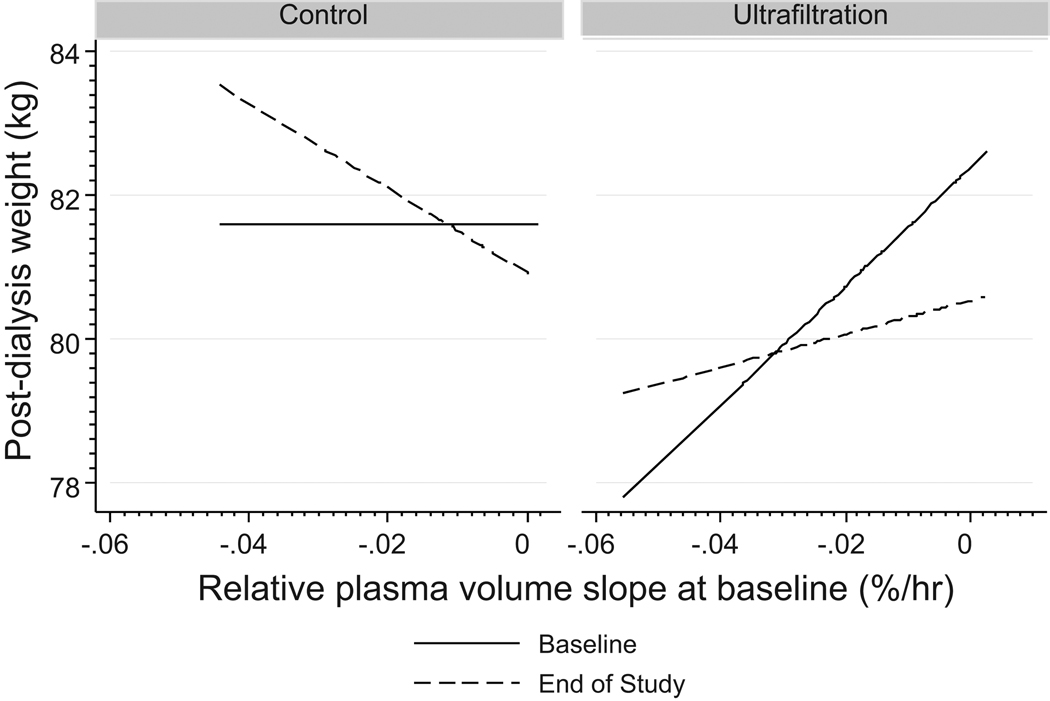
If flat RPV slopes denote expanded extracellular fluid (ECF) volume, then those with flat slopes would have decline in weight. Conversely, if steeper RPV slopes denote a contracted ECF volume, then those with steeper slopes would have increase in weight. Figure 2 shows weight loss as a function of baseline RPV slope. The mean weight overall was 81.6 kg. Those assigned to the UF group were 0.55 kg lighter (p>0.2). In the control group, an increase of 0.15 kg in post-dialysis weight was noted (p>0.2). Compared to the change in the control group, those in the UF group had 1.0 kg decline in weight (p=0.028). Regardless of the study group, patients with steep (more negative) slopes gained weight and those with flatter slopes lost weight. Consideration of RPV slopes in the model improved the estimation of weight loss over the course of the trial (p=0.005).
Figure 2.
Relationship between post-dialysis weight in the two groups by phases of trial and baseline RPV slopes. Weight loss was predicted by baseline RPV slopes. Regardless of the study group, patients with steep (more negative) slopes gained weight and those with flatter slopes lost weight. Consideration of RPV slopes in the model improved the estimation of weight loss over the course of the trial (p=0.005).
Table 2 shows RPV slopes by quartiles of weight change. Quartile 1 had the least weight loss and Quartile 4 the greatest weight loss over 8 weeks. The baseline RPV slopes in the control group were similar at baseline. On the other hand the baseline RPV slopes in the ultrafiltration group were dissimilar depending on the quartile; those in the higher quartiles had flatter baseline slopes. The change from baseline in RPV slope was dependent on quartiles of weight loss in the control group. The change from baseline in RPV slope was also dependent on quartiles of weight loss in the ultrafiltration group. Even when the change from baseline in RPV slope in the control group was subtracted from change from baseline in RPV slope in the ultrafiltration group, the changes remained highly significant and dependent upon quartiles of weight change.
Table 2.
RPV slopes (%/hr) by quartiles of weight change.
| Group/Time | Q1 | Q2 | Q3 | Q4 | Heterogeneity between quartiles (p) |
|---|---|---|---|---|---|
| Control Baseline | 1.62 (0.22) | 1.02 (0.25) | 1.61 (0.3) | 1.51 (0.57) | >0.2 |
| CFB control | −0.07 (0.02) | 0.23 (0.01) | −0.41 (0.02) | 0.08 (0.03) | <0.0001 |
| pairwise p for change | <0.0001 | <0.0001 | <0.0001 | <0.001 | |
| UF Baseline | 1.69 (0.17) | 1.92 (0.26) | 1.55 (0.21) | 1.15 (0.18) | 0.06 |
| CFB UF | 0.57 (0.01) | −0.18 (0.03) | −0.76 (0.03) | 0.54 (0.02) | <0.0001 |
| pairwise p for change | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| CFB UF- CFB control | 0.64 (0.02) | −0.41 (0.03) | −0.35 (0.03) | 0.46 (0.04) | <0.0001 |
| p for delta-delta | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Quartiles of weight change (Q1–Q4) from baseline to 8 weeks. Q4 experience the greatest reduction in weight. CFB = change from baseline, UF = ultrafiltration, RPV = Relative plasma volume.
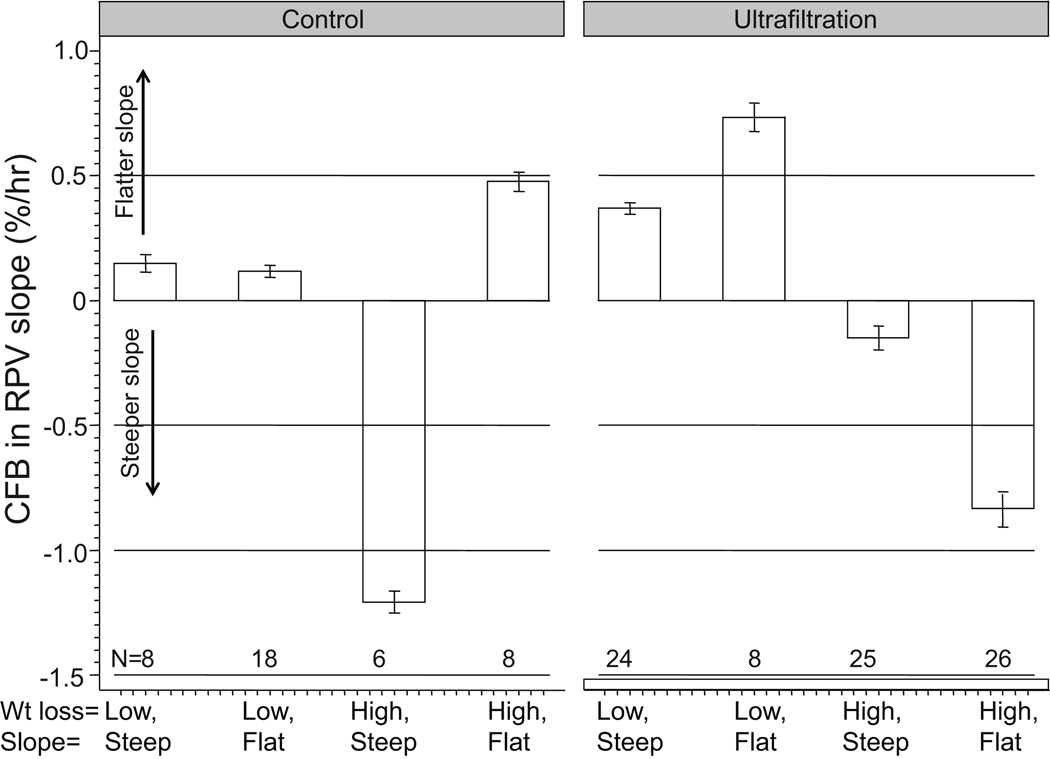
The change from baseline in RPV slope was also dependent on quartiles of weight loss in the ultrafiltration group; those with the steepest slopes at baseline had flattening of slope whereas those with the flattest slopes at baseline had the steepest RPV slope at the end of trial (Table S1 (please see http://hyper.ahajournals.org)). The combined effect of baseline RPV slopes and weight loss on subsequent change in RPV slopes are shown in Figure 3. Those who had low weight loss had flattening of slopes regardless of whether their slopes were steep or flat at onset. Those who had higher weight loss had steepening of slopes only when probed for dry weight. Those in the control group, who did not have their dry-weights probed had discordant responses. The high weight loss and steep slope group had limited number of patients; the steepening in RPV slope observed is based on the outcomes of only 6 patients. The interaction effect between baseline RPV slope and weight loss on change in RPV slopes was highly significant (p<0.001). Compared to the steep slope group, the odds of losing weight in the UF group was 3.12 (95% CI 1.08–9.5, p=0.019) in the flat RPV slope group. The odds of losing weight in the control group was 0.59 (95% 0.13 –2.85, p=0.44). The test of homogeneity of odds ratio was significant (p<0.05).
Figure 3.
Weight loss and baseline RPV slope are both important in modulating the change from baseline (CFB) in relative plasma volume (RPV) slopes. Low wt loss represents wt loss of <0.3 kg from baseline to end of trial. Flat slopes represent slopes flatter than 1.33%/hr. A significant interaction effect was seen between baseline RPV slope and weight loss suggesting that effect of these two factors is multiplicative on subsequent change in RPV slopes. Error bars represent the standard errors of means. The row just above the X-axis represents the number of individuals in that group.
Table S2 (please see http://hyper.ahajournals.org) shows 44-hour interdialytic ambulatory systolic blood pressure in the control and ultrafiltration groups by quartiles of baseline RPV slopes. The changes from baseline BP over 8 weeks are also shown. Both in control and ultrafiltration groups, baseline systolic BP was similarly elevated. This difference did not differ between quartiles. A fall in systolic BP was seen in control group; this fall occurred independent of baseline quartile of RPV slope. A greater fall in systolic BP was seen in ultrafiltration group. However, this fall in systolic BP had a linear trend with increasing quartiles. Thus, those with the flattest RPV slopes at baseline experienced the greatest decline in BP upon probing dry-weight.
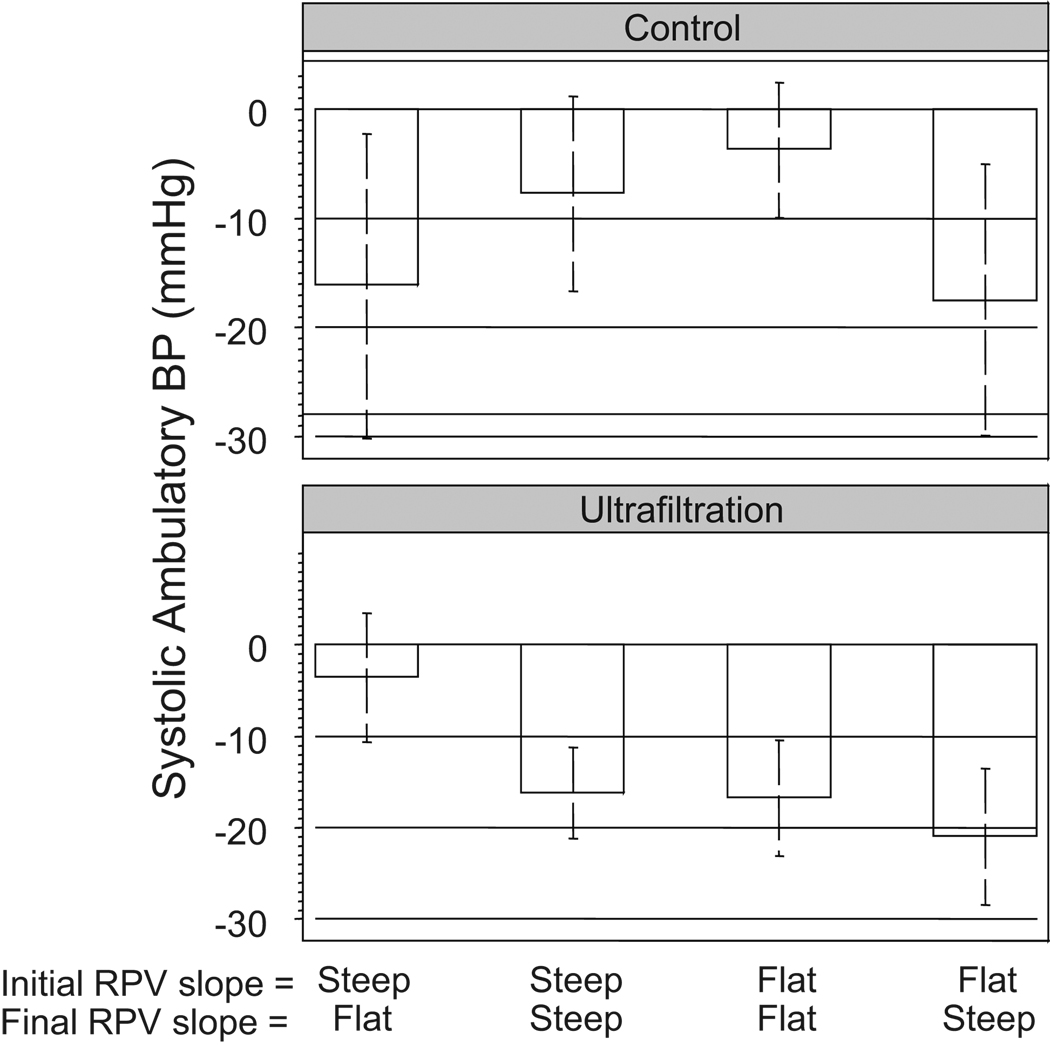
Figure 4 shows the effect of initial and final RPV slope on changes in systolic ambulatory BP. BP did not change when the slope was steep initially and became flatter subsequently. The most profound effect on BP reduction was seen when the initial RPV slope was flat and then steepened subsequently. When dry weight was not probed (control group) patients who had no change in slopes had no significant change in BP (note that the confidence interval of the change goes through zero). However, those who went from flat to steep slope had a significant reduction in systolic BP. Models that excluded initial RPV slope or final RPV slopes or both were significantly worse than nested models.
Figure 4.
Magnitude of change in 44-hour interdialytic ambulatory systolic BP with ultrafiltration is dependent on the initial RPV slope and the final RPV slope. Mean changes and their 95% confidence intervals are shown. If the confidence interval crosses zero, the mean is statistically insignificant at the 5% level.
Finally, we noted that RPV slopes directly affected the frequency of cramps during dialysis, the need for saline boluses and the need to reduce ultrafiltration. The frequency of these events was accelerated when dry-weight was probed in patients who had steeper slopes (data not shown).
Discussion
Among hypertensive individuals on long-term hemodialysis, we found the following reasons that support the use of RPV slopes as markers of dry-weight. 1) RPV slopes are responsive to probing dry-weight (Figure 1); patients who have dry-weight probed (ultrafiltration group) have steeping of slopes. 2) When dry-weight is probed, RPV slopes predict the magnitude of weight change (Figure 2). 3) Baseline RPV slopes reflect volume status. Flat RPV slopes suggest a volume overloaded state; probing dry-weight in these patients leads to subsequent steeper slopes (Table 2). In contrast, steepening of RPV slopes occurs less often in those who have steeper RPV slopes at baseline (Table S1). 4) Both baseline RPV slopes and the intensity of weight loss are important for subsequent changes in RPV slopes (Table S2 and Figure 3). For example, patients with flatter slopes and above median weight loss steepen their RPV slopes. This steepening of RPV slopes is dependent upon probing dry-weight (assignment to ultrafiltration group); the steepening of RPV slopes is much less in those who do not have dry-weight probed (the control group). 5) RPV slopes predict the subsequent reduction in interdialytic ambulatory systolic BP; those with the flattest slopes had the greatest decline in BP upon probing dry-weight. Thus, flat slopes identify volume-responsive hypertension. 6) Both baseline RPV slopes and the change in RPV slopes are important for subsequent changes in ambulatory systolic BP (Figure 4). 7) Baseline RPV predicts the time to onset of intradialytic cramps, need to stop ultrafiltration and administer saline boluses.
Currently, the change over time in post-dialysis weight is taken as a marker of volume reduction. We found that greater weight loss is associated with a greater steepening of slope regardless of randomization. However, weight loss alone was insufficient to explain changes in RPV slopes evoked by probing dry-weight. This supports the notion that post-dialysis weight alone is a poor proxy of dry-weight. Dry-weight can be better estimated with RPV slope monitoring. This is so because those with the flattest RPV slopes experienced the greatest steeping of slopes on probing dry-weight. More importantly, those with the flattest RPV slopes who had the greatest steepening of slopes also experienced the greatest declines in 44-hour systolic ambulatory BP upon probing dry-weight. Furthermore, those with the steepest slopes had more symptoms and interventions upon probing dry-weight.
Although the changes in RPV slopes are related to the magnitude of weight loss, the relationship between weight loss and RPV slope is inconsistent (Figure 3 and Table 2). Changes in post-dialysis weight accounted for some changes in RPV slopes but were not sufficient to account for all the changes. Some possibilities for the lack of relationship between post-dialysis weight change and change in RPV slopes may be as follows: 1) post-dialysis weight may not reflect the true change in ultrafiltration volume during dialysis because often patients eat and drink during dialysis; 2) post-dialysis weight changes may not be accurately capture alteration in body-composition and therefore fluid-volume compartments over 8 weeks of the study.
Our results support the observations of Lopot et al who were among the first to suggest that RPV monitoring may be valuable in the assessment of dry-weight11. They reported that RPV monitor-guided reduction in dry weight reduced echocardiographic inferior vena cava diameter among patients who were found to be volume overloaded. Similarly, Rodriguez et al reported in a cohort study of 28 patients that RPV monitoring lead to changes in dry-weight in all patients 21. Steuer et al reported that 18% of the patients in a dialysis unit had less than 5% reduction in relative blood volume 22. Over 6 weeks, they reduced the weight by an average 0.8kg which resulted in larger decrease in relative blood volume with low incidence of symptoms22. The median RPV slope in our study was 1.33%/hr which over 4 hours would lead to reduction in RPV of a magnitude similar to reported by Steuer et al. Thus, nearly half the patients in our study were volume overloaded by Steuer et al’s definition probably because we studied only those patients who were hypertensive. The mean weight loss over 8 weeks in our study was 1 kg—also similar to Steuer et al. We also found steepening of RPV slope when fluid was removed similar to their study. Our data also support the work of Dasselaar et al who evaluated the role of blood volume tracking compared to standard therapy in the management of hypertension in hemodialysis patients by reducing dry-weight 15. They reported that among 14 patients randomized to blood volume tracking-guided dry-weight reduction predialysis BP was reduced by 22.5/8.3 mmHg; ECF water and cardiothoracic ratio was also reduced. Among pediatric hemodialysis patients, RPV monitoring has been used to guide dry-weight reduction; this results in lower interdialytic ambulatory BP 23, 24 and reduced the rate of hospitalizations 25. Our data also confirm the observations of Zellweger et al who demonstrated that dry-weight reduction is more likely when relative blood volume changes are lower 26. Taken together, these data support the notion that therapy guided by RPV slope may serve as a valid tool to assess volume and prescribe augmented volume reduction therapy among hemodialysis patients.
Our study has some limitations. We conducted RPV monitoring only once at baseline and once at the end of the trial. Multiple recordings may have improved the precision of RPV slopes for individual patients. Although the analysis of RPV monitoring was pre-specified, patients were not randomized based on RPV. Thus, a cause and effect relationship between RPV slopes and subsequent improvement in interdialytic ambulatory BP may be premature. Finally, there were few non-African American patients in our study. Whether the results of our study are generalizable to non-African American patients will need to be demonstrated in future trials.
Perspective
The assessment of dry-weight in patients on long-term hemodialysis has been a long-term challenge. Our study provides a simple and a widely available tool that can aid the evaluation of dry-weight. RPV slopes derived by this measurement can predict the success of subsequent weight loss and improvement in BP. Periodic monitoring of RPV may assist in the management of dry-weight and control of hypertension among long-term hemodialysis patients. The median RPV slope at baseline seen in our study was 1.33% per hour. Patients with flatter RPV slopes may thus be volume overloaded. Although RPV slope may serve as a marker of volume, its utility needs to be confirmed in clinical trials.
Supplementary Material
Acknowledgments
Source of Funding:
NIH 2R01-DK062030-06A109
Dr Sinha is supported by a National Kidney Foundation Fellowship Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
None
Reference List
- 1.Ishibe S, Peixoto AJ. Methods of assessment of volume status and intercompartmental fluid shifts in hemodialysis patients: implications in clinical practice. Semin Dial. 2004;17:37–43. doi: 10.1111/j.1525-139x.2004.17112.x. [DOI] [PubMed] [Google Scholar]
- 2.Thomson GE, Waterhouse K, McDonald HP, Jr, Friedman EA. Hemodialysis for chronic renal failure. Clinical observations. Arch Intern Med. 1967;120:153–167. [PubMed] [Google Scholar]
- 3.Agarwal R, Andersen MJ, Pratt JH. On the importance of pedal edema in hemodialysis patients. Clin J Am Soc Nephrol. 2008;3:153–158. doi: 10.2215/CJN.03650807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khosla UM, Johnson RJ. Hypertension in the hemodialysis patient and the "lag phenomenon": insights into pathophysiology and clinical management. Am J Kidney Dis. 2004;43:739–751. doi: 10.1053/j.ajkd.2003.12.036. [DOI] [PubMed] [Google Scholar]
- 5.Wizemann V, Wabel P, Chamney P, Zaluska W, Moissl U, Rode C, Malecka-Masalska T, Marcelli D. The mortality risk of overhydration in haemodialysis patients. Nephrol Dial Transplant. 2009;24:1574–1579. doi: 10.1093/ndt/gfn707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kouw PM, Kooman JP, Cheriex EC, Olthof CG, de Vries PM, Leunissen KM. Assessment of postdialysis dry weight: a comparison of techniques. J Am Soc Nephrol. 1993;4:98–104. doi: 10.1681/ASN.V4198. [DOI] [PubMed] [Google Scholar]
- 7.Jaeger JQ, Mehta RL. Assessment of dry weight in hemodialysis: an overview. J Am Soc Nephrol. 1999;10:392–403. doi: 10.1681/ASN.V102392. [DOI] [PubMed] [Google Scholar]
- 8.Satyan S, Light RP, Agarwal R. Relationships of N-terminal pro-B-natriuretic peptide and cardiac troponin T to left ventricular mass and function and mortality in asymptomatic hemodialysis patients. Am J Kidney Dis. 2007;50:1009–1019. doi: 10.1053/j.ajkd.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheriex EC, Leunissen KM, Janssen JH, Mooy JM, Van Hooff JP. Echography of the inferior vena cava is a simple and reliable tool for estimation of 'dry weight' in haemodialysis patients. Nephrol Dial Transplant. 1989;4:563–568. [PubMed] [Google Scholar]
- 10.Kuhlmann MK, Zhu F, Seibert E, Levin NW. Bioimpedance, dry weight and blood pressure control: new methods and consequences. Curr Opin Nephrol Hypertens. 2005;14:543–549. doi: 10.1097/01.mnh.0000185983.48319.00. [DOI] [PubMed] [Google Scholar]
- 11.Lopot F, Kotyk P, Blaha J, Forejt J. Use of continuous blood volume monitoring to detect inadequately high dry weight. Int J Artif Organs. 1996;19:411–414. [PubMed] [Google Scholar]
- 12.Leypoldt JK, Cheung AK, Steuer RR, Harris DH, Conis JM. Determination of circulating blood volume by continuously monitoring hematocrit during hemodialysis. J Am Soc Nephrol. 1995;6:214–219. doi: 10.1681/ASN.V62214. [DOI] [PubMed] [Google Scholar]
- 13.Dasselaar JJ, Huisman RM, de Jong PE, Franssen CF. Measurement of relative blood volume changes during haemodialysis: merits and limitations. Nephrol Dial Transplant. 2005;20:2043–2049. doi: 10.1093/ndt/gfi056. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal R, Kelley K, Light RP. Diagnostic utility of blood volume monitoring in hemodialysis patients. Am J Kidney Dis. 2008;51:242–254. doi: 10.1053/j.ajkd.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 15.Dasselaar JJ, Huisman RM, de Jong PE, Burgerhof JG, Franssen CF. Effects of relative blood volume-controlled hemodialysis on blood pressure and volume status in hypertensive patients. ASAIO J. 2007;53:357–364. doi: 10.1097/MAT.0b013e318031b513. [DOI] [PubMed] [Google Scholar]
- 16.de Vries JP, Kouw PM, van der Meer NJ, Olthof CG, Oe LP, Donker AJ, de Vries PM. Non-invasive monitoring of blood volume during hemodialysis: its relation with post-dialytic dry weight. Kidney Int. 1993;44:851–854. doi: 10.1038/ki.1993.321. [DOI] [PubMed] [Google Scholar]
- 17.Kooman JP, Van der Sande FM, Leunissen KM. Wet or dry in dialysis--can new technologies help? Semin Dial. 2009;22:9–12. doi: 10.1111/j.1525-139X.2008.00533.x. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal R, Alborzi P, Satyan S, Light RP. Dry-weight reduction in hypertensive hemodialysis patients (DRIP): a randomized, controlled trial. Hypertension. 2009;53:500–507. doi: 10.1161/HYPERTENSIONAHA.108.125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charra B, Laurent G, Chazot C, Calemard E, Terrat JC, Vanel T, Jean G, Ruffet M. Clinical assessment of dry weight. Nephrol Dial Transplant. 1996;11 Suppl 2:16–19. doi: 10.1093/ndt/11.supp2.16. [DOI] [PubMed] [Google Scholar]
- 20.Steuer RR, Bell DA, Barrett LL. Optical measurement of hematocrit and other biological constituents in renal therapy. Adv Ren Replace Ther. 1999;6:217–224. doi: 10.1016/s1073-4449(99)70017-8. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez HJ, Domenici R, Diroll A, Goykhman I. Assessment of dry weight by monitoring changes in blood volume during hemodialysis using Crit-Line. Kidney Int. 2005;68:854–861. doi: 10.1111/j.1523-1755.2005.00467.x. [DOI] [PubMed] [Google Scholar]
- 22.Steuer RR, Germain MJ, Leypoldt JK, Cheung AK. Enhanced fluid removal guided by blood volume monitoring during chronic hemodialysis. Artif Organs. 1998;22:627–632. doi: 10.1046/j.1525-1594.1998.06036.x. [DOI] [PubMed] [Google Scholar]
- 23.Candan C, Sever L, Civilibal M, Caliskan S, Arisoy N. Blood volume monitoring to adjust dry weight in hypertensive pediatric hemodialysis patients. Pediatr Nephrol. 2009;24:581–587. doi: 10.1007/s00467-008-0985-9. [DOI] [PubMed] [Google Scholar]
- 24.Patel HP, Goldstein SL, Mahan JD, Smith B, Fried CB, Currier H, Flynn JT. A standard, noninvasive monitoring of hematocrit algorithm improves blood pressure control in pediatric hemodialysis patients. Clin J Am Soc Nephrol. 2007;2:252–257. doi: 10.2215/CJN.02410706. [DOI] [PubMed] [Google Scholar]
- 25.Goldstein SL, Smith CM, Currier H. Noninvasive interventions to decrease hospitalization and associated costs for pediatric patients receiving hemodialysis. J Am Soc Nephrol. 2003;14:2127–2131. doi: 10.1097/01.asn.0000076077.05508.7e. [DOI] [PubMed] [Google Scholar]
- 26.Zellweger M, Querin S, Madore F. Measurement of blood volume during hemodialysis is a useful tool to achieve safely adequate dry weight by enhanced ultrafiltration. ASAIO J. 2004;50:242–245. doi: 10.1097/01.mat.0000123571.98351.73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.