Abstract
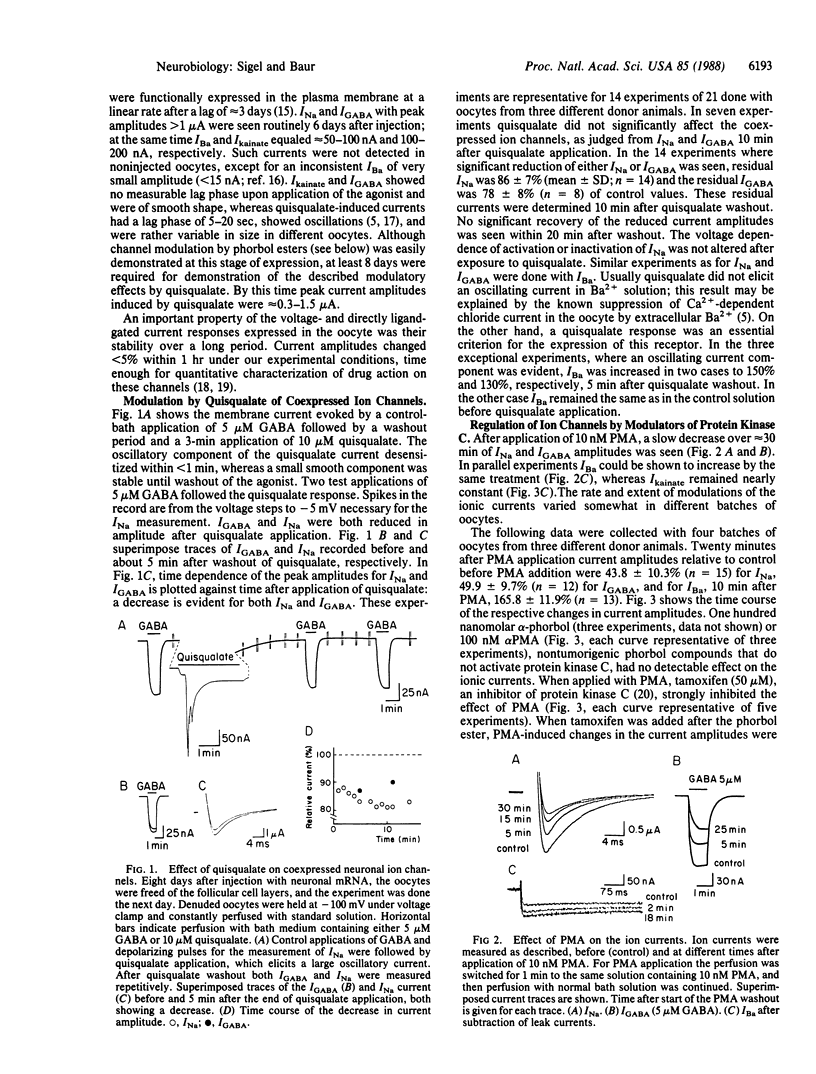
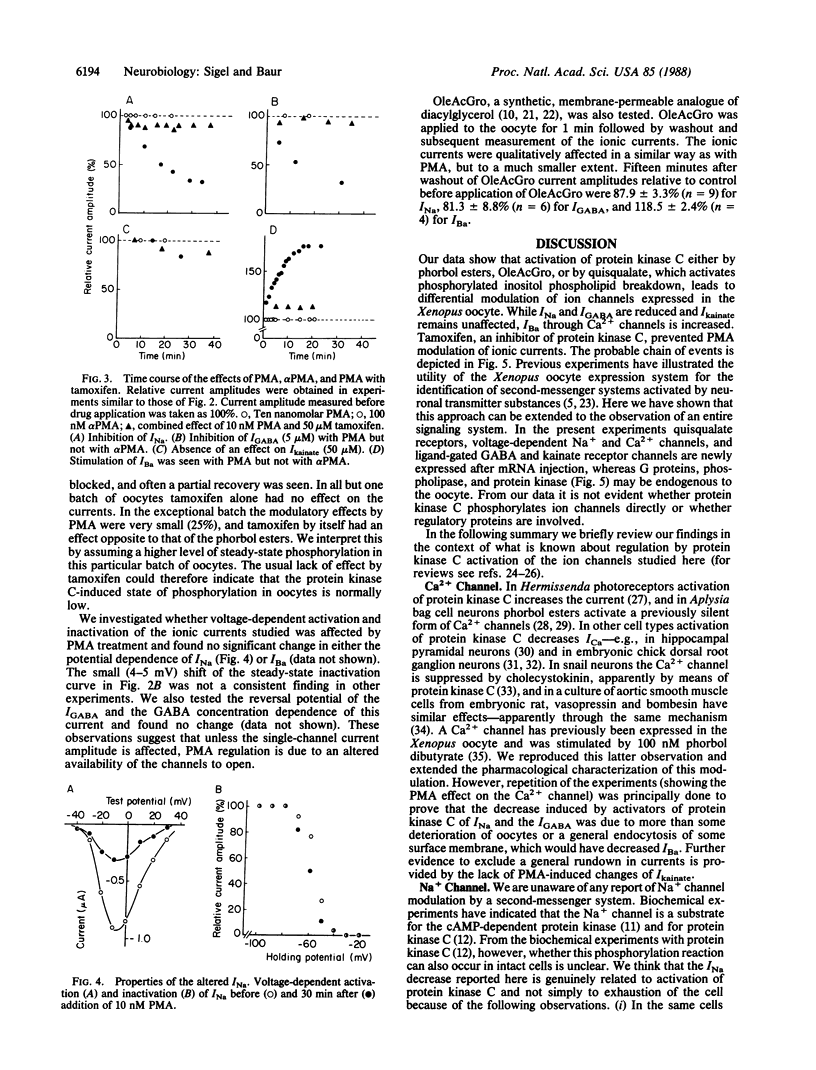
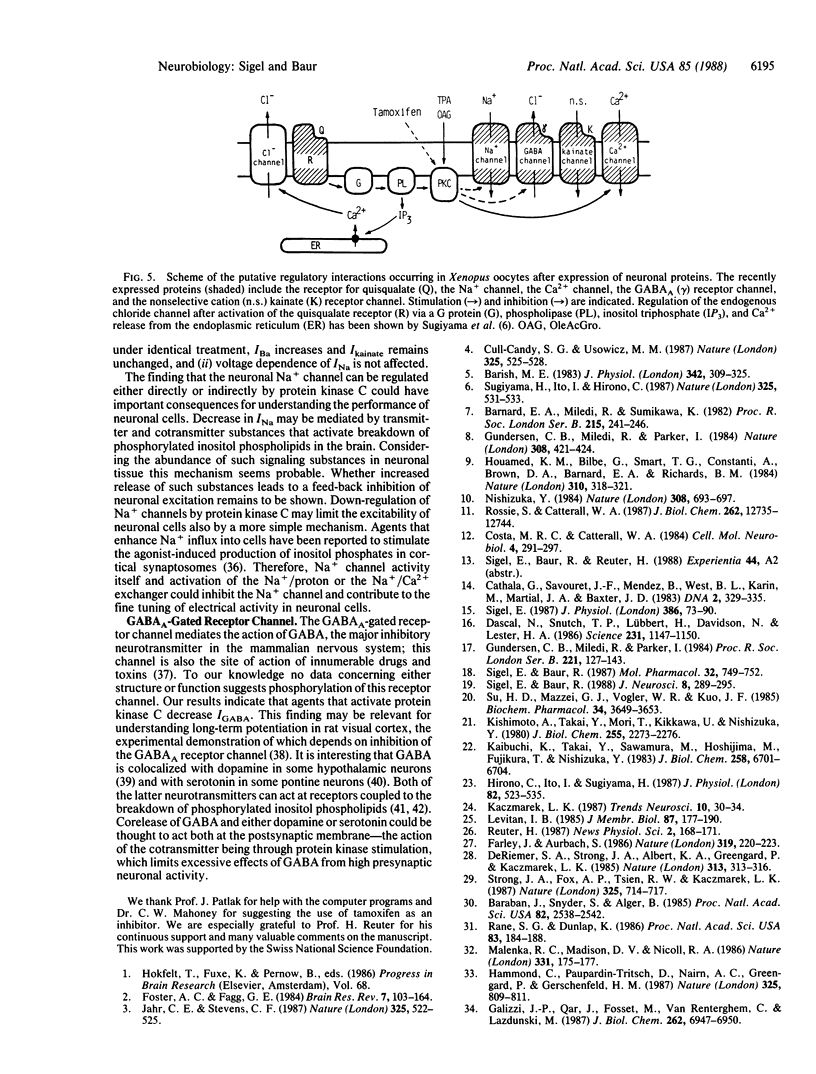
Xenopus oocytes were used to study the interaction of neuronal quisqualate receptors with neuronal ion channels. Total mRNA was isolated from chick forebrain and injected into Xenopus oocytes. This technique led to the expression of functional voltage-gated Na+ and Ca2+ channels, of ligand-gated gamma-aminobutyrate and kainate receptor channels, and of quisqualate receptors that could activate endogenous chloride channels by means of inositol trisphosphate-mediated Ca2+ release. Exposure of the oocytes to quisqualate decreased the amplitude of the Na+ current and of the gamma-aminobutyrate type A-gated current and increased the amplitude of the Ba2+ current through Ca2+ channels. This modulation of neuronal ion channels by quisqualate could be mimicked by the protein kinase C activator phorbol 12-myristate 13-acetate and the diacylglycerol analogue 1,2-oleoylacetylglycerol. The kainate-gated channel was not affected by these agents. Phorbol esters that do not activate protein kinase C, alpha-phorbol 12-myristate 13-acetate and alpha-phorbol, were without effect. The inhibitor of protein kinase C, tamoxifen, prevented the modulatory effects of phorbol 12-myristate 13-acetate. The present evidence suggests that the activity of the neuronal Na+ and Ca2+ channels and the ligand-gated gamma-aminobutyrate type A receptor channel are under the control of protein kinase C and that neurotransmitters that activate protein kinase C could profoundly affect neuronal signaling.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artola A., Singer W. Long-term potentiation and NMDA receptors in rat visual cortex. Nature. 1987 Dec 17;330(6149):649–652. doi: 10.1038/330649a0. [DOI] [PubMed] [Google Scholar]
- Baraban J. M., Snyder S. H., Alger B. E. Protein kinase C regulates ionic conductance in hippocampal pyramidal neurons: electrophysiological effects of phorbol esters. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2538–2542. doi: 10.1073/pnas.82.8.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barish M. E. A transient calcium-dependent chloride current in the immature Xenopus oocyte. J Physiol. 1983 Sep;342:309–325. doi: 10.1113/jphysiol.1983.sp014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard E. A., Miledi R., Sumikawa K. Translation of exogenous messenger RNA coding for nicotinic acetylcholine receptors produces functional receptors in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1982 May 22;215(1199):241–246. doi: 10.1098/rspb.1982.0040. [DOI] [PubMed] [Google Scholar]
- Belin M. F., Nanopoulos D., Didier M., Aguera M., Steinbusch H., Verhofstad A., Maitre M., Pujol J. F. Immunohistochemical evidence for the presence of gamma-aminobutyric acid and serotonin in one nerve cell. A study on the raphe nuclei of the rat using antibodies to glutamate decarboxylase and serotonin. Brain Res. 1983 Sep 26;275(2):329–339. doi: 10.1016/0006-8993(83)90994-0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Costa M. R., Catterall W. A. Phosphorylation of the alpha subunit of the sodium channel by protein kinase C. Cell Mol Neurobiol. 1984 Sep;4(3):291–297. doi: 10.1007/BF00733592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. Multiple-conductance channels activated by excitatory amino acids in cerebellar neurons. Nature. 1987 Feb 5;325(6104):525–528. doi: 10.1038/325525a0. [DOI] [PubMed] [Google Scholar]
- Dascal N., Snutch T. P., Lübbert H., Davidson N., Lester H. A. Expression and modulation of voltage-gated calcium channels after RNA injection in Xenopus oocytes. Science. 1986 Mar 7;231(4742):1147–1150. doi: 10.1126/science.2418503. [DOI] [PubMed] [Google Scholar]
- DeRiemer S. A., Strong J. A., Albert K. A., Greengard P., Kaczmarek L. K. Enhancement of calcium current in Aplysia neurones by phorbol ester and protein kinase C. Nature. 1985 Jan 24;313(6000):313–316. doi: 10.1038/313313a0. [DOI] [PubMed] [Google Scholar]
- Farley J., Auerbach S. Protein kinase C activation induces conductance changes in Hermissenda photoreceptors like those seen in associative learning. Nature. 1986 Jan 16;319(6050):220–223. doi: 10.1038/319220a0. [DOI] [PubMed] [Google Scholar]
- Foster A. C., Fagg G. E. Acidic amino acid binding sites in mammalian neuronal membranes: their characteristics and relationship to synaptic receptors. Brain Res. 1984 May;319(2):103–164. doi: 10.1016/0165-0173(84)90020-1. [DOI] [PubMed] [Google Scholar]
- Galizzi J. P., Qar J., Fosset M., Van Renterghem C., Lazdunski M. Regulation of calcium channels in aortic muscle cells by protein kinase C activators (diacylglycerol and phorbol esters) and by peptides (vasopressin and bombesin) that stimulate phosphoinositide breakdown. J Biol Chem. 1987 May 25;262(15):6947–6950. [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Glutamate and kainate receptors induced by rat brain messenger RNA in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1984 Apr 24;221(1223):127–143. doi: 10.1098/rspb.1984.0027. [DOI] [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Messenger RNA from human brain induces drug- and voltage-operated channels in Xenopus oocytes. 1984 Mar 29-Apr 4Nature. 308(5958):421–424. doi: 10.1038/308421a0. [DOI] [PubMed] [Google Scholar]
- Gusovsky F., Hollingsworth E. B., Daly J. W. Regulation of phosphatidylinositol turnover in brain synaptoneurosomes: stimulatory effects of agents that enhance influx of sodium ions. Proc Natl Acad Sci U S A. 1986 May;83(9):3003–3007. doi: 10.1073/pnas.83.9.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C., Paupardin-Tritsch D., Nairn A. C., Greengard P., Gerschenfeld H. M. Cholecystokinin induces a decrease in Ca2+ current in snail neurons that appears to be mediated by protein kinase C. 1987 Feb 26-Mar 4Nature. 325(6107):809–811. doi: 10.1038/325809a0. [DOI] [PubMed] [Google Scholar]
- Hirono C., Ito I., Sugiyama H. Neurotensin and acetylcholine evoke common responses in frog oocytes injected with rat brain messenger ribonucleic acid. J Physiol. 1987 Jan;382:523–535. doi: 10.1113/jphysiol.1987.sp016382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houamed K. M., Bilbe G., Smart T. G., Constanti A., Brown D. A., Barnard E. A., Richards B. M. Expression of functional GABA, glycine and glutamate receptors in Xenopus oocytes injected with rat brain mRNA. 1984 Jul 26-Aug 1Nature. 310(5975):318–321. doi: 10.1038/310318a0. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Glutamate activates multiple single channel conductances in hippocampal neurons. Nature. 1987 Feb 5;325(6104):522–525. doi: 10.1038/325522a0. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Sawamura M., Hoshijima M., Fujikura T., Nishizuka Y. Synergistic functions of protein phosphorylation and calcium mobilization in platelet activation. J Biol Chem. 1983 Jun 10;258(11):6701–6704. [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Leonard J. P., Nargeot J., Snutch T. P., Davidson N., Lester H. A. Ca channels induced in Xenopus oocytes by rat brain mRNA. J Neurosci. 1987 Mar;7(3):875–881. doi: 10.1523/JNEUROSCI.07-03-00875.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan I. B. Phosphorylation of ion channels. J Membr Biol. 1985;87(3):177–190. doi: 10.1007/BF01871217. [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Madison D. V., Nicoll R. A. Potentiation of synaptic transmission in the hippocampus by phorbol esters. Nature. 1986 May 8;321(6066):175–177. doi: 10.1038/321175a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Olsen R. W. Drug interactions at the GABA receptor-ionophore complex. Annu Rev Pharmacol Toxicol. 1982;22:245–277. doi: 10.1146/annurev.pa.22.040182.001333. [DOI] [PubMed] [Google Scholar]
- Rane S. G., Dunlap K. Kinase C activator 1,2-oleoylacetylglycerol attenuates voltage-dependent calcium current in sensory neurons. Proc Natl Acad Sci U S A. 1986 Jan;83(1):184–188. doi: 10.1073/pnas.83.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossie S., Catterall W. A. Cyclic-AMP-dependent phosphorylation of voltage-sensitive sodium channels in primary cultures of rat brain neurons. J Biol Chem. 1987 Sep 15;262(26):12735–12744. [PubMed] [Google Scholar]
- Sekar M. C., Hokin L. E. The role of phosphoinositides in signal transduction. J Membr Biol. 1986;89(3):193–210. doi: 10.1007/BF01870664. [DOI] [PubMed] [Google Scholar]
- Sigel E., Baur R. Allosteric modulation by benzodiazepine receptor ligands of the GABAA receptor channel expressed in Xenopus oocytes. J Neurosci. 1988 Jan;8(1):289–295. doi: 10.1523/JNEUROSCI.08-01-00289.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel E., Baur R. Effect of avermectin B1a on chick neuronal gamma-aminobutyrate receptor channels expressed in Xenopus oocytes. Mol Pharmacol. 1987 Dec;32(6):749–752. [PubMed] [Google Scholar]
- Sigel E. Properties of single sodium channels translated by Xenopus oocytes after injection with messenger ribonucleic acid. J Physiol. 1987 May;386:73–90. doi: 10.1113/jphysiol.1987.sp016523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong J. A., Fox A. P., Tsien R. W., Kaczmarek L. K. Stimulation of protein kinase C recruits covert calcium channels in Aplysia bag cell neurons. Nature. 1987 Feb 19;325(6106):714–717. doi: 10.1038/325714a0. [DOI] [PubMed] [Google Scholar]
- Su H. D., Mazzei G. J., Vogler W. R., Kuo J. F. Effect of tamoxifen, a nonsteroidal antiestrogen, on phospholipid/calcium-dependent protein kinase and phosphorylation of its endogenous substrate proteins from the rat brain and ovary. Biochem Pharmacol. 1985 Oct 15;34(20):3649–3653. doi: 10.1016/0006-2952(85)90225-4. [DOI] [PubMed] [Google Scholar]
- Sugiyama H., Ito I., Hirono C. A new type of glutamate receptor linked to inositol phospholipid metabolism. Nature. 1987 Feb 5;325(6104):531–533. doi: 10.1038/325531a0. [DOI] [PubMed] [Google Scholar]


