Abstract
Because youth with aggressive conduct disorder (CD) often inflict pain on others, it is important to determine if they exhibit atypical empathic responses to viewing others in pain. In this initial functional magnetic resonance imaging (fMRI) study, 8 adolescents with aggressive CD and 8 matched controls were scanned while watching animated visual stimuli depicting other people experiencing pain or not experiencing pain. Furthermore, these situations involved either an individual whose pain was caused by accident or an individual whose pain was inflicted on purpose by another person. After scanning, participants rated how painful the situations were. In both groups the perception of others in pain was associated with activation of the pain matrix, including the ACC, insula, somatosensory cortex, supplementary motor area and periaqueductal gray. The pain matrix was activated to a significantly greater extent in participants with CD, who also showed strong amygdala, ventral striatum, and temporal pole activation. When watching situations in which pain was intentionally inflicted, control youth also exhibited signal increase in the medial prefrontal frontal cortex, lateral obitofrontal cortex, and temporoparietal junction, whereas youth with CD only exhibited activation in the insula. Furthermore, connectivity analyses demonstrated that youth with CD exhibited less amygdala/prefrontal coupling when watching pain inflicted by another than did control youth. These preliminary findings suggest that youth with aggressive CD exhibit an atypical pattern of neural response to viewing others in pain that should be explored in further studies.
Keywords: Perception of Pain, Empathy, Emotion Regulation, Conduct Disorder, Aggression
1. Introduction
Conduct disorder (CD) is a serious mental disorder of childhood and adolescence that is characterized by a longstanding pattern of violations of rules and laws. Symptoms of CD include physical aggression, manipulative lying, theft, forced sex, bullying, running away from home overnight, and destruction of property. CD is a major public health problem because youth with conduct disorder not only inflict serious physical and psychological harm on others, but they are at greatly increased risk for incarceration, injury, depression, substance abuse, and death by homicide and suicide themselves (Loeber et al., 1998). Furthermore, CD is important because it is the major childhood precursor to antisocial personality disorder in adulthood (Lahey, Loeber, Burke, & Applegate, 2005). Thus there is a pressing need to understand the biopsychological processes at multiple levels of analysis that give rise to CD. Biological studies of antisocial behavior can lead to new approaches to the treatment of psychiatric conditio ns associated with aggression (Van Goozen & Fairchild, 2008).
Empathy, the capacity to understand and appreciate the emotional states and needs of others in reference to oneself, has been one psychological characteristic repeatedly proposed as a core deficit in CD (see Lovett & Sheffield, 2007 for a review). Here we consider empathy as a construct accounting for a sense of similarity in feelings experienced by the self and the other, without confusion between the two individuals (Batson, Fultz & Schoenrade, 1987; Decety & Batson, 2007; Decety & Jackson, 2004; Decety & Moriguchi, 2007; Eisenberg, Spinrad & Sadovsky, 2006). The experience of empathy can lead to sympathy or empathic concern for another based on the apprehension or comprehension of the other’s emotional state or condition, or to personal distress, i.e., an aversive, self-focused emotional reaction to the emotional state or condition of another (Batson, Fultz & Schoenrade, 1987; Decety & Lamm, 2008; Eisenberg & Eggum, 2008; Lamm, Batson & Decety, 2007).
Interestingly, some developmental psychologists have hypothesized that empathy and sympathetic concern for others is an essential factor inhibiting aggression toward others (Eisenberg 2005, Hoffman, 2000). Empathy may be regarded as a proximate factor motivating prosocial rather than antisocial behavior (Batson, 1991). It is commonly defined as an affective reaction that is appropriate to someone else’s situation rather than one’s own. Some researchers have theorized that there should be a relation between aggressive behavior and a lack of empathy (e.g., Zahn-Waxler et al., 1995). Similarly, other scholars have proposed that individual differences in callous disregard for the welfare of others is an important risk factor for CD (Frick et al., 2005; Lahey & Waldman, 2003).
The propensity for aggressive behavior has been hypothesized to reflect a blunted empathic response to the suffering of others (Blair, 2005). Such a lack of empathy in aggressive individuals may be a consequence of a failure to be aroused to the distress of others (Raine et al., 1997). In line with this hypothesis, it has been suggested that aggressive behavior arises from an abnormal processing of affective information, resulting in a deficiency in experiencing fear, empathy, and guilt, which in normally inhibit the acting out of violent impulses (Davidson et al., 2000; Herpertz & Sass, 2000). Consistent with this hypothesis, one functional MRI study found reduced left amygdala response in 13 adolescents with CD in response to the visual presentation of pictures with strong negative emotional valence compared to 14 control adolescents (Sterzer et al., 2005).
An alternative hypothesis can be drawn from research on aggression that has shown that negative affect is generally associated with aggression (Anderson & Bushman, 2002; Berkowitz, 2003), suggesting that empathic mimicry in conjunction with poor emotion regulation might produce distress that increases aggression (Campbell, 1990; Gill & Calkins, 2003). For instance, there are many empirical studies that documented that physical pain often instigates aggressive inclinations (Berkowitz, 1983, 1993). This is particularly interesting in the light of recent work in cognitive neuroscience of empathy for pain.
Indeed, a growing number of fMRI studies have demonstrated striking similarities in the neural circuits involved in the processing of both the first-hand experience of pain and the experience of observing other individuals in pain (Jackson, Rainville & Decety, 2006). These studies have consistently shown that the perception of pain in others elicits activation of the neural circuit subserving the processing of the affective and motivational dimension of pain in oneself (Botvinick et al., 2005; Cheng et al., 2007a; Gu & Han, 2007; Jackson et al., 2005, 2006; Lamm et al., 2007a, Moriguchi et al., 2007, Morrison et al., 2004; Ogino et al., 2007; Saarela et al. 2007, Singer et al., 2004). This circuit includes the dorsal anterior cingulate cortex (ACC), the anterior midcingulate cortex (aMCC), the supplementary motor area (SMA), and anterior insula (Derbyshire, 2000; Price, 2000). In addition, somatosensory-evoked potentials (Bufalari et al., 2007), and fMRI studies (Cheng et al., 2007a; Lamm et al., 2007b; Moriguchi et al., 2007) have demonstrated that areas processing the sensory dimension of pain (posterior insula/somatosensory cortex) may also be elicited by the visual perception of pain in others.
Recently, one functional MRI study investigated empathy and intentionality in typically developing middle-school children (Decety, Michalska & Akitsuki, 2008) while they watched dynamic visual stimuli depicting either a person whose pain was accidentally caused or a person whose pain was intentionally inflicted by another individual. Interestingly, when watching a person inflicting pain on another, regions that are engaged in representing social interaction and moral behavior including the temporo-parietal junction, the paracingulate, orbital medial frontal cortices, and amygdala (see Moll et al., 2003, 2007) were additionally recruited, and increased their connectivity with the frontoparietal attention network.
There also is evidence that specifically associates the amygdala and paralimbic prefrontal regions, including the dorsal and ventral/orbital medial prefrontal cortex (dMPFC and vMPFC/OFC, respectively), with human aggression (Coccarro et al., 2007; Davidson et al., 2000). In humans, amygdala atrophy and/or lesions have been associated with impulsively aggressive behaviors (van Elst et al., 2000). Specific damage to the OFC is associated with impulsive and aggressive behavior (Izquierdo et al., 2005), and individuals with such damage show little control over their emotions as well as limited awareness of the moral implications of their actions (Anderson et al., 1999). Since the amygdala and OFC are anatomically and functionally connected (Amaral and Price 1984), their interactions may be critical for interpreting emotionally significant information and guiding goal directed behaviors (Saddoris et al., 2005). Furthermore, the OFC is hypothesized to play a key role in modulating limbic reactivity to threat (Davidson et al., 2000; Izquierdo et al., 2005), and in general is important for the interpretation of social cues.
Thus, there is evidence that perceiving others in pain triggers an automatic somatic and sensorimotor resonance between other and self, which activates almost the entire neural pain matrix including the periaqueductal gray (PAG) a major site in pain transmission and for processing fear and anxiety (Jenk et al., 1995), the SMA that programs defensive skeletomotor impulses to avoid the stimulus in the context of nociceptive information (Morrison et al., 2006), and thalamus. Such a mechanism provides a functional bridge between first-person and third person information, which allows for analogical reasoning, and offers a possible, yet partial, route to understanding others (Decety & Sommerville, 2003). It also provides a clear signal of the other’s distress that usually inhibits aggressive behavior.
So far, there is no published work on how youth with CD react to viewing others in pain. If the blunted empathic emotional response hypothesis is correct, adolescents with aggressive CD should react less to stimuli depicting others in pain than healthy controls. Furthermore, this lack of signal from the pain matrix and amygdala could account for impairment in recognizing information about the distress of others. If the pain-aggression hypothesis is correct, youth with aggressive CD should exhibit greater activation than healthy controls in the amygdala, PAG, and related areas in response to stimuli depicting others in pain. This strong ac tivation of structures subserving negative emotion and less functional amygdala/OFC connectedness could reflect a general tendency to experience personal distress that elicits aggression in some circumstances. In addition, having a condition in which pain was intentionally inflicted into another allows us to examine the respective contribution of mechanisms that contribute to theory of mind and moral reasoning in the context of pain perception (see Decety, Michalska & Akitsuki, 2008). This is particularly interesting to investigate with respect to antisocial behavior given that these individuals have been reported to lack guilt and empathic concern.
2. Methods and Materials
2.1. Participants
Two groups of 16–18 year-old adolescents (exactly matched on age, sex, and race-ethnicity) were scanned. The participants were purposively selected from a 9-year longitudinal study of 127 adolescents with attention-deficit/hyperactivity disorder and a matched healthy comparison group of 125 youth (Lahey et al., 2005). The study included 8 structured diagnostic assessments of CD using the DSM-IV field trials version of the Diagnostic Interview Schedule for Children (Lahey et al., 1994; Shaffer, Fisher, Piacentini, Schwab-Stone, & Wicks, 1993) conducted over 9 years beginning at 4–6 years of age. Information on symptoms of the disruptive behavior disorders was obtained from teachers using the DSM-IV version of the DBD Checklist (Pelham et al., 1992). Beginning in the 6th annual assessment, the DISC was also administered to the youth. Youth were considered to exhibit each symptom if any informant reported it (Piacentini, Cohen, & Cohen, 1992).
In the 6th annual assessment, parents also completed the Child and Adolescent Dispositions Scale (CADS, Lahey et al, in press), which quantifies three socioemotional dispositions. Prosociality is defined by sympathetic concern for others, helping and sharing, respect for social rules, and guilt over misdeeds. It is similar to Eisenberg’s construct of dispositional sympathy (Eisenberg et al., 1989), which is inversely related to CD. Daring is defined by the descriptors of daring, brave, and adventurous, and by enjoyment of risky and loud activities and rough games and sports. It is based on Farrington and West’s (1993) observation that parent ratings of the single item of ‘daring’ during childhood was a robust predictor of future criminal offending and is similar to the construct of sensation seeking, which is correlated with CD (Russo et al., 1993). Children rated high on negative emotionality are easily and intensely upset by frustrations, threats, and losses.
An ad hoc measure of sadism was created from items that did not factor onto the three main factors of the CADS. The sadism measure was composed of the items: enjoys bothering or hurting other children, thinks it’s funny when other children are upset; likes to scare other children, and thinks it would be fun to watch two dogs fight.
2.2. Experimental groups
The aggressive CD group was selected to be the 8 adolescents in the sample with the most persistent and aggressive histories of CD over the 9 years. They met criteria for CD 1–7 times in the 8 assessments (mean = 2.25) and exhibited a total of 2–18 aggression symptoms (starting fights; bullying using a weapon; theft with confrontation of the victim; physical cruelty to people; cruelty to animals and forced sex) over the 8 assessments (mean = 7.5). In contrast, the comparison group was selected to be 8 adolescents who never exhibited any symptoms of CD or aggression during the 8 assessments and who matched the CD youth in age, sex, and race-ethnicity. Parental written informed consent and adolescent oral assent were obtained. Participants were paid for their participation. The study was approved by the University of Chicago Institutional Review Board and conducted in accordance with the Declaration of Helsinki.
2.3. Stimuli preparation and validation
The task consisted of the successive presentation of animated visual images of hands and feet in blocks depicting painful and non-painful situations. A series of 96 stimuli were created and validated for this study. Validation of the material was conducted with a group of 222 healthy male and female participants (age range 12–38 yrs; middle school to college educated) who were shown these dynamic stimuli and asked to estimate how painful these situations were and whether they believed that the pain was caused intentionally (Estabrook, 2007). Each animation consisted of three digital color pictures, which were edited to the same size (600 × 480 pixels). The durations of the first, second and third pictures were 1000 ms, 200 ms and 1000 ms respectively. These animated stimuli contained scenes of various types of painful and non-painful everyday situations.
Each animation displayed one or two persons whose right hands or right feet are visible but not their faces (see Figure 1). When presented, the two people are distinguished from one another in clothing or shoe type. These 96 stimuli belong to four categories (24 each) of pain and involved person types, including:
Figure 1.
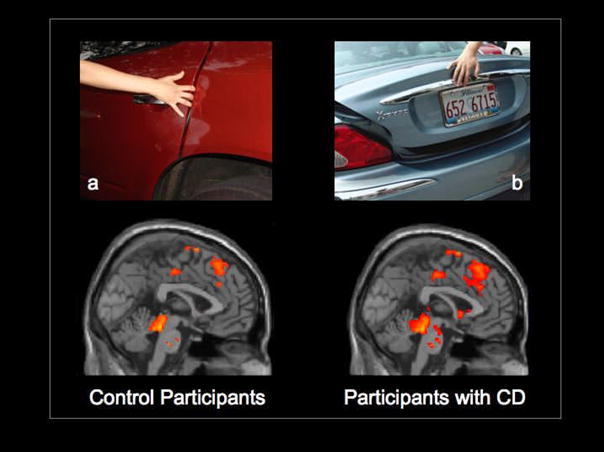
Selective activation of the ACC, aMCC and PAG overlaid onto a sagittal MRI section of the MNI brain when control participants and participants with CD observed dynamic visual stimuli that depict painful situations caused by accident (a) compared with not painful situations (b). Activation of areas that belong to the pain matrix (including the anterior insula and somatosensory cortex, not shown here) was stronger in the adolescents with CD. Note that the amygdala and ventral striatum were also activated in the CD group.
Only one person is in a painful situation caused by accident, e.g., a person dropping a heavy bowl on her hand (PCA).
Only one person is involved in a non-painful situation, e.g., opening a door (NPS).
One person is in a painful situation caused by another, e.g., stepping purposely on someone’s toe (PCO).
One person is in a painful situation at first but this pain is alleviated by the other, e.g., helping another get his or her hand out of a door (APO).
2.4. Training in the mock scanner
Prior to MRI scanning, adolescents were acclimated to the procedures in a mock scanner. They were asked to lie in the mock scanner while a documentary movie was played (Jacques Cousteau’s Pacific Explorations). When the adolescents felt comfortable, then they were presented with 24 stimuli (6 per condition) depicting situations similar to, but not the same as, those they would watch in the actual scanning sessions. MRI noise was simulated through a recording played during the mock session, which lasted approximately 10–15 minutes with their parents remained in the room.
2.5. Scanning session
Stimuli were presented with E-prime software (Psychology Software Tools, Inc. Pittsburgh, PA, USA) and a back-projection system. A block-design paradigm was used with 9 baseline blocks (duration 17.6 s each) during which fixation cross was presented and 8 active blocks (duration 19.8 s each) during which stimuli from one of the four categories were presented. The presentation order was counterbalanced across runs and across subjects. Each block consisted of 6 stimuli (2200 ms each) with 5 inter-stimulus intervals (1100 ms each) during which a black fixation cross was presented against a gray background. Adolescents were shown the stimuli in two short sessions (6 min each) to maintain their attention. To avoid confounding motor-related activation in the ACC and pre-SMA/SMA, no overt response was required. Instead, adolescents were instructed to watch the stimuli carefully.
Magnetic resonance imaging was performed on a GE 3T magnet (Horizon LX). Functional images were obtained using T2*-weighted gradient echo spiral in/out pulse sequence (Glover and Lai, 2001). Thirty six coronal slices of 5 mm slice thickness without spatial gap were obtained for 160 repetitions (including 16 discarded acquisitions at the onset of each of two runs) using the following parameters: TR = 2200 ms, TE = 26 ms, flip angle = 81°, FOV = 24 cm, matrix = 64 × 64, and in-plane resolution = 3.75 × 3.75 mm. The spiral-in/out sequence was shown to be effective in recovering blood oxygenation level-dependent (BOLD) signal in frontal regions important to this study (Preston et al., 2004). An axial T1-weighted 3D magnetization-prepared rapid acquisition gradient echo (MP-RAGE) anatomical scan was also acquired for 3D localization (TR = 8 ms, TE = 3.2 ms, flip angle = 6°, FOV = 24 cm, matrix = 256 × 192, slice thickness = 1.5 mm, 124 slices).
2.6. Image processing and analysis
Image processing was carried out with SPM5 (Wellcome Department of Imaging Neuroscience, London, UK), implemented in MATLAB 7.0 (Mathworks Inc. Sherborn, MA). Preprocessing included slice-timing correction, correction for head motion, normalization to the EPI template provided in SPM5, and smoothing using a 6-mm full-width half-maximum isotropic Gaussian kernel. Images were realigned and normalized using standard SPM procedures. All 17 subjects had less than 0.5 voxels of in-plane motion. A two-level approach for block-design fMRI data was adopted using SPM5. A voxel-by-voxel multiple regression analysis of expected signal changes for each of the four block categories, which were constructed using the hemodynamic response function provided by SPM5, was applied to the preprocessed images for each subject. Individual subject data were analyzed using a fixed-effects model. Group data were analyzed using a random-effects model using one-sample t tests. Condition effects at the subject level were modeled by box-car regressors representing the occurrence of each of the 4 block types. Except where noted, a voxel-level threshold of P < 0.005 for group contrasts, uncorrected for multiple comparisons (with an extent threshold of 10 contiguous voxels) was used for to identify significant activity changes in pain-related regions and other regions of a priori interest. These included regions associated with theory of mind (TPJ, PCC), and emotion regulation (OFC, dACC). For other regions, a threshold of P < 0.005 corrected for multiple comparisons was used. Activations were overlaid on a representative high-resolution structural T1-weighted image from a single subject from the SPM5 canonical image set, co-registered to Montreal Neurological Institute (MNI) space.
Pain related activation was identified using the contrast between stimuli depicting “pain caused by accident vs. no pain stimulus” (PCA - NPS). Perception of agency-related activation was identified using the contrast between “pain caused by other vs. pain caused by accident” (PCO - PCA).
2.7. Analyses of effective connectivity
To investigate group difference in the context-dependent contributions of left amygdala activity during painful and non-painful trials, psycho-physiological interaction (PPI; Friston et al., 1997) analyses were performed. The goal of PPI analyses is to assess whether the influence two neural networks exert over each other is modulated by certain psychological factors. The PPI analysis is used to compare the functional ‘coupling’ of different brain regions (physical component) during different tasks (psychological component); it can capture the modulation of activity in one brain region by activity in another brain region dependent on specific active tasks. In a PPI analysis, a design matrix contains three columns of variables: (i) stimuli; (ii) a time-series ‘physiological’ variable that represents the time course of the source (seed) region; (iii) an interaction variable that represents the interaction of psychological and physiological variables, (i) and (ii) respectively. In our case, we were interested in one specific modulation: (2) whether the pain was caused by another individual (PCO) or caused by accident (PCA). The regression coefficient for the interaction term provides a measure of PPI; a correlation in activity between the seed region and the identified ‘coupled’ region (or regions) that is significantly different between tasks (i.e., pain caused by other versus pain caused by accident) yields a significant PPI effect. As such, a PPI analysis examines differences in context-specific functional connectivity between regions of interest. Based upon a priori hypotheses, we were specifically interested in amygdala–PFC/OFC interactions while viewing painful situations. We therefore chose the amygdala as a potential source region of interest. To obtain data for the physiological variable, we extracted the individual time series from a 6-mm spherical region centered on the coordinates of subject-specific activations in the left amygdala. To perform an unbiased contrast analysis, we first performed a conjunction analysis with SPM5 to identify amygdala activation that was present across all subjects in both PCO and PCA blocks. The conjunction (i.e., [PCO > Baseline] and [PCA > Baseline]) analysis identified that the left amygdala cluster was active during both.
PPI analyses were performed in the following way: 1) extraction of the time-series data of the first eigenvalue of the seed VOI (2) generating a vector contrasting the time-series of the estimated neural response for the targeted conditions (the PPI regressor), a second vector representing the main effect of the selected contrast (the psychological variable), and a third vector representing the VOI timecourse (the physiological variable); and 3) forward-convolving these regressors with the canonical hemodynamic response function in order to estimate the effects of the PPI regressor. The resulting Statistical Parametric Maps (SPMs) showed clusters for which connectivity differed in the chosen conditions.
As in the segregation analyses, the two target contrasts were Pain Caused by Accident > No Pain Self, and Pain Caused by Other > Pain Caused by Accident. The resulting individual PPI-SPMs were then entered into a random-effects group analysis (two-sample t-tests) to look at group differences. Results were second-level rfx analyses, thresholded at P = 0.001, k = 10 uncorrected for areas with no a priori hypotheses, and P = 0.005, k = 10 uncorrected for areas with a priori hypotheses (i.e., areas of the pain matrix, dmPFC, OFC and temporo-parietal junction, which all have been associated with empathy for pain and mentalizing in previous studies (Cheng et al., 2007a; Decety et al., 2008; Jackson et al., 2006; Lamm et al., 2007).
2.8. Post-scan pain ratings
After the fMRI sessions, the same stimuli that had been shown during scanning were presented to the adolescents on a computer screen. They were asked to rate how painful the each situation was using a computer-based visual analogue scale (VAS) ranging from “no pain” to “extreme pain.” Adolescents were also asked to report what they felt when watching the stimuli in the scanner. Mean ratings for each of the four conditions were assessed by repeated measures ANOVA. A significance threshold of P < 0.05 was used for all comparisons.
2.9. Correlation analyses
To assess correlations between the youth’s post-scanning pain intensity ratings and hemodynamic responses, random-effects correlation analyses were performed. Each individual’s average scores for “pain caused by accident” on the VAS ratings was correlated with parameter estimates of the contrast “pain caused by accident” > Baseline. A significance threshold of P < 0.005 (uncorrected) and k > 10 was selected for these analyses. To reduce chances of false positives associated with the multitude of possible analyses, significant correlations were only interpreted if they were located in a priori defined regions of the pain matrix (Derbyshire, 2000).
Parametric analyses were also performed to determine the regions whose activity varied with of the 8 youths’ number of aggression symptoms. Each individual’s mean number of aggressive symptoms, and mean score on each of the three CADS scores, negative emotionality, daring/sensation seeking and prosociality, as well as adolescents’ average for the sadism score were correlated with parameter estimates of the contrast PCO > PCA. A significance threshold of P < 0.005 (uncorrected) and k > 10 was selected for these analyses.
3. Results
3.1. Post-scanning pain ratings
A repeated-measures ANOVA on the pain ratings indicated that both healthy adolescents and adolescents with CD rated the painful situations as depicting significantly greater pain (F1,14 = 98.9) p < 0.001) (PCA: 57 ± 7, and PCO: 59 ± 7 for healthy adolescents; PCA: 61 ± 5 and PCO: 62 ± 4 for adolescents with CD) than the neutral situations (APO: 27 ± 8, and NPS: 0.4 ± 0.2 for healthy adolescents; APO: 29 ± 7 and NPS: 2 ± 1 for adolescents with CD). Although watching hands and feet in painful situations resulted in the highest pain ratings for PCO than the other three conditions in both groups of adolescents, there was no statistical difference between this condition and the PCA condition (P > 0.26).
3.2. Functional MRI results
When watching others in pain caused by accident versus others in non-painful situations, the pain matrix (including the anterior insula, aMCC, somatosensory cortex, and PAG), was selectively activated in both groups (see Tables 1 and 2). In addition, in adolescents with CD, significant signal increase was detected bilaterally in the amygdala, ventral striatum, medial orbitofrontal cortex and temporal pole. A two-sample t-test for this contrast (PCA > NPS) showed a significant group effect in anterior midcingulate cortex, left amygdala, right caudate, and temporal pole bilaterally (see Table 3).
Table 1.
Brain regions showing significant activation (p < 0.005, uncorrected) in the control participants when they watched dynamic visual stimuli depicting painful situations caused by accident and painful situations intentionally caused by another individual.
| MNI Coordinates |
||||
|---|---|---|---|---|
| Regions of Interest | X | Y | Z | t Value |
| Pain Caused by Accident vs. No Pain Self | ||||
| R Anterior Insula | 38 | 24 | 2 | 5.09 |
| R Middle Insula | 38 | 4 | −2 | 4.63 |
| L Anterior Midcingulate Cortex | −8 | 16 | 30 | 5.35 |
| L Dorsal Anterior Cingulate Cortex | −8 | 8 | 34 | 5.45 |
| R Pre-Supplementary Motor Area | 6 | 22 | 54 | 4.66 |
| L Supplementary Motor Area | −6 | 4 | 70 | 5.26 |
| L Somatosensory Cortex | −10 | −36 | 74 | 3.93 |
| R Periaqueductal Gray | 2 | −30 | −8 | 5.42 |
| L Periaqueductal Gray | −2 | −30 | −8 | 5.42 |
| Pain Caused by Other vs. Pain Caused by Accident | ||||
| R Anterior Paracingulate Cortex | 2 | 64 | 10 | 6.56 |
| L Lateral Orbitofrontal Cortex | −22 | 64 | −2 | 3.79 |
| R Temporo-Parietal Junction | 50 | −46 | 20 | 4.76 |
| R Amygdala | 14 | 0 | −10 | 3.44 |
| R Middle Temporal Gyrus | 50 | −54 | 12 | 4.82 |
| R Superior Frontal Gyrus | 14 | 66 | 4 | 5.14 |
| R Medial Prefrontal Cortex | 12 | 56 | 8 | 3.68 |
Table 2.
Brain regions showing significant signal increase (p<0.005, uncorrected) in the CD participants for both pain caused by accident and pain caused by other conditions.
| MNI Coordinates |
||||
|---|---|---|---|---|
| Regions of Interest | X | Y | Z | t Value |
| Pain Caused by Accident vs. No Pain Self | ||||
| R Anterior Insula | 32 | 32 | 4 | 11.65 |
| L Middle Insula | −40 | −2 | 8 | 5.30 |
| L Anterior Midcingulate Cortex | −2 | 12 | 24 | 10.95 |
| R Middle Cingulate Cortex | 6 | 6 | 42 | 9.30 |
| R Pre-Supplementary Motor Area | 4 | 6 | 48 | 7.69 |
| L Pre-Supplementary Motor Area | −4 | 2 | 52 | 5.68 |
| L Somatosensory Cortex | −58 | −29 | 36 | 4.70 |
| R Medial Orbital Gyrus | 10 | 46 | −14 | 8.05 |
| L Medial Orbital Gyrus | −6 | 54 | −6 | 8.75 |
| L Temporal Pole | −56 | 10 | −2 | 7.71 |
| R Temporal Pole | 38 | 6 | −38 | 3.65 |
| R Ventral Striatum (putamen) | 26 | 12 | −4 | 8.12 |
| R Ventral Striatum (head of caudate) | 8 | 4 | 10 | 4.88 |
| R Periaqueductal Gray | 2 | −30 | −7 | 3.09 |
| L Periaqueductal Gray | −2 | −30 | −7 | 4.17 |
| L Amygdala | −18 | −8 | −10 | 11.60 |
| R Amygdala | 15 | −8 | −9 | 9.42 |
| Pain Caused by Other vs. Pain Caused by Acccident | ||||
| L Temporal Pole | −34 | 16 | −28 | 6.64 |
| L Middle Temporal Gyrus | −54 | −68 | 14 | 5.60 |
| R Middle Temporal Gyrus | 62 | −34 | 0 | 5.13 |
| R Precuneus | 8 | −62 | 48 | 10.24 |
| L Inferior Temporal Gyrus | −32 | −2 | −44 | 9.01 |
| R Superior Frontal Gyrus | 16 | 42 | 34 | 5.97 |
| L Medial Orbitofrontal Cortex | −4 | 30 | −17 | 4.79 |
Table 3.
Brain regions showing greater activation in participants with CD compared with healthy control participants in response to the PCA > NPS contrast. Whole-Brain Voxel-Wise Analysis (p < 0.005, uncorrected).
| MNI Coordinates |
||||
|---|---|---|---|---|
| Regions of Interest | X | Y | Z | t Value |
| Pain Caused by Accident >No Pain | ||||
| L Middle Insula | −37 | 6 | 10 | 3.30 |
| R Anterior Midcingulate Cortex | 2 | 9 | 26 | 3.90 |
| R Middle Cingulate Cortex | 6 | 6 | 40 | 3.84 |
| R Supplementary Motor Area | 4 | 6 | 48 | 2.08 |
| R Medial Orbital Gyrus | 2 | 54 | −10 | 3.41 |
| L Medial Orbital Gyrus | −4 | 56 | −8 | 3.57 |
| L Temporal Pole | −50 | −4 | 0 | 3.37 |
| R Temporal Pole | 62 | −2 | −6 | 3.34 |
| L Amygdala | −18 | −8 | −10 | 6.10 |
| R caudate (striatum) | 12 | 18 | 4 | 3.74 |
Watching painful situations intentionally caused by another individual versus pain caused by accident was associated with different patterns of activation in the two groups. The PCC, lateral OFC, TPJ, and superior frontal gyrus were activated in the control group. The left temporal pole, medial OFC and middle temporal gyrus were activated in the CD group.
Direct comparison (two sample t test) between the control and CD groups when viewing situations depicting an individual intentionally inflicting harm shows greater activation in adolescents with CD than controls in the left insula, SMA, precentral gyrus, and left medial OFC (see Table 4). Conversely, activation of the DLPFC [−42, 42, 22] was greater in HC than CD subjects. Participants with conduct disorder showed diminished BOLD response in the anterior PCC, TPJ and the lateral orbitofrontal cortex.
Table 4.
Brain regions showing greater activation in participants with CD compared with healthy control participants in response to the PCO > PS contrast. Whole-Brain Voxel-Wise Analysis (p < 0.005, uncorrected).
| MNI Coordinates |
||||
|---|---|---|---|---|
| Regions of Interest | X | Y | Z | t Value |
| Pain Caused by Other > Pain Caused by Accident | ||||
| L Anterior Insula | −26 | 24 | −4 | 3.33 |
| L Middle Cingulate Cortex | −10 | −6 | 46 | 3.83 |
| R Supplementary Motor Area | 12 | −10 | 60 | 3.83 |
| L Precentral Gyrus | −30 | −16 | 54 | 3.47 |
| L Middle Orbitofrontal Cortex | −12 | 34 | −12 | 3.88 |
The degree of activation in the anterior TP in the CD group was linearly correlated with the post-scanning VAS ratings of the pain experienced by the persons in the stimuli (r = 0.91, P < 0.001). Furthermore, a similar and nearly perfect correlation (r = 0.93, P < 0.001) was found with hemodynamic activity in the aMCC and right anterior and middle insula (r = 0.89, P < 0.001).
3.3. Effective connectivity analysis
The PPI analysis indicated that the CD group differed from the controls in the extent to which activity in the amygdala covaried with frontal cortical regions and the insula during the PCO condition compared to the PCA condition. For the healthy adolescents, PPI analyses showed that activity in the left amygdala was accompanied by condition-dependent (pain caused by other > pain caused by accident) functional interaction with a number of areas in the prefrontal cortex (see Table 5). The pattern of coupling was observed only in the pain caused by other > pain caused by accident contrast. In contrast, the CD group showed no significant effective connectivity between amygdala and these frontal and parietal regions. However, in this group, the PCO condition did modulate the effective connectivity between the amygdala and the left insula [−32, 12, −18], which was not the case for the healthy controls. Direct comparisons (2 sample t tests) confirmed the above differences between these two groups (P < 0.005). Interestingly, the effective connectivity between the left amygdala and the temporal pole was stronger for PCA > NPS in participants with CD. No significant modulation was detected with frontal regions.
Table 5.
Brain regions showing greater functional connectivity with the left amygdala in healthy control participants than participants with CD in response to the PCO condition (relative to the PCA condition): Whole-Brain Voxel-Wise Analysis.
| MNI Coordinates |
||||
|---|---|---|---|---|
| Regions of Interest | X | Y | Z | t Value |
| Pain Caused by Other > Pain Caused by Accident | ||||
| R Middle Frontal Gyrus | 34 | 42 | 24 | 5.08 |
| L Middle Frontal Gyrus | −34 | 26 | 36 | 2.38 |
| R Superior Frontal Gyrus | 26 | 40 | 34 | 3.41 |
| L Superior Frontal Gyrus | −12 | 46 | 28 | 4.49 |
| R Anterior Cingulate Cortex | 10 | 38 | 10 | 3.38 |
| L Anterior Cingulate Cortex | 0 | 18 | 28 | 3.34 |
| R Middle Cingulate Cortex | 2 | 20 | 32 | 3.61 |
| L Middle Cingulate Cortex | −4 | −6 | 50 | 3.86 |
3.4. Correlation analyses between hemodynamic response and traits related to psychopathy
Mean number of aggressive CD symptoms and ratings on the CADS daring dimension both correlated with amygdala, inferior frontal gyrus, right PAG and right middle cingulate cortex [12, 38, 32] activations but prosociality did not. Interestingly, adolescents’ sadism scores were highly correlated with activity in right amygdala [28, −12, −8], bilateral precuneus [0, −56, 46; 4, −60, 64], and left fusiform gyrus [−24, −52, −14] (P < 0.0001).
4. Discussion
The goal of this study was to explore differences in neural response to empathy-eliciting stimuli in adolescents with and without aggressive CD. As predicted, when the control participants observed painful situations accidentally caused, regions of the pain matrix were selectively activated, including the insula, aMCC, dorsal ACC, SMA, PAG and somatosensory cortex. This result fits well with previous functional neuroimaging studies on pain empathy (see Jackson, Rainville & Decety, 2006 for a review) that consistently showed that attending to the other people’ pain triggers an automatic somatic sensorimotor resonance mechanism between other and self, which activates almost the entire neural pain matrix in the observer including the periaqueductal gray (PAG) and the SMA.
Interestingly, the pattern of activation in the adolescents with CD showed both similarities as well as striking differences when observing these painful situations. In the CD group, hemodynamic signal increase was detected in the insula, aMCC, SMA, PAG, and somatosensory cortex (see Figure 1). In addition, strong activation was observed bilaterally in the amygdala, ventral striatum, and temporal poles. The dorsal portion of the TP projects to the hypothalamus, a neuromodulatory region important for autonomic regulation. Electrical stimulation of the TP produces changes in heart rate, respiration, and blood pressure (Gloor et al., 1982). These regions (i.e., amygdala, striatum and TP) were not activated in the control participants. This result suggests that individuals with CD actually react to the pain of others to a greater extent than youth without CD. Direct comparison of the two groups further indicates that participants with CD have a stronger signal response in the aMCC, striatum and left amygdala than the control participants when viewing painful situations that have been accidentally caused.
The finding that aggressive adolescents with CD exhibit greater response in the pain matrix when viewing accidental pain than controls is interesting given the finding of previous fMRI studies that reported reduced amygdala response in youth with CD during the viewing of pictures with negative emotional valence (Marsh et al., 2008; Sterzer et al., 2005), as well as reduced amygdala volume (Sterzer et al., 2007). The present findings suggest that youth with CD do not exhibit reduced amygdala response to all negatively valenced stimuli; indeed, they appear to exhibit enhanced response to images of people in pain, including a specific activation of the ventral striatum.
Our results suggest that there may be no deficit in neural response to distress of others (as reflected by the strong activation in the amygdala, temporal poles, and other structures in the pain matrix) in youth with CD. In fact, this somatic sensorimotor resonance was significantly greater (P < 0.005) in participants with CD than without CD. We also observed that the extent of amygdala activation to painful situations in subjects with CD was significantly related in a positive direction to their number of aggressive acts and ratings of daring and sadism score on the CADS (Lahey et al., in press).
The present findings generate at least two hypotheses for testing in future studies. First, it is important to note that the amygdala is involved in the processing of more than just negative affect. Numerous studies point to a role for the amygdala in positive affect, and its coupling with the ventral striatum enables a general arousing effect of reward (Murray, 2007). It is possible, therefore, that the robust hemodynamic response in the amygdala/ventral striatum to viewing others in pain in youth with aggressive CD reflects a positive affective response (e.g., “excitement”). That is, highly aggressive antisocial youth may enjoy hurting others and, coupled with diminished PFC/amygdala connectivity may not effectively regulate positively reinforced aggressive impulses. The finding that CADS ratings on the daring dimension (which reflects sensation seeking) and sadism items correlate with amygdala response is consistent with this hypothesis. The ventral striatum (nucleus accumbens) plays an important role in reward, pleasure, but also in fear. It is located at the head of the caudate nucleus and anterior portion of the putamen and receives major input (excitatory fibers) from the amygdala and the hippocampal formation. It can be viewed as a functional interface between the limbic and motor systems (Mogenson, Jones & Yim, 1980). In humans, the striatum is activated by stimuli associated with reward, but also aversive, novel or intense stimuli. A common property linking these stimuli is saliency (Groenewegen, 2007). Attending to the pain of others may lead to either approach or avoidance. The instrumental aspects of avoidance unlike the Pavlovian elicited responses require connections between the amygdala and the ventral striatum for their acquisition and/or expression. In particular, the nucleus accumbens of the ventral striatum may be a crucial area for the initiation and control of instrumental responses motivated by either appetitive or aversive responses, resulting from its innervation by dopaminergic pathways (LeDoux, 2002). It is thus difficult to decide whether the amygdala/ventral striatum response in CD participants is associated with enjoyment or repulsion when watching the pain of others. The fact that this condition was also associated with strong activation of the PAG may be a clue. This region of the midbrain receives input from the amygdala and its stimulation triggers aversive or defensive responses and anxiety. It is also worth noting that lesion to the ventral striatum is associated with selective impairment of anger processing, both in the recognition of signals of anger and the experience of this emotion (Calder et al., 2004).
Second, many studies indicate that individuals with CD have a lower threshold for sensitivity to negative affect than other youth (Lahey & Waldman, 2003). This is potentially important as their negative affect may increase the likelihood of aggression, especially in the absence of effective emotion regulation (Berkowitz, 1993, 2003). This interpretation fits well with the hypothesis of a dysfunction in the neural circuitry of emotion regulation (Davidson et al., 2000) and is consistent with our analyses of effective PFC/amygdala connectivity. Aggression may be related to affective instability and poor impulse control (Raine, 2002). Children with aggressive behavior problems have difficulties regulating negative emotions, which may result in harmful patterns of interpersonal behavior (Lewis, Granic & Lamm, 2006). Often triggered by hypersensitivity to specific stimuli, aggressive adults experience escalating agitation followed by an abrupt outburst of aggressive and threatening behavior (Gollan, Lee, & Coccaro, 2005). Failure to discriminate between pain to others and to oneself may further lead to personal distress. The fact that the anterior TP was specifically and highly activated in youth with CD provides support for the distress interpretation. It has been suggested that the TP is part of a system that modulates visceral functions in response to emotionally evocative stimuli based on its anatomical connectivity (Kondo et al., 2003). A number of neuroimaging studies have reported activation in the left anterior TP in response to negative visual and auditory stimuli, such as aversive sounds (Olson et al., 2007). Importantly, one fMRI study found that TP activation correlates with personal distress scores, a measure of how much one personally feels upset when viewing another’s negative emotions (Moriguchi et al., 2006).
The stimuli that depicted pain intentionally caused by another individual were associated, in the control group, with additional activation of temporoparietal junction, PCC and lateral OFC. The same pattern of activity was recently reported in a functional MRI study with typically developing children, and interpreted with relation to the perception of social interaction and intentionality (Decety, Michalska & Akitsuki, 2008). Functional neuroimaging studies have consistently supported the existence of a distributed neural network underlying the ability to understand other people as intentional and emotional agents (theory of mind mechanism). This network comprises the superior temporal sulcus, the TPJ, and the medial prefrontal/anterior paracingulate cortex (e.g., Ciaramidaro et al., 2007; Decety & Lamm, 2007). Specifically, research indicates that the anterior PCC is implicated in understanding the mental states of an agent involved in social interaction, regardless of whether this interaction is observed, taking place online or even imagined (e.g., Walter et al., 2004).
Different patterns of response were detected in the orbitofrontal cortex across the two groups. While the lateral OFC was selectively activated in the control participants when observing pain inflicted by another, activation of the medial OFC was found in the participants with CD. Direct comparison between the groups confirmed this finding. The OFC/MPFC has been specifically implicated in a variety of areas relevant to CD and aggression, including the regulation of negative affect (Phan et al., 2005). The finding that the response to these situations in the lateral OFC was attenuated in CD subjects relative to controls suggests an impairment in the CD group in the regulation of negative affect. This interpretation supports the findings of a recent study that observed an attenuated response in this region to angry faces in adults with intermittent explosive disorder (Coccaro et al., 2007).
The PPI analyses corroborated the functional MRI findings, demonstrating an amygdala/PFC coupling specifically while watching pain being intentionally caused by another individual and only for the control group. In particular, in healthy adolescents, left amygdala activity covaried with activity in the prefrontal cortex to a greater extent while watching situations of pain being intentionally caused compared to viewing of pain caused by accident. Adolescents with CD showed no functional connectivity between frontal regions and amygdala, which is in line with a recent study that demonstrated greater functional connectivity between the amygdala and PFC in comparison subjects than a group of aggressive youth (Marsh et al., 2008).
We posit that the condition in which another individual is inflicting pain intentionally elicits in the normal controls a certain degree of regulation/inhibition. Such regulatory process depends upon posterior STS and medial prefrontal cortex (Harenski & Hamann, 2006). A meta-analysis shows that frontal regions become active when subjects engage in cognitive strategies (such as reappraisal or detachment) to modulate negative affect (Ochsner & Gross, 2005). This is consistent with the role of the prefrontal cortex in cognitive inhibition and executive function, processes that are important for the regulation of affect (Fuster, 2001).
Overall, our results suggest a complex relation between the neural correlates of empathy and CD. The functional MRI data seem to indicate that adolescents with CD are at least as responsive to the pain of others than the adolescents without CD. The fact that activation of the posterior insula, somatosensory cortex, and PAG are involved in the observation of others in painful situations such an interpretation. However, unlike the adolescents without CD, and the group of typically developing children in our first preliminary study (Decety, Michalska & Akitsuki, 2008), there was no activation in adolescents with CD in the neural regions that contribute to self-regulation and metacognition (including moral reasoning), such as the DLPFC, PCC, TPJ, dorsal and medial ACC and OFC.
Research with non-humans demonstrates that physical pain often elicits aggression (Berkowitz, 2003). It has been hypothesized that aggressive persons are disposed to experience negative affect (Anderson & Bushman, 2002; Lahey & Waldman, 2003). This suggests that, in certain situations, empathic mimicry might produce high levels of distress in youth predisposed to be aggressive that, ironically, increases their aggression. It is possible that strong activation of neural circuits that underpin actual pain processing is associated with negative affect in youth with CD. This, in conjunction with reduced activation in areas associated with emotion regulation, could result in a dysregulated negative affective state, which may instigate aggression under some circumstances (Berkowitz, 1983). For example, youth with CD who see an injured friend (or fellow member of a gang) may be more likely to respond aggressively than other youth for this reason.
Finally, the strong and specific activation of the amygdala and ventral striatum in the aggressive adolescents with CD during the perception of pain in others is an important and intriguing finding, which necessitates additional research in order to understand its role in aggression and empathic dysfunction.
4.1. Conclusion
This study is to our knowledge the first functional neuroimaging investigation of brain response to pain empathy-eliciting stimuli in aggressive adolescents with CD. In the future it will be important, given the limited size of our sample, to examine whether these findings replicate with larger samples. We believe that such investigations are critical to move beyond self-report measures of empathy. They will also provide a better understanding of the computational and neural mechanism underpinning empathy as well as their dysfunction in high-risk juvenile populations.
Acknowledgments
The study was supported by NSF (BCS-0718480), a seed grant to Jean Decety from the University of Chicago Center for Integrative Neuroscience and Neuroengineering Research, and grant R01 MH053554 to Benjamin Lahey.
References
- Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) Journal of Comparative Neurology. 1984;230:465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nature Neuroscience. 1999;2:1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Anderson CA, Bushman BJ. Human aggression. Annual Review of Psychology. 2002;53:27–51. doi: 10.1146/annurev.psych.53.100901.135231. [DOI] [PubMed] [Google Scholar]
- Batson CD. Empathic joy and the empathy altruism hypothesis. Journal of Personality and Social Psychology. 1991;61:413–426. doi: 10.1037//0022-3514.61.3.413. [DOI] [PubMed] [Google Scholar]
- Batson CD, Fultz J, Schoenrade PA. Distress and empathy: two quantitatively distinct vicarious emotions with different motivational consequences. Journal of Personality. 1987;55:19–39. doi: 10.1111/j.1467-6494.1987.tb00426.x. [DOI] [PubMed] [Google Scholar]
- Berkowitz L. Aversively stimulated aggression: some parallels and differences in research with animals and humans. American Psychologist. 1983;38:1135–1144. doi: 10.1037//0003-066x.38.11.1135. [DOI] [PubMed] [Google Scholar]
- Berkowitz L. Pain and aggression: some findings and implications. Motivation and Emotion. 1993;17:277–293. [Google Scholar]
- Berkowitz L. Affect, aggression, and antisocial behavior. In: Davidson RJ, Scherer KR, Goldsmith HH, editors. Handbook of Affective Sciences. New York: Oxford University Press; 2003. pp. 804–823. [Google Scholar]
- Blair RJR. Responding to the emotions of others: Dissociating forms of empathy through the study of typical and psychiatric populations. Consciousness and Cognition. 2005;14:698–718. doi: 10.1016/j.concog.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Jha AP, Bylsma LM, Fabian SA, Solomon PE, Prkachin KM. Viewing facial expressions of pain engages cortical areas involved in the direct experience of pain. NeuroImage. 2005;25:312–319. doi: 10.1016/j.neuroimage.2004.11.043. [DOI] [PubMed] [Google Scholar]
- Bufalari I, Aprile T, Avenanti A, Di Russo F, Aglioti SM. Empathy for pain and touch in the human somatosensory cortex. Cerebral Cortex. 2007;17:2553–2561. doi: 10.1093/cercor/bhl161. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Keane J, Lawrence AD, Manes F. Impaired recognition of anger following damage of the ventral striatum. Brain. 2004;127:1958–1969. doi: 10.1093/brain/awh214. [DOI] [PubMed] [Google Scholar]
- Campbell SB. Behavior problems in preschool children: Clinical and developmental issues. New York: Guilford Press; 1990. [Google Scholar]
- Cheng Y, Lin C, Liu HL, Hsu Y, Lim K, Hung D, Decety J. Expertise modulates the perception of pain in others. Current Biology. 2007a;17:1708–1713. doi: 10.1016/j.cub.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Ciaramidaro A, Adenzato M, Enrici I, Erk S, Pia L, Bara BG, Walter H. The intentional network: How the brain reads varieties of intentions. Neuropsychologia. 2007;45:3105–3113. doi: 10.1016/j.neuropsychologia.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Coccaro E, McCloskey M, Fitzgerald D, Phan K. Amygdala and Orbitofrontal Reactivity to Social Threat in Individuals with Impulsive Aggression. Biological Psychiatry. 2007;62:168–178. doi: 10.1016/j.biopsych.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation-a possible prelude to violence. Science. 2000;289:591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- Decety J, Michalska KJ, Akitsuki Y. Who caused the pain? A functional MRI investigation of empathy and intentionality in children. Neuropsychologia. 2008;46:2607–2614. doi: 10.1016/j.neuropsychologia.2008.05.026. [DOI] [PubMed] [Google Scholar]
- Decety J, Batson CD. Social neuroscience approaches to interpersonal sensitivity. Social Neuroscience. 2007;2(3–4):151–157. doi: 10.1080/17470910701506060. [DOI] [PubMed] [Google Scholar]
- Decety J, Jackson PL. The functional architecture of human empathy. Behavioral and Cognitive Neuroscience Reviews. 2004;3:71–100. doi: 10.1177/1534582304267187. [DOI] [PubMed] [Google Scholar]
- Decety J, Lamm C. Empathy versus personal distress - Recent evidence from social neuroscience. In: Decety J, Ickes W, editors. The Social Neuroscience of Empathy. Cambridge: MIT press; 2008. in press. [Google Scholar]
- Decety J, Lamm C. The role of the right temporoparietal junction in social interaction: How low-level computational processes contribute to meta-cognition. The Neuroscientist. 2007;13:580–593. doi: 10.1177/1073858407304654. [DOI] [PubMed] [Google Scholar]
- Decety J, Moriguchi Y. The empathic brain and its dysfunction in psychiatric populations: implications for intervention across different clinical conditions. BioPsychoSocial Medicine. 2007;1:22–65. doi: 10.1186/1751-0759-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Sommerville JA. Shared representations between self and others: A social cognitive neuroscience view. Trends in Cognitive Sciences. 2003;7:527–533. doi: 10.1016/j.tics.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Derbyshire SWG. Exploring the pain “neuromatrix”. Current Review of Pain. 2000;4:467–477. doi: 10.1007/s11916-000-0071-x. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Miller PA, Schaller M, Fabes RA, Fultz J, Shell R. The role of sympathy and altruistic personality traits in helping: A reexamination. Journal of Personality. 1989;57:41–67. doi: 10.1111/j.1467-6494.1989.tb00760.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg N. Age changes in prosocial responding and moral reasoning in adolescence and early adulthood. Journal of Research on Adolescence. 2005;15:235–260. doi: 10.1111/j.1532-7795.2005.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Eggum ND. Empathic responding: sympathy and personal distress. In: Decety J, Ickes W, editors. The Social Neuroscience of Empathy. Cambridge: MIT press; 2008. in press. [Google Scholar]
- Eisenberg N, Spinrad TL, Sadovsky A. Empathy-related responding in children. In: Killen M, Smetana J, editors. Handbook of Moral Development. Mahwah, NJ: Lawrence Erlbaum Associates; 2006. pp. 517–549. [Google Scholar]
- Estabrook S. Master of Art Thesis under direction of Dr. Jean Decety. University of Chicago; 2007. Does context modulate empathy for pain? [Google Scholar]
- Farrington DP, West DJ. Criminal, penal and life histories of chronic offenders: Risk and protective factors and early identification. Criminal Behaviour and Mental Health. 1993;3:492–523. [Google Scholar]
- Frick PJ, Stickle TR, Dandreaux DM, Farrell JM, Kimonis ER. Callous-unemotional traits in predicting the severity and stability of conduct problems and delinquency. Journal of Abnormal Child Psychology. 2005;33:471–487. doi: 10.1007/s10648-005-5728-9. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interaction in neuroimaging. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex - An update: review time is of the essence. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Gill KL, Calkins SD. Do aggressive/destructive toddlers lack concern for others? Behavioral and physiological indicators of empathic responding in 2-year-old children. Development and Psychopathology. 2003;15:55–71. doi: 10.1017/s095457940300004x. [DOI] [PubMed] [Google Scholar]
- Glover GH, Lai TF. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magnetic Resonance Medicine. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Gloor P, Olivier A, Quesney LF, Andermann F, Horowitz S. The role of the limbic system in experiential phenomena of temporal lobe epilepsy. Annals of Neurology. 1982;12:129–144. doi: 10.1002/ana.410120203. [DOI] [PubMed] [Google Scholar]
- Gollan JK, Lee R, Coccaro EF. Developmental psychopathology and neurobiology of aggression. Development and Psychopathology. 2005;17:1151–1171. doi: 10.1017/s0954579405050546. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ. The ventral striatum as an interface between the limbic and motor systems. CNS Spectrums. 2007;12:887–889. doi: 10.1017/s1092852900015650. [DOI] [PubMed] [Google Scholar]
- Gu X, Han S. Attention and reality constraints on the neural processes of empathy for pain. NeuroImage. 2007;36:256–267. doi: 10.1016/j.neuroimage.2007.02.025. [DOI] [PubMed] [Google Scholar]
- Harenski CL, Hamann S. Neural correlates of regulating negative emotions related to moral violations. NeuroImage. 2006;30:313–324. doi: 10.1016/j.neuroimage.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Sass H. Emotional deficiency and psychopathy. Behavioral Science and Law. 2000;18:317–323. doi: 10.1002/1099-0798(200010)18:5<567::aid-bsl410>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Hoffman ML. Empathy and Moral Development. New York: Cambridge University Press; 2000. [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Comparison of the effects of bilateral orbitofrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. Journal of Neuroscience. 2005;25:8534–8542. doi: 10.1523/JNEUROSCI.1232-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson PL, Brunet E, Meltzoff AN, Decety J. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain: An event-related fMRI study. Neuropsychologia. 2006;44:752–61. doi: 10.1016/j.neuropsychologia.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others: a window into the neural processes involved in empathy. NeuroImage. 2005;24:771–779. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Rainville P, Decety J. To what extent do we share the pain of others? Insight from the neural bases of pain empathy. Pain. 2006;125:5–9. doi: 10.1016/j.pain.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Jenck F, Moreau JL, Martin JR. Dorsal periaqueductal gray-induced aversion as a simulation of panic anxiety: Elements of face and predictive validity. Psychiatry Research. 1995;57:181–191. doi: 10.1016/0165-1781(95)02673-k. [DOI] [PubMed] [Google Scholar]
- Kondo H, Saleem KS, Price JL. Differential connections of the temporal pole with the orbital and medial prefrontal networks in macaque monkeys. Journal of Comparative Neurology. 2003;465:499–523. doi: 10.1002/cne.10842. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Applegate B, Barkley RA, Garfinkel B, McBurnett K, Kerdyk L, Greenhill L, Hynd GW, Frick PJ, Newcorn J, Biederman J, Ollendick T, Hart EL, Perez D, Waldman I, Shaffer D. DSM-IV field trials for oppositional defiant disorder and conduct disorder in children and adolescents. American Journal of Psychiatry. 1994;151:1163–1171. doi: 10.1176/ajp.151.8.1163. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Waldman ID. A developmental propensity model of the origins of conduct problems during childhood and adolescence. In: Lahey BB, Moffitt TE, Caspi A, editors. Causes of conduct disorder and juvenile delinquency. New York: Guilford Press; 2003. pp. 76–117. [Google Scholar]
- Lahey BB, Loeber R, Burke JD, Applegate B. Predicting future antisocial personality disorder in males from a clinical assessment in childhood. Journal of Consulting and Clinical Psychology. 2005;73:389–399. doi: 10.1037/0022-006X.73.3.389. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Applegate B, Chronis AM, Jones HA, Williams SH, Loney J, Waldman ID. Psychometric characteristics of a measure of emotional dispositions developed to test a developmental propensity model of conduct disorder. Journal of Clinical Child and Adolescent Psychology. doi: 10.1080/15374410802359635. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Batson CD, Decety J. The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. Journal of Cognitive Neuroscience. 2007a;19:42–58. doi: 10.1162/jocn.2007.19.1.42. [DOI] [PubMed] [Google Scholar]
- Lamm C, Nusbaum H, Meltzoff AN, Decety J. What are you feeling? Using functional magnetic resonance imaging to assess the modulation of sensory and affective responses during empathy for pain. PLoS ONE. 2007b;12:e1292. doi: 10.1371/journal.pone.0001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion: Clue from the brain. In: Cacioppo JT, et al., editors. Foundations in Social Neuroscience. Cambridge: MIT press; 2002. pp. 389–410. [Google Scholar]
- Lewis MD, Granic I, Lamm C. Behavioral differences in aggressive children linked with neural mechanisms of emotion regulation. Annals of the New York Academy of Science. 2006;1094:164–177. doi: 10.1196/annals.1376.017. [DOI] [PubMed] [Google Scholar]
- Loeber R, Farrington DP, Stouthamer-Loeber M, Van Kammen WB. Antisocial behavior and mental health problems: Explanatory factors in childhood and adolescence. Mahwah, NJ: Lawrence Erlbaum Associates; 1998. [Google Scholar]
- Lovett BJ, Sheffield RA. Affective empathy deficits in aggressive children and adolescents: A critical review. Clinical Psychology Review. 2007;27:1–13. doi: 10.1016/j.cpr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DGV, Reid ME, Sims C, Kosson D, Towbin KE, Leibenluft E, Pine DS, Blair RJR. Reduced amygdala response to fearful expressions in children and adolescents with callous unemotional traits and disruptive behavior disorders. American Journal of Psychiatry. 2008 doi: 10.1176/appi.ajp.2007.07071145. Epub ahead of time. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Kim CY. From motivation to action: functional interface between the limbic system and the motor system. Progress in Neurobiology. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliviera-Souza R, Eslinger P. Morals and the human brain. NeuroReport. 2003;14:299–305. doi: 10.1097/00001756-200303030-00001. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliviera-Souza R, Garrido GJ, Bramati IE, Caparelli-Daquer EMA, Paiva ML, Zahn R, Grafman J. The self as a moral agent: Linking the neural bases of social agency and moral sensitivity. Social Neuroscience. 2007;2(3–4):336–352. doi: 10.1080/17470910701392024. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y, Decety J, Ohnishi T, Maeda M, Matsuda H, Komaki G. Empathy and judging other’s pain: An fMRI study of alexithymia. Cerebral Cortex. 2007;17:2223–2234. doi: 10.1093/cercor/bhl130. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y, Ohnishi T, Lane RD, Maeda M, Mori T, Nemoto K, Matsuda H, Komaki G. Impaired self-awareness and theory of mind: an fMRI study of mentalizing in alexithymia. NeuroImage. 2006;32:1472–1482. doi: 10.1016/j.neuroimage.2006.04.186. [DOI] [PubMed] [Google Scholar]
- Morrison I, Lloyd D, di Pellegrino G, Roberts N. Vicarious responses to pain in anterior cingulate cortex: is empathy a multisensory issue? Cognitive and Affective Behavioral Neuroscience. 2004;4:270–278. doi: 10.3758/cabn.4.2.270. [DOI] [PubMed] [Google Scholar]
- Morrison I, Poliakoff E, Gordon L, Downing PE. Response specific effects of pain observation on motor behavior. Cognition. 2006;104:407–416. doi: 10.1016/j.cognition.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Murray EA. The amygdala, reward and emotion. Trends in Cognitive Sciences. 2007;11:489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Gnagy EM, Greenslade KE, Milich R. Teacher ratings of DSM-III-R symptoms for the disruptive behavior disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31:210–218. doi: 10.1097/00004583-199203000-00006. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biological Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biological Psychiatry. 2003;54:504–14. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Piacentini JC, Cohen P, Cohen J. Combining discrepant diagnostic information from multiple sources: Are complex algorithms better than simple ones? Journal of Abnormal Child Psychology. 1992;20:51–63. doi: 10.1007/BF00927116. [DOI] [PubMed] [Google Scholar]
- Preston AR, Thomason ME, Ochsner KN, Cooper JC, Glover GH. Comparison of spiral-in/out and spiral-out BOLD fMRI at 1.5 T and 3 T. NeuroImage. 2004;21:291–301. doi: 10.1016/j.neuroimage.2003.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- Raine A. Biosocial Studies of Antisocial and Violent Behavior in Children and Adults: A Review. Journal of Abnormal Child Psychology. 2002;30:311–326. doi: 10.1023/a:1015754122318. [DOI] [PubMed] [Google Scholar]
- Raine A, Venables P, Mednick S. Low resting heart rate at age three years predisposes to aggression at age 11 years: Evidence from the Mauritius Child Health Project. Journal of the Academy of Child and Adolescent Psychiatry. 1997;36:1457–1464. doi: 10.1097/00004583-199710000-00029. [DOI] [PubMed] [Google Scholar]
- Russo MF, Stokes GS, Lahey BB, Christ MAG, McBurnett K, Loeber R. A sensation seeking scale for children: Further refinement and psychometric development. Journal of Psychopathology and Behavioral Assessment. 1993;15:69–86. [Google Scholar]
- Saarela MV, Hlushchuk Y, Williams AC, Schurmann M, Kalso E, Hari R. The compassionate brain: humans detect pain intensity from another’s face. Cerebral Cortex. 2007;17:230–237. doi: 10.1093/cercor/bhj141. [DOI] [PubMed] [Google Scholar]
- Saddoris MP, Gallagher M, Schoenbaum G. Rapid associative encoding in basolateral amygdala depends on connections with orbitofrontal cortex. Neuron. 2005;46:321–331. doi: 10.1016/j.neuron.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Piacentini J, Schwab-Stone M, Wicks J. Diagnostic Interview Schedule for Children. New York: Columbia University; 1993. [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not the sensory components of pain. Science. 2004;303:1157–1161. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Stadler C, Krebs A, Kleinschmidt A, Poutska F. Abnormal neural responses to emotional visual stimuli in adolescents with conduct disorder. Biological Psychiatry. 2005;57:7–15. doi: 10.1016/j.biopsych.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Stadler C, Poustka F, Kleinschmidt A. A structural neural deficit in adolescents with conduct disorder and its association with lack of empathy. NeuroImage. 2007;37:335–342. doi: 10.1016/j.neuroimage.2007.04.043. [DOI] [PubMed] [Google Scholar]
- van Elst LT, Woermann FG, Lemieux L, Thompson PJ, Trimble MR. Affective aggression in patients with temporal lobe epilepsy: a quantitative MRI study of the amygdala. Brain. 2000;123:234–243. doi: 10.1093/brain/123.2.234. [DOI] [PubMed] [Google Scholar]
- Van Goozen SHM, Fairchild G. How can the study of biological processes help design new interventions for children with severe antisocial behavior? Development and Psychopathology. 2008;20:941–973. doi: 10.1017/S095457940800045X. [DOI] [PubMed] [Google Scholar]
- Walter H, Adenzato M, Ciaramidaro A, Enrici I, Bara BG. Understanding intentions in social interaction: the role of the anterior paracingulate cortex. Journal of Cognitive Neuroscience. 2004;16:1854–1863. doi: 10.1162/0898929042947838. [DOI] [PubMed] [Google Scholar]
- Zahn-Waxler C, Cole PM, Welsh JD, Fox NA. Psychophysiological correlates of empathy and prosocial behaviors in preschool children with behavior problems. Development and Psychopathology. 1995;7:27–48. [Google Scholar]


