Cardiac fibrosis is characterized by net accumulation of extracellular matrix in the myocardium and is an integral component of most cardiac pathologic conditions. Fibrotic remodeling of the ventricle has profound consequences on cardiac function. Increased deposition of interstitial collagen in the perimysial space is initially associated with a stiffer ventricle and diastolic dysfunction. At a later stage, accumulation of extracellular matrix proteins in the cardiac interstitium activates proteolytic pathways leading to the development of ventricular dilation and systolic failure. Disturbance of the matrix network in the fibrotic heart may cause systolic dysfunction through several distinct mechanisms. First, loss of fibrillar collagen may impair transduction of cardiomyocyte contraction into myocardial force development resulting in uncoordinated contraction of cardiomyocyte bundles. Second, disruption of key interactions between endomysial matrix proteins (such as laminin and collagen) and their receptors in cardiomyocytes may promote cardiomyocyte death1. Finally, fibrosis may result in sliding displacement (slippage) of cardiomyocytes leading to a decrease in the number of muscular layers in the ventricular wall and subsequent left ventricular dilation. Beyond its effects on cardiac function, fibrotic ventricular remodeling also promotes arrhythmogenesis through impaired anisotropic conduction and subsequent generation of reentry circuits.
Extensive evidence suggests that hemodynamic overload activates the rennin-angiotensin system (RAS) triggering potent fibrogenic signals that stimulate cardiac fibroblasts and enhance collagen deposition in the myocardium. Angiotensin II, the central effector molecule of the RAS, stimulates fibroblast proliferation and promotes matrix protein synthesis through interactions involving the AT1 receptor. Angiotensin II-induced fibrosis appears to be mediated, at least in part, through activation of Transforming Growth Factor (TGF)-β signaling pathways2. Angiotensin II upregulates TGF-β synthesis by cardiac fibroblasts and induces expression of the matricellular protein Thrombospondin-1 (TSP)-1, a crucial activator of latent TGF-β. Increased levels of bioactive TGF-β in the cardiac interstitium modulate fibroblast phenotype promoting collagen synthesis and enhancing matrix preservation through upregulation of tissue inhibitors of metalloproteinases (TIMP). Active TGF-β binds to the constitutively active type II receptor (TβRII) at the cell surface. The complex subsequently interacts with, and transphosphorylates the cytoplasmic domain of the type I receptor (TβRI), Phosphorylation of the TβRI propagates downstream intracellular signals, through the Smad proteins, essential components of the TGF-β signaling pathway. Activation of the Smad2/3 pathway is crucial for TGF-β-mediated synthesis of matrix proteins and TIMPs by cardiac fibroblasts3.
It is becoming increasingly appreciated that regulation of angiotensin II/TGF-β signaling involves interactions with extracellular matrix proteins and proteoglycans. Highlighting the complexity of the molecular circuitry involved in transducing angiotensin II-mediated fibrogenic actions, Schellings and co-workers identified the heparan sulfate proteoglycan (HSPG) syndecan-1 as an essential mediator in angiotensin II-induced cardiac fibrosis4. Angiotensin II treatment resulted in marked upregulation of syndecan-1 in the mouse heart, predominantly localized in fibrotic areas. Angiotensin II-induced fibrosis and dysfunction was attenuated in syndecan-1 null hearts; the protective effects of syndecan-1 loss were associated with blunted expression of the TGF-β-inducible gene Connective Tissue Growth Factor (CTGF). Absence of syndecan-1 reduced matrix protein synthesis in angiotensin II-stimulated cardiac fibroblasts; these in vitro effects of syndecan-1 loss were associated with decreased activation of the Smad2 pathway. On the other hand, adenoviral overexpression of syndecan-1 accentuated TGF-β1- and angiotensin II-mediated Smad2 phosphorylation and enhanced CTGF induction, suggesting that syndecan-1 augments responses to fibrogenic mediators. In contrast, fibroblasts treated with recombinant syndecan-1 ectodomain without heparin sulfate groups had no effect on Smad2 phosphorylation and CTGF expression indicating that syndecan-1 heparan sulfates are involved in fibrogenic signal transduction. The findings provide the first demonstration of a role for syndecan-1 in the pathogenesis of cardiac fibrosis. In addition, the study contributes new insights into the significance of proteoglycan-mediated interactions in fibrotic tissue remodeling.
A growing body of evidence suggests that, beyond their structural role, proteoglycans are directly involved in regulating cell:cell and cell:matrix interactions. The syndecan family of heparan sulfate proteoglycans is comprised of four members (syndecan-1, -2, -3 and -4), each consisting of an extracellular domain with covalently attached heparan sulfate or chondroitin sulfate chains, a transmembrane domain, and a short cytoplasmic tail5. Syndecans act as co-receptors for growth factor binding and are involved in regulation of integrin-mediated cell adhesion, proliferation and angiogenesis. Although studies using genetically targeted mice suggested that other matrix HSPGs may compensate for the absence of syndecans during development and in tissue homeostasis, experiments using models of injury revealed essential roles for the members of the syndecan family in reparative and fibrotic processes.
Extensive evidence suggests that syndecan-1 is induced in injured tissues and may regulate inflammatory and reparative responses. The biology of syndecan-1 in tissue injury is complex and context-dependent. Extracellular domain shedding, due to activation of proteases in the injured area, is believed to play a key role in regulating syndecan-1-mediated interactions5. Shed ectodomains may compete with intact syndecan for extracellular ligands and may regulate growth factor bioavailability during wound repair6. In addition, studies in models of lung injury demonstrated that shed and exogenous syndecan-1 ectodomains induce neutrophil chemotaxis7 and that shed syndecan-1 binds to and regulates chemokine activity contributing to the development of a transepithelial chemotactic gradient8.
The role of syndecan-1 in cardiac pathobiology appears to be dependent on the context and on the mechanism of injury. Experiments using syndecan-1 null mice showed that following myocardial infarction, endogenous syndecan-1 is an essential protective mechanism that prevents uncontrolled inflammation and reduces adverse cardiac remodeling and dysfunction9. These surprising anti-inflammatory effects of syndecan-1 were attributed to its presumed role as a “barrier” against infiltrating inflammatory leukocytes. This concept was supported by the observation that syndecan-1 null neutrophils exhibited increased adhesion to endothelial cells and enhanced transendothelial migration. In contrast, in the angiotensin II-treated heart, syndecan-1 promotes fibrous tissue deposition and cardiac dysfunction; these effects may be mediated in part through accentuation of angiotensin II/TGF-β signaling (Figure). On the basis of these observations, it is tempting to hypothesize that the disparate functional consequences of syndecan-1 loss in these two models of cardiac injury may be mediated through regulation of TGF-β signaling. In the infarcted heart, syndecan-1-mediated TGF-β activation may suppress the intense inflammatory reaction triggered by the death of a large number of cardiomyocytes. On the other hand, in angiotensin II-treated hearts where inflammatory cell recruitment is less prominent, syndecan-1-induced accentuation of the profibrotic actions of the TGF-β axis may enhance cardiac fibrosis. However, considering the diverse effects of syndecan-1 on chemokine and growth factor signaling, and the potential consequences of ectodomain shedding, syndecan-1-mediated interactions following cardiac injury may affect a wide range of molecular pathways.
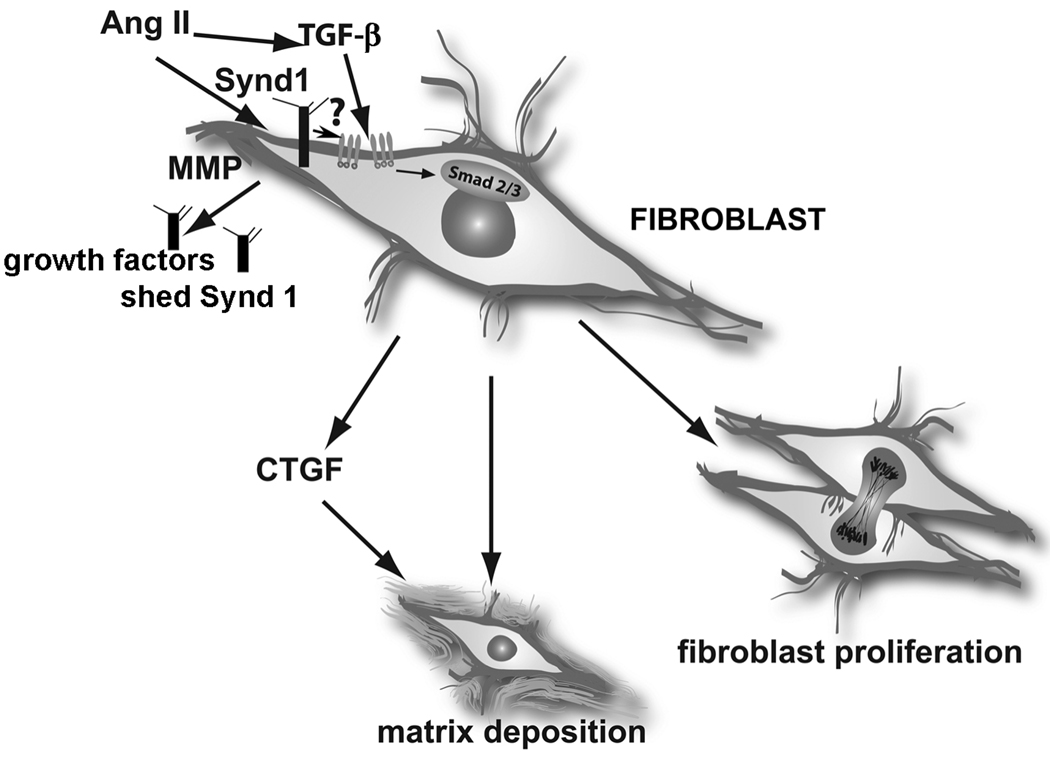
Figure.
The role of Syndecan-1 (Synd1) in cardiac fibrosis. Synd1 may act by presenting growth factors to their receptors, may increase TGF-β receptor levels, may promote TGF-β activation, or may activate downstream TGF-β signaling pathways. Understanding the role of syndecan-1 in cardiac injury is complicated by the possible presence of shed ectodomains (released due to increased Matrix Metalloproteinase [MMP] activity), that may sequester growth factors, or promote chemotactic gradients (Ang II, angiotensin II).
The intriguing effects of syndecan-1 on TGF-β and angiotensin II-mediated activation of fibroblasts raise an important question. What is the molecular basis for the observed effects of syndecan-1 on angiotensin II/TGF-β signaling? Although the mechanisms for these effects have not been explored, several possibilities should be considered (Figure). First, syndecan-1 may regulate TGF-β-induced matrix synthesis by modulating expression of TGF-β receptors; such effects have been demonstrated for syndecan-210. Second, syndecan-1 may regulate availability and activity of TGF-β in vivo. Third, interactions between syndecan-1 and the TGF-β receptors may result in direct modulation of TGF-β/Smad2/3 signaling. Because syndecans appear to be critically involved in regulating fundamental pathways in tissue injury, repair, and fibrosis, dissection of their role in the injured heart will undoubtedly provide new insight into the mechanistic basis of heart disease.
Acknowledgments
SOURCES OF FUNDING:
Dr Frangogiannis’ laboratory is funded by NIH grants R01 HL-76246 and R01 HL-85440, the Alkek endowment and the Medallion foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES: None
REFERENCES
- 1.Wang J, Hoshijima M, Lam J, Zhou Z, Jokiel A, Dalton ND, Hultenby K, Ruiz-Lozano P, Ross J, Jr, Tryggvason K, Chien KR. Cardiomyopathy associated with microcirculation dysfunction in laminin alpha4 chain-deficient mice. J Biol Chem. 2006;281:213–220. doi: 10.1074/jbc.M505061200. [DOI] [PubMed] [Google Scholar]
- 2.Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007;74:184–195. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bujak M, Ren G, Kweon HJ, Dobaczewski M, Reddy A, Taffet G, Wang XF, Frangogiannis NG. Essential Role of Smad3 in Infarct Healing and in the Pathogenesis of Cardiac Remodeling. Circulation. 2007;116:2127–2138. doi: 10.1161/CIRCULATIONAHA.107.704197. [DOI] [PubMed] [Google Scholar]
- 4.Schellings M, Vanhoutte D, Van Almen GC, Swinnen M, Leenders J, Kubben N, Van Leeuwen RE, Hofstra L, Heymans S, Pinto Y. Syndecan-1 amplifies Angiotensin II-induced cardiac fibrosis. Hypertension. 2009 doi: 10.1161/HYPERTENSIONAHA.109.137885. in press. [DOI] [PubMed] [Google Scholar]
- 5.Fears CY, Woods A. The role of syndecans in disease and wound healing. Matrix Biol. 2006;25:443–456. doi: 10.1016/j.matbio.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Alexopoulou AN, Multhaupt HA, Couchman JR. Syndecans in wound healing, inflammation and vascular biology. Int J Biochem Cell Biol. 2007;39:505–528. doi: 10.1016/j.biocel.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 7.Kliment CR, Englert JM, Gochuico BR, Yu G, Kaminski N, Rosas I, Oury TD. Oxidative stress alters syndecan-1 distribution in lungs with pulmonary fibrosis. J Biol Chem. 2009;284:3537–3545. doi: 10.1074/jbc.M807001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111:635–646. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- 9.Vanhoutte D, Schellings MW, Gotte M, Swinnen M, Herias V, Wild MK, Vestweber D, Chorianopoulos E, Cortes V, Rigotti A, Stepp MA, Van de Werf F, Carmeliet P, Pinto YM, Heymans S. Increased expression of syndecan-1 protects against cardiac dilatation and dysfunction after myocardial infarction. Circulation. 2007;115:475–482. doi: 10.1161/CIRCULATIONAHA.106.644609. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Klass C, Woods A. Syndecan-2 regulates transforming growth factor-beta signaling. J Biol Chem. 2004;279:15715–15718. doi: 10.1074/jbc.C300430200. [DOI] [PubMed] [Google Scholar]