Abstract
Background
Heart failure with preserved ejection fraction is one consequence of hypertension and caused by impaired cardiac diastolic relaxation. Nitric oxide (NO) is a known modulator of cardiac relaxation. Hypertension can lead to a reduction in vascular NO, in part because nitric oxide synthase (NOS) becomes uncoupled when oxidative depletion of its co-factor tetrahydrobiopterin (BH4) occurs.Similar events may occur in the heart leading to uncoupled NOS and diastolic dysfunction.
Methods and Results
In a hypertensive mouse model, diastolic dysfunction was accompanied by cardiac oxidation, a reduction in cardiac BH4, and uncoupled NOS. Compared to sham-operated animals, male mice with unilateral nephrectomy, with subcutaneous implantation of a controlled release deoxycorticosterone acetate (DOCA) pellet, and given 1% saline to drink were mildly hypertensive and had diastolic dysfunction in the absence of systolic dysfunction or cardiac hypertrophy. The hypertensive mouse hearts showed increased oxidized biopterins, NOS-dependent superoxide production, reduced NO production, and phosphorylated phospholamban. Feeding hypertensive mice BH4 (5 mg/day), but not treating with hydralazine or tetrahydroneopterin, improved cardiac BH4 stores, phosphorylated phospholamban levels, and diastolic dysfunction. Isolated cardiomyocyte experiments revealed impaired relaxation that was normalized with acute BH4 treatment. Targeted cardiac overexpression of angiotensin converting enzyme also resulted in cardiac oxidation, NOS uncoupling, and diastolic dysfunction in the absence of hypertension.
Conclusions
Cardiac oxidation, independent of vascular changes, can lead to uncoupled cardiac NOS and diastolic dysfunction. BH4 may represent a possible treatment for diastolic dysfunction.
Keywords: diastole, heart failure, nitric oxide synthase
Introduction
Heart failure with preserved ejection fraction as a result of diastolic dysfunction accounts for significant mortality and healthcare expenditures, especially amongst hypertensive individuals.1,2 The incidence of this type of heart failure is increasing1.
There are no specific treatments for diastolic dysfunction, partly because of a relative lack of the mechanistic understanding of this disorder3. There is a strong epidemiological association between hypertension and diastolic dysfunction.2,4,5 In the vasculature, hypertension and activation of the renin-angiotensin system (RAS) leads to reduced vascular nitric oxide (NO), in part because nitric oxide synthase (NOS) becomes uncoupled when oxidative depletion of its co-factor tetrahydrobiopterin (BH4) occurs, leading to production of superoxide (O2•−) instead of NO.6 NO and NOS have been identified recently as having a role in the modulation of cardiac relaxation.7 Therefore, we hypothesized that hypertension and or activation of the RAS in the absence of an increase in blood pressure may lead to NOS uncoupling in the heart and to diastolic dysfunction.
Methods
All experiments were approved by the appropriate Institutional Animal Care and Use Committees.
Hypertensive mouse model
We used a mouse model of hypertension to mimic the most common human risk factor for diastolic dysfunction (see Supplement).2,4,5,8,8 Previously, we have shown that this model leads to vascular oxidative stress, BH4 depletion, and NOS uncoupling6. A subset of hypertensive mice were randomly selected on post-operative day one to receive 5 mg/day of either BH4 (AXXORA, San Diego, CA) or tetrahydroneopterin (H4N), an enzymatically inactive analog of BH4 with equivalent antioxidant properties (Schricks Laboratories, Jona, Switzerland).9,10 A cohort of hypertensive DOCA-salt mice was randomized on post-operative day 11, a time when all DOCA-salt mice have evidence of diastolic dysfunction, to either 12–14 d of BH4 feeding as described above or treatment with hydralazine (25–30 mg/kg/d in 1% saline; Sigma-Aldrich, St. Louis, MO).
Echocardiography was performed on a subset of mice 24–48 h prior to terminal procedures. These were done on postoperative day 14 for hypertensive DOCA-salt, BH4 prevention, H4N, and control mice and on postoperative days 22–24 for mice in BH4 or hydralazine treatment groups (see Supplement).
Angiotensin converting enzyme (ACE) 1/8 mouse model
Male compound heterozygous ACE 1/8 mice and age matched littermate controls were used to test if cardiac oxidation could lead to diastolic dysfunction (see Supplement).11 These mice have increased cardiac ACE, minimal extra-cardiac ACE expression, and preserved ejection fraction.11
Noninvasive assessment of diastolic dysfunction
Mice were studied by pulsed-wave tissue Doppler from the apical four-chamber view.12 Results correlated with invasive measures (see Supplement).
Invasive assessment of diastolic dysfunction
(see Supplement).13
Cardiac biopterin content
Cardiac biopterins were measured as previously reported (see Supplement).6,14–16
Measurement of cardiac superoxide
Cardiac O2•− was measuredusing a dihydroethidium based HPLC assay as previously described.17 Tissue was either kept in plain buffer, treated with 1 mM of the non-selective NOS inhibitor Nω-Nitro-L-arginine methyl ester hydrochloride (L-NAME; Sigma) or 10 µM of the neuronal NOS (nNOS) inhibitor 7-nitroinidazole (7N) (AXXORA, San Diego, CA; see Supplement).18
NOS activity and expression
NOS activity was determined by measuring the conversion of [14C] labeled arginine to citrulline in myocardial homogenates (Calbiochem, San Diego, CA). Neuronal NOS (nNOS) and endothelial NOS (eNOS) monomers were assayed using cold SDS-PAGE western blot analysis under reducing conditions (see Supplement).
Phospholamban (PLB) expression
Frozen samples were homogenized in protease and phosphatase inhibitors (Sigma). SDS-PAGE was performed using a 15% acrylamide gel (see Supplement).
Myocyte isolation and cell shortening
Cardiac ventricular myocytes were isolated and analyzed from the hearts of DOCA-salt mice 11–14 days post-operatively and of age matched controls using a previously described protocol (see Supplement).19,20
Histology
Heart tissue was stained with hematoxylin and eosin or Masson’s trichrome to determine myocyte cross-sectional diameter and interstitial fibrosis (see Supplement).
Statistical Analysis
Data are presented as mean ± SE unless otherwise specified. Comparison between two groups was done with a two-tailed Student’s t-test. Multiple groups were compared with a one-way ANOVA and a post-hoc test (Student–Newman–Keuls or Bonferroni).
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Hypertensive mice show diastolic dysfunction
Tail-cuff blood pressure measurements and echocardiograms were obtained on postoperative days 11 to 13 in DOCA-salt mice. Mean systolic blood pressure and heart rate were mildly elevated in conscious, acclimated hypertensive mice as compared to age matched controls (Table 1).
Table 1.
Comparison of control and hypertensive mice.
| control | DOCA | p value | |
|---|---|---|---|
| (n=10) | (n=9) | ||
| Systolic | 98 ± 5 | 114 ± 2 | 0.01 |
| HR (beats/min) | 564 ± 15 | 609 ± 14 | 0.03 |
| Diastolic | (n=4) | (n=6) | |
| E’ (cm/s) | 5.6 ± 0.2 | 2.9 ± 0.2 | <0.001 |
| E’/A’ | 1.5 ± 0.1 | 0.7 ± 0.1 | <0.001 |
| Vp (cm/s) | 50.8 ± 1.9 | 29.8 ± 1.4 | <0.001 |
| E/E’ | 16.1 ± 0.8 | 29.0 ± 1.5 | <0.001 |
| E/A | 1.4 ± 0.4 | 1.5 ± 0.1 | NS |
| Systolic | |||
| (n=6) | (n=5) | ||
| %FS (%) | 29 ± 1.9 | 33 ± 2.0 | NS |
| %EF (%) | 56 ± 2.9 | 60 ± 0.2 | NS |
| Sm (cm/s) | 3.3 ± 0.4 | 2.9 ± 0.1 | NS |
| LV dimensions | |||
| (n=6) | (n=5) | ||
| LVPWd (mm) | 0.74 ± 0.01 | 0.76 ± 0.01 | NS |
| LVEDd (mm) | 3.8 ± 0.1 | 3.7 ± 0.2 | NS |
| Myocyte | |||
| (n=9) | (n=9) | ||
| Diameter (µm) | 16.9 ± 0.5 | 16.8 ± 0.3 | NS |
| (n=6) | (n=6) | ||
| Length (µm) | 123.2 ± 6.1 | 111.3 ± 12.9 | NS |
BP, blood pressure; HR, heart rate; E’, early septal mitral annulus velocity measured by tissue Doppler imaging (TDI); A’, late diastolic septal mitral annulus velocity (TDI);Sm, systolic septal mitral annulus velocity (TDI); Vp, left ventricular inflow propagation velocity by color M-mode Doppler; E, early diastolic filling velocity measured by conventional Doppler; A, late diastolic filling velocity measured by conventional Doppler; %FS, percent fractional shortening; %LVEF, percent ejection fraction; LV, left ventricle; LVPWd, LV posterior wall thickness; LVEDd, LV end diastolic dimension.
Hypertensive mice had echocardiographic evidence of diastolic dysfunction21,22 (Figure 1). They had significant reduction in tissue mitral annulus early longitudinal (E’) velocities, ratio of tissue early to atria (E’/A’) velocities, and left ventricle (LV) inflow propagation velocity (Vp) compared to controls. The ratio of early diastolic filling velocity to the early diastolic mitral annulus velocity (E/E’) has been reported to have the highest correlation with invasive hemodynamic measures of diastolic dysfunction23. Hypertensive mice had a higher E/E’ compared to controls, consistent with impaired relaxation. The mitral inflow velocities, E and A, were similar between groups. The normal E/A ratio in conjunction with abnormal tissue Doppler imaging and color M-mode measurements implied that this value was “pseudonormal,” a pattern associated with advanced diastolic dysfunction.24 The changes in relaxation parameters occurred in the absence of valvular regurgitation, LV wall motion abnormalities, or hypertrophy. Systolic function including LVEF(%), fractional shortening (FS, %), and septal annulus systolic velocity (Sm) were statistically indistinguishable between groups.
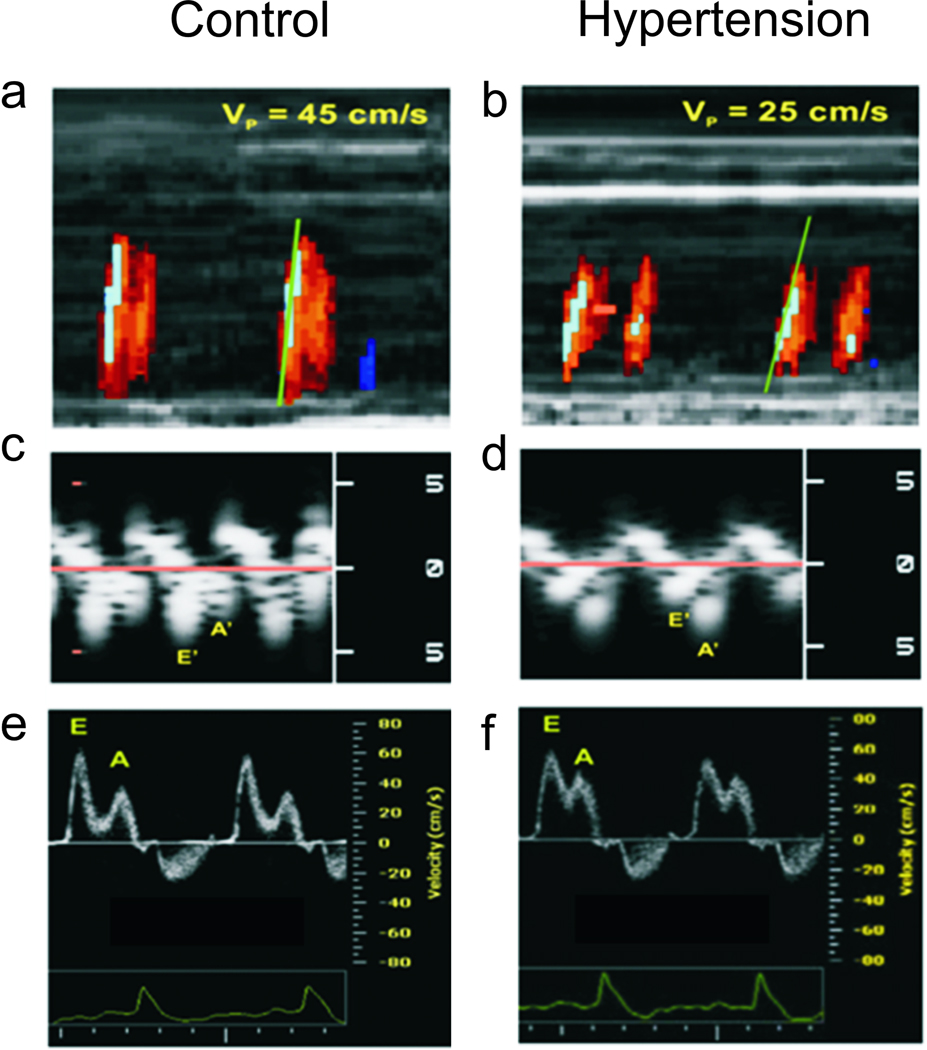
Figure 1.
Representative echocardiographic assessments of LV diastolic function.Panels a and b: Left ventricular inflow propagation velocity (Vp) interrogated with color M-mode Doppler. A control mouse shows a steeper isovelocity line slope, corresponding to a higher Vp compared with a hypertensive DOCA mouse. Panels c and d: Septal mitral annulus velocities interrogated with tissue Doppler imaging (TDI). The control animal has a higher E’ (early diastolic velocity), and lower A’ (late diastolic velocity) than the hypertensive animal. Panels e and f: Conventional pulsed wave Doppler shows a normal E/A (early to late diastolic filling velocity ratio) of >1 and <2 for both the control and DOCA mice, a pseudonormal” pattern.
Invasive hemodynamic evaluation confirmed the echocardiographic findings (Figure 2). As expected, LV end systolic pressure and LV end diastolic pressure were mildly elevated in hypertensive mice as compared to controls (108 ± 3 vs. 95 ± 2 mmHg, p=0.002; 7.2 ± 0.7 vs. 4.5 ± 0.4 mmHg, p=0.004; Figure 2, a–c ), respectively. Compared to controls, hypertensive mice had prolonged time constants for isovolumic relaxation calculated by two standard methods, τWeiss (10.3 ± 0.08 vs. 8.1 ± 0.03 ms, p = 0.02) and τGlantz (5.9 ± 0.02vs. 4.9 ± 0.02 ms, p=0.03; Figure 2, d).25 The best fit for the end-diastolic pressure volume-relation (EDPVR) was described by the linear function Pressure end diastole = EDPVR * Volume end diastole + intercept (median r value 0.99, range 0.91–0.99, for both groups combined; Figure 2, e & f).Hypertensive DOCA-salt mice had a steeper EDPVR compared to controls (1.3 ± 0.1 vs. 0.67 ± 0.1 mmHg/µL, p=0.0004; Figure 2, e).
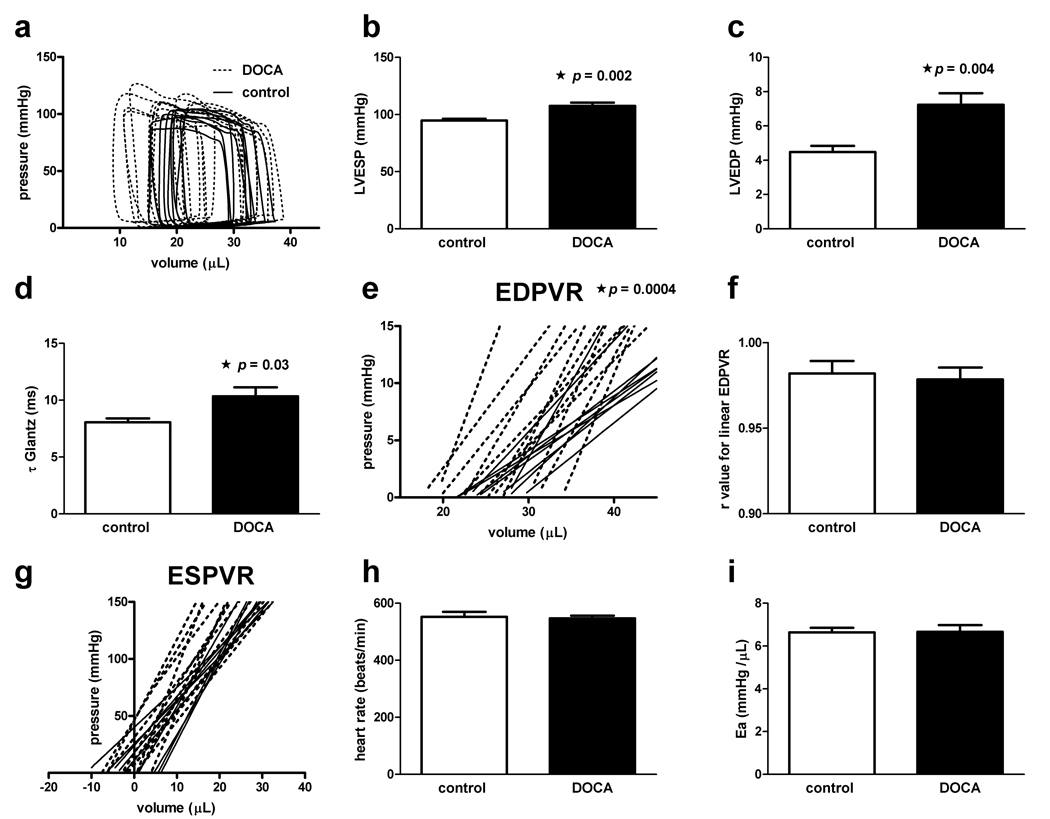
Figure 2.
Invasive hemodynamic assessment of LV diastolic dysfunction. Panel a: Baseline pressure-volume loops for hypertensive and control animals. Panel b: Comparison of LVESP for hypertensive and control animals (p=0.002). Panel c: Comparison of LVEDP for hypertensive and control animals (7.2 ± 0.7 vs. 4.5 ± 0.4 mmHg, p=0.004).Panel d: The time constant for isovolemic relaxation (τGlantz) is increased in hypertensive mice compared to controls (p=0.03).Panel e: The end-diastolic pressure-volume relation (EDPVR) slope is steeper in hypertensive mice as compared to controls (p=0.0004).Panel f: Pearson correlation coefficients for linear fitting of the EDPVR.Panel g: LV contractility assessed by the end-systolic pressure-volume relation (ESPVR) slope (p=NS) and the volume axis intercept Vo (p=NS) are similar between DOCA and control groups. Panel h: Mean heart rate between groups (p=NS).Panel i: Arterial elastance (Ea) a measure of vascular stiffness is similar between hypertensive and control mice (p=NS).
Diastolic dysfunction did not appear to be the result of changes in myocardial systolic contractile properties. LV systolic function was preserved in hypertensive mice compared to controls based on multiple invasive indices including: the slope of the end-systolic pressure-volume relation (ESPVR; 6.7 ± 0.6 vs. 5.3 ± 0.5 mmHg/µL, p=NS; Figure 2, f and g ) and its volume axis intercept (Vo; −2.6 ± 1.3 vs. −1.6 ± 2.1 mmHg/µL, p=NS), LV ejection fraction (52 ± 2.0 vs. 45 ± 1.1%, p=NS), stroke volume (16.0 ± 0.4 vs. 14.4 ± 0.4 µL, p=0.009), and peak rate of pressure rise (dp/dtmax; 10690 ± 459 vs. 11680 ± 470 mmHg/s, p=NS).Body weight was similar between the two groups (23.1 ± 0.2 vs. 23.3 ± 0.2 g, p=NS). These changes were unexplained by differences in heart rate (553 ± 17 vs. 547 ± 10 beats/min, p=NS; Figure 2, h) and arterial elastance, a measure of vascular stiffness that is calculated by dividing the end-systolic pressure by stroke volume, was similar between hypertensive mice and controls (6.64 ± 0.2 vs. 6.67 ± 0.3 mmHg/µL, p=NS; Figure 2, i).26
At a cellular level, LV tissue from mildly hypertensive mice did not show an increase in collagen staining with Mason’s trichrome (Supplemental Figure 2). Myocytes from DOCA-salt mice had similar cell diameters and length measurements in comparison to control cells (P=NS; Table 1). Fractional shortening % in isolated myocytes was similar between groups (P=NS; Supplemental Figure 3).
In the DOCA-salt model, the changes in diastolic properties were dependent on the presence of mild hypertension. Mice implanted with a DOCA pellet in the absence of unilateral nephrectomy and salt supplementation had similar mean systolic blood pressure compared to controls (103.3 ± 3 vs. 99.5 ± 3 mmHg, p=NS). There were no significant differences in ventricular relaxation in these mice compared to controls: E’ (5.0 ± 0.6 vs. 5.6 ± 0.1cm/s), Vp (47.3 ± 3.3 vs. 50.8 ± 1.9 cm/sec), and E/E’ (18.2 ± 1.9 vs. 16.1 ± 0.8; n=5 per group).
Diastolic dysfunction is associated with cardiac oxidation
LV tissue obtained from hypertensive mice on postoperative day 14 showed a two-fold increase in O2•− as measured by 2-oxyethidium (2HO-ET) levels (0.09 ± 0.01 vs. 0.18 ± 0.02 µM/mg, p=0.02). Administering BH4 to hypertensive mice on postoperative days 1–14 significantly blunted the increase in cardiac 2HO-ET compared to that observed in the untreated DOCA-salt group (0.12 ± 0.01 vs. 0.18 ± 0.02 µM/mg, p=.0.02; Figure 3, a–b).
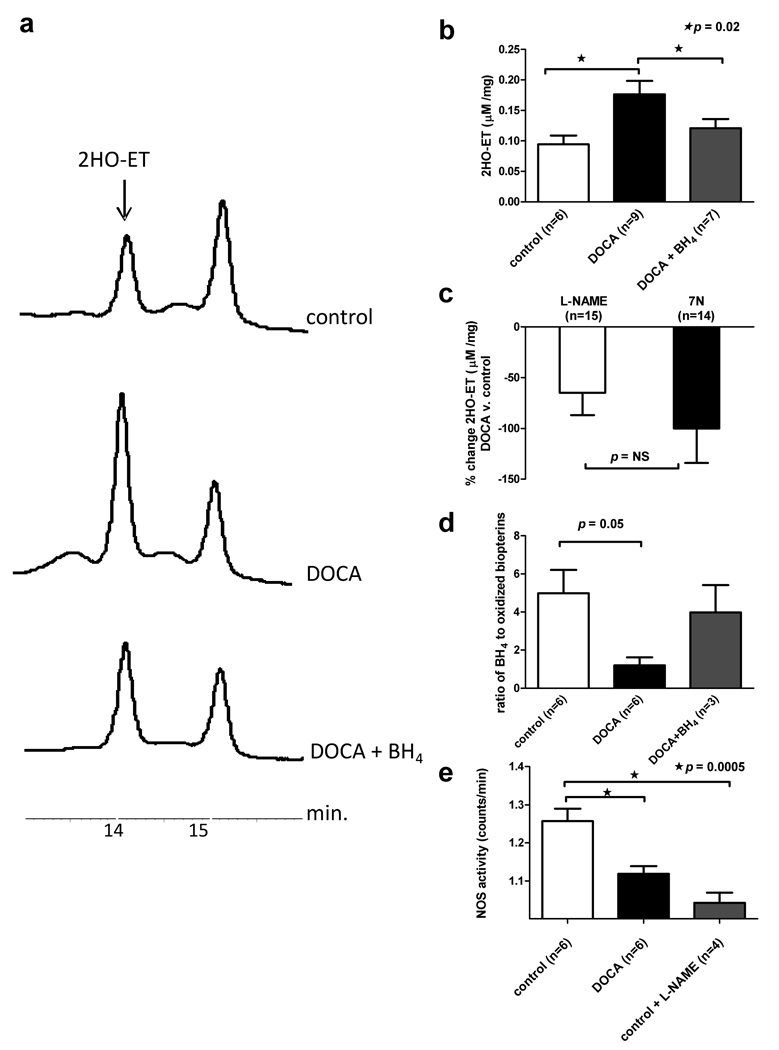
Figure 3.
Hypertensive mice show cardiac oxidation and reduced BH4. Panel a: Typical HPLC spectra obtained from a control (top), DOCA (middle), BH4 treated DOCA mice (bottom) show a larger oxyethidium (2HO-ET) peak in the DOCA animal.Panel b: Mean 2HO-ET levels are significantly higher in DOCA mice as compared to control and BH4 treated mice (days 1–14). Panel c: The percent O2•− reduction between control and DOCA LV tissue treated with the non-selective NOS inhibitor, L-NAME, and the selective neuronal NOS inhibitor, 7-nitroinidazole (7N).Panel d: BH4 and oxidized biopterins are quantified using the differential oxidation method and HPLC. The ratio of reduced BH4 to oxidized pterins in the heart is lower in DOCA mice versus controls. Feeding BH4 to hypertensive mice (days 1–14) increases the cardiac reduced BH4 to oxidized pterins ratio. Panel e: Total NOS activity is reduced to a similar degree in DOCA and L-NAME treated control hearts.
O2•− production in diastolic dysfunction could be suppressed by NOS inhibitors
Hypertension is known to cause increased oxidative stress in the vasculature, in part, as a result of O2•− production from uncoupled NOS.6 Both eNOS and neuronal nNOS have been reported in heart.10,27 In order to examine whether uncoupled NOS enzymes were contributing to cardiac O2•− production in hypertensive mice, LV tissue was incubated with the non-selective NOS inhibitor, L-NAME, or the selective nNOS inhibitor, 7N.18 The percent reduction in O2•− production between control and hypertensive animals was 65 ± 22% for L-NAME- and 100 ± 34% for 7N-treated tissue (p=NS), suggesting that nNOS uncoupling was responsible for most of the increase in O2•− production in hypertensive mice (Figure 3, c).
Cardiac oxidation was associated with less cardiac BH4 and reduced NO
BH4 is an essential cofactor for NOS28. When BH4 is oxidized, NOS becomes uncoupled.6,10 Cardiac biopterins were quantified in LV tissue obtained on postoperative day 14. Consistent with the increase in O2•− and evidence of NOS uncoupling, oxidized pterins were increased in hypertensive mice compared to controls (0.97 ± 0.1 vs. 0.46 ± 0.3 pmol/mg protein, p= 0.04).Total biopterins (1.8 ± 0.2 vs. 1.9 ± 0.3 pmol/mg protein) and reduced BH4 (1.4 ± 0.2 vs. 0.94 ± 0.2 pmol/mg protein) were unchanged between the groups (p=NS). Therefore, the ratio of reduced BH4 to oxidized pterins, which reflects the amount of BH4 available for NOS, was significantly lower in hypertensive mice compared to controls (1.2 ± 0.4 vs. 5.0 ± 1.2, p=0.02).Feeding BH4 to DOCA-salt mice (n=3; days 1–14) increased cardiac BH4 and total biopterins (p< 0.01 vs. DOCA-salt and controls), demonstrating that oral administration could deliver reduced BH4 to the myocardium. A 3.3 fold increase in the ratio of reduced BH4 to oxidized pterins was seen in BH4 fed DOCA-salt animals as compared to hypertensive mice without BH4 feeding (p=NS; Figure 3, d). NO production by NOS was reduced to similar degrees in DOCA-salt and in L-NAME treated control hearts when compared to untreated controls (1.1 ± 0.02 vs. 1.0 ± 0.03 vs. 1.3 ± 0.03 counts/min respectively, p=0.0005; Figure 3, e) without changes in NOS isoform levels (p=NS; Supplemental Figure 4).
BH4 prevented or reversed diastolic dysfunction in hypertensive mice
We examined whether BH4 could prevent diastolic function in mice treated with DOCA-salt. BH4 administration prevented the hypertension associated with DOCA-salt treatment (Figure 4, a). Assessment of diastolic function by echocardiography showed preserved LV relaxation with BH4 based on all indices (Figure 4, b–d).BH4 prevented the increase in LV end diastolic pressure seen with hypertensive DOCA-salt mice (6.3 ± 0.9 mmHg; p=NS compared to controls). The slope of the EDPVR was significantly lower in BH4 prevention mice compared to DOCA-salt mice and was statistically indistinguishable from controls (Figure 4, e–f).Measures of systolic function including the ESPVR and its volume axis intercept (Vo) were statistically equivalent between groups, and arterial elastance was unchanged, suggesting that neither arterial nor systolic function alterations explained the prevention of diastolic dysfunction seen with BH4 (Supplemental Table 1).Moreover, BH4 did not affect heart rate. The mean heart rate was 550–576 beats/min during non-invasive and invasive studies for all groups (p=NS).
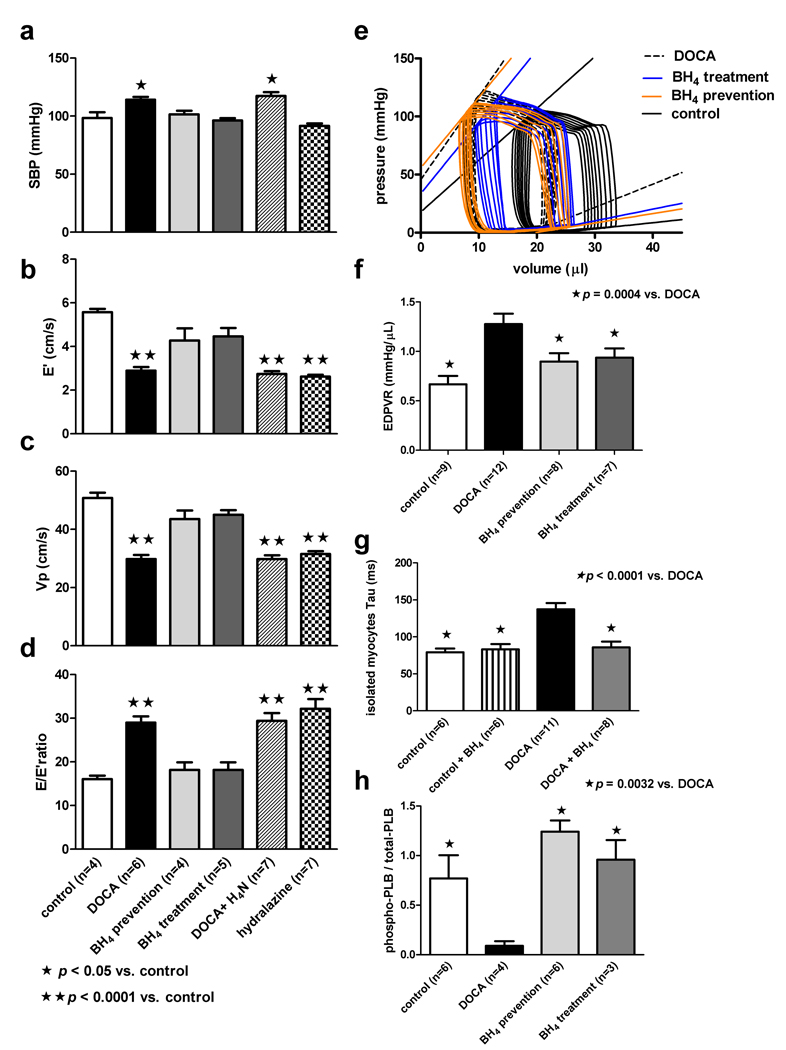
Figure 4.
Tetrahydrobiopterin (BH4) prevents or reverses diastolic dysfunction. Panel a: DOCA mice treated with BH4 or hydralazine (days 12–24) show a reduction in systolic blood pressure (SBP) to levels observed in control animals. Treatment with tetrahydroneopterin (H4N; days 12–24), an enzymatically inactive analog of BH4 with equivalent antioxidant properties, has no effect on blood pressure in hypertensive DOCA mice. Panels b, c, and d: Assessment of diastolic function using echocardiography: E’ (early septal mitral annulus velocity measured with tissue Doppler imaging), Vp (left ventricular inflow propagation velocity interrogated with color M-mode Doppler) and E/E’ ratio show BH4, but not H4N or hydralazine, prevents or reverses diastolic dysfunction in hypertensive DOCA mice. Panel e: Sample pressure-volume loops obtained during inferior vena cava occlusion demonstrate the EDPVR and ESPVR in control-, DOCA-, and BH4-treated mice.Panel f: The mean EDPVR is steeper in DOCA mice versus control, BH4 prevention, and BH4 treatment groups.Panel g: Isolated myocytes from DOCA mice have a prolonged relaxation constant (τ) compared to control animals. The addition of exogenous BH4 to isolated DOCA myocytes normalizes relaxation kinetics. Panel g: DOCA mice have a reduced phosphorylated to total PLB ratio compared to control, BH4 prevention, and BH4 treatment groups.
BH4 could also reverse diastolic dysfunction once established. Hypertensive DOCA-salt mice were treated on postoperative day 11, a time when all hypertensive mice had evidence of diastolic dysfunction, with a 12–14 day course of BH4. Mean systolic blood pressure (96 ± 2 and 99 ± 3 mmHg in control; p=NS) and the invasively measured LV end systolic pressure (LVESP) were normalized in BH4 treated mice. LV end diastolic pressure (LVEDP) did not increase compared to controls (6.2 ± 0.9 mmHg; p=NS). Echocardiographic indices of diastolic function in the BH4 treatment group mirrored those observed in the BH4 prevention group and controls (Figure 4, b–d). The EDPVR was significantly improved in the BH4 treated mice compared to DOCA mice (Figure 4, e–f). Systolic function, arterial elastance, and heart rate were similar in BH4 treated mice as compared to all other groups (Supplemental Table 1). The lack of improvement in blood pressure or lusitropy after treatment of DOCA-salt mice with tetrahydroneopterin, a free radical scavenger without NOS catalytic activity, implied the effects were specific to BH4 (Figure 4, b–d).6,10
Since lower blood pressures were observed in BH4 treated mice, it was unclear whether the improvements observed in diastolic function were a result of reduced blood pressure or improvement in NOS coupling. Hydralazine, an antihypertensive agent that lowers blood pressure without re-coupling NOS, was used to evaluate these possibilities.29 Mice treated with hydralazine had a similar reduction in blood pressure to that observed with BH4. Nevertheless, diastolic dysfunction did not improve in hydralazine treated mice, suggesting that the effect of BH4 on DOCA-salt mice was independent of blood pressure lowering (Figure 4, a–d).
A possible mechanism for the BH4 effect
In order to determine if abnormalities in diastolic function or the beneficial lusitropic effects seen with BH4 in hypertensive mice were mediated at the myocyte level, cell shortening was measured in isolated myocytes. Cell length and fractional shortening were similar between groups (P=NS; Table 1 and Supplemental Figure 3), but the relaxation constant τ was prolonged in myocytes from hypertensive hearts compared to controls (137.3 ± 8.5 vs. 79.3 ± 4.9 ms, p < 0.0001; Figure 4, g).Incubating myocytes from hypertensive mice with 10 µM BH4 for 15 min normalized τ (86.0 ± 7.6 ms, p = NS vs. control and p < 0.0001 vs. DOCA; Figure 4, h).Addition of BH4 to control myocytes had no effect on τ or fractional shortening (p=NS; Figure 4, g and Supplemental Figure 3).
Recently nNOS-mediated oxidation changes to the phosphorylation state of phospholamban (PLB), a key protein controlling sarcoplasmic reticulum Ca2+ pump activity and cytosolic Ca2+ levels, have been described.30,31 The ratio of phosphorylated-PLB to total PLB was quantified in DOCA-salt, control, BH4 prevention, and BH4 treatment groups (Figure 4, h). DOCA-salt mice had a marked reduction in the phosphorylated-PLB to total PLB ratio compared to all other groups (0.09 ± 0.04 vs. 0.76 ± 0.2 vs. 1.2 ± 0.1 vs. 1.0 ± 0.2; p= 0.003), respectively. Feeding BH4 to control mice had no effect on the phosphorylated-PLB to total PLB ratio (Supplemental Figure 7).
Cardiac oxidation and diastolic dysfunction in ACE 1/8 mice
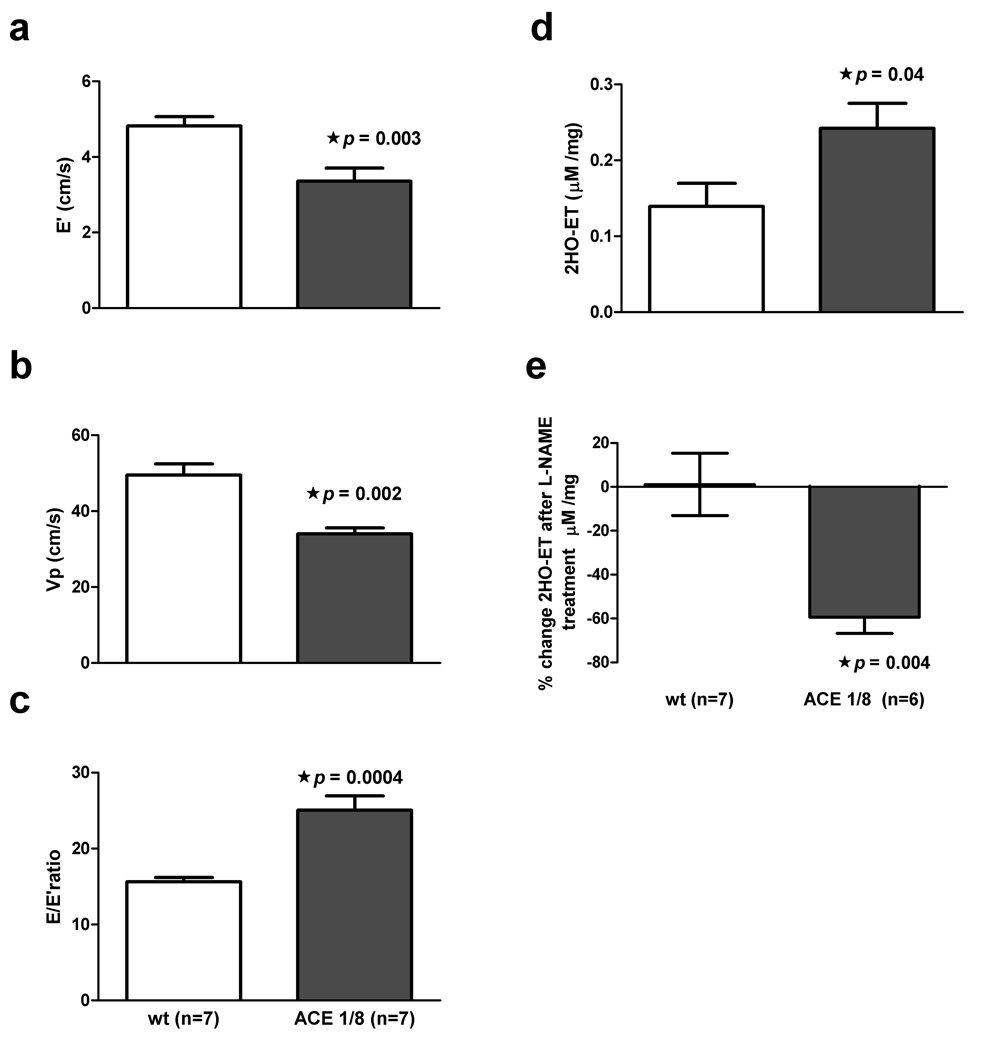
We investigated the role of cardiac oxidation in the pathogenesis of diastolic dysfunction using ACE 1/8 mice. Because these mice express ACE only in the heart, their blood pressure is unchanged from controls. Moreover, these mice have normal systolic function.11 Since cardiac AngII levels are increased in ACE 1/8 mice and AngII causes increase oxidative stress, we evaluated whether cardiac oxidation in the absence of hypertension resulted in diastolic dysfunction.32,33 Ten-week old ACE 1/8 mice showed multiple indices of diastolic dysfunction when compared to controls (Figure 5, a–c). O2•− levels were higher in ACE 1/8 mice compared to control littermates (0.24 ± 0.03 vs. 0.14 ± 0.3 µM/mg tissue, p= 0.04; Figure 5, d).O2•− production was suppressed by 60 ± 17% in ACE 1/8 mice by L-NAME. NOS inhibition had no effect on O2•− production in control littermates, suggesting that uncoupled NOS was involved in the cardiac oxidation seen in ACE 1/8 mice (Figure 5, e).
Figure 5.
Cardiac-specific ACE overexpression results in increased oxidation and diastolic dysfunction.Panels a, b, and c: ACE 1/8 mice have reduced E’, lower Vp, and an increased E/E’ ratio when compared to wild-type (wt) controls.Panel d: Mean oxyethidium (2HO-ET) levels are significantly higher in ACE 1/8 mice versus controls.Panel e: L-NAME suppresses O2•− production in ACE 1/8 mice (p=0.004).
Discussion
Heart failure with preserved systolic function is growing in prevalence and has no specific therapies.1,2 One of the most common associated risk factors for diastolic dysfunction is hypertension.4,34 Above, we showed that mild hypertension resulted in diastolic dysfunction. This relaxation defect was associated with cardiac oxidation and reduced NOS NO production. NOS inhibitors suggested that the oxidative stress was, in large part, the result of uncoupled NOS. Further suggesting uncoupled NOS was the increase in oxidized cardiac pterins and concomitant decrease in reduced cardiac BH4. Prevention or reversal of diastolic dysfunction by BH4 treatment but not HN4 implied that NOS uncoupling played a pathogenic role in the diastolic changes observed. The impaired relaxation observed in isolated myocytes from hypertensive animals coupled with the beneficial lusitropic effect observed by acutely treating these cells with BH4 supports the role of myocyte-specific uncoupled NOS as a mediator of diastolic dysfunction. Therefore, it appears that hypertension can cause diastolic dysfunction by oxidizing cardiac BH4 leading to myocyte uncoupled NOS.
Our results are consistent with those of Takimoto and Moens et al.10,35 These investigators showed that transaortic constriction with resultant systolic dysfunction was associated with cardiac BH4 depletion and NOS uncoupling. Exogenous BH4 treatment halted the progression of ventricular remodeling, reversed fibrosis, and improved calcium handling in this model. In our case, the lusitropic role for NOS was suggested in the absence of systolic dysfunction, marked ventricular remodeling, or increased arterial elastence.
Based on inhibitor experiments, nNOS was the largest contributor to cardiac O2•− production in diastolic dysfunction. eNOS and nNOS isoforms are known to be present in heart, and BH4 depletion appears to result in uncoupling of either form.7,10,36,37 A growing body of work points to nNOS as an important modulator of cardiac nitroso-redox balance and function.38–41 For example, Casadei and her co-workers have show that nNOS modulates cardiac relaxation via effects on phospholamban phosphorylation.31 On the other hand, interpretation that nNOS generates most of the increased oxidative stress in hypertensive mice may be misleading if 7N is less specific than reported.18
Our data suggest that reduced NO leads to PLB changes that are consistent with increased cytosolic Ca2+ and diastolic dysfunction. The beneficial effects of BH4 correlate with the phosphorylation state of PLB, consistent with work demonstrating NO is a mediator of cardiac lusitropy and the finding that Ca2+ handling and the phosphorylation state of PLB are subject to oxidative regulation.7,30,31,35,42 Other possible targets of oxidative modification that were not examined in the current study include the SR Ca2+ release channel, SR Ca2+ pump, or the sarcolemmal L-type Ca2+ channel. The troponin T- I79N mutation leads to diastolic dysfunction as a result of enhanced calcium myofilament sensitivity,43 suggesting oxidative stress-dependent changes in myofilament Ca2+ sensitivity may be another possible mechanism.
Previously, it has been difficult to resolve the contributions of changes in the vasculature versus changes in the myocardium in the pathogenesis of diastolic dysfunction.44 We attempted to address this quandary with isolated myocyte experiments and gene-targeted animals. The abnormal relaxation pattern observed in cardiomyocytes isolated from hypertensive mice combined with the ability BH4 to normalize relaxation in these cells support the concept that diastolic dysfunction in this model is in large measure myocyte dependent. The ability of the RAS to lead to vascular oxidation and uncoupling of NOS is well documented.6 Using a gene-targeted mouse that overexpressed ACE in the heart while simultaneously eliminating ACE elsewhere, we created a mouse that has increased cardiac oxidation and diastolic dysfunction in the absence of hypertension, suggesting cardiac oxidation is sufficient to cause diastolic dysfunction. This notion is further supported by the lack of change in vascular elastance in the mildly hypertensive mice with diastolic dysfunction.
Because there is a debate about the proper methodology to assess diastolic dysfunction, we chose to use both noninvasive and invasive means.25,45 Diastolic function was assessed using the combination of three echocardiographic modalities.46 Two of these modalities, tissue Doppler imaging and color M-mode assessment of left ventricular inflow propagation velocity, are relatively independent of cardiac loading conditions.21,47 Moreover, the invasively-derived EDPVR was used, generally thought to be the standard for lusitropic assessment.25 The strong correlation observed between E’, Vp, and E/E’ and the invasively-derived EDPVR suggests that these techniques assess similar myocardial properties. Measures indicated perturbations in active and passive stiffness. The abnormal phosphorylation state of PLB observed in hypertensive mice would be expected to impact active relaxation predominately. Since increased collagen deposition and hypertrophy were not observed, the increased passive stiffness may have resulted from enhanced myofilament Ca2+ sensitivity, changes in titin isoform expression, or changes in the composition of collagen.
Oral BH4, but not HN4, was able to raise cardiac BH4 levels and prevent or reverse the diastolic parameter changes seen in hypertensive mice. This suggests that the BH4 effect was not the result of nonspecific radical scavenging. Nevertheless, BH4 lowered blood pressure, in accordance with previously published data from human and animal studies, and this might prevent or ameliorate diastolic dysfunction,29,48 experiments with hydralazine, a direct vasodilator that does not re-couple NOS, suggest otherwise.29 In this case, hydralazine lowered blood pressure identically to BH4 but did not prevent diastolic dysfunction.
In summary, this study demonstrates that cardiac oxidation can lead to BH4 depletion, NOS uncoupling, and diastolic dysfunction. This dysfunction can be ameliorated by BH4 treatment. Diastolic dysfunction can occur in the absence of changes in the vasculature. Finally, these results suggest that BH4 may be of value in the treatment of diastolic dysfunction.
Clinical Summary
Heart failure with preserved ejection fraction as a result of diastolic dysfunction accounts for significant morbidity, mortality, and healthcare expenditures. Although many proven therapies exist for heart failure patients with reduced ejection fraction, specific treatments for diastolic dysfunction are lacking. In the vasculature, hypertension and activation of the renin-angiotensin system (RAS) leads to reduced vascular nitric oxide (NO), in part because nitric oxide synthase (NOS) becomes uncoupled. This dysfunctional state of NOS is characterized by oxidative depletion of its co-factor tetrahydrobiopterin (BH4) which leads to production of superoxide instead of NO. NO and NOS are also modulators of cardiac relaxation. We hypothesized that hypertension or activation of the RAS leads cardiac NOS uncoupling and diastolic dysfunction. Using a mouse model of mild hypertension to mimic the most common risk factor for diastolic dysfunction in humans, we showed that isolated impaired cardiac relaxation is associated with uncoupled NOS. Local cardiac activation of RAS demonstrated that cardiac oxidation was sufficient to cause diastolic dysfunction without hypertension. Diastolic dysfunction could be treated or prevented by oral administration of BH4, which recoupled cardiac NOS. These results suggest that BH4 may be of value in the treatment of diastolic dysfunction.
Supplementary Material
Acknowledgments
Funding Sources
SCD: NIH: R01 HL085520, R01 HL085558, R01 HL073753, an American Heart Association Established Investigator Award 0440164N, and a Veterans Affairs MERIT grant.
KEB: NIH: R01 DK39777, R01 HL085558, and R01 DK51445
GAS: NIH: 5 F32 HL086232-02
HDX: American Heart Association Scientist Development Award
BMW: NIH: RO1 HL79032
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Subject codes: [148] Heart failure - basic studies, [130] Animal models of human disease, [91] Oxidant stress
Disclosures
S.C.D., T.M.F., D.G.H.: patent, Methods and Compositions for Treating Diastolic Dysfunction, 60/840,368.
References
- 1.Schocken DD, Benjamin EJ, Fonarow GC, Krumholz HM, Levy D, Mensah GA, Narula J, Shor ES, Young JB, Hong Y. Prevention of heart failure: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2008;117:2544–2565. doi: 10.1161/CIRCULATIONAHA.107.188965. [DOI] [PubMed] [Google Scholar]
- 2.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 3.Ouzounian M, Lee DS, Liu PP. Diastolic heart failure: mechanisms and controversies. Nat Clin Pract Cardiovasc Med. 2008;5:375–386. doi: 10.1038/ncpcardio1245. [DOI] [PubMed] [Google Scholar]
- 4.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 5.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 6.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ziolo MT, Kohr MJ, Wang H. Nitric oxide signaling and the regulation of myocardial function. J Mol Cell Cardiol. 2008;45:625–632. doi: 10.1016/j.yjmcc.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Obst M, Gross V, Luft FC. Systemic hemodynamics in non-anesthetized L-NAME and DOCA-salt-treated mice. J Hypertens. 2004;22:1889–1894. doi: 10.1097/00004872-200410000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Heitzer T, Brockhoff C, Mayer B, Warnholtz A, Mollnau H, Henne S, Meinertz T, Munzel T. Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers : Evidence for a dysfunctional nitric oxide synthase. Circ Res. 2000;86:E36–E41. doi: 10.1161/01.res.86.2.e36. [DOI] [PubMed] [Google Scholar]
- 10.Takimoto E, Champion HC, Li M, Ren S, Rodriguez ER, Tavazzi B, Lazzarino G, Paolocci N, Gabrielson KL, Wang Y, Kass DA. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest. 2005;115:1221–1231. doi: 10.1172/JCI21968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao HD, Fuchs S, Bernstein EA, Li P, Campbell DJ, Bernstein KE. Mice expressing ACE only in the heart show that increased cardiac angiotensin II is not associated with cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2008;294:H659–H667. doi: 10.1152/ajpheart.01147.2007. [DOI] [PubMed] [Google Scholar]
- 12.Ho CY, Solomon SD. A clinician's guide to tissue Doppler imaging. Circulation. 2006;113:e396–e398. doi: 10.1161/CIRCULATIONAHA.105.579268. [DOI] [PubMed] [Google Scholar]
- 13.Yang B, Larson DF, Beischel J, Kelly R, Shi J, Watson RR. Validation of conductance catheter system for quantification of murine pressure-volume loops. J Invest Surg. 2001;14:341–355. doi: 10.1080/089419301753435710. [DOI] [PubMed] [Google Scholar]
- 14.Boulden BM, Widder JD, Allen JC, Smith DA, Al-Baldawi RN, Harrison DG, Dikalov SI, Jo H, Dudley SC., Jr Early determinants of H2O2-induced endothelial dysfunction. Free Radic Biol Med. 2006;41:810–817. doi: 10.1016/j.freeradbiomed.2006.05.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Widder JD, Chen W, Li L, Dikalov S, Thony B, Hatakeyama K, Harrison DG. Regulation of tetrahydrobiopterin biosynthesis by shear stress. Circ Res. 2007;101:830–838. doi: 10.1161/CIRCRESAHA.107.153809. [DOI] [PubMed] [Google Scholar]
- 16.Fukushima T, Nixon JC. Analysis of reduced forms of biopterin in biological tissues and fluids. Anal Biochem. 1980;102:176–188. doi: 10.1016/0003-2697(80)90336-x. [DOI] [PubMed] [Google Scholar]
- 17.Fink B, Laude K, McCann L, Doughan A, Harrison DG, Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am J Physiol Cell Physiol. 2004;287:C895–C902. doi: 10.1152/ajpcell.00028.2004. [DOI] [PubMed] [Google Scholar]
- 18.Bland-Ward PA, Moore PK. 7-Nitro indazole derivatives are potent inhibitors of brain, endothelium and inducible isoforms of nitric oxide synthase. Life Sci. 1995;57:L131–L135. doi: 10.1016/0024-3205(95)02046-l. [DOI] [PubMed] [Google Scholar]
- 19.Dias FA, Walker LA, Arteaga GM, Walker JS, Vijayan K, Pena JR, Ke Y, Fogaca RT, Sanbe A, Robbins J, Wolska BM. The effect of myosin regulatory light chain phosphorylation on the frequency-dependent regulation of cardiac function. J Mol Cell Cardiol. 2006;41:330–339. doi: 10.1016/j.yjmcc.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Sheehan KA, Ke Y, Wolska BM, Solaro RJ. Expression of active p21-activated kinase-1 induces Ca2+ flux modification with altered regulatory protein phosphorylation in cardiac myocytes. Am J Physiol Cell Physiol. 2009;296:C47–C58. doi: 10.1152/ajpcell.00012.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De KG, Leite-Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 22.De Boeck BW, Oh JK, Vandervoort PM, Vierendeels JA, van der Aa RP, Cramer MJ. Colour M-mode velocity propagation: a glance at intra-ventricular pressure gradients and early diastolic ventricular performance. Eur J Heart Fail. 2005;7:19–28. doi: 10.1016/j.ejheart.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Kasner M, Westermann D, Steendijk P, Gaub R, Wilkenshoff U, Weitmann K, Hoffmann W, Poller W, Schultheiss HP, Pauschinger M, Tschope C. Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler-conductance catheterization study. Circulation. 2007;116:637–647. doi: 10.1161/CIRCULATIONAHA.106.661983. [DOI] [PubMed] [Google Scholar]
- 24.Aurigemma GP, Gaasch WH. Clinical practice. Diastolic heart failure. N Engl J Med. 2004;351:1097–1105. doi: 10.1056/NEJMcp022709. [DOI] [PubMed] [Google Scholar]
- 25.Kass DA. Assessment of diastolic dysfunction. Invasive modalities. Cardiol Clin. 2000;18:571–586. doi: 10.1016/s0733-8651(05)70162-4. [DOI] [PubMed] [Google Scholar]
- 26.Frenneaux M, Williams L. Ventricular-arterial and ventricular-ventricular interactions and their relevance to diastolic filling. Prog Cardiovasc Dis. 2007;49:252–262. doi: 10.1016/j.pcad.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Casadei B. The emerging role of neuronal nitric oxide synthase in the regulation of myocardial function. Exp Physiol. 2006;91:943–955. doi: 10.1113/expphysiol.2006.035493. [DOI] [PubMed] [Google Scholar]
- 28.Moens AL, Kass DA. Therapeutic potential of tetrahydrobiopterin for treating vascular and cardiac disease. J Cardiovasc Pharmacol. 2007;50:238–246. doi: 10.1097/FJC.0b013e318123f854. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto E, Kataoka K, Shintaku H, Yamashita T, Tokutomi Y, Dong YF, Matsuba S, Ichijo H, Ogawa H, Kim-Mitsuyama S. Novel mechanism and role of angiotensin II induced vascular endothelial injury in hypertensive diastolic heart failure. Arterioscler Thromb Vasc Biol. 2007;27:2569–2575. doi: 10.1161/ATVBAHA.107.153692. [DOI] [PubMed] [Google Scholar]
- 30.Wang H, Kohr MJ, Traynham CJ, Wheeler DG, Janssen PM, Ziolo MT. Neuronal nitric oxide synthase signaling within cardiac myocytes targets phospholamban. Am J Physiol Cell Physiol. 2008;294:C1566–C1575. doi: 10.1152/ajpcell.00367.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang YH, Zhang MH, Sears CE, Emanuel K, Redwood C, El-Armouche A, Kranias EG, Casadei B. Reduced phospholamban phosphorylation is associated with impaired relaxation in left ventricular myocytes from neuronal NO synthase-deficient mice. Circ Res. 2008;102:242–249. doi: 10.1161/CIRCRESAHA.107.164798. [DOI] [PubMed] [Google Scholar]
- 32.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 33.Harrison DG, Cai H, Landmesser U, Griendling KK. Interactions of angiotensin II with NAD(P)H oxidase, oxidant stress and cardiovascular disease. J Renin Angiotensin Aldosterone Syst. 2003;4:51–61. doi: 10.3317/jraas.2003.014. [DOI] [PubMed] [Google Scholar]
- 34.Owan TE, Redfield MM. Epidemiology of diastolic heart failure. Prog Cardiovasc Dis. 2005;47:320–332. doi: 10.1016/j.pcad.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Moens AL, Takimoto E, Tocchetti CG, Chakir K, Bedja D, Cormaci G, Ketner EA, Majmudar M, Gabrielson K, Halushka MK, Mitchell JB, Biswal S, Channon KM, Wolin MS, Alp NJ, Paolocci N, Champion HC, Kass DA. Reversal of cardiac hypertrophy and fibrosis from pressure overload by tetrahydrobiopterin: Efficacy of recoupling nitric oxide synthase as a therapeutic strategy. Circulation. 2008;117:2626–2636. doi: 10.1161/CIRCULATIONAHA.107.737031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seddon M, Shah AM, Casadei B. Cardiomyocytes as effectors of nitric oxide signalling. Cardiovasc Res. 2007;75:315–326. doi: 10.1016/j.cardiores.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 37.Sun J, Druhan LJ, Zweier JL. Dose dependent effects of reactive oxygen and nitrogen species on the function of neuronal nitric oxide synthase. Arch Biochem Biophys. 2008;471:126–133. doi: 10.1016/j.abb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Landmesser U, Harrison DG, Drexler H. Oxidant stress—a major cause of reduced endothelial nitric oxide availability in cardiovascular disease. Eur J Clinical Pharmacol. 2006;62:13–19. [Google Scholar]
- 39.Takimoto E, Kass DA. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension. 2007;49:241–248. doi: 10.1161/01.HYP.0000254415.31362.a7. [DOI] [PubMed] [Google Scholar]
- 40.Khan SA, Lee K, Minhas KM, Gonzalez DR, Raju SV, Tejani AD, Li D, Berkowitz DE, Hare JM. Neuronal nitric oxide synthase negatively regulates xanthine oxidoreductase inhibition of cardiac excitation-contraction coupling. Proc Natl Acad Sci USA. 2004;101:15944–15948. doi: 10.1073/pnas.0404136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ashley EA, Sears CE, Bryant SM, Watkins HC, Casadei B. Cardiac nitric oxide synthase 1 regulates basal and beta-adrenergic contractility in murine ventricular myocytes. Circulation. 2002;105:3011–3016. doi: 10.1161/01.cir.0000019516.31040.2d. [DOI] [PubMed] [Google Scholar]
- 42.Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part II: causal mechanisms and treatment. Circulation. 2002;105:1503–1508. doi: 10.1161/hc1202.105290. [DOI] [PubMed] [Google Scholar]
- 43.Westermann D, Knollmann BC, Steendijk P, Rutschow S, Riad A, Pauschinger M, Potter JD, Schultheiss HP, Tschope C. Diltiazem treatment prevents diastolic heart failure in mice with familial hypertrophic cardiomyopathy. Eur J Heart Fail. 2006;8:115–121. doi: 10.1016/j.ejheart.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 44.Doi R, Masuyama T, Yamamoto K, Doi Y, Mano T, Sakata Y, Ono K, Kuzuya T, Hirota S, Koyama T, Miwa T, Hori M. Development of different phenotypes of hypertensive heart failure: systolic versus diastolic failure in Dahl salt-sensitive rats. J Hypertens. 2000;18:111–120. doi: 10.1097/00004872-200018010-00016. [DOI] [PubMed] [Google Scholar]
- 45.Yellin EL, Meisner JS. Physiology of diastolic function and transmitral pressure-flow relations. Cardiol Clin. 2000;18:411–433. doi: 10.1016/s0733-8651(05)70153-3. vii. [DOI] [PubMed] [Google Scholar]
- 46.Lester SJ, Tajik AJ, Nishimura RA, Oh JK, Khandheria BK, Seward JB. Unlocking the mysteries of diastolic function: deciphering the Rosetta Stone 10 years later. J Am Coll Cardiol. 2008;51:679–689. doi: 10.1016/j.jacc.2007.09.061. [DOI] [PubMed] [Google Scholar]
- 47.Takatsuji H, Mikami T, Urasawa K, Teranishi J, Onozuka H, Takagi C, Makita Y, Matsuo H, Kusuoka H, Kitabatake A. A new approach for evaluation of left ventricular diastolic function: spatial and temporal analysis of left ventricular filling flow propagation by color M-mode Doppler echocardiography. J Am Coll Cardiol. 1996;27:365–371. doi: 10.1016/0735-1097(96)81240-x. [DOI] [PubMed] [Google Scholar]
- 48.Porkert M, Sher S, Reddy U, Cheema F, Niessner C, Kolm P, Jones DP, Hooper C, Taylor WR, Harrison D, Quyyumi AA. Tetrahydrobiopterin: a novel antihypertensive therapy. J Hum Hypertens. 2008;22:401–407. doi: 10.1038/sj.jhh.1002329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.