Abstract
The secreted goblet cell-derived protein resistin-like molecule β (RELMβ) has been implicated in divergent functions, including a direct effector function against parasitic helminths and a pathogenic function in promoting inflammation in models of colitis and ileitis. However, whether RELMβ influences CD4+ T cell responses in the intestine is unknown. Using a natural model of intestinal inflammation induced by chronic infection with gastrointestinal helminth Trichuris muris, we identify dual functions for RELMβ in augmenting CD4+ Th1 cell responses and promoting infection-induced intestinal inflammation. Following exposure to low-dose Trichuris, wild-type C57BL/6 mice exhibit persistent infection associated with robust IFN-γ production and intestinal inflammation. In contrast, infected RELMβ−/− mice exhibited a significantly reduced expression of parasite-specific CD4+ T cell-derived IFN-γ and TNF-α and failed to develop Trichuris-induced intestinal inflammation. In in vitro T cell differentiation assays, recombinant RELMβ activated macrophages to express MHC class II and secrete IL-12/23p40 and enhanced their ability to mediate Ag-specific IFN-γ expression in CD4+ T cells. Taken together, these data suggest that goblet cell-macrophage cross-talk, mediated in part by RELMβ, can promote adaptive CD4+ T cell responses and chronic inflammation following intestinal helminth infection.
Intestinal epithelial cells (IEC)3 have numerous adaptations that provide a physical barrier between the host and the external environment, including elaborate tight junctions and a mucin-rich microvilli brush border (1). Recent reports using IEC-specific knockout mice demonstrated that IEC also play a fundamental role in intestinal immune homeostasis through direct regulation of innate and adaptive immune responses (2, 3). Intestinal epithelial stem cells are pluripotent and can differentiate into at least four distinct cell lineages including enterocytes, Paneth cells, enteroendocrine cells, and goblet cells (4, 5). Goblet cells secrete a panel of bioactive molecules that include mucins, trefoil peptides, and resistin-like molecule β (RELMβ) and their differentiation is a hallmark of chronic inflammation in a range of diseases (6–8).
RELMβ belongs to the family of resistin-like molecules, a group of small, cysteine-rich secreted mammalian proteins (9–12). RELMβ is highly expressed in several disease settings including murine models of helminth infection, cystic fibrosis, and inflammatory bowel disease (7, 13–16). We previously reported that exposure to the intestinal-dwelling helminth pathogen Trichuris promotes RELMβ expression and that RELMβ could disrupt parasite sensory functions in an in vitro assay, suggesting a potential antiparasite effector function (13). More recent studies suggested an additional role for RELMβ in promoting intestinal inflammation both in response to chemical-induced colonic injury and in a genetic model of ileitis (7, 15, 17). However, whether RELMβ is necessary for resistance to Trichuris or can directly influence innate or adaptive immune cell function following enteric infection remains unknown. To address this question, we used RELMβ−/− mice in both acute and chronic Trichuris infection. Trichuris is a natural parasite of mice and provides a well-established model where resistance to infection is critically dependent on the development of an adaptive Th2 cytokine response, while susceptibility is associated with a Th1 cytokine response (18–20). In C57BL/6 mice, the development of an acute or chronic Trichuris infection is achieved through manipulation of the infection dose (21). In response to high-dose infection, a parasite-specific protective Th2 cytokine response is generated, allowing efficient parasite expulsion while low-dose infection, which is more representative of a natural infection in humans, mediates the generation of a Th1 cytokine-dominated response and chronic parasite establishment (21–25).
In this study, using RELMβ−/− mice in acute infection with Trichuris, we demonstrate that goblet cell-derived RELMβ is not required for the generation of a protective Th2 cytokine response and the expulsion of Trichuris. However, in low-dose Trichuris infection, RELMβ−/− mice exhibited reduced expression of parasite-specific IFN-γ, failed to develop infection-induced intestinal inflammation, and did not develop persistent infection. In vitro, recombinant RELMβ augmented Th1 cell differentiation through the direct activation of macrophages to up-regulate MHC class II expression and production of IL-12/23p40. Collectively, these data identify dual functions for RELMβ in enhancing Trichuris-induced intestinal inflammation and adaptive CD4+ Th1 cell responses, and support a role for intestinal goblet cells in influencing innate and adaptive immune responses through the regulation of intestinal macrophage function.
Materials and Methods
Animals, parasites, Ags, and infections
RELMβ−/− mice were generated as described previously (15). C57BL/6 mice were obtained from The Jackson Laboratory. Mice transgenic for the OVA323–339-specific TCR (OT-II) on a C57BL/6 background were bred and maintained in a specific pathogen-free environment. All experiments were performed following the guidelines of the University of Pennsylvania Institutional Animal Care and Use Committee. Trichuris was maintained in genetically susceptible animals. Isolation of Trichuris excretory-secretory Ag and eggs was performed as described previously (26). Mice were infected orally with 30 (low-dose) or 200 (high-dose) embryonated eggs, and parasite burdens in ceca were determined on various days after infection. For BrdU incorporation assays, mice were injected i.p. with 0.8 mg of BrdU (Sigma-Aldrich) in PBS at 1 and 3 days before sacrifice.
In vitro T cell differentiation assays
Splenocytes were isolated from naive animals, labeled with CFSE (5 μg/ml; Molecular Probes) and cultured for 4 days with anti-CD3/anti-CD28 (1 μg/ml each, eBioscience) in the presence or absence of rRELMβ (1–10 μg/ml; PeproTech). CD4+ T cells, purified by negative depletion using homegrown supernatants (cell lines RAE, 2.43, M5114, and 2.4G2) and goat anti-rat magnetic beads (Qiagen) allowing >90% purity were CFSE labeled and activated with plate-bound anti-CD3/anti-CD28 in the presence or absence of rRELMβ (10 μg/ml). Four days later, cells were stimulated with PMA (50 ng/ml), ionomycin (500 ng/ml), and brefeldin A (10 μg/ml) (all from Sigma-Aldrich). Cells were surface stained with CD4, followed by intracellular staining with IFN-γ and IL-4 (all from eBioscience) allowing analysis by flow cytometry. Cell-free supernatants were harvested and analyzed for cytokine secretion by ELISA. For macrophage/dendritic cell (DC) coculture assays, BM macrophages and DC were generated as described previously (3, 27). Macrophages or DC were cultured overnight with medium alone or OVA protein (500 μg/ml; Worthington) in the presence or absence of rRELMβ (10 μg/ml) followed by two washes with warm medium to remove stimulus and coculture with CFSE-labeled OTII CD4+ T cells, purified by negative depletion as above, at a 4:1 T cell: macrophage ratio. Four days later, cells were pulsed with PMA, ionomycin, and brefeldin A as above, followed by cell recovery for flow cytometric analysis, and recovery of supernatants for cytokine analysis by ELISA.
Macrophage activation assays
For analysis of MAPK signaling, 1 × 106 BM macrophages were pulsed with rRELMβ (40 μg/ml), followed by lysis at time points indicated in HNTG buffer (0.1% Triton X-100, 20 mM HEPES, 10% glycerol, 150 mM NaCl, and 1 mM DTT, pH7.5) supplemented with a mixture of protease inhibitors (Roche). Whole cell lysates were resolved by SDS-PAGE, followed by immunoblotting with antiphosphorylated ERK1/2 and β-actin (all from Cell Signaling Technology). For analysis of surface activation markers and cytokine secretion, 1 × 106 macrophages were cultured overnight in medium alone, treated with rRELMβ (10 μg/ml) or LPS (100 ng/ml) followed by recovery of supernatants for measurement of cytokine secretion by ELISA and flow cytometric analysis of cells with Abs to F4/80 and MHC class II (all from eBioscience).
Analysis of Trichuris-induced immune responses
Mesenteric lymph node (MLN) or cecal patch cells from naive or infected mice were pulsed for 4–6 h with PMA, ionomycin, and brefeldin A followed by intracellular cytokine analysis by flow cytometry. For BrdU incorporation, MLN cells were stained ex vivo according to the BD Pharmingen BrdU staining protocol. For Ag-specific cytokine responses, MLN cells were plated in medium alone or in the presence of Trichuris ES Ag (50 μg/ml). Cell-free supernatants were harvested and analyzed for cytokine secretion by ELISA. Trichuris-specific IgG1 and IgG2a were measured by ELISA of serum dilutions from naive and infected mice using Trichuris Ag-coated plates (5 μg/ml).
RELMβ responses and histological analysis
RELMβ (Retnlb) gene expression analysis was performed by intestinal tissue RNA isolation using the Qiagen RNeasy Kit (Qiagen) followed by cDNA generation and real-time PCR using SYBR Green technology (Applied Biosystems) with RELMβ- and β-actin-customized primers (Qiagen). Reactions were run on the GeneAmp 5700 Sequence Detection System (Applied Biosystems). Similar to RELMβ, expression of Il18, Il12a, Ifng, and Il4 in the colons was measured using customized primers. Western blot analysis was performed as described previously (13). In brief, 20 μg of protein was isolated from fecal pellets and resolved by SDS-PAGE followed by immunoblotting for RELMβ with a polyclonal rabbit antimurine RELMβ Ab. For immunofluorescence and histological analysis, paraffin-embedded 4% paraformaldehyde (PFA)-fixed cecal tissue sections were stained for RELMβ as described previously (13) or with H&E or periodic acid-Schiff (PAS)/Alcian blue.
Statistical analysis
Results, representative of three or more separate experiments, represent the mean ± SEM of individual animals or conditions. Statistical significance was determined by the two-tailed Student’s t test. Results were considered significant when p < 0.05.
Results
Expulsion of Trichuris can occur in the absence of RELMβ expression
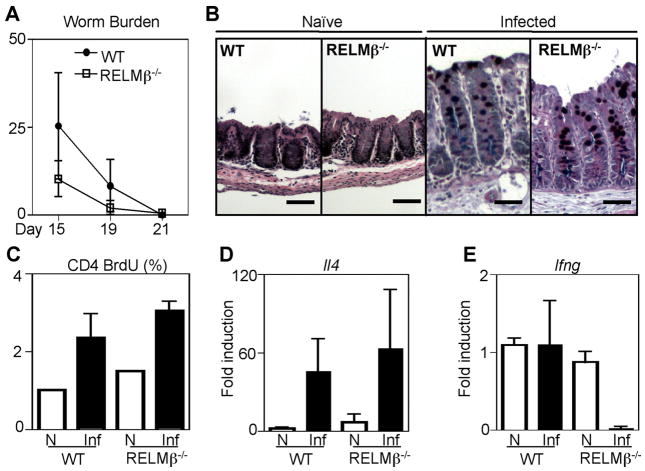
Our previous studies demonstrated that RELMβ was expressed following infection with the gastrointestinal helminth Trichuris and that in vitro RELMβ could bind to the parasite sensory apparatus and inhibit chemotaxis (13). To investigate a potential antiparasitic effector function for RELMβ in vivo, wild-type (WT) and RELMβ−/− mice were infected with 200 Trichuris eggs, and the kinetics of parasite clearance were examined. By day 15 after infection, both WT and RELMβ−/− mice exhibited equivalent worm expulsion and sterile immunity was achieved by day 21 in both genotypes (Fig. 1A). Since RELMβ was recently implicated in promoting mucus secretion (28), an important antiparasitic effector response (29), we examined goblet cell responses in naive and Trichuris-infected WT and RELMβ−/− mice. Histological analysis of PAS/Alcian blue-stained cecal tissue sections revealed that Trichuris infection induced equivalent intestinal crypt elongation and mucus production by goblet cells in both WT and RELMβ−/− mice, demonstrating that RELMβ deficiency did not noticeably alter goblet cell responses. Additionally, worm expulsion was associated with the equivalent expansion of CD4+ T cells from the draining MLN, examined by BrdU incorporation (Fig. 1C), and the induction of IL-4 mRNA in the colons of infected WT and RELMβ−/− mice at day 21 postinfection (Fig. 1D). Consistent with a polarized Th2 cytokine response, infected WT mice did not exhibit elevated levels of IFN-γ mRNA in the intestine compared with naive mice (Fig. 1E). Strikingly, basal levels of IFN-γ mRNA were reduced in the colons of infected RELMβ−/− mice (Fig. 1E). Taken together, these findings demonstrate that although goblet cell-derived RELMβ is not essential for the development of infection-induced Th2 cytokine responses or sterile immunity, it may influence IFN-γ responses in the intestinal microenvironment.
FIGURE 1.
RELMβ is not essential for expulsion of Trichuris. WT and RELMβ−/− mice were infected orally with 200 eggs and the kinetics of worm expulsion examined by parasite counts in the infected ceca at days 15, 19, and 21 after infection (A). B, PFA-fixed cecal tissue sections from naive and day 19-infected WT and RELMβ−/− mice were stained with PAS/Alcian blue for histological analysis. C, Frequency of proliferating CD4+ T cells from the draining MLN of naive (N; □) and day 19-infected (Inf; ■) mice was examined by BrdU incorporation. D and E, Expression of IL-4 (D) and IFN-γ (E) in the colons of naive and day 19-infected WT and RELMβ−/− mice was measured by real-time PCR. Results represent fold induction over naive WT mice ± SEM Bar, 50 μm.
RELMβ is expressed during chronic Trichuris infection and promotes expression of proinflammatory cytokines in vivo
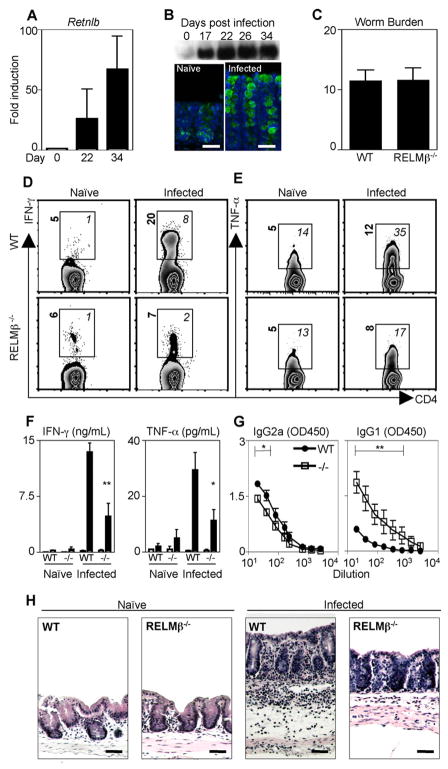
RELMβ was recently implicated in influencing host inflammatory responses. Specifically, we and others showed that RELMβ−/− mice exhibit reduced intestinal inflammation in a murine model of colitis (7, 15). Based on these findings, and the reduced levels of IFN-γ mRNA in the colons of infected RELMβ−/− mice (Fig. 1E), we hypothesized that goblet cell-derived RELMβ may influence infection-induced inflammatory responses during chronic infection. Infection with a low dose of Trichuris results in the production of IFN-γ, which promotes persistent infection in normally resistant hosts (21, 23). Following exposure to Trichuris, RELMβ (Retnlb) mRNA levels in the large intestine were up-regulated 25- and 60-fold over levels in naive mice at days 22 and 34 postinfection, respectively (Fig. 2A). Western blot analysis of luminally secreted RELMβ in the feces of infected mice revealed that RELMβ secretion was detectable by day 17 and sustained at day 34 (Fig. 2B, upper panel), and immunofluorescent microscopy analysis demonstrated that goblet cells were the cellular source of RELMβ in chronically infected mice (Fig. 2B, lower panel). To test whether RELMβ could influence expression of proinflammatory cytokines and the development of persistent Trichuris infection, WT and RELMβ−/− mice were infected with low-dose Trichuris. At day 22 after infection, WT and RELMβ−/− mice displayed similar parasite burdens (Fig. 2C). Ex vivo analysis of CD4+ T cells isolated from the cecal patch of naive and infected WT mice revealed an infection-induced elevation in the frequency of CD4+ T cells producing IFN-γ and TNF-α (Fig. 2, D and E, top panels, italics). This included an 8-fold increase in the frequency of IFN-γ -positive CD4+ T cells and a 2.5-fold increase in the frequency of TNF-α -positive CD4+ T cells. In addition to increased frequencies of cytokine-positive cells, infected WT mice exhibited elevated levels of IFN-γ and TNF-α produced per cell, as indicated by an elevation in mean fluorescent intensity (MFI) values (Fig. 2, D and E, top panels, bold). In contrast, the frequency and MFI of CD4+ T cells producing IFN-γ and TNF-α were reduced in infected RELMβ−/− mice compared with WT mice (Fig. 2, D and E, bottom panels). This included a 4-fold reduction in the percentage of IFN-γ -positive CD4+ T cells and a 2-fold reduction in TNF-α -positive CD4+ T cells compared with CD4+ T cells in infected WT mice. Upon Ag-specific restimulation, the MLN cells from infected RELMβ−/− mice also secreted significantly lower levels of IFN-γ and TNF-α than cells isolated from infected WT mice (Fig. 2F). Associated with the reduction in proinflammatory cytokine expression, infected RELMβ−/− mice also exhibited significantly reduced Trichuris-specific IgG2a Ab titers but elevated levels of Trichuris-specific IgG1 (Fig. 2G), indicative of a switch from a nonprotective type 1 response to a protective type 2 response in the absence of RELMβ. Given that IFN-γ and TNF-α can promote intestinal inflammation (30), we sought to test the hypothesis that RELMβ−/− mice may exhibit less severe infection-induced intestinal inflammation than WT mice. Histological analysis of H&E-stained cecal tissue revealed that WT mice developed severe infection-induced intestinal inflammation characterized by edema, influx of inflammatory cells to the lamina propria, and thickening of the muscularis (Fig. 2H). In contrast, despite similar levels of infection (Fig. 2C), infected RELMβ−/− mice did not exhibit demonstrable intestinal inflammation in comparison to naive controls. Taken together, these data implicate a role for RELMβ in promoting IFN-γ responses and infection-induced intestinal inflammation.
FIGURE 2.
RELMβ is expressed during chronic Trichuris infection and promotes expression of proinflammatory cytokines. A, C57BL/6 mice were infected orally with 30 Trichuris eggs and RELMβ (Retnlb) expression in the colon was measured as fold induction over naive by real-time PCR at days 22 and 34 after infection. Graph represents the mean fold induction over naive of four infected mice. B, Fecal samples pooled from four infected mice were collected on the days indicated and analyzed for RELMβ protein by Western blot. PFA-fixed cecal tissue from naive and day 34-infected mice was examined for RELMβ expression by immunofluorescence (green, RELMβ; blue, DAPI). C–H, C57BL/6 (WT) and RELMβ−/− mice were infected orally with 30 Trichuris eggs and sacrificed at day 22 for analysis. C, Worm counts in the ceca of eight WT and RELMβ−/− mice. D and E, Cecal patch cells were isolated from naive or infected WT or RELMβ−/− mice and the frequency of CD4+ T cells expressing IFN-γ (D) or TNF-α (E) was determined. Numbers represent the percentage of cytokine-positive CD4+ T cells. F, MLN cells isolated from naive or infected mice were cultured for 48 h with medium (□) or Trichuris Ag (■) and production of IFN-γ or TNF-α determined by ELISA. G, Sera from infected WT and RELMβ−/− mice were recovered for measurement of Ag-specific IgG2a and IgG1 by ELISA. H, PFA-fixed cecal tissue sections from naive and infected WT and −/− mice were stained with H&E for histological analysis. Bar, 50 μm. Error bars represent mean ± SEM from four mice in each infected group.*, p <0.05 and **, p < 0.01.
RELMβ−/− mice are resistant to chronic Trichuris infection and severe intestinal inflammation
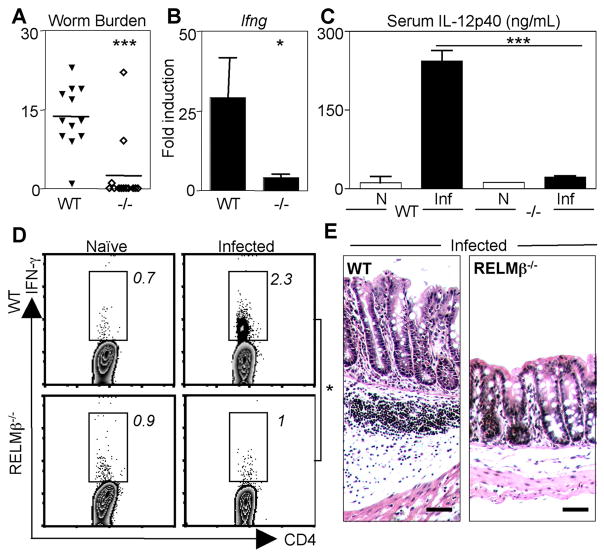
Expression of IFN-γ is associated with the establishment of chronic Trichuris infection and intestinal inflammation (21, 23, 31). Given the striking reduction in IFN-γ levels in infected RELMβ−/− mice at day 22 after infection, we sought to test the hypothesis that RELMβ−/− mice may be resistant to establishment of chronic infection. WT and RELMβ−/− mice were infected with low-dose Trichuris and sacrificed at day 34 for measurement of parasite burdens. Although WT mice harbored persistent infection, 10 of 12 RELMβ−/− mice had cleared infection (Fig. 3A). Resistance to chronic infection was associated with a significant reduction in IFN-γ mRNA expression in the colon of infected RELMβ−/− mice (Fig. 3B). Consistent with reduced Th1 cell responses, we observed a significant reduction in IL-12p40 protein in the serum of infected RELMβ−/− mice (Fig. 3C). Ex vivo analysis of the MLN cells from infected mice revealed that RELMβ−/− mice had a significantly reduced frequency of IFN-γ + CD4+ T cells in comparison to WT mice (Fig. 3D). Finally, infected RELMβ−/− mice exhibited minimal infection-induced inflammation in comparison to WT mice (Fig. 3E). Taken together, these data implicate goblet cell-derived RELMβ in the promotion of chronic Trichuris infection, expression of proinflammatory cytokines, and the development of severe intestinal inflammation.
FIGURE 3.
RELMβ−/− mice fail to establish chronic Trichuris infection. WT and RELMβ−/− mice were infected orally with 30 Trichuris eggs and mice were sacrificed at day 34 for analysis. A, Worm counts in the infected ceca of 12 WT and RELMβ−/− mice. B, Real-time PCR analysis as fold induction over naive of infected colons for Ifng. C, IL-12p40 levels in naive (N) and day 34-infected (Inf) WT and RELMβ−/− mice were measured by ELISA. D, MLN cells were isolated from naive or infected mice, and the frequency of CD4+ T cells expressing IFN-γ was determined by flow cytometry. Numbers represent the mean frequency of cytokine-positive CD4+ T cells from four individual mice. E, PFA-fixed cecal tissue sections from infected WT and RELMβ−/− mice were stained with H&E for histological analysis. Bar, 50 μm.*, p < 0.05 and ***, p < 0.001.
RELMβ augments IFN-γ expression in CD4+ T cells
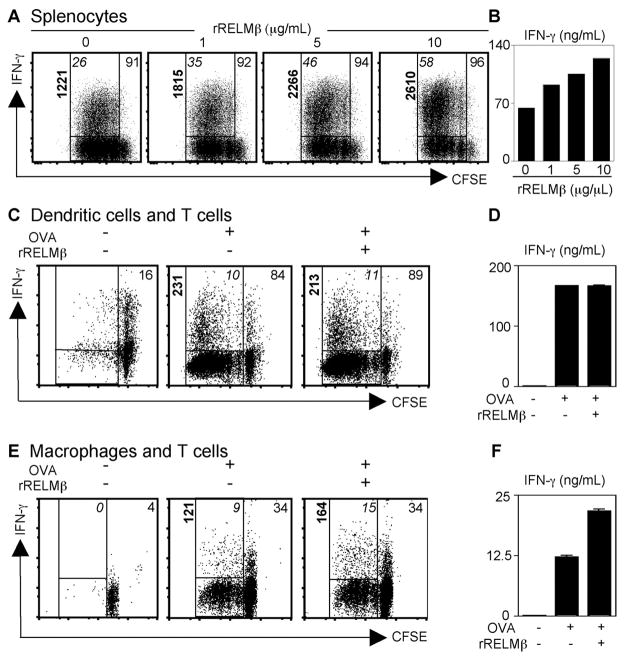
Given the striking reduction in IFN-γ expression in Trichuris-infected RELMβ−/− mice, we sought to test the hypothesis that RELMβ could directly influence T cell proliferation or IFN-γ production. CFSE-labeled splenocytes from naive C57BL/6 mice were stimulated with anti-CD3/anti-CD28 for 4 days in the presence or absence of increasing doses of rRELMβ. rRELMβ treatment had no effect on proliferation or cytokine production in un-stimulated CD4+ T cells, demonstrating that rRELMβ could not initiate T cell proliferation or Th1 cell differentiation (data not shown). However, following polyclonal stimulation, rRELMβ treatment resulted in a dose-dependent augmentation of IFN-γ production by CD4+ T cells (Fig. 4A; frequency of proliferating cells expressing IFN-γ denoted in upper left corner). The effect of rRELMβ on IFN-γ expression was independent of any effects on T cell proliferation (Fig. 4A, frequency of CFSE dim proliferating cells denoted in upper right corner). At maximal concentration, rRELMβ treatment resulted in a 2.2-fold up-regulation in the frequency of proliferating CD4+ T cells that expressed IFN-γ (Fig. 4A, upper left italics) and a greater than 2-fold increase in the amount of IFN-γ produced per cell as determined by the increase in MFI (Fig. 4A, bold). Finally, the dose-dependent elevation in IFN-γ secretion following treatment with rRELMβ was confirmed by ELISA (Fig. 4B). It is important to note that since the rRELMβ utilized was bacterially derived, the concentrations of protein used in vitro (1–10 μg/ml) may not reflect the concentration of native RELMβ in vivo.
FIGURE 4.
RELMβ stimulates CD4+ T cell-derived IFN-γ production through effects on macrophages. A, CFSE-labeled splenocytes were stimulated with anti-CD3/anti-CD28 in the presence of increasing doses of rRELMβ, followed by flow cytometric analysis. Numbers represent the percentage of CFSEdim CD4+ T cells (top right corner), the frequency of proliferated CD4+ T cells expressing IFN-γ (italics), and the MFI (bold). B, The dose-dependent effect of rRELMβ on IFN-γ secretion by anti-CD3/anti-CD28-stimulated splenocytes was measured by ELISA. C–E, Untreated or OVA-pulsed control or rRELMβ-treated DC (C and D) or macrophages (E and F) were cultured with CFSE-labeled OVA-specific CD4+ T cells. Numbers represent the percentage of CFSEdimCD4+ T cells (top right corner), the frequency of proliferated CD4+ T cells expressing IFN-γ (italics), and the MFI (bold) (C and E). Supernatants were recovered for measurement of IFN-γ secretion by ELISA (D and F). Results reflect cultures pooled from six to eight replicate wells per condition.
In vitro assays with purified CD4+ T cells demonstrated that rRELMβ did not directly act on CD4+ T cells (data not shown). Thus, to test whether RELMβ augmented CD4+ T cell production of IFN-γ through effects on accessory cells, BM-derived DC and macrophages were pulsed with OVA protein in the presence or absence of rRELMβ, followed by coculture for 4 days with CFSE-labeled OVA-specific CD4+ T cells. OVA-pulsed DC and macrophages promoted CD4+ T cell proliferation and IFN-γ production, as monitored by CFSE dilution and IFN-γ staining, demonstrating Ag-specific helper T cell differentiation (Fig. 4, C and E, middle panel). Although rRELMβ treatment had no noticeable effect on the ability of DC to drive Ag-specific T cell proliferation and IFN-γ production (Fig. 4, C, right panel, and D), treatment of OVA-pulsed macrophages with rRELMβ augmented their ability to drive Th1 cell differentiation. This included a 1.7-fold increase in the percentage of IFN-γ -positive CD4+ T cells (Fig. 4E, italic), a 1.6-fold augmentation in the amount of IFN-γ expressed per cell (Fig. 4E, bold), and elevated secretion of IFN-γ (Fig. 4F). Taken together, these results identify that goblet cell-derived RELMβ can augment Th1 cell differentiation in vitro through selective regulatory effects on macrophages. Furthermore, they suggest that one mechanism underlying reduced Th1 cell responses in RELMβ−/− mice may be through dysregulated macrophage responses.
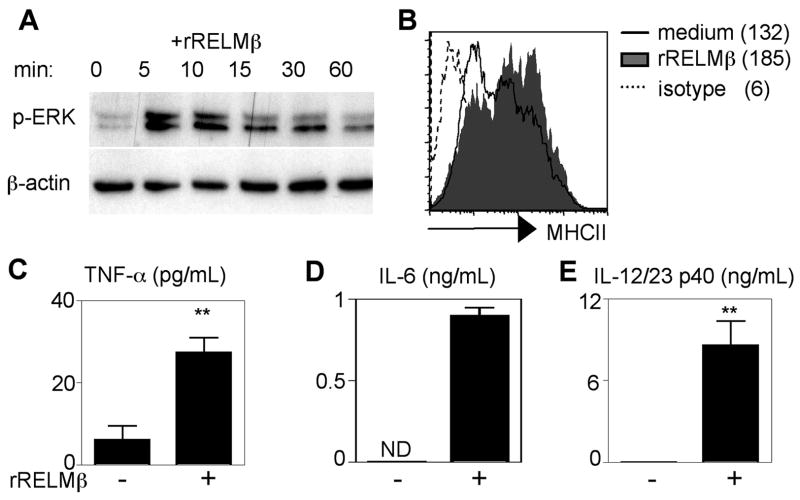
RELMβ activates macrophages to express MHC class II and proinflammatory cytokines
To examine the mechanism through which rRELMβ-treated macrophages augmented CD4+ Th1 cell responses, BM macrophages were stimulated with rRELMβ. rRELMβ treatment of macrophages induced ERK1/2 phosphorylation (Fig. 5A), demonstrating that macrophages are responsive to RELMβ. rRELMβ-treated macrophages also exhibited increased surface expression of MHC class II (Fig. 5B) and significant elevation in the secretion of TNF-α (Fig. 5C), IL-6 (Fig. 5D), and IL-12/23p40 (Fig. 5E). Taken together, these in vitro studies suggest that one mechanism by which rRELMβ augments CD4+ Th1 cell responses is through direct activation of macrophages to up-regulate MHC class II and IL-12/23p40 and provide a likely basis for the reduced Th1 responses and resistance to chronic infection and intestinal inflammation observed in Trichuris-infected RELMβ−/− mice (Fig. 6).
FIGURE 5.
RELMβ activates macrophages to express MHC class II and proinflammatory cytokines. A, BM macrophages were pulsed with rRELMβ and analyzed for MAPK activation by Western blot of cell lysates at the time points indicated for phospho-ERK (p-ERK) and β-actin. B, Control macrophages (bold) or rRELMβ-treated macrophages (shaded histogram) were recovered 18 h after exposure and surface stained for MHC class II or isotype controls (dashed). Numbers represent MFI. C–E, Supernatants from triplicate wells were recovered and analyzed by ELISA for TNF-α (C), IL-6 (D), and IL-12/23p40 secretion (E) ± SEM; **, p <0.01; ND, Not detected.
FIGURE 6.
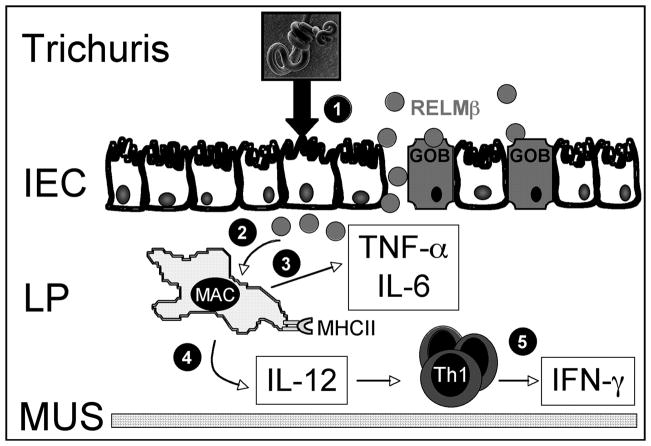
Model depicting the proposed role of RELMβ in promoting Th1 cytokine production and intestinal inflammation. Exposure of the intestinal epithelium (IEC) to Trichuris results in the production of RELMβ by goblet cells (GOB; (1)). Infection-induced injury and inflammation could lead to a breach in the intestinal barrier and consequently leakage of RELMβ into the lamina propria (LP; (2)). Exposure of recruited lamina propria macrophages (MAC) to RELMβ results in the production of proinflammatory cytokines TNF-α and IL-6, which will promote intestinal inflammation (3). Additionally, RELMβ-exposed macrophages up-regulate MHC class II (MHC II) and secrete IL-12 (4), which will promote IFN-γ production by effector Th1 cells recruited to the site of inflammation (5). MUS, Muscularis.
Discussion
Collectively, these studies identify a previously unappreciated role for goblet cell-derived RELMβ in the regulation of CD4+ T cell responses following enteric infection and support the growing concept that multiple lineages of IEC not only serve as a physical barrier to pathogens, but play dynamic roles in directly modulating innate and adaptive immune cell function in the gastrointestinal microenvironment (3, 32). Previous studies have reported that alterations in goblet cell function, in particular altered production of mucus or trefoil factors, are associated with increased susceptibility to intestinal inflammation (6, 33). We and others have also previously demonstrated that RELMβ promotes intestinal inflammation in chemically induced colitis (7, 15), and one mechanism of action may be through activation of macrophages to produce proinflammatory cytokines (7, 17). Using low dose Trichuris infection as a natural model of intestinal inflammation, we demonstrate that in addition to promoting innate inflammatory responses, RELMβ has dual functions in augmenting infection-induced inflammation and parasite-specific CD4+ Th1 cell responses. rRELMβ promoted expression of MHC class II and IL-12/23p40 in macrophages and enhanced their ability to drive Ag-specific Th1 cell differentiation. Macrophages are a dominant cell type recruited to the intestinal lamina propria following gastrointestinal helminth infection (34), and if activated by protective Th2 cytokines can mediate parasite expulsion (35). By augmenting IL-12 secretion and MHC class II expression, goblet cell-derived RELMβ may enhance the ability of infiltrating macrophages to promote or maintain Th1 effector cell function, thereby impeding Th2 cytokine-dependent parasite expulsion. In addition to a role in influencing naive T cell differentiation, IL-12 can also act on effector T cells to up-regulate IFN-γ production (36), thus increased IL-12 levels could influence previously activated T cells recruited into the lamina propria. As yet, there is no evidence that in addition to luminal secretion, RELMβ is secreted basolaterally. However, macrophage exposure to RELMβ may occur from a breach in the integrity of the IEC barrier due to colonic injury or through direct macrophage sampling of the luminal contents in a similar manner to lamina propria DC (37, 38).
Most gastrointestinal helminth infections provoke polarized Th2 cytokine responses, and we and others have previously shown that maximal Th2 cytokine production is associated with RELMβ expression (13, 14). In this study, we show that RELMβ expression is also induced and maintained in a Th1 cytokine-biased setting following establishment of chronic Trichuris infection, where it can act as a susceptibility factor by promoting CD4+ Th1 cell responses, chronic infection, and intestinal inflammation. Sequence analysis of the RELMβ promoter has revealed that in addition to STAT6-responsive elements implicating regulation by Th2 cytokines, RELMβ expression may also be regulated by Cdx2 and NFκB signaling, broadening the scope of factors that induce RELMβ (14, 39). Indeed, colonization of the intestine with commensal bacteria can promote RELMβ expression (39). Since Trichuris adult parasites actively embed within IEC (40), the resulting damage to this barrier may allow exposure to commensal bacteria and subsequent RELMβ expression. Supporting this hypothesis, LPS treatment of human goblet cell lines up-regulates RELMβ expression (39). Intriguingly, mice deficient in TLR4 and Myd88 signaling exhibit a similar phenotype to RELMβ−/− mice and are resistant to chronic Trichuris infection (22). Our findings implicate that RELMβ expression may be downstream of TLR4 signaling, potentially induced following exposure to commensal bacteria, and may provide the link between TLR4 signaling and the establishment of chronic Trichuris infection. Although RELMβ expression is detrimental to the host and promotes both parasite establishment and intestinal inflammation, this goblet cell-specific gene may have evolved to protect the intestinal barrier from invasion of both commensal bacteria as well as other intestinal pathogens that may require an adaptive Th1 cytokine response for clearance. Notwithstanding this, these findings support a critical role for IEC-macrophage interactions in shaping CD4+ T cell responses in the gut and suggest that the manipulation of goblet cell differentiation or RELMβ expression may offer therapeutic prospects for the treatment of infection-induced intestinal inflammation as well as other mucosal inflammatory diseases associated with dysregulated T cell responses.
Acknowledgments
We acknowledge Jackie Perrigoue, Amy Troy, and Betsy Taylor for critical reading of this manuscript.
Footnotes
Abbreviations used in this paper: IEC, intestinal epithelial cell; RELMβ, resistin-like molecule β; BM, bone marrow; MLN, mesenteric lymph node; WT, wild type; MFI, mean fluorescent intensity; DC, dendritic cell.
Disclosures
The authors have no financial conflict of interest.
This work was supported by National Institutes of Health Grants AI61570 and AI74878 (to D.A.), the Burroughs Wellcome Fund’s Investigator in Pathogenesis of Infectious Disease Award (to D.A.), the Crohn’s and Colitis Foundation of America’s William and Shelby Modell Family Foundation Research Award (to D.A.), and pilot grants from the University of Pennsylvania Center for Infectious Diseases and University Research Fund (to D.A.). C.Z. is funded by the Irvington Institute Fellowship Program of the Cancer Research Institute.
References
- 1.Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331–341. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 2.Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, Huth M, Nikolaev A, Neufert C, Madison B, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 3.Zaph C, Troy AE, Taylor BC, Berman-Booty LD, Guild KJ, Du Y, Yost EA, Gruber AD, May MJ, Greten FR, et al. Epithelial-cell-intrinsic IKK-β expression regulates intestinal immune homeostasis. Nature. 2007;446:552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 4.van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, Clevers H. Notch/γ-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 5.Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- 6.Mashimo H, Wu DC, Podolsky DK, Fishman MC. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science. 1996;274:262–265. doi: 10.1126/science.274.5285.262. [DOI] [PubMed] [Google Scholar]
- 7.McVay LD, Keilbaugh SA, Wong TM, Kierstein S, Shin ME, Lehrke M, Lefterova MI, Shifflett DE, Barnes SL, et al. Absence of bacterially induced RELMβ reduces injury in the dextran sodium sulfate model of colitis. J Clin Invest. 2006;116:2914–2923. doi: 10.1172/JCI28121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 9.Steppan CM, Brown EJ, Wright CM, Bhat S, Banerjee RR, Dai CY, Enders GH, Silberg DG, Wen X, Wu GD, Lazar MA. A family of tissue-specific resistin-like molecules. Proc Natl Acad Sci USA. 2001;98:502–506. doi: 10.1073/pnas.98.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel SD, Rajala MW, Rossetti L, Scherer PE, Shapiro L. Disulfide-dependent multimeric assembly of resistin family hormones. Science. 2004;304:1154–1158. doi: 10.1126/science.1093466. [DOI] [PubMed] [Google Scholar]
- 11.Yang RZ, Huang Q, Xu A, McLenithan JC, Eisen JA, Shuldiner AR, Alkan S, Gong DW. Comparative studies of resistin expression and phylogenomics in human and mouse. Biochem Biophys Res Commun. 2003;310:927–935. doi: 10.1016/j.bbrc.2003.09.093. [DOI] [PubMed] [Google Scholar]
- 12.Nair MG, I, Gallagher J, Taylor MD, Loke P, Coulson PS, Wilson RA, Maizels RM, Allen JE. Chitinase and Fizz family members are a generalized feature of nematode infection with selective upregulation of Ym1 and Fizz1 by antigen-presenting cells. Infect Immun. 2005;73:385–394. doi: 10.1128/IAI.73.1.385-394.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Artis D, Wang ML, Keilbaugh SA, He W, Brenes M, Swain GP, Knight PA, Donaldson DD, Lazar MA, Miller HR, et al. RELMβ/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc Natl Acad Sci USA. 2004;101:13596–13600. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang ML, Shin ME, Knight PA, Artis D, Silberg DG, Suh E, Wu GD. Regulation of RELM/FIZZ isoform expression by Cdx2 in response to innate and adaptive immune stimulation in the intestine. Am J Physiol. 2005;288:G1074–G1083. doi: 10.1152/ajpgi.00442.2004. [DOI] [PubMed] [Google Scholar]
- 15.Hogan SP, Seidu L, Blanchard C, Groschwitz K, Mishra A, Karow ML, Ahrens R, Artis D, Murphy AJ, Valenzuela DM, et al. Resistin-like molecule β regulates innate colonic function: barrier integrity and inflammation susceptibility. J Allergy Clin Immunol. 2006;118:257–268. doi: 10.1016/j.jaci.2006.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mishra A, Wang M, Schlotman J, Nikolaidis NM, Debrosse CW, Karow ML, Rothenberg ME. Resistin-like molecule-β is an allergen-induced cytokine with inflammatory and remodeling activity in the murine lung. Am J Physiol. 2007;293:L305–L313. doi: 10.1152/ajplung.00147.2007. [DOI] [PubMed] [Google Scholar]
- 17.Barnes SL, Vidrich A, Wang ML, Wu GD, Cominelli F, Rivera-Nieves J, Bamias G, Cohn SM. Resistin-like molecule β (RELMβ/FIZZ2) is highly expressed in the ileum of SAMP1/YitFc mice and is associated with initiation of ileitis. J Immunol. 2007;179:7012–7020. doi: 10.4049/jimmunol.179.10.7012. [DOI] [PubMed] [Google Scholar]
- 18.Bancroft AJ, Else KJ, Sypek JP, Grencis RK. Interleukin-12 promotes a chronic intestinal nematode infection. Eur J Immunol. 1997;27:866–870. doi: 10.1002/eji.1830270410. [DOI] [PubMed] [Google Scholar]
- 19.Bancroft AJ, McKenzie AN, Grencis RK. A critical role for IL-13 in resistance to intestinal nematode infection. J Immunol. 1998;160:3453–3461. [PubMed] [Google Scholar]
- 20.Else KJ, Finkelman FD, Maliszewski CR, Grencis RK. Cytokine-mediated regulation of chronic intestinal helminth infection. J Exp Med. 1994;179:347–351. doi: 10.1084/jem.179.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bancroft AJ, Else KJ, Grencis RK. Low-level infection with Trichuris muris significantly affects the polarization of the CD4 response. Eur J Immunol. 1994;24:3113–3118. doi: 10.1002/eji.1830241230. [DOI] [PubMed] [Google Scholar]
- 22.Helmby H, Grencis RK. Essential role for TLR4 and MyD88 in the development of chronic intestinal nematode infection. Eur J Immunol. 2003;33:2974–2979. doi: 10.1002/eji.200324264. [DOI] [PubMed] [Google Scholar]
- 23.Helmby H, Takeda K, Akira S, Grencis RK. Interleukin (IL)-18 promotes the development of chronic gastrointestinal helminth infection by downregulating IL-13. J Exp Med. 2001;194:355–364. doi: 10.1084/jem.194.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bancroft AJ, Humphreys NE, Worthington JJ, Yoshida H, Grencis RK. WSX-1: a key role in induction of chronic intestinal nematode infection. J Immunol. 2004;172:7635–7641. doi: 10.4049/jimmunol.172.12.7635. [DOI] [PubMed] [Google Scholar]
- 25.Hayes KS, Bancroft AJ, Grencis RK. Immune-mediated regulation of chronic intestinal nematode infection. Immunol Rev. 2004;201:75–88. doi: 10.1111/j.0105-2896.2004.00193.x. [DOI] [PubMed] [Google Scholar]
- 26.Artis D, Villarino A, Silverman M, He W, Thornton EM, Mu S, Summer S, Covey TM, Huang E, Yoshida H, et al. The IL-27 receptor (WSX-1) is an inhibitor of innate and adaptive elements of type 2 immunity. J Immunol. 2004;173:5626–5634. doi: 10.4049/jimmunol.173.9.5626. [DOI] [PubMed] [Google Scholar]
- 27.Perrigoue JG, Li J, Zaph C, Goldschmidt M, Scott P, de Sauvage FJ, Pearce EJ, Ghilardi N, Artis D. IL-31-IL-31R interactions negatively regulate type 2 inflammation in the lung. J Exp Med. 2007;204:481–487. doi: 10.1084/jem.20061791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krimi RB, Kotelevets L, Dubuquoy L, Plaisancie P, Walker F, Lehy T, Desreumaux P, Van Seuningen I, Chastre E, Forgue-Lafitte ME, Marie JC. Resistin-like molecule β regulates intestinal mucous secretion and curtails TNBS-induced colitis in mice. Inflamm Bowel Dis. 2008;14:931–941. doi: 10.1002/ibd.20420. [DOI] [PubMed] [Google Scholar]
- 29.Finkelman FD, Shea-Donohue T, Morris SC, Gildea L, Strait R, Madden KB, Schopf L, Urban JF., Jr Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol Rev. 2004;201:139–155. doi: 10.1111/j.0105-2896.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- 30.Plevy SE, Landers CJ, Prehn J, Carramanzana NM, Deem RL, Shealy D, Targan SR. A role for TNF-α and mucosal T helper-1 cytokines in the pathogenesis of Crohn’s disease. J Immunol. 1997;159:6276–6282. [PubMed] [Google Scholar]
- 31.Artis D, Potten CS, Else KJ, Finkelman FD, Grencis RK. Trichuris muris: host intestinal epithelial cell hyperproliferation during chronic infection is regulated by interferon-γ. Exp Parasitol. 1999;92:144–153. doi: 10.1006/expr.1999.4407. [DOI] [PubMed] [Google Scholar]
- 32.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Buller HA, Dekker J, Van Seuningen I, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 34.deSchoolmeester ML, Little MC, Rollins BJ, Else KJ. Absence of CC chemokine ligand 2 results in an altered Th1/Th2 cytokine balance and failure to expel Trichuris muris infection. J Immunol. 2003;170:4693–4700. doi: 10.4049/jimmunol.170.9.4693. [DOI] [PubMed] [Google Scholar]
- 35.Anthony RM, Urban JF, Jr, Alem F, Hamed HA, Rozo CT, Boucher JL, Van Rooijen N, Gause WC. Memory TH2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med. 2006;12:955–960. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mullen AC, High FA, Hutchins AS, Lee HW, Villarino AV, Livingston DM, Kung AL, Cereb N, Yao TP, Yang SY, Reiner SL. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292:1907–1910. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 37.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 38.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–2852. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He W, Wang ML, Jiang HQ, Steppan CM, Shin ME, Thurnheer MC, Cebra JJ, Lazar MA, Wu GD. Bacterial colonization leads to the colonic secretion of RELMβ/FIZZ2, a novel goblet cell-specific protein. Gastroenterology. 2003;125:1388–1397. doi: 10.1016/j.gastro.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 40.Worley DE, Meisenhelder JE, Sheffield HG, Thompson PE. Experimental studies on Trichuris muris in mice with an appraisal of its use for evaluating anthelmintics. J Parasitol. 1962;48:433–437. [PubMed] [Google Scholar]