Abstract
Peripheral T-cell lymphomas are a heterogeneous group that often requires the use of ancillary testing for accurate diagnosis. This is particularly applicable to the diagnosis of angiommunoblastic T-cell lymphoma (AITL) and peripheral T-cell lymphoma, unclassified (PTCLU), because of their histologic and immunophenotypic overlap with reactive lymphoid proliferations. Recently, immunohistochemistry for programmed death-1 (PD-1), a marker of follicular helper T-cells, was shown to be sensitive in the detection of AITL and PTCLU. The sensitivity of this marker in reactive entities, however, has not been adequately evaluated. We confirm that PD-1 staining is a highly sensitive marker in the diagnosis of peripheral T-cell lymphomas: increased extrafollicular PD-1-positive cells were seen in 93% (76/82) of AITL, 62% (16/26) of PTCLU, and 11% (2/18) of ALK-negative anaplastic large cell lymphomas. The majority of reactive lymphadenopathies including Cat-scratch disease, Kikuchi lymphadenitis, Castleman disease and reactive follicular hyperplasia showed no PD-1 staining outside follicles. Some reactive lymph nodes, showed increased extrafollicular PD-1-positive cells in a pattern similar to AITL and PTCLU, and include progressive transformation of germinal centers, viral lymphadenitis (EBV and HIV) and Rosai-Dorfman disease. This study demonstrates that PD-1-positive cells may be increased in a number of settings other than T-cell lymphomas. We conclude that staining for PD-1 in reactive and atypical lymphadenopathies should be interpreted with caution and in the context of other ancillary immunophenotypic and molecular studies before a diagnosis of AITL or PTCLU is entertained.
Keywords: PD-1, T-cell lymphoma, viral, lymphadenitis, immunohistochemistry, reactive lymphadenopathy
INTRODUCTION
Peripheral T-cell lymphomas are a heterogeneous group of disorders largely derived from post-thymic T-cells. Of these, the most common types encountered in Western populations include angioimmunoblastic T-cell lymphoma (AITL) and peripheral T-cell lymphoma, unclassified (PTCLU). Historically, distinguishing among the categories of T-cell lymphomas was based on a combination of clinical and histopathologic features. Immunohistochemistry can be a useful adjunct in separating some categories of T-cell lymphomas (ie. ALK-positive anaplastic large cell lymphoma and gamma-delta T-cell lymphomas), however, it can be a less sensitive modality with respect to AITL and PTCLU.11
Recently, gene expression profiling has shown that among CD4+ T-cells, the germinal center or T-follicular helper cell (Tfh) subset exhibits a distinct genetic signature.2,7 Among the genes that are differentially expressed in Tfh cells are members of the programmed death family of CD28-related molecules: PD-1, CXCL13 and Bcl-6. PD-1 (and its ligands PD-L1 and PD-L2) serves to deliver signals that help balance T-cell activation and regulate peripheral tolerance of self-reactive T-cells. Additionally, it is thought that induction of PD-1 by T-follicular helper cells within the germinal center promotes survival and selection of B-cells with high affinity immunoglobulin receptor.5 Several authors have shown that CXCL13 and PD-1 are preferentially expressed by the atypical T-cells in AITL, and to a lesser extent, PTCLU.3,4,9,10,15 In addition, PD-1 is also a useful marker in nodular lymphocyte predominant Hodgkin lymphoma (NLPHL) where it highlights the presence of characteristic T-cell rosettes that encircle the neoplastic B-cells.8,10
Even as PD-1 immunohistochemistry is gaining popularity as an aid in the diagnosis of peripheral T-cell lymphomas, its utility is not well defined in non-neoplastic settings where the specificity of expanded populations of PD-1-positive Tfh cells is largely unexplored. The broad spectrum of cytologic features and associated milieu of reactive cells that typify most T-cell proliferations confound their separation from reactive entities and often require additional ancillary studies for T-cell clonality. Moreover, experience with PD-1 staining is limited in reactive as well as neoplastic settings in which disruption of the germinal center is encountered. Thus, the purpose of this study was to investigate the pattern of immunoreactivity of PD-1 in T-cell lymphomas and various reactive entities to better define its utility in the separation of T-cell lymphomas from morphologic mimics in their differential diagnosis.
MATERIALS AND METHODS
Case selection
Cases were selected from the archives of Stanford University Department of Pathology, from June 2006 to December 2008. All cases were reviewed by two pathologists (CK and YN) and diagnoses were assigned according to the revised WHO 2008 classification.11 Cases in the various diagnostic categories were selected prior to evaluating PD-1 staining. Institutional Review Board approval was obtained for these studies.
One hundred and thirty nine peripheral T-cell lymphomas (104 from the consultation files and 35 from a previously constructed tissue microarray) were evaluated. The 104 consultation cases included 80 AITL, 14 PTCLU and 10 ALK-negative ALCL; whole sections from these cases were used for all immunophenotypic studies and details of these cases are summarized in Table 1. Tissue microarrays were used to screen for PD-1 positivity in a wide range of hematolymphoid diagnoses and al so included: 2 AITL, 12 PTCLU, 8 ALK-negative ALCL and 13 T-lymphoblastic lymphoma. Among PTCLU consult cases, 8 were nodal and 6 were extra-nodal (breast, bone marrow, palate, small & large bowel). ALK-positive ALCL cases were not included as these are usually more easily separated from other T-cell lymphomas and reactive entities by their positivity for ALK1. Cases of AITL were further classified using nomenclature described by Attylgale et al.1 In cases with sufficient material to adequately evaluate a morphologic subtype, type I morphology occurred in 16/79 (20%) cases, type II morphology occurred in 7/79 (9%) cases, and type III morphology occurred in 56/79 (71%) cases. For PTCLU cases, both nodal (8) and extranodal (6) cases were included and there were CD4-positive (5) and negative (4) cases; CD4 information was not available in 5 cases. Additional features of the AITL, PTCLU and ALK-negative ALCL are provided in Table 1.
Table 1.
Characteristic features of consultation cases representing AITL, PTCLU and ALK (−) ALCL
| AITL | PTCLU | ALK (−) ALCL | |
|---|---|---|---|
| TOTAL CASES | 80 | 14 | 10 |
| AGE | 65 | 61 | 60 |
| SEX | 40F / 40M | 11 M / 3 F | 6 M / 4 F |
| T-CELL CLONE DETECTED | 24 / 43 | 5 / 6 | 1 / 1 |
| 56% | 83% | 100% | |
| B-CELL CLONE DETECTED | 11 / 30 | 2 / 3 | N.D. |
| 37% | 67% | N/A | |
| EBV POSITIVE BY ISH | 46 / 72 | 2 / 11 | 1 / 5 |
| 64% | 18% | 20% | |
| LARGE B-CELL PROLIFERATION | 34 / 80 | 1 / 14 | 0 / 10 |
| 43% | 7% | 0% | |
| EXPANDED FDCS | 66 / 70 | 0/14 | N.D. |
| 94% | 0% | N/A | |
| CXCL13 | 68 / 76 | 1 / 4 | 0 / 2 |
| 89% | 25% | 0% | |
| PD-1 | 74 / 80 | 10 /14 | 2 / 10 |
| 93% | 71% | 20% | |
Abbreviations: AITL – angioimmunoblastic T-cell lymphoma; PTCLU – peripheral T-cell lymphoma, unclassified; ALCL – anaplastic large cell lymphoma; ISH – in-situ hybridization; FDCS – follicular dendritic cells. In-situ hybridization for the presence of EBV was performed as described in the materials and methods section. Presence of a large B-cell proliferation was determined by a combination of immunohistochemistry, detection of EBV RNA by in situ hybridization in large cells and the presence of a B-cell clone by molecular methods.12,13 Only 7 of 34 instances where AITL was complicated by a large B-cell proliferation lacked detectable EBV within neoplastic cells by in-situ hybridization. Expanded FDCs refer to the presence of expanded follicular dendritic cell networks as detected by CD21 immunohistochemistry.
Reactive lymphoid disorders included 26 reactive follicular hyperplasia (15 with and 11 without follicle lysis), 4 cat scratch disease, 5 Kikuchi lymphadenitis, 14 Castleman disease (11 hyaline vascular type and 3 plasma cell variants), 35 progressive transformation of germinal centers (PTGC), 6 atypical paracortical hyperplasia, 10 EBV lymphadenitis, 2 HIV lymphadenitis and 3 Rosai-Dorfman disease. Information on HIV status was not available for all cases with follicle lysis. Only cases of cat scratch disease in which the presence of B. henslae organisms were detected by immunohistochemistry, were included. One PTGC case showed a small focus of NLPHL within a background of florid PTGC nodules. Non-neoplastic tissues employed in this study included 5 tonsils showing reactive hyperplasia and 10 bone marrow biopsies without involvement by a hematolymphoid process (negative staging bone marrow biopsies).
Immunohistochemistry
Immunohistochemical analysis was performed on 4 micron, formalin-fixed, paraffin-embedded sections, after microwave-assisted antigen retrieval in 0.1 M citrate buffer (pH 6.0), and subsequent incubation with 3% hydrogen peroxide. Staining for PD-1 antibody (clone NAT105, 1:40 dilution; courtesy of G. Roncador, Centro Nacional de Investigaciones Oncologicas, Madrid, Spain), using an automated stainer (Dako Autostainer, Carpinteria, CA, USA).
Cases were considered to have a “normal” PD-1 staining pattern if PD-1 reactivity was confined to intrafollicular cells only and no PD-1-positive cells were found outside follicles; the positive cells were often localized to the periphery of the germinal centers.3,10 Cases were considered to have an “abnormal” PD-1 reactivity if PD-1-positive cells were present outside follicles (extrafollicular). This abnormal pattern of extrafollicular PD-1 expression was scored positive for the purposes of this study. When appropriate, qualitative description of PD-1 staining (weak vs. strong) intensity was made using the intensity of normal Tfh cells as a baseline. All other markers used for the work-up of T-cell lymphomas at our center and the conditions used for immunohistochemistry are summarized in Table 2; note that not all markers were used in all cases. For example, CD21 was used in all cases to evaluate the presence and the extent of follicular dendritic cell meshworks. Immunohistologic markers selected to highlight T-cells (CD2, CD3, CD5, CD4, CD8, CD43) varied depending on the availability of flow cytometry data. CD10 was performed only in a minority of cases, as we find that the sensitivity of CXCL13 and PD-1 to detect Tfh cells is superior.
Table 2.
Reagents and conditions used for immunohistochemistry
| Antibody | Clone | Automated stainer, retrieval and detection methods |
Dilution | Manufacturer |
|---|---|---|---|---|
| CD20 | L26 | Ventana; Standard retrieval | 1:1000 | Dako Corporation Capinteria, CA |
| CD3 | Rabbit polyclonal |
Microwave, Tris 0.5 m, pH 10 |
1:50 | Cell Marque, Hot Springs, AZ |
| CD5 | 4C7 | Ventana; Standard retrieval | 1:200 | Leica/Novocastra Newcastle Upon Tyne, UK |
| CD2 | AB7S | Ventana; Standard retrieval | 1:200 | Leica |
| CD7 | CD7-272 | Ventana; Standard retrieval | 1:50 | Leica |
| CD4 | IF6 | Ventana; Standard retrieval | 1:20 | Leica |
| CD8 | C8/144B | Ventana; Standard retrieval | 1:200 | Dako |
| CD30 | BerH2 | Ventana; Mild retrieval | 1:40 | Dako |
| CD15 | Carb3 | Dako; citrate retrieval with MACH2 polymer detection |
1:1500 | Dako |
| CD43 | L60 | Ventana; Standard retrieval | 1:2000 | BD Biosciences San Jose, CA |
| CD246 | ALK-1 | Ventana; Standard retrieval | 1:100 | Dako |
| TIA-1 | 2G9 | Dako; citrate retrieval with MACH2 polymer detection |
1:20 | Becton-Coulter Cedex, France |
| CD56 | 123C3 | Dako; citrate retrieval with MACH2 polymer detection |
1:20 | Invitrogen Camarillo, CA |
| CD21 | IF8 | Ventana; Protease retrieval | 1:20 | Dako |
| CXCL13 | 53610 | Ventana; Mild retrieval | 1:200 | R&D Systems Inc Minneapolis, MN |
| CD10 | 56C6 | Ventana; Standard retrieval | 1:20 | Leica |
| TCR beta | BF1/8A3 | Dako proteinase K; Envision | 1:50 | Thermo Scientific Rockford, IL |
| Ki67 | Polyclonal | Microwave, Tris 0.5 m, pH 10 |
1:1000 | Dako |
Note: Not all stains were performed on all cases. Immunohistologic panels were selected based on H&E morphology and other available ancillary data such as flow cytometry and moleculae studies for T- and B-cell clonality.
In-situ hybridization
EBV-encoded small RNA (EBER) was detected from 4 micron, formalin-fixed, paraffin-embedded tissue sections by in situ hybridization using a Ventana Bench-Mark instrument running a standardized program incorporating deparaffinization, hybridization to the Inform EBER probe cocktail, and staining with ISH iVIEW nitro blue tetrazolium (Ventana Medical Systems, Tucson, AZ).
Polymerase chain reaction for B-cell and T-cell clonality
DNA was obtained from formalin-fixed paraffin sections by cutting four to eight 20-µm-thick sections and deparaffinizing by extracting three times in 1.0 ml of xylene (Fisher Scientific, Pittsburgh, PA). The extracted tissue was washed two times in 1.0 ml of 100% ethanol (Gold Shield Chemical Co., Hayward, CA) and then dried at 65°C. The tissue was resuspended in 2 volumes (50 to 200 µl) of a mixture of 4× PCR buffer II (Applied Biosystems, Foster City, CA), 0.1% sodium dodecyl sulfate, and 0.6 mg/ml proteinase K (Stratagene, La Jolla, CA) and incubated at 65°C overnight. Samples were further purified using a DNeasy Tissue kit (Qiagen, Valencia, CA) according to the manufacturer’s protocols and eluting the DNA in 100 to 200 µl of DNase/RNase-free water (Invitrogen, Carlsbad, CA).
Cases were evaluated for TRG and IGH clonality using commercially available PCR-based kits (InVivoScribe Technologies, San Diego, CA) based on a European collaborative study (BIOMED-2 Concerted Action).13 The PCRs were performed according to the manufacturer’s protocols and by the methods described by Tan et al.12 Briefly, a 5-µl aliquot of DNA sample was added to 45 µl of each reaction mixture and 1.25 units of AmpliTaq Gold (Applied Biosystems). Amplification was performed using a PTC-200 thermocycler (M&J Research, Waltham, MA) by initially heating at 95°C for 7 minutes, followed by 35 cycles of 95°C for 45 seconds, 60°C for 45 seconds, and 72°C for 90 seconds. The final step was incubation at 72°C for 10 minutes.
After amplification, 1 µl of PCR product was added to 10 µl of Hi-Di Formamide (Applied Biosystems) and 1 µl of ROX-500 internal size standard (Applied Biosystems). The mixture was then denatured at 95°C for 5 minutes, chilled on ice for 5 minutes, and resolved by capillary electrophoresis on an ABI 3100 instrument using performance optimized polymer-4 (Applied Biosystems). The data were stored electronically and analyzed using GeneScan software (Applied Biosystems). Criteria for assigning a positive or negative result were similar to those used for clinical specimens at the Stanford Molecular Pathology laboratory. Briefly, the products of a primer set were considered clonal if one or two distinct peaks were present within the expected size range. To be considered clonal, the height of at least one distinct peak had to exceed that of the polyclonal background by at least twofold.
RESULTS
PD-1 staining in normal hematopoietic tissue
In normal tonsils, PD-1 staining showed consistent membrane and cytoplasmic reactivity in lymphocytes restricted to the germinal center. In some cases, previously cut sections from older paraffin embedded tissue samples (generally greater than 4–5 years in storage) showed weaker staining intensity or lack of reactivity in the cells of interest. This phenomenon was less pronounced in newly cut tissue sections. The 10 uninvolved staging bone marrow biopsies of patients with non-Hodgkin lymphomas and acute myeloid leukemias, showed no PD-1 staining among normal bone marrow hematopoietic cells (data not shown). A subset of megakaryocytes showed cytoplasmic staining in occasional marrow biopsies; the significance of this finding is unclear. One bone marrow containing a reactive secondary follicle showed a normal distribution of PD-1-positive cells within the follicle.
PD-1 staining in T-cell lymphomas
The expression of extrafollicular PD-1-positive cells was essentially restricted to mature T-cell lymphomas and was seen in 93% (76/82) of AITL, 62% (16/26) of PTCLU and 11% (2/18) of ALK-negative ALCL.
The 79 cases of AITL from consultation lymph node material showed variably expanded paracortical zones containing atypical mononuclear cells, a concomitant increase in the number of arborizing high-endothelial venules and expanded follicular dendritic cell meshworks as highlighted by CD21 immunohistochemistry. The pattern of PD-1 reactivity ranged from focal areas of increased numbers of extrafollicular T-cells (usually less than 5% of interfollicular cells), to diffuse reactivity throughout the lesion (Figure 1). This latter pattern was the most common, and was present in 64 of 79 (81%) lymph node AITL cases. Both PD-1 and CXCL13 showed a similar frequency in highlighting expanded populations of extrafollicular Tfh-cells (93% vs 89%; Table 3). In the single AITL case involving the bone marrow, PD-1 reactivity was seen in large atypical lymphoma cells in one focus, but was not disseminated throughout the marrow.
Figure 1. Typical morphologic and immunohistochemical findings in Angioimmunoblastic T-cell lymphoma.
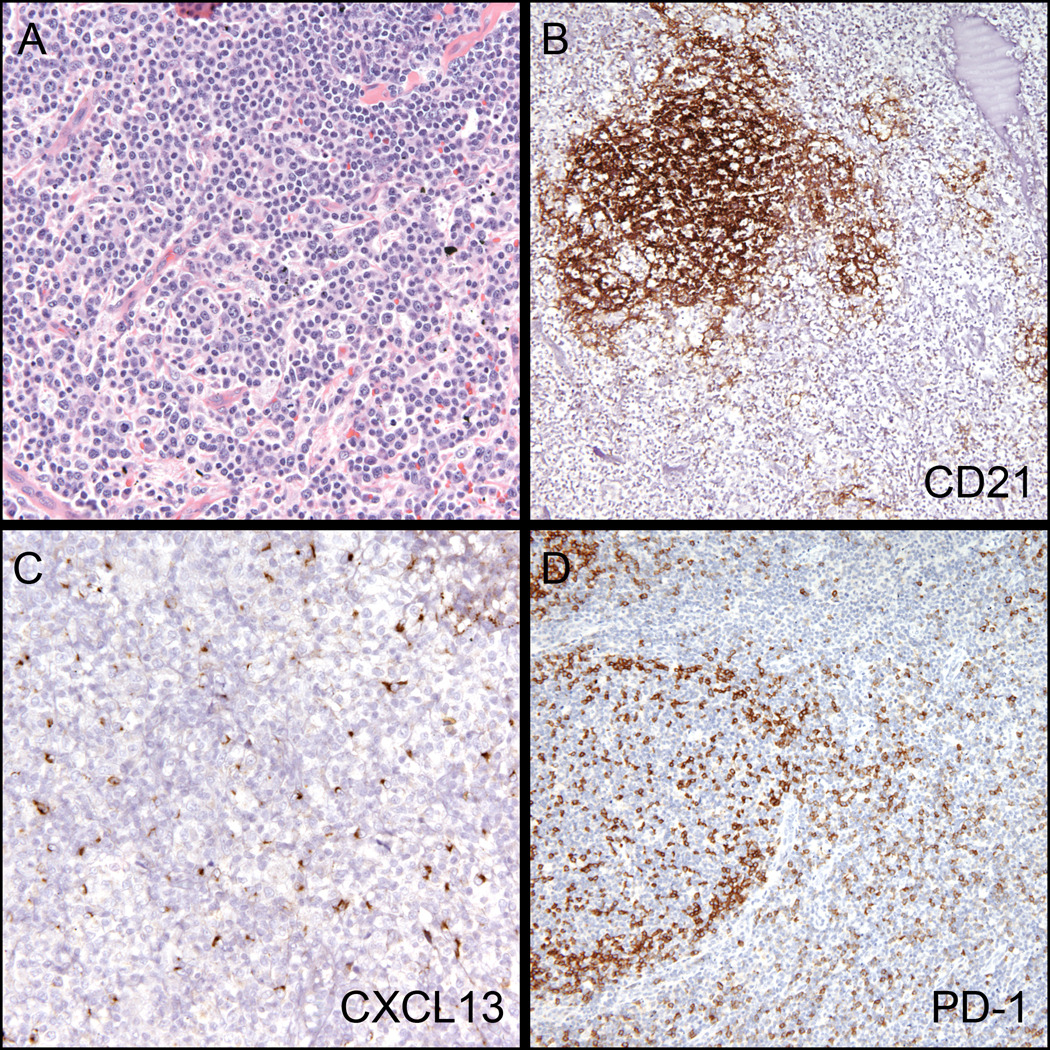
(A) Diffuse paracortical expansion comprised of atypical lymphocytes, eosinophils and prominent blood vessels (H&E, 200x). (B) Expanded follicular dendritic cell network (CD21, 100x). (C) Increased labeling with CXCL13 within the paracortex (CXCL13, 200x). (D) Increased labeling with PD-1 within the paracortex – note normal pattern in germinal center. (PD-1, 200x).
Table 3.
PD-1 immunoreactivity in T-cell lymphomas
| PD-1 clone |
AITL | PTCLU | ALK (−) ALCL |
T-ALL | |
|---|---|---|---|---|---|
| Dorfman et al.3 | EH12 | 100% (23/23) |
0% (0/14) |
0% (0/12) |
0% (0/5) |
| Roncador et al.10 | NAT | 86% (42/49) |
17% (5/30) |
0% (0/1) |
0% (0/20) |
| Rodriguez-Pinilla et al.9 | NAT | 95% (56/59) |
30% (24/81) |
17% (1/6) |
N/A |
| Krishnan et al. (current study) |
NAT | 93% (76/82) |
62% (16/26) |
11% (2/18) |
0% (0/13) |
| TOTALS | 93% (197/213) |
30% (45/151) |
8% (3/37) |
0% (0/38) |
|
Abbreviations: ATIL – angioimmunoblastic T-cell lymphoma; PTCLU – peripheral T-cell lymphoma, unclassified; ALCL – anaplastic large cell lymphoma; T-ALL – T-cell lymphoblastic lymphoma. The data provided for the current study include all whole section and tissue microarray T-cell cases studied.
In contrast to our cases of AITL, the 16 cases of PTCLU displayed diffuse effacement of normal lymph node architecture without preservation of secondary follicles. There was a predominance of relatively monotonous, medium-to-large atypical lymphocytes with variably prominent nucleoli. Scattered areas within the lesion displayed heterogeneity with respect to lymphocyte size, presence of histocytes and prominence of high-endothelial venules. The latter was not a consistent finding throughout these cases. Expanded or extrafollicular dendritic cell meshworks, as highlighted by CD21, were absent. Two cases contained Reed-Sternberg-like (RS-like) cells that stained with CD30 and CD15. These RS-like cells were not present in any of our AITL cases. One case was complicated by a large B-cell proliferation, as identified by a combination of morphologic and immunohistochemical findings.6,12,14 Among the PTCLU cases, 71% (10/14) showed strong PD-1 staining in increased numbers of extrafollicular cells.
Likewise, among our 10 cases of ALK-negative ALCL, a predominant population of large, atypical lymphocytes with irregular nuclear contours was observed. Most of these cases showed near-complete effacement of the normal lymph node architecture, or discrete partial involvement within the lymph node in a sinusoidal pattern. High-endothelial venules were not as prominent, and extrafollicular expansions of follicular dendritic cells were absent. PD-1 labeled increased numbers of extrafollicular cells in a minority of cases, 10% (2/10).
The cases of T-lymphoblastic lymphoma did not show an abnormal pattern of reactivity. Most of these cases showed effacement of the lymph node architecture by the lymphoma and seldom provided normal residual follicles for evaluation. Nevertheless, aside from the rare PD-1-positive cell admixed within the lymphoma, PD-1 expression was minimal in these cases.
PD-1 staining in reactive and non-neoplastic conditions
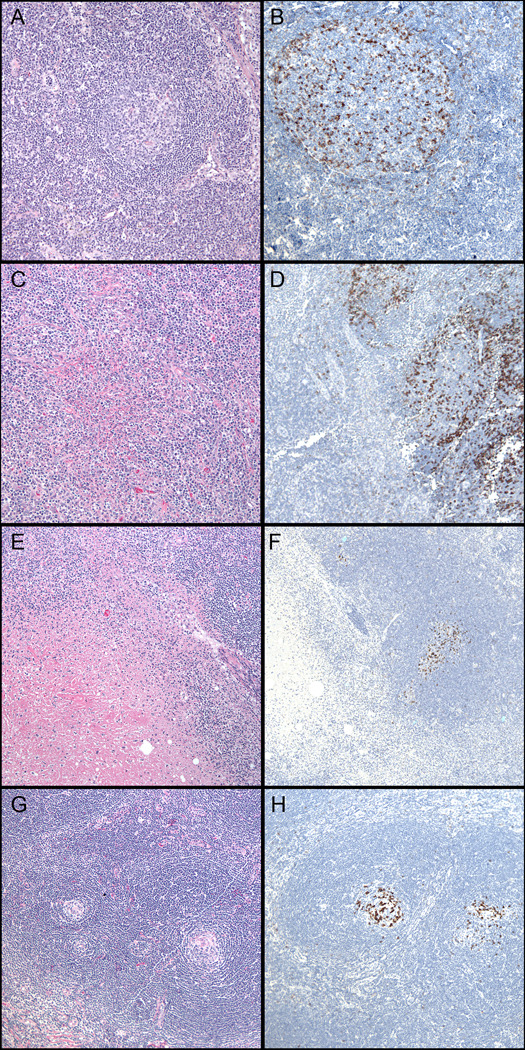
A normal pattern of PD-1 staining was observed in the subset of reactive and non-neoplastic conditions that we studied (Table 4). The expected pattern of PD-1-positive Tfh-cells that was localized primarily to the periphery of germinal centers, was seen. This was the pattern consistently encountered in uncomplicated cases of reactive follicular hyperplasia (Figure 2A,B). Similarly, in Cat-scratch disease, a normal PD-1 pattern was observed and increased numbers of PD-1-positive cells were not seen in association with granulomas or microabscesses (Figure 2C,D). Kikuchi lymphadenitis showed a normal pattern of PD-1 expression in the unaffected portions of the lymph node; PD-1-positive Tfh-cells were not present in areas of geographic necrosis or apoptotic debris (Figure 2E,F). On occasion, scattered PD-1-positive cells were present at the periphery of these lesions, and likely represent cells associated with disrupted remnants of germinal centers. Both hyaline-vascular and plasma cell variants of Castleman disease showed few PD-1-positive Tfh-cells associated with atrophic follicles. Only rare, small lymphocytes labeled with PD-1 in the extrafollicular areas (Figure 2G,H).
Table 4.
Reactive conditions showing a normal* pattern of PD-1 expression
| Diagnosis | N | Description of PD-1 Staining |
|---|---|---|
| Reactive lymphoid tissue |
15 | PD-1-positive Tfh cells are present along the periphery of germinal centers |
| Reactive lymphoid tissue with follicle lysis |
11 | PD-1-positive Tfh cells are restricted to germinal centers. There is no increase in PD-1 staining in areas of follicle lysis |
| Cat Scratch Disease |
4 | No PD-1 reactivity associated with granulomas or abscesses |
| Kikuchi Lymphadenitis |
5 | No PD-1 reactivity associated with necrotizing areas |
| Castleman Disease | 14 | Few PD-1-positive cells are present within atrophic follicles |
Abbreviations: Tfh – follicular helper T-cells; N – number of cases studied.
A normal PD-1 staining pattern refers to the presence of PD-1-positive cells that are essentially localized within germinal centers of secondary follicles.
Figure 2. PD-1 staining patterns in non-neoplastic or reactive lymphoid conditions.
(A,B) Reactive follicular hyperplasia (H&E, left, 100x). Note accentuation at the periphery of the germinal center and few positive cells in the mantle zones (PD-1, right, 200x). (C,D) Cat-scratch disease (H&E, left, 100x & PD-1, right, 100x). (E,F) Kikuchi lymphadenitis (H&E, left, 100x). Note absence of staining within regions of necrosis (PD-1, right, 100x). (G,H) Castleman Disease, hyaline vascular type (H&E, left 100x). Note atrophic follicles with dense collection of PD-1 staining (PD-1, right, 100x).
In contrast, we did encounter several non-neoplastic settings in which the pattern of PD-1 staining closely resembled the pattern observed in AITL or PTCLU. These disorders included cases of progressive transformation of germinal centers (PTGC), atypical paracortical hyperplasia (without evidence of T-cell lymphoma), lymphadenopathy associated with viral infections including examples of HIV, EBV and CMV lymphadenitis, and Rosai-Dorfman disease (Table 5). Overall, these cases showed a wide range of PD-1 staining from less intense staining in few interfollicular cells (5–10% of cells) to diffuse staining in the majority of interfollicular cells (>80% of cells).
Table 5.
Reactive conditions showing an abnormal* pattern of PD-1 expression
| N | Age (years) | PD-1 | Comment | |
|---|---|---|---|---|
| Atypical paracortical hyperplasia |
6 | 34.1 (13 – 73) |
83% (5/6) |
All cases showed a paracortical expansion with variable numbers of atypical cells, but minimal histologic suggestion of IM-like changes |
| Progressive transformation of germinal centers |
35 | 36.5 (11 – 69) |
100% (35/35) |
All cases show increased numbers of PD-1-positive cells in affected nodules, as well as increased numbers of extrafollicular PD-1- positive cells in between affected nodules |
| EBV Lymphadenitis |
10 | 16.4 (2 – 37) |
80% (8/10) |
Diffuse PD-1-positive cells within the paracortex is seen in all positive cases; 5 of 8 cases showed weak staining intensity |
| HIV lymphadenitis |
2 | 47.5 (38 – 57) |
100% (2/2) |
Paracortical hyperplasia with increased PD-1- positive; one case contained granulomas and one case also showed CMV infection |
| Rosai-Dorfman Disease | 3 | 44.6 (39 – 54) |
67% (2/3) |
Both positive cases showed diffuse PD-1-positive T-cells. PD-1 does not label characteristic histiocytes exhibiting emperipolesis |
Abbreviations: IM: infectious mononucleosis; EBV – Epstein Barr Virus; HIV – Human immunodeficiency virus; CMV – cytomegalovirus; N – number of cases studied.
An abnormal PD-1 staining pattern refers to the presence of PD-1-positive cells in extrafollicular (interfollicular) areas.
Lymph nodes exhibiting PTGC showed a unique pattern of PD-1 expression: both a marked increase in the number of follicle Tfh-cells and increased extrafollicular PD-1-positive cells were present in the affected portions of the lymph node (Figure 4A–C). This pattern of PD-1 staining was not uniformly distributed in the unaffected regions of the lymph node. One case of PTGC was notable in that after review of the PD-1 staining, it was determined that a focus of NLPHL was present. This case showed both increased numbers of PD-1-positive cells in the areas adjacent to PTGC nodules, as well as Tfh-cell rosettes encircling the characteristic large B-cells of NLPHL (Figure 4D).
Figure 4. PD-1 patterns in PTGC and NLPHL.
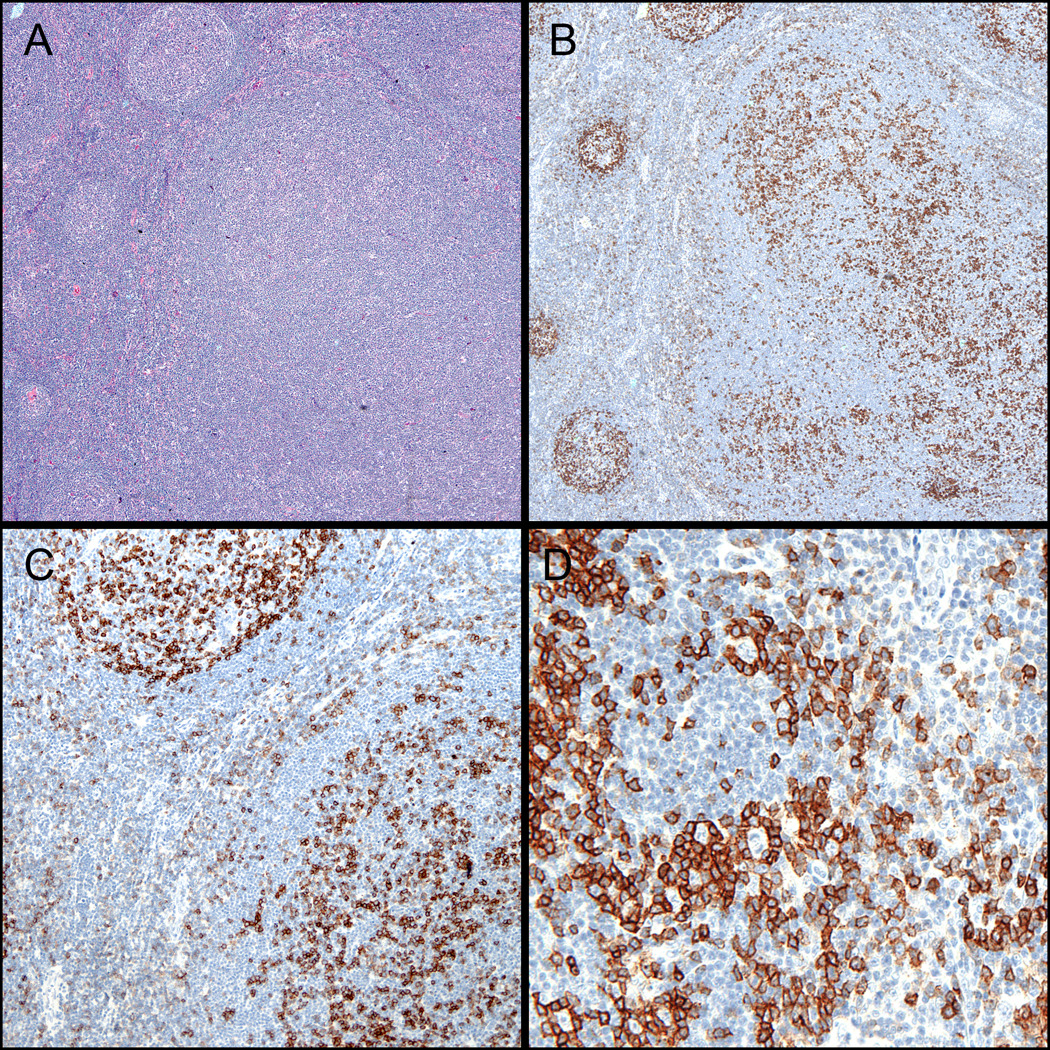
(A) PTGC – note the prominent nodule with disrupted germinal center adjacent to reactive follicles (H&E, 100x). (B) PD-1 staining is increased within the PTGC nodules (100X). (C) PD-1 staining is also increased in the paracortical zones surrounding the PTGC nodules (200x). (D) PD-1-positive GC-T-cell rosettes are clearly highlighted in a case previously diagnosed as florid PTGC but was found to have focal involvement by NLPHL (400X).
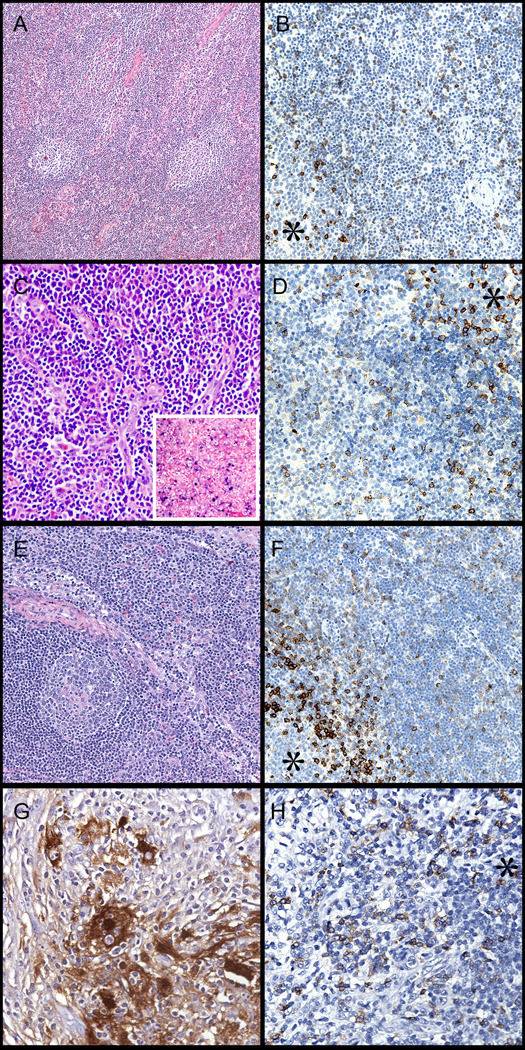
A large proportion of cases with viral lymphadenitis (HIV, EBV or CMV) and paracortical hyperplasia demonstrated expanded populations of extrafollicular PD-1-positive cells (Figure 5A, B; Table 5). The staining pattern closely mimicked the pattern seen in peripheral T-cell lymphomas; however, a peripheral T-cell lymphoma was excluded based on the combination of clinical findings and ancillary immunophenotypic and molecular studies. Overall, 83% of cases with an atypical paracortical hyperplasia displayed extrafollicular PD-1-positive cells; all but one of these cases showed strong PD-1 staining in the extrafollicular compartment. Lymph nodes demonstrating infectious mononucleosis (confirmed by in-situ hybridization for EBV RNA) showed the most striking examples of extensive extrafollicular PD-1 staining (Fig. 5C, D). Eighty percent of these cases demonstrated extensive extrafollicular PD-1-positive cell populations, albeit with variable staining intensity. These cases encompassed a mixture of lymph nodes and tonsils from children and young adults with unifocal lymphadenopathy. A similar pattern of PD-1 staining was seen in two lymph nodes from HIV-positive patients (Figure 5E, F). One of these nodes also demonstrated foci of necrosis with CMV-positive cells (confirmed by immunohistochemistry). Another case contained scattered granulomas in a background of paracortical hyperplasia and was associated with increased numbers of extrafollicular PD-1-positive cells.
Figure 5. Reactive conditions showing an abnormal pattern of PD-1 expression.
* Asterisk designates a germinal center. (A,B) Atypical paracortical hyperplasia in 22 year old female. Note the presence of reactive germinal centers and increased vasculature in the paracortex (H&E, left, 100x). PD-1 labels many cells outside of follicles. (PD-1, right, 200x). (C,D) Infectious mononucleosis in 19 year old male (H&E, left, 200x & EBV in-situ hybridization, inset). PD-1 labeling in the paracortex, with germinal center (asterisk) for comparison (right, 200x). (E,F) Lymph node in HIV infected patient (H&E, left, 100x). PD-1 labeling in paracortex with germinal center for comparison (right, 200x). (G,H) Rosai-Dorfman Disease in the soft tissue of a 41-year old man (S100 stain, left, 400x). Note the typical histiocyte of this disorder and the presence of emperipolesis. PD-1 stains many cells throughout the lesion (right, 200x).
Expanded PD-1-positive cells were seen in a lymph node and soft tissue mass exhibiting Rosai-Dorfman disease (sinus histiocytosis with massive lymphadenopathy). Both cases displayed a diffuse proliferation of predominantly T-cells, in addition to other features of Rosai-Dorfman disease such as fibrosis and increased plasma cells. No reactivity for PD-1 was noted in the characteristic hitiocytes exhibiting emperipolesis (Figure 5G, H).
DISCUSSION
Recent gene expression profiling and immunohistologic studies have shown that a subset of peripheral T-cell lymphoma is derived from follicular helper T-cells with specific immunoreactivity for CD10, CXCL13 and PD-1.2,3,7,9,10,15 Given the growing support that PD-1 is a sensitive marker of Tfh-cells, PD-1 can be a powerful diagnostic tool for identifying lymphomas derived from that cell type, most specifically, AITL.3,9,10,15 In an effort to better delineate its diagnostic utility, we evaluated the immunoreactivity of PD-1 in a variety of reactive and non-neoplastic conditions in comparison to peripheral T-cell lymphomas. Here, we report our experience with PD-1 as a sensitive, but not specific, marker of AITL and PTCLU. Our findings that an abnormal PD-1 staining pattern defined by the presence of extrafollicular PD-1-positive cells can be seen in several reactive conditions, particularly, viral lymphadenitis, points to the need for caution in the interpretation of PD-1 staining patterns in the evaluation of lymphadenopathy mimicking lymphoma.
A common histologic feature of both AITL and PTCLU is the presence of expanded paracortical zones by atypical lymphocytes.11 The presence of increased numbers of extrafollicular PD-1-positive cells allows the focus of further ancillary tests to confirm or rule out a neoplastic T-cell proliferation. While this pattern serves to alert the diagnostician to the possibility of an underlying T-cell process, unfortunately, it can also be seen in the setting of a variety of reactive conditions. Usually, in the appropriate clinical setting (ie. young age, history of active viral infection, unifocal lymphadenopathy), the histologic, immunophenotypic and molecular findings provide sufficient data to arrive at an appropriate diagnosis; however, this distinction becomes more difficult when clinical information or tissue is limited. The practical utility of PD-1 staining would be greatly enhanced if it could reliably distinguish peripheral T-cell lymphomas from its non-neoplastic mimics.
In cases of AITL, our observations are consistent with findings reported in the literature and show extrafollicular expansions of PD-1-positive cells in a high proportion (93% in our cohort) of cases3,9,10,15 PD-1-positive cells were particularly enriched in areas unassociated with lymphoid follicles. We also encountered a higher frequency of PTCLU cases (62%) that exhibit expanded PD-1-positive cells than has been reported in three other studies.3,9,10 Factors that may account for this difference include the lack of reproducibility in morphologic criteria used to separate AITL from PTCLU. To this end, morphologic overlap between classic AITL and PTCLU is becoming increasingly recognized.9 In addition, variation in laboratory methods used for PD-1-staining may have resulted in differences in sensitivity in detecting PD-1-positive cells. We also surveyed a broad spectrum of T- and NK-cell lymphomas and confirmed that PD-1 staining is not found in the majority of neoplasms that do not meet criteria for AITL or PTCLU. Thus, it appears that among neoplastic T-cell lymphoproliferative disorders, PD-1-staining is a sensitive marker of AITL or PTCLU.
Prior studies of PD-1 immunostaining in “normal” tissues include several examples each of normal and reactive lymph nodes, tonsils, spleen and thymic tissue. In the initial studies by Dorfman et al., the expected germinal center T cell-specific pattern of PD-1 staining was described in lymph nodes and splenic tissue, using double-labeling for CD3 and CD20.3 Roncador and colleagues reported a similar distribution of PD-1-positive T-cells in tonsillar tissue.10 Together, these studies established a well recognized and expected localization of PD-1-positive T-cells in secondary lymphoid follicles in normal tissues. Our findings of PD-1 reactivity confirmed previously published observations. In addition, several cases of staging bone marrow biopsies uninvolved by disease showed that normal hematopoietic tissues lack PD-1 reactivity.
Information is limited about PD-1 reactivity in non-neoplastic conditions. From a diagnostic standpoint, the use of PD-1 immunoreactivity in the differential diagnosis of peripheral T-cell lymphomas requires confident exclusion of non-neoplastic causes based on the staining pattern. Therefore, we evaluated several reactive lesions that bear resemblance to peripheral T-cell lymphomas that often enter the morphologic differential diagnosis, particularly in cases where an expanded paracortical zone with variably increased vasculature is present. In our experience, this pattern of paracortical hyperplasia often serves to alert the diagnostician to the possibility of an underlying T-cell lymphoproliferative process.
Overall, we observed three patterns of PD-1 staining among our reactive cases: normal, PTGC and PTCL-like. A normal PD-1 pattern in which the PD-1-positive cells were confined to secondary follicles was observed in most cases of reactive lymphadenopathy. Entities in this category include cat-scratch disease, Kikuchi lymphadenitis, Castleman disease and reactive follicular hyperplasia (including those exhibiting follicle lysis). Somewhat unexpectedly, we also found that a significant number of non-neoplastic conditions that we studied showed an abnormal PD-1 staining pattern which included PTGC and paracortical expansions of unknown etiology. The staining pattern in the setting of PTGC was originally described by Nam-Cha and colleagues, and is characterized by increased PD-1-positive cells in affected nodules.8 We observed increased PD-1 immunoreactivity in all 35 cases of PTGC that we studied. Specifically, markedly increased numbers of PD-1-positive cells were seen in affected PTGC nodules (at least 2–3x the number of Tfh cells), as compared to unaffected germinal centers. In addition, we noted that there were increased numbers of extrafollicular PD-1 positive cells within the surrounding paracortical areas adjacent to the PTGC nodules. These interfollicular regions showed a similar pattern of PD-1 reactivity to that of AITL or PTCLU, and may be a source of misinterpretation, especially on a small or core needle biopsy. One case of PTGC was particularly informative in that an area within the enlarged lymph node showed features of NLPHL that were not previously recognized. While both the PTGC and NLPHL areas contained increased numbers of PD-1-positive cells, rosettes of PD-1-positive cells encircling large atypical cells were only seen in the NLPHL component. These findings underscore the value of PD-1 staining in the interpretation of PTGC and NLPHL, where the spectrum of histologic changes show significant overlap and lead to diagnostic difficulty.
Expanded extrafollicular PD-1-positive cells were also particularly prominent in viral lymphadenitis. There is significant morphologic overlap between reactive viral adenopathies and peripheral T-cell lymphomas, and in some instances, patients with a peripheral T-cell lymphoma may also harbor secondary viral infections or viral-associated lymphoid proliferations. In such cases, it is very difficult to ascertain whether an expanded PD-1-positive population represents a T-cell lymphoma or viral-related changes. We studied cases of unifocal lymphadenopathy in children or young adults that largely represented changes secondary to viral infections (infectious mononucleosis, HIV or CMV lymphadenitis), and where the possibility of a peripheral T-cell lymphoma was less likely.
These cases showed expanded populations PD-1-postive cells in keeping with expansion of Tfh-cells induced by active viral infections.5 Nonetheless, the pattern of PD-1 staining in this setting underscores the importance of recognizing that extrafollicular PD-1 staining is not specific for peripheral T-cell lymphomas. Three cases of Rosai-Dorfman disease occurring in the soft-tissue also showed increased numbers of PD-1-positive cells. The significance of this finding is unclear, but may represent disruption of germinal centers or an association between expansion of Tfh-cells in conditions involving immune dysregulation. Nonetheless, additional studies in Rosai-Dorfman disease as well as related histiocytic disorders are needed to further assess the significance of this finding and the diagnostic overlap these disorders may share with peripheral T-cell and other lymphoproliferative processes.
In summary, our findings show that increased numbers of PD-1-positive Tfh-cells appear to be a relatively specific finding among T-cell lymphomas that is seen in ATIL and PTCLU. This finding, however, can be encountered in non-neoplastic settings and include viral-lymphadenitis, non-viral paracortical hyperplasias, PTGC and Rosai-Dorfman disease. Most other reactive lymphadenopathies that we studied did not show an abnormal PD-1 pattern. Similarly, normal bone marrow precursors lack PD-I staining, although PD-1-positive cells are seen in bone marrows involved by AITL or within reactive lymphoid aggregates. Our studies indicate that extrafollicular PD-1 staining should be interpreted with caution and correlated with clinical, morphologic and other ancillary studies to assess for a peripheral T-cell lymphoma. This study therefore provides a much-needed framework for the interpretation of PD-1 staining and an assessment of its specificity in a variety of diagnostic settings.
Figure 3. PD-1 staining in bone marrow biopsies.
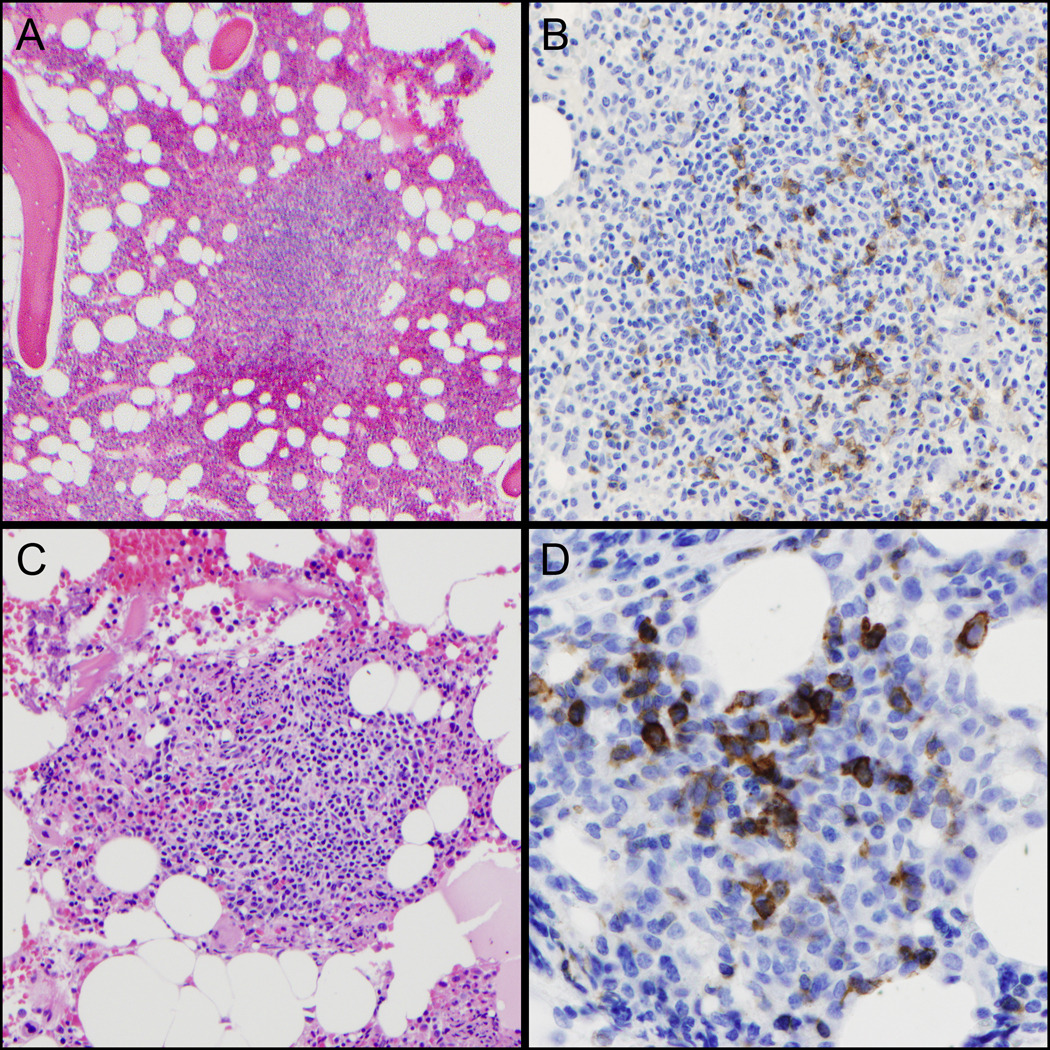
(A,B) Reactive lymphoid aggregate with germinal center. PD-1 (right) stains scattered small GC-T-cells in the area of the germinal center (H&E, left, 100x & PD-1, right, 200x). (C,D) Bone marrow involvement by AITL. The lymphoid aggregate contains a mixture of eosinophils and branching vessels (H&E, left, 200x). PD-1 (right) is strongly positive in the large, atypical lymphoma cells (right, 400x).
Acknowledgments
Grant Support: YN is supported i n part by NIH P0I CA34233 and NIH R01CA122105
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Attygalle AD, Kyriakou C, Dupuis J, et al. Histologic evolution of angioimmunoblastic T-cell lymphoma in consecutive biopsies: clinical correlation and insights into natural history and disease progression. Am. J. Surg. Pathol. 2007;31(7):1077–1088. doi: 10.1097/PAS.0b013e31802d68e9. [DOI] [PubMed] [Google Scholar]
- 2.Chtanova T, Tangye SG, Newton R, et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173(1):68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 3.Dorfman DM, Brown JA, Shahsafaei A, Freeman GJ. Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. Am. J. Surg. Pathol. 2006;30(7):802–810. doi: 10.1097/01.pas.0000209855.28282.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dupuis J, Boye K, Martin N, et al. Expression of CXCL13 by neoplastic cells in angioimmunoblastic T-cell lymphoma (AITL): a new diagnostic marker providing evidence that AITL derives from follicular helper T cells. Am. J. Surg. Pathol. 2006;30(4):490–494. doi: 10.1097/00000478-200604000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008 Aug;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 6.Higgins JP, van de Rijn M, Jones CD, et al. Peripheral T-cell lymphoma complicated by a proliferation of large B cells. Am. J. Clin. Pathol. 2000;114(2):236–247. doi: 10.1309/72CM-KAXF-66DE-4XVA. [DOI] [PubMed] [Google Scholar]
- 7.Kim CH, Lim HW, Kim JR, et al. Unique gene expression program of human germinal center T helper cells. Blood. 2004;104(7):1952–1960. doi: 10.1182/blood-2004-03-1206. [DOI] [PubMed] [Google Scholar]
- 8.Nam-Cha S, Roncador G, Sanchez-Verde L, et al. PD-1, a Follicular T-cell Marker Useful for Recognizing Nodular Lymphocyte-predominant Hodgkin Lymphoma. Am. J. Surg. Pathol. 2008;32(8):1252–1257. doi: 10.1097/PAS.0b013e318165b0d6. [DOI] [PubMed] [Google Scholar]
- 9.Rodríguez-Pinilla SM, Atienza L, Murillo C, et al. Peripheral T-cell lymphoma with follicular T-cell markers. Am J Surg Pathol. 2008;32(12):1787–1799. doi: 10.1097/PAS.0b013e31817f123e. [DOI] [PubMed] [Google Scholar]
- 10.Roncador G, García Verdes-Montenegro J, Tedoldi S, et al. Expression of two markers of germinal center T cells (SAP and PD-1) in angioimmunoblastic T-cell lymphoma. Haematologica. 2007;92(8):1059–1066. doi: 10.3324/haematol.10864. [DOI] [PubMed] [Google Scholar]
- 11.Swerdlow S, Campo E, Harris N, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue. Fourth. World Health Organization; 2008. [Google Scholar]
- 12.Tan BT, Warnke RA, Arber DA. The frequency of B- and T-cell gene rearrangements and epstein-barr virus in T-cell lymphomas: a comparison between angioimmunoblastic T-cell lymphoma and peripheral T-cell lymphoma, unspecified with and without associated B-cell proliferations. The Journal of Molecular Diagnostics: JMD. 2006;8(4):466–475. doi: 10.2353/jmoldx.2006.060016. quiz 527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Dongen JJM, Langerak AW, Brüggemann M, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003;17(12):2257–2317. doi: 10.1038/sj.leu.2403202. [DOI] [PubMed] [Google Scholar]
- 14.Warnke RA, Jones D, Hsi ED. Morphologic and immunophenotypic variants of nodal T-cell lymphomas and T-cell lymphoma mimics. Am. J. Clin. Pathol. 2007;127(4):511–527. doi: 10.1309/QBLAMA321K9AD2XK. [DOI] [PubMed] [Google Scholar]
- 15.Yu H, Shahsafaei A, Dorfman DM. Germinal-Center T-Helper-Cell Markers PD-1 and CXCL13 Are Both Expressed by Neoplastic Cells in Angioimmunoblastic T-Cell Lymphoma. Am J Clin Pathol. 2009;131(1):33–41. doi: 10.1309/AJCP62WRKERPXDRT. [DOI] [PubMed] [Google Scholar]