Abstract
Objective
To determine the levels of two endogenous inhibitors of angiogenesis, thrombospondin-1 (TSP1) and pigment epithelium-derived factor (PEDF), in the vitreous fluids from patients with and without diabetes.
Methods
The levels of TSP1 and PEDF in vitreous samples from diabetic and age-matched nondiabetic patients were determined by Western blot analysis.
Results
We observed significant amounts of TSP1 and PEDF in the vitreous samples of control eyes. The TSP1 levels varied in samples from patients with diabetes. In contrast, PEDF levels showed little or no change in vitreous samples from patients with or without diabetes. However, the PEDF protein exhibited a variation in its molecular weight among the samples. We consistently observed lower levels of TSP1 in diabetic patients that expressed the higher molecular weight PEDF (H) isoform.
Conclusions and Clinical Relevance
Our results suggest that in diabetes the changes in the TSP1 level may play a role in shifting the angiogenic balance and contributing to the pathogenesis of diabetic retinopathy. Although the PEDF level did not change, the diabetic samples with the PEDF (H) isoform consistently showed lower levels of TSP1. Thus, the presence of the PEDF (H) isoform may be associated with a greater risk for severe diabetic retinopathy.
Introduction
Diabetic retinopathy is a serious microvascular complication and is a major cause of adult blindness when it progresses to the proliferative stage with active neovascularization. It is characterized by early microvascular damage and capillary non-perfusion resulting in retinal ischemia and retinal neovascularization (1-3). The retinal neovascularization is driven by ischemia, which results in increased production of several stimulators of angiogenesis, and perhaps decreased production of inhibitors of angiogenesis. Thus, alterations in the balanced production of positive and negative regulators of angiogenesis may determine the pathogenesis of diabetic retinopathy. Many studies have focused on the role of positive factors, such as vascular endothelial growth factor (VEGF). However, the potential role of the endogenous inhibitors of angiogenesis in the pathogenesis of diabetic retinopathy remains poorly understood.
Endogenous inhibitors of angiogenesis including thrombospondin-1 (TSP1) and pigment epithelium-derived factor (PEDF), which are present at ocular avascular sites such as vitreous, may play a key role in retinal vascular homeostasis (4, 5). TSP1 is a member of the thrombospondin family of the matricellular proteins with potent antiangiogenic activity (6). TSP1 was the first endogenous inhibitor of angiogenesis identified whose expression was down-regulated during malignant transformation (7). We have shown that expression of TSP1 is essential for appropriate development of retinal vasculature. Mice deficient in TSP1 fail to undergo appropriate remodeling and pruning of the developing retinal vasculature and as a result exhibit increased retinal vascular density (4). We also observed high levels of TSP1 in vitreous samples prepared from normal eyes of various species including human, bovine, rat, and mouse (8). We also showed increased expression of TSP1 in mouse eyes attenuates normal retinal vascular development and retinal neovascularization during oxygen-induced ischemic retinopathy (OIR) (9). Thus, altered production of TSP1 may play a significant role in the development and progression of diabetic retinopathy.
The role of TSP1 in the development and progression of diabetic retinopathy remains elusive. We previously showed that TSP1 is present at high levels in vitreous samples prepared from control rats, while it was absent in vitreous samples prepared from diabetic rats (8). This was associated with significant early vasculopathies observed in diabetic animals. We also showed exposure of microvascular endothelial cells (EC) including retinal EC to high glucose results in decreased production of TSP1, and enhanced migration of retinal EC (8, 10). Furthermore, retinal EC prepared from TSP1-deficient mice maintain a pro-angiogenic phenotype in culture (11, 12). Together these studies indicate that TSP1 plays a critical role in retinal vascular homeostasis, whose decreased production during diabetes may contribute to the pathogenesis of diabetic retinopathy. However, the level of TSP1 in the eyes of patients with diabetes has not been previously evaluated and its contribution to the development and progression of diabetic retinopathy requires investigation.
PEDF, a 50 kDa neurotrophic glycoprotein, is also an endogenous inhibitor of angiogenesis and may play a role in retinal vascular homeostasis (13-16). We recently showed that the development of retinal vasculature proceeds at a faster rate in PEDF-deficient mice and the retinal vasculature is more sensitive to hyperoxia-mediated vessel obliteration during OIR (5). This is in contrast to what we observed in the TSP1-deficient mice, which exhibited protection from the vessel obliteration in response to hyperoxia (4). Therefore, the roles these molecules play in retinal vascular homeostasis are distinct and need further evaluation.
PEDF, like TSP1, inhibits angiogenesis in a number of models and inhibits EC proliferation and migration in culture (14, 17-19). It has been previously reported that the vitreous level of PEDF is lower in patients with proliferative diabetic retinopathy (PDR) compared with nondiabetic controls (20, 21). In contrast, others have reported that the vitreous levels of PEDF are higher in diabetic patients with active proliferative retinopathy than in those with no neovascularization (22, 23). Therefore, for an improved management of diabetic retinopathy, a better understanding of the pathogenesis of this disease is crucial. The aim of this study was to examine whether the alterations in TSP1 and PEDF levels occur during diabetes.
Materials and Methods
Patients
Patients were recruited from the Department of Ophthalmology and Visual Sciences of the University of Wisconsin School of Medicine and Public Health Hospital (UWSMPH). Patients with diabetic retinopathy and age-matched controls were recruited from November 2001 to July 2003. The diagnosis of diabetes was made by the criteria of the American Diabetes Association reported in 1997. Patient history and duration of diabetes was gathered from medical charts. All patients signed written informed consent for their participation in the study. The study design and protocol was approved by the UWSMPH IRB.
Vitreous sample collection
At the beginning of vitrectomy surgery, 0.1-0.5 ml of undiluted vitreous was removed via a vitrectomy cutting instrument attached to a sterile 1-ml syringe before turning on the infusion. Each syringe was capped and immediately placed on ice. Vitreous samples were immediately centrifuged at 16,000 ×g for 10 minutes at 4°C to remove cellular debris, transferred to a clean tube, and stored at -80°C until analyzed.
Western blotting
Western blot analysis of vitreous samples was performed to determine the levels of TSP1 and PEDF. Vitreous samples (50 μl) were combined with 10 μl of 6x SDS loading buffer, boiled, electrophoresed in 4-20% polyacrylamide gel (Invitrogen, Carlsbad, CA) under non-reducing condition, and transferred to a nitrocellulose membrane. The membrane was blocked for 1 h in blocking solution (3% BSA and 3% nondairy creamer), prepared in Tris-Buffered Saline (TBS, 20 mM Tris pH 7.6, 150 mM NaCl) containing 0.1% Tween 20 (TBST), and then incubated with a mouse anti-human TSP1 antibody (A6.1; Neo Marker, Fremont, CA) for 1 h at room temperature. We have shown that this antibody reacts with TSP1 under non-reducing conditions in conditioned medium prepared from wild type but not TSP1-/- retinal endothelial cells (24). The immune complexes were detected with a goat-anti-mouse horseradish peroxide (HRP)-conjugated secondary antibody (1:10,000; Pierce, Rockford, IL) followed by ECL detection (Amersham Biosciences, Piscataway, NJ). The same blot was re-probed with an anti-human PEDF antibody (1:1,000; Millipore, Temecula, CA) as described above. For re-probing, blots were washed once in TBST and stripped by two washes with 25 ml of pre-warmed stripping solution (62.5 mM Tris-HCl pH 6.8, 2% SDS, 100 mM β-mercaptoethanol) at 65°C. Blots were then washed twice with TBST (5 min each) at room temperature before proceeding with blotting.
Data analysis
The Western blots were evaluated in a masked fashion and the bands were evaluated by their intensities using Image J software (NIH). For quantitative assessment of the data the TSP1 (the upper band corresponding to the intact protein) and PEDF band intensities were determined for each sample and the level of TSP1 is reported relative to PEDF for each sample, since PEDF level was minimally affected among the samples. The data were scored based on the TSP1 level relative to PEDF level: ++++ for TSP1 values 2- 2.5, +++ for TSP1 values 1.0-2.0, ++ for TSP1 values 0.5-1.0, and + or +/- for TSP1 values <0.5. The PEDF level was minimally changed among the samples and is shown as ++++, to indicate the high level and minimal change among the samples.
Results
Baseline characteristics
This study involved a total of 21 patients without diabetes mean (SD) age 69.7 (7.73) years with idiopathic macular hole or epiretinal membrane that served as controls (Table 1) and 35 diabetic patients with diabetic retinopathy with a mean (SD) age of 62.9 (7.57) years (Table 2). The mean duration of diabetes for diabetic patients was 22.2 (10.0) years. All diabetic patients had proliferative retinopathy. Gender and age were comparable between diabetic and control groups (Tables 1 and 2).
Table 1.
Characterization of Control Patients
| Patient Number | Sex | Age | Diagnosis | Duration of Diabetes | Previous PRP | TSP1 | PEDF |
|---|---|---|---|---|---|---|---|
| 1 | F | 73 | MH | NA | No | +++ | ++++(H) |
| 2 | M | 64 | ERM | NA | No | +++ | ++++(H) |
| 3 | F | 72 | ERM | NA | No | +++ | ++++(H) |
| 4 | F | 54 | MH | NA | No | ++++ | ++++(L) |
| 5 | M | 71 | ERM | NA | No | ++++ | ++++(L) |
| 6 | F | 61 | ERM | NA | No | ++ | ++++(H) |
| 7 | F | 76 | ERM | NA | No | +++ | ++++(L) |
| 8 | M | 78 | ERM | NA | No | +++ | ++++(L) |
| 9 | M | 72 | ERM | NA | No | +++ | ++++(H) |
| 10 | M | 76 | ERM | NA | No | +++ | ++++(L) |
| 11 | F | 75 | ERM | NA | No | +++ | ++++(H) |
| 12 | F | 87 | ERM | NA | No | +++ | ++++(H) |
| 13 | M | 65 | ERM | NA | No | ++++ | ++++(L) |
| 14 | F | 60 | MH | NA | No | ++++ | ++++(L) |
| 15 | M | 77 | MH | NA | No | ++++ | ++++(L) |
| 16 | M | 72 | MH | NA | No | ++++ | ++++(L) |
| 17 | F | 65 | MH | NA | No | +++ | ++++(H) |
| 18 | M | 72 | MH | NA | No | +++ | ++++(L) |
| 19 | F | 60 | MH | NA | No | ++++ | ++++(L) |
| 20 | F | 63 | MH | NA | No | ++++ | ++++(L) |
| 21 | F | 71 | MH | NA | No | ++++ | ++++(H) |
MH: Macular Hole
ERM: Epiretinal Membrane
NA: Not Applicable
H: Higher Molecular Weight PEDF
L: Lower Molecular Weight PEDF
PRP: Panretinal photocoagulation
Table 2.
Characterization of Diabetic Patients
| Patient Number | Sex | Age | Diagnosis | Duration of Diabetes | Previous PRP | TSP1 | PEDF |
|---|---|---|---|---|---|---|---|
| 22 | M | 59 | PDR | 39 | Yes | +++ | ++++(L) |
| 23 | M | 57 | PDR | 16 | Yes | +++ | ++++(L) |
| 24 | M | 62 | PDR | 20 | Yes | +++ | ++++(L) |
| 25 | M | 77 | PDR | 15 | Yes | +++ | ++++(L) |
| 26 | F | 65 | PDR | 8 | Yes | +++ | ++++(L) |
| 27 | F | 65 | PDR | 20 | No | ++ | ++++(H) |
| 28 | M | 56 | PDR | 32 | Yes | +++ | ++++(L) |
| 29 | M | 72 | PDR | 14 | No | +++ | ++++(L) |
| 30 | M | 66 | PDR | 35 | Yes | + | ++++(H) |
| 31 | M | 54 | PDR | 10 | Yes | ++ | ++++(H) |
| 32 | F | 71 | PDR | 20 | Yes | +++ | ++++(L) |
| 33 | F | 53 | PDR | 27 | Yes | +/- | ++++(H) |
| 34 | F | 51 | PDR | 36 | Yes | ++++ | ++++(L) |
| 35 | M | 66 | PDR | 12 | Yes | +++ | ++++(L) |
| 36 | M | 77 | PDR | 15 | Yes | ++++ | ++++(L) |
| 37 | M | 68 | PDR | 30 | Yes | ++++ | ++++(L) |
| 38 | M | 62 | PDR | 18 | Yes | + | ++++(H) |
| 39 | M | 55 | PDR | 31 | Yes | ++ | ++++(H) |
| 40 | M | 55 | PDR | 20 | Yes | ++ | ++++(H) |
| 41 | F | 56 | PDR | 20 | Yes | + | ++++(H) |
| 42 | M | 62 | PDR | 24 | Yes | ++ | ++++(L) |
| 43 | M | 56 | PDR | 20 | No | +++ | ++++(L) |
| 44 | M | 63 | PDR | 15 | Yes | +++ | ++++(H) |
| 45 | M | 79 | PDR | 35 | Yes | +++ | ++++(L) |
| 46 | F | 64 | PDR | 35 | Yes | +++ | ++++(L) |
| 47 | M | 57 | PDR | 12 | Yes | +++ | ++++(L) |
| 48 | M | 69 | PDR | 12 | Yes | +++ | ++++(L) |
| 49 | F | 54 | PDR | 30 | Yes | +++ | ++++(L) |
| 50 | F | 60 | PDR | 51 | No | ++++ | ++++(L) |
| 51 | M | 57 | PDR | 13 | Yes | ++++ | ++++(L) |
| 52 | M | 63 | PDR | 18 | Yes | +++ | ++++(H) |
| 53 | M | 65 | PDR | 25 | Yes | +/- | ++++(H) |
| 54 | M | 67 | PDR | 10 | Yes | ++++ | ++++(L) |
| 55 | M | 78 | PDR | 15 | Yes | +++ | ++++(L) |
| 56 | M | 61 | PDR | 24 | Yes | ++++ | ++++(L) |
PDR: Proliferative Diabetic Retinopathy
H: Higher Molecular Weight PEDF
L: Lower Molecular Weight PEDF
Alterations in TSP1 level in vitreous samples from patients with diabetes
We previously showed that TSP1 is present at high levels in vitreous samples from normal rats, while it was significantly diminished with diabetes (8). We examined the level of TSP1 in vitreous samples from all patients by Western blotting. Figure 1A (top panel) shows a representative TSP1 western blot of vitreous samples from control and diabetic patients. The quantitative assessment of the date is shown in Figure 1B. The data for the remaining samples are summarized in Tables 1 and 2. Our results showed that a significant amount of TSP1 is present in vitreous samples from control eyes. However, the level of TSP1 in vitreous samples varied among patients with diabetes. To our knowledge this is the first report of detection of TSP1 and its potential changes in vitreous samples from patients with diabetes.
Figure 1.
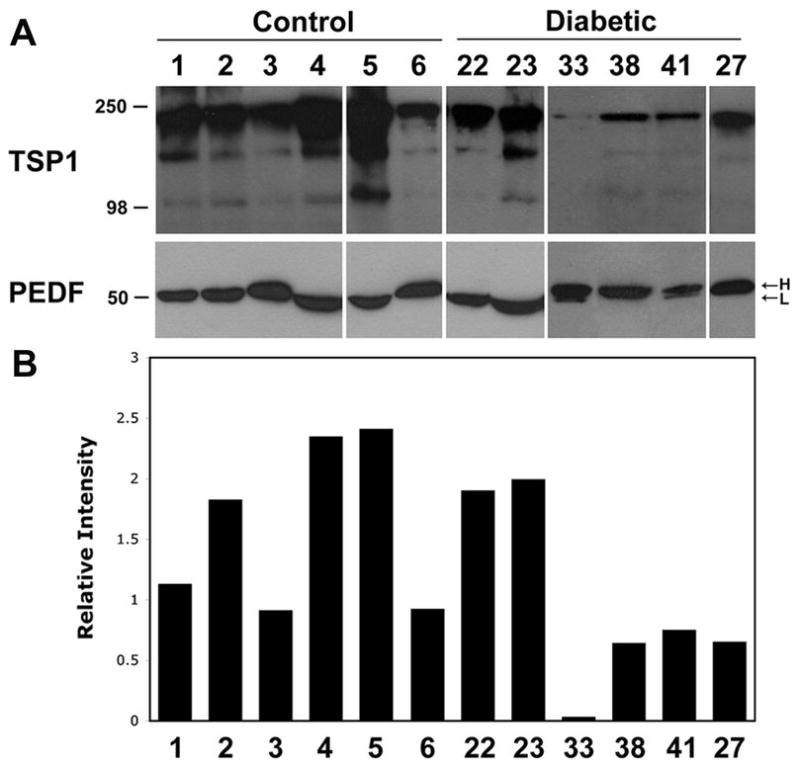
Determination of TSP1 and PEDF levels in vitreous samples. The level of TSP1 and PEDF, in vitreous samples prepared from control or diabetic patients was determined by Western blot analysis. A representative set of samples from control and diabetic groups is shown in A. Please note the wide variation in the levels of TSP1 in diabetic samples compared to control. Minimal variation was observed in the level of PEDF. PEDF varied in its molecular weight among the samples (L, lower molecular weight; H, high molecular weight). Please note samples from diabetic patients with high molecular weight PEDF isoform express significantly less TSP1 compared to samples expressing the low molecular weight isoform of PEDF. For quantitative assessment of the data shown in A, the TSP1 band intensities relative to PEDF were determined as described in Methods and shown in B. The results from the remaining samples are presented in a summary format in Tables 1 and 2.
Lack of alterations in PEDF level in vitreous samples from patients with diabetes
We next determined whether PEDF level changed in vitreous samples of diabetic patients compared to control. Figure 1A (lower panel) shows a representative PEDF Western blot of vitreous samples from control and diabetic patients. The results from the remaining samples are summarized in Tables 1 and 2. We observed little or no differences in the level of PEDF detected in vitreous samples from diabetic patients compared to controls. However, PEDF exhibited a variation in its molecular weight among the samples (H, higher molecular weight vs. L, Lower molecular weight; Figure 1A and Tables 1 and 2). Thus, our data suggest that there are two isoforms of PEDF present in vitreous samples from control and diabetic patients, which vary in their molecular weight. This may be, in part, due to various polymorphisms previously reported in human PEDF gene (25-27) or its glycosylation (28). We consistently observed lower levels of TSP1 in vitreous samples of diabetic patients with higher molecular weight isoform of PEDF.
Discussion
In the present study we analyzed the levels of two major endogenous inhibitors of angiogenesis, TSP1 and PEDF, in the vitreous samples from patients with diabetes compared with those from controls. Our hypothesis is that decreased production of TSP1 occurs during diabetes and contributes to the development and progression of diabetic retinopathy. We observed variation in the levels of TSP1 in vitreous samples prepared from diabetic patients compared to control. This ranged from minimal changes in TSP1 levels to little or no detectable TSP1 in vitreous samples from diabetic patients. In contrast, we detected significant levels of PEDF in all vitreous samples with minimal effects on PEDF level with diabetes. However, we observed two major isoforms of PEDF in human vitreous samples, which migrated differently on SDS-PAGE. We observed that TSP1 level was consistently lower in vitreous samples prepared from diabetic patients with higher molecular weight isoform of PEDF. Thus, the neuro-protective and/or antiangiogenic activity of PEDF, rather than its level, may contribute to the development and progression of diabetic retinopathy. The exact identity and the neuro-protective and/or antiangiogenic activity of these isoforms are subject of future investigation.
The tight regulation of angiogenesis is achieved by a balanced production of positive and negative factors. Alterations in this balance, under various pathological conditions such as diabetes, result in angiogenesis. Vascular endothelial growth factor (VEGF) acts as an EC mitogen in vitro and promotes vascular permeability and angiogenesis in vivo (29-31). Intraocular VEGF concentrations are increased during the periods of active intraocular neovascularization in patients with PDR (2). However, little is known about the potential changes in expression and/or activity of endogenous inhibitors of angiogenesis during diabetes.
Many endogenous inhibitors of angiogenesis, including TSP1, and its closely related family member TSP2, angiostatin, endostatin, PEDF, interferon-α/β, and platelet factor 4 have been reported (32, 33). TSP1 was the first endogenous inhibitor of angiogenesis identified whose expression is suppressed during progression of many solid tumors is associated with the activation of angiogenic switch (7, 34). We consider TSP1 to be an angiogenic inhibitor associated with the development and progression of diabetic retinopathy (8). TSP1 is present in vitreous and aqueous humor at high levels and produced by almost all known cell types in the eye including retinal endothelial cells, astrocytes, pericytes, and elsewhere including retinal pigmented epithelial cell, corneal epithelial and endothelial cells, and trabecular meshwork cells (10, 11, 35-38). It specifically inhibits EC proliferation and migration, and blocks angiogenesis and tumor growth (34, 39). TSP1 and its antiangiogenic peptides effectively inhibit new blood vessel growth during OIR (9, 40). However, the possibility that administration of TSP1 and/or its antiangiogenic peptides may inhibit the development and progression of PDR needs evaluation. Production of TSP1 and TSP2 by astrocytes are recently shown to be essential for appropriate synaptogenesis and retinal neurons functions (41). Thus, alterations in TSP1 levels may also impact retinal neuronal functions contributing to visual dysfunctions associated with diabetes (42, 43). Furthermore, decreased production of TSP1, as well as PEDF and endostatin, observed in eyes with age-related macular degeneration (38, 44) suggest an important role for these angiogenesis inhibitors in modulation of choroidal vascular homeostasis.
In diabetic retinopathy, the total protein concentration of vitreous is generally increased perhaps due to the loss of retinal EC barrier function, an early dysfunction associated with diabetes. Since changes in total vitreous protein content is a likely concern, an important early characteristic of diabetic retinopathy, we utilized volumes of vitreous for our analysis rather that total protein concentration in order to normalize across all samples. In addition, to circumvent the possibility of vitreous hemorrhage affecting our results, we removed cell debris and platelets, a major source of TSP1, before further analysis of the samples. However, this does not rule out the potential release of TSP1 from activated platelets resulting in increased levels of TSP1. Although obtaining vitreous samples from patients in early stages of diabetic retinopathy may be challenging, it will help to address these concerns. Alternatively, one can evaluate TSP1 levels following laser treatment and quiescence of retinal vasculature. However, the limited changes observed in vitreous levels of PEDF suggest a minimal contribution from contaminating serum.
TSP1 concentration was unaffected in some diabetic patients and was dramatically down regulated in others. Since we didn't further classify the patients with diabetes into active (highly active neovascularization with rapidly proliferative membrane and fresh vitreous hemorrhage or retinal detachment) or quiescent (chronic neovascularization with extensive panretinal laser photocoagulation and minimal background retinopathy), there are three possible explanations for the results seen here. First, diabetic retinopathy was active in patients with low TSP1 in the vitreous fluid. Second, diabetic retinopathy may be quiescent in patients with a high level of TSP1 in the vitreous fluid. Third, retinopathy may be active or inactive when TSP1 is high or low, respectively, depending on the concentration of stimulators such as VEGF. Thus, the activity of diabetic retinopathy rather than its severity more accurately reflects the effects of angiogenic stimulators and inhibitors. Unfortunately, the lack of sufficient patient information was prohibitory in delineating these possibilities. Further investigation of VEGF levels and better classification retinopathies are needed for more accurate assessment of the TSP1 changes during diabetes and its impact on the development and progression of diabetic retinopathy.
PEDF is also a potent inhibitor of ocular angiogenesis (13). Alterations in PEDF levels in patients with diabetes compared with nondiabetic patients have been controversial and not clearly resolved. It was reported that the level of PEDF in vitreous was lower in patient with diabetes (20, 21). There are also reports that PEDF concentration was higher in the vitreous from patients with diabetes (22, 23). Our study showed that PEDF was at consistently high levels in the eyes of diabetic and control groups. However, we detected two different isoforms of PEDF in human vitreous samples with different migration/molecular weight (Figure 1). One isoform migrated faster than other isoform on SDS-PAGE (L, lower molecular weight). We consistently observed lower levels of TSP1 in patient with higher molecular weight PEDF isoform.
Multiple polymorphisms have been reported in human PEDF gene (27) and an association between some of these polymorphisms (the promoter and exon 3 coding region) and diabetic retinopathy and age-related macular degeneration have been reported (25, 26). However, it is not known whether these polymorphisms result in production of proteins with different molecular weight or expression level. The identity and the level of transcripts generated by these polymorphisms, and the identity/activity of their potential product require further investigation. A potential polymorphism reported in intron 5 (just upstream from the splice acceptor site) may be a potential candidate for generation of an alternatively spliced transcript producing a different size protein (27). Thus, the identity of the PEDF polymorphisms and their protein products, and their association with the severity of diabetic retinopathy, will be very informative. Furthermore, this may allow the development of a screening method for identification of diabetic patients that are at greater risk for the development of PDR.
TSP1 and PEDF, as endogenous inhibitors of angiogenesis, exhibit different expression patterns and functions in retinal vascular development and angiogenesis (4, 45). Although the level of PEDF was not dramatically changed in the eyes of patients with diabetes the involvement of different PEDF isoform in the pathogenesis of diabetic retinopathy needs further investigation, especially in regards to its effect on TSP1 expression. It is possible that different isoforms of PEDF have different antiangiogenic potential during development and progression of diabetic retinopathy (26), perhaps through modulation of TSP1 level. Although vitamin A is shown to increase expression of TSP1 and PEDF in retinal pigmented epithelial cells (46) nothing is known about the regulation of TSP1 by PEDF. Thus, further investigations are needed to address the relationship of proangiogenic factors such as VEGF and, isoforms of PEDF and TSP1 expression in the pathogenesis of diabetic retinopathy.
In summary, we demonstrated that the vitreous TSP1 levels varied among diabetic samples and was consistently lower in the diabetic patients which expressed the higher molecular weight isoform of PEDF. The vitreous levels of PEDF, however, did not vary significantly among diabetic and control patients. Thus, decreased production of TSP1, along with expression of the high molecular weight isoform of PEDF, in patients with diabetes may indicate a greater risk for development of severe retinopathies.
Acknowledgments
This work was supported in part by grants EY16995 (NS) and DK67120 (CMS) from the National Institutes of Health. NS is a recipient of a Research Award from American Diabetes Association, 1-06-RA-123, and Retina Research Foundation.
References
- 1.Frank RN. Diabetic Retinopathy. N Engl J Med. 2004;350:48–58. doi: 10.1056/NEJMra021678. [DOI] [PubMed] [Google Scholar]
- 2.Aiello LP. Angiogenic pathways in diabetic retinopathy. N Engl J Med. 2005;353:839–841. doi: 10.1056/NEJMe058142. [DOI] [PubMed] [Google Scholar]
- 3.Gariano RF, Gardner TW. Retinal angiogenesis in development and disease. Nature. 2005;438:960–966. doi: 10.1038/nature04482. [DOI] [PubMed] [Google Scholar]
- 4.Wang S, Wu Z, Sorenson CM, Lawler J, Sheibani N. Thrombospondin-1-deficient mice exhibit increased vascular density during retinal vascular development and are less sensitive to hyperoxia-mediated vessel obliteration. Dev Dyn. 2003;228:630–642. doi: 10.1002/dvdy.10412. [DOI] [PubMed] [Google Scholar]
- 5.Huang Q, Wang S, Sorenson CM, Sheibani N. PEDF-deficient mice exhibit an enhanced rate of retinal vascular expansion and are more sensitive to hyperoxia-mediated vessel obliteration. Exp Eye Res. 2008 doi: 10.1016/j.exer.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheibani N, Frazier WA. Thrombospondin-1, PECAM-1, and regulation of angiogenesis. Histol Histopathol. 1999;14:285–294. doi: 10.14670/HH-14.285. [DOI] [PubMed] [Google Scholar]
- 7.Rastinejad F, Polverini PJ, Bouck NP. Regulation of the activity of a new inhibitor of angiogenesis by a cancer suppressor gene. Cell. 1989;56:345–355. doi: 10.1016/0092-8674(89)90238-9. [DOI] [PubMed] [Google Scholar]
- 8.Sheibani N, Sorenson CM, Cornelius LA, Frazier WA. Thrombospondin-1, a natural inhibitor of angiogenesis, is present in vitreous and aqueous humor and is modulated by hyperglycemia. Biochem Biophys Res Commun. 2000;267:257–261. doi: 10.1006/bbrc.1999.1903. [DOI] [PubMed] [Google Scholar]
- 9.Wu Z, Wang S, Sorenson CM, Sheibani N. Attenuation of retinal vascular development and neovascularization in transgenic mice over-expressing thrombospondin-1 in the lens. Dev Dyn. 2006;235:1908–1920. doi: 10.1002/dvdy.20837. [DOI] [PubMed] [Google Scholar]
- 10.Huang Q, Sheibani N. High glucose promotes retinal endothelial cell migration through activation of Src, PI3K/Akt1/eNOS, and ERKs. Am J Physiol Cell Physiol. 2008;295:C1647–1657. doi: 10.1152/ajpcell.00322.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su X, Sorenson CM, Sheibani N. Isolation and characterization of murine retinal endothelial cells. Mol Vis. 2003;9:171–178. [PubMed] [Google Scholar]
- 12.Wang Y, Wang S, Sheibani N. Enhanced proangiogenic signaling in thrombospondin-1-deficient retinal endothelial cells. Microvasc Res. 2006;71:143–151. doi: 10.1016/j.mvr.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Dawson DW, Volpert OV, Gillis P, et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 14.Stellmach V, Crawford SE, Zhou W, Bouck N. Prevention of ischemia-induced retinopathy by the natural ocular antiangiogenic agent pigment epithelium-derived factor. Proc Natl Acad Sci U S A. 2001;98:2593–2597. doi: 10.1073/pnas.031252398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mori K, Gehlbach P, Yamamoto S, et al. AAV-mediated gene transfer of pigment epithelium-derived factor inhibits choroidal neovascularization. Invest Ophthalmol Vis Sci. 2002;43:1994–2000. [PubMed] [Google Scholar]
- 16.Tombran-Tink J, Barnstable CJ. PEDF: a multifaceted neurotrophic factor. Nat Rev Neurosci. 2003;4:628–636. doi: 10.1038/nrn1176. [DOI] [PubMed] [Google Scholar]
- 17.Kanda S, Mochizuki Y, Nakamura T, Miyata Y, Matsuyama T, Kanetake H. Pigment epithelium-derived factor inhibits fibroblast-growth-factor-2-induced capillary morphogenesis of endothelial cells through Fyn. J Cell Sci. 2005;118:961–970. doi: 10.1242/jcs.01686. [DOI] [PubMed] [Google Scholar]
- 18.Mori K, Duh E, Gehlbach P, et al. Pigment epithelium-derived factor inhibits retinal and choroidal neovascularization. J Cell Physiol. 2001;188:253–263. doi: 10.1002/jcp.1114. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Zhang SS, Barnstable CJ, Tombran-Tink J. PEDF induces apoptosis in human endothelial cells by activating p38 MAP kinase dependent cleavage of multiple caspases. Biochem Biophys Res Commun. 2006;348:1288–1295. doi: 10.1016/j.bbrc.2006.07.188. [DOI] [PubMed] [Google Scholar]
- 20.Funatsu H, Yamashita H, Nakamura S, et al. Vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology. 2006;113:294–301. doi: 10.1016/j.ophtha.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 21.Ogata N, Nishikawa M, Nishimura T, Mitsuma Y, Matsumura M. Unbalanced vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor in diabetic retinopathy. Am J Ophthalmol. 2002;134:348–353. doi: 10.1016/s0002-9394(02)01568-4. [DOI] [PubMed] [Google Scholar]
- 22.Ogata N, Matsuoka M, Matsuyama K, et al. Plasma concentration of pigment epithelium-derived factor in patients with diabetic retinopathy. J Clin Endocrinol Metab. 2007;92:1176–1179. doi: 10.1210/jc.2006-2249. [DOI] [PubMed] [Google Scholar]
- 23.Bhutto IA, McLeod DS, Hasegawa T, et al. Pigment epithelium-derived factor (PEDF) and vascular endothelial growth factor (VEGF) in aged human choroid and eyes with age-related macular degeneration. Exp Eye Res. 2006;82:99–110. doi: 10.1016/j.exer.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun J, Williams J, Yan HC, Amin KM, Albelda SM, DeLisser HM. Platelet endothelial cell adhesion molecule-1 (PECAM-1) homophilic adhesion is mediated by immunoglobulin-like domains 1 and 2 and depends on the cytoplasmic domain and the level of surface expression. J Biol Chem. 1996;271:18561–18570. doi: 10.1074/jbc.271.31.18561. [DOI] [PubMed] [Google Scholar]
- 25.Lin JM, Wan L, Tsai YY, et al. Pigment epithelium-derived factor gene Met72Thr polymorphism is associated with increased risk of wet age-related macular degeneration. Am J Ophthalmol. 2008;145:716–721. doi: 10.1016/j.ajo.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Iizuka H, Awata T, Osaki M, et al. Promoter polymorphisms of the pigment epithelium-derived factor gene are associated with diabetic retinopathy. Biochem Biophys Res Commun. 2007;361:421–426. doi: 10.1016/j.bbrc.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 27.Koenekoop R, Pina AL, Loyer M, et al. Four polymorphic variations in the PEDF gene identified during the mutation screening of patients with Leber congenital amaurosis. Mol Vis. 1999;5:10. [PubMed] [Google Scholar]
- 28.Duh EJ, Yang HS, Suzuma I, et al. Pigment epithelium-derived factor suppresses ischemia-induced retinal neovascularization and VEGF-induced migration and growth. Invest Ophthalmol Vis Sci. 2002;43:821–829. [PubMed] [Google Scholar]
- 29.Miller JW. Vascular endothelial growth factor and ocular neovascularization. Am J Pathol. 1997;151:13–23. [PMC free article] [PubMed] [Google Scholar]
- 30.Gerber HP, Dixit V, Ferrara N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem. 1998;273:13313–13316. doi: 10.1074/jbc.273.21.13313. [DOI] [PubMed] [Google Scholar]
- 31.Nor JE, Christensen J, Mooney DJ, Polverini PJ. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am J Pathol. 1999;154:375–384. doi: 10.1016/S0002-9440(10)65284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 33.Folkman J. Endogenous angiogenesis inhibitors. APMIS. 2004;112:496–507. doi: 10.1111/j.1600-0463.2004.apm11207-0809.x. [DOI] [PubMed] [Google Scholar]
- 34.Lawler J. The functions of thrombospondin-1 and-2. Curr Opin Cell Biol. 2000;12:634–640. doi: 10.1016/s0955-0674(00)00143-5. [DOI] [PubMed] [Google Scholar]
- 35.Scheef E, Wang S, Sorenson CM, Sheibani N. Isolation and characterization of murine retinal astrocytes. Mol Vis. 2005;11:613–624. [PubMed] [Google Scholar]
- 36.Scheef EA, Huang Q, Wang S, Sorenson CM, Sheibani N. Isolation and characterization of corneal endothelial cells from wild type and thrombospondin-1 deficient mice. Mol Vis. 2007;13:1483–1495. [PubMed] [Google Scholar]
- 37.Liu X, Wu Z, Sheibani N, Brandt CR, Polansky JR, Kaufman PL. Low dose latrunculin-A inhibits dexamethasone-induced changes in the actin cytoskeleton and alters extracellular matrix protein expression in cultured human trabecular meshwork cells. Exp Eye Res. 2003;77:181–188. doi: 10.1016/s0014-4835(03)00118-0. [DOI] [PubMed] [Google Scholar]
- 38.Uno K, Bhutto IA, McLeod DS, Merges C, Lutty GA. Impaired expression of thrombospondin-1 in eyes with age related macular degeneration. Br J Ophthalmol. 2006;90:48–54. doi: 10.1136/bjo.2005.074005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tolsma SS, Volpert OV, Good DJ, Frazier WA, Polverini PJ, Bouck N. Peptides derived from two separate domains of the matrix protein thrombospondin-1 have anti-angiogenic activity. J Cell Biol. 1993;122:497–511. doi: 10.1083/jcb.122.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shafiee A, Penn JS, Krutzsch HC, Inman JK, Roberts DD, Blake DA. Inhibition of retinal angiogenesis by peptides derived from thrombospondin-1. Invest Ophthalmol Vis Sci. 2000;41:2378–2388. [PubMed] [Google Scholar]
- 41.Christopherson KS, Ullian EM, Stokes CC, et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 42.Vanguilder HD, Brucklacher RM, Patel K, Ellis RW, Freeman WM, Barber AJ. Diabetes downregulates presynaptic proteins and reduces basal synapsin I phosphorylation in rat retina. Eur J Neurosci. 2008;28:1–11. doi: 10.1111/j.1460-9568.2008.06322.x. [DOI] [PubMed] [Google Scholar]
- 43.Kern TS, Barber AJ. Retinal ganglion cells in diabetes. J Physiol. 2008;586:4401–4408. doi: 10.1113/jphysiol.2008.156695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhutto IA, Uno K, Merges C, Zhang L, McLeod DS, Lutty GA. Reduction of endogenous angiogenesis inhibitors in Bruch's membrane of the submacular region in eyes with age-related macular degeneration. Arch Ophthalmol. 2008;126:670–678. doi: 10.1001/archopht.126.5.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang Q, Wang S, Sorenson CM, Sheibani N. PEDF-deficient mice exhibit an enhanced rate of retinal vascular expansion and are more sensitive to hyperoxia-mediated vessel obliteration. Exp Eye Res. 2008;87:226–241. doi: 10.1016/j.exer.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uchida H, Hayashi H, Kuroki M, et al. Vitamin A up-regulates the expression of thrombospondin-1 and pigment epithelium-derived factor in retinal pigment epithelial cells. Exp Eye Res. 2005;80:23–30. doi: 10.1016/j.exer.2004.08.004. [DOI] [PubMed] [Google Scholar]


