Abstract
Cellular interaction in blood vessels is maintained by multiple communication pathways, including gap junctions. They consist of intercellular channels ensuring direct interaction between endothelial and smooth muscle cells and the synchronization of their behavior along the vascular wall. Gap-junction channels arise from the docking of two hemichannels or connexons, formed by the assembly of six connexins, and achieve direct cellular communication by allowing the transport of small metabolites, second messengers, and ions between two adjacent cells. Physiologic variations in connexin expression are observed along the vascular tree, with most common connexins being Cx37, Cx40, and Cx43. Changes in the level of expression of connexins have been correlated to the development of vascular disease, such as hypertension, atherosclerosis, or restenosis. Recent studies on connexin-deficient mice highlighted key roles of these communication pathways in the development of these pathologies and confirmed the need for targeted pharmacologic approaches for their prevention and treatment. The aim of this issue is to review the current knowledge on the implication of gap junctions in vascular function and most common cardiovascular diseases. Antioxid. Redox Signal. 11, 267–282.
Introduction
Coordination of vascular responses is essential for the control of normal vascular function. Cell-to-cell coupling via gap junctions forms a vital component in this coordination (4, 23, 30). Gap junctions allow the exchange of metabolites, ions, and other messenger molecules between adjacent cells (98). In vascular cells, gap junctions enable changes in membrane potential to be propagated electrotonically via coupling between endothelial cells (ECs), smooth muscle cells (SMCs), or ECs and SMCs, or a combination of these, via the myoendothelial junction (MEJ). Gap junctions may also play a role in slower physiologic processes, such as cell growth, differentiation, and development.
Gap junctions consist of connexins (Cx), a family of proteins that form channels linking the cytoplasm of adjacent cells (98). Six radially arranged connexins form single-membrane channels, hemichannels or connexons, which align with their counterparts in adjacent cell membranes to form a complete intercellular channel. All connexin molecules have four membrane-spanning domains, two extracellular domains, and a cytoplasmic carboxy-terminal tail of varying length that has an important role in the regulation of the gating properties of the channel. The opening and closing of gap-junction channels can be controlled posttranslationally by various growth factors (64), and the permeabilities of gap-junction channels are unique for each connexin isoform. Hemichannels can contain a single connexin subtype (a homomeric connexon) or multiple connexin subtypes (a heteromeric connexon). Homomeric connexons can form homotypic intercellular channels consisting of the same connexon subunits in neighboring cells, or a heterotypic intercellular channel that consists of different connexon subunits. Given that 21 mammalian connexins have been characterized (113), a large number of physiologically distinct channel types may be formed, thus providing potential for diversity of gap-junctional intercellular communication (GJIC).
In the vascular wall, only four connexins have been found in ECs and SMCs: Cx37, Cx40, Cx43, and Cx45 (33, 48, 59, 73, 119, 132). Before the characterization of connexin subtypes, earlier studies suggested that the size and abundance of gap junctions vary in different regions of the vascular tree and change with disease states such as atherosclerosis and hypertension (106, 107). Interestingly, the distribution of connexins has also been shown to vary between individual vascular beds of one species, between the same vascular beds of different species, as well as during embryologic development and the progress of disease (46, 107). In this article, we review the current knowledge on the implication of gap junctions in vascular function and the most common cardiovascular diseases.
Gap Junctions in Large Arteries
Transmission and scanning electron microscopy (TEM and SEM), as well as immunohistochemistry, demonstrate that the ECs of the large arteries are particularly well coupled with connexins. In general, Cx40 and Cx37 are abundantly expressed in elastic (aorta) and muscular (coronary) arteries of various species (11, 119, 132), whereas the expression of Cx43 is restricted to the ECs at branch points of these arteries (33). Functionally, the large-vessel ECs have been demonstrated to be coupled with dyes such as lucifer yellow and carboxyfluoresceine, indicating open gap-junction channels (111). Several studies have detected changes in connexin expression or changes in dye-transfer–mediated intercellular communication in response to different flow patterns across the ECs (26); however, the functional significance of these observations is not yet known.
Within the SMCs of the large arteries, gap-junction linkage of the cells allows the coordination of intracellular calcium-mediated contraction along the length of the vessel, a concept that has been extensively demonstrated (28). This is in contrast to the fact that gap junctions between SMCs are usually found between small sections of plasma membrane and do not resemble those of ECs (i.e., large gap-junction plaques between tightly sealed opposing plasma membranes). Besides coordination of vessel constriction, other roles for gap junctions in the large arteries may also exist, as suggested by Reidy et al. (93). Their experiments consisted of inserting a catheter at the anterior end of the aorta, which they found to cause an increase in thymidine (a marker for cellular mitosis) uptake in the SMCs down the length of the thoracic and abdominal aorta (93). This suggested that long-distance communication along SMCs in the larger vessels might play a role in regulation of mitosis. To test the idea that the factors inducing SMC proliferation were not paracrine mediated (i.e., carried in the direction of blood flow), they alternatively placed the indwelling catheter through the iliac to the posterior base of the abdominal aorta and again found increased thymidine uptake along the length of the vessel up to the thoracic aorta. Data that appear to correlate this were recently obtained from mice with SMC-targeted deletion of Cx43. In this mouse model, the removal of Cx43 in the SMCs caused a significant increase in SMC proliferation (70). Similar to the role for Cx43 in migration of neural crest cells (76), the regulation of SMC proliferation by Cx43 might also critically depend on the level of GJIC, because reducing Cx43 expression by half did not induce an increase in SMC proliferation, but reduced SMC proliferation instead (15). Although the mechanism remains to be elucidated, these data suggest that Cx43-based communication along the SMCs in the large vessels may be responsible for dictating cell-division rates, and thus also possibly SMC differentiation.
Gap Junctions in Resistance Vessels
Anatomic and immunohistochemistry evidence implicates ECs of the resistance vessels as a highly coupled tissue, similar to the ECs of large arteries. Some of the initial work to address the functional presence of gap junctions was performed by Little et al. (74), in which they demonstrated that biocytin, lucifer yellow, carboxyfluorescein, and ethidium bromide could rapidly move between ECs in hamster cheek pouch arterioles (74). Both Cx40 and Cx43 were found to be the potential mediators of the dye transfer (73). Cx43 appears to be especially important for calcium communication in ECs, as convincingly demonstrated in vivo from mouse lung ECs that used caged second-messenger compounds that were UV flashed to release IP3 or Ca2+. They found that release of the caged compounds induced a calcium wave among ECs, but that the calcium communication between the ECs was severely diminished in mice with EC-specific deletion of Cx43 (85). Taken together, extensive data from the literature [for review (23)] indicate that ECs of the resistance vessels are a highly coupled tissue.
In arteriolar SMCs, it is thought that GJIC allows coordination of vessel constriction. This has shown to be the case by electrical coupling in multiple experiments (3). However, experiments to show coupling in terms of dye transfer within resistance vessels have given mixed results (74, 105). Although, as previously mentioned, gap-junctional plaques between SMCs are not similar to those between ECs, making immunofluorescent punctate detection, and thus verification of traditional gap-junction plaques, very difficult in vivo (77). It is clear that more work on the role of gap junctions in SMCs is required to clarify this issue.
A unique aspect of resistance-vessel physiology is the degree to which the ECs and SMCs are integrated via direct cell contact (as demonstrated in Fig. 1 with F-actin distribution) to control vascular function. The integration occurs via numerous paracrine factors [e.g., prostaglandins and nitric oxide (NO)], as well as through gap junctions. The gap junctions reside in structures termed MEJ, which are not found in the large vessels (e.g., aorta, carotid). These cellular extensions from the ECs (mostly), or the SMCs, break through the extracellular matrix (ECM)-enriched internal elastic lamina so that direct cell–cell contact is achieved (see Fig. 2 for an example). Anatomically, these structures are prominent in all arteriole beds examined; however, they have been for the most part too small (∼0.5 × 0.5 μm) and at a too-confined location for studies at the light-microscopy level. For this reason, any study on the proteins expressed (including connexins) at the MEJ has had to rely exclusively on TEM. This has limited studies on the function of the MEJ and is likely responsible for some of the current controversy that has begun regarding the function of gap junctions at the MEJ.
FIG. 1.
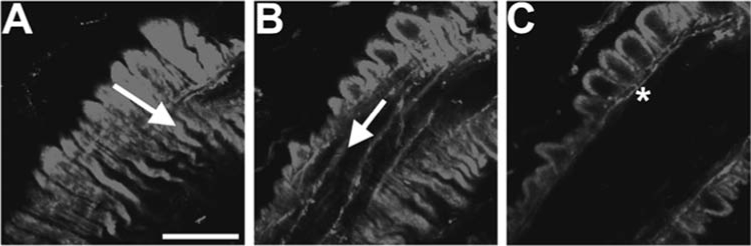
Focal planes of in situ mouse cremaster arteriole. Mouse cremaster arterioles were stained with phalloidin to mark actin in endothelium and smooth muscle and were observed under an Olympus Fluoview confocal microscope in different focal planes. (A) SMCs overlay the arteriole, with the arrow indicating orientation. (B) In the same field of view as A, ECs can be seen running perpendicular to the orientation of SMCs. (C) Another focal plane from the same arteriole in A and B, where the image is viewed transversely. In this orientation, actin can be seen linking the ECs and SMCs, presumably at the MEJ (*). With these different focal planes, one is able to detect with certainty the connexin localization within each cell type. Bar is 15 μm.
FIG. 2.
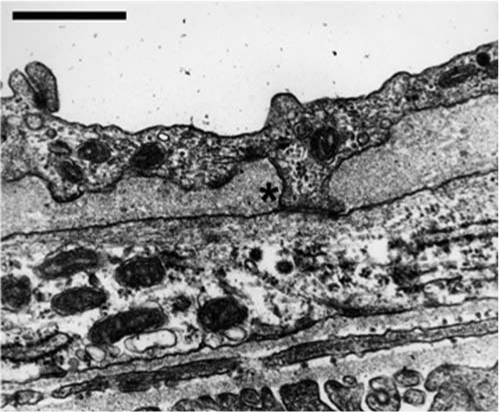
Myoendothelial junction in mouse cremaster arteriole. Usually, the use of electron microscopy is required to identify the MEJ in arterioles. In this electron micrograph, an EC extension is seen breaking through the internal elastic lamina and making contact with an SMC at the MEJ (*). Bar is 1 μm.
Evidence from TEM (100, 115) and dye-transfer experiments (74) confirmed that gap junctions are found in MEJs across species and tissue beds. Connexin subtypes have been more difficult to detect for the reason cited earlier, but recent data found Cx37 and Cx40 in rat brain arterioles (37), Cx40 and Cx37 separately or in combination in rat mesentery (79, 101), Cx40 and Cx43 in mice arterioles (47) (also in vivo; B.E. Isakson, unpublished observation), and Cx43 in human subcutaneous resistance arteries (66). This indicates that the connexins composing the gap junctions at the MEJs are likely dependent on the species and the vascular bed being examined.
Physiologically, multiple studies on an endothelium-derived hyperpolarizing factor (EDHF) provided the vast majority of the evidence for functional gap junctions between ECs and SMCs at the MEJs. The EDHF phenomenon is based on the observation that stimulation of ECs with acetylcholine produces a vascular dilatory molecule (the “EDHF”) that moves from ECs to SMCs through gap junctions (presumably), even when inhibitors for prostaglandins and NO are present. This process appears to be dependent on an increase in EC [Ca2+]i, but not necessarily a change in EC membrane potential (82, 81). Surprisingly, as might be expected for gap junction–based communication after an increase in [Ca2+]i (8), the SMCs do not display a corresponding increase in [Ca2+]i (which would cause dilation), but instead respond by dilating. Controversial information still remains as to the nature of the EDHF signal. It has been demonstrated that Ca2+ “sparks” can induce SMC hyperpolarization and relaxation (83), and although a Ca2+ spark in SMCs in response to EC has not been demonstrated, this could possibly occur if the current moves from EC through gap junctions to SMCs (81), or possibly Ca2+ itself (50), activating ryanodine receptors, the characteristic receptors of a Ca2+ spark. Multiple factors have also been implicated, including most recently hydrogen peroxide (H2O2) (75). The complexity of the MEJ environment has made this work difficult and limited with standard in vivo techniques and, as such, has required innovative new techniques, which have only recently begun to show some interesting results (79).
Although the previously described early EDHF work demonstrated interactions between ECs and SMCs, the direct evidence for gap junctions in EDHF comes from a series of articles demonstrating that application of connexin-mimetic peptides selectively blocked SMC response (16, 20, 54). This observation is based on data indicating that peptides derived from the extracellular loop of Cx37, Cx40, and Cx43 could specifically and reversibly block gap junctions at the MEJ. With another technique, ECs were loaded in vivo by a pinocytotic method (47) by using hypotonic and hypertonic solutions that introduced a Cx40 antibody into the cells (79). The authors demonstrated an EDHF block, possibly indicating that the Cx40 antibody in the ECs blocked gap junctions at the MEJ. Both observations are consistent with the idea that gap junctions play a role in EDHF coupling. These data correlate strongly with observations of a diminishing EDHF response as the artery size increases (100, 108), a pattern that is mimicked by the appearance of the MEJs in arteries (4, 94).
Evidence for functional gap junctions at the MEJ is not always readily apparent. Recent experiments have questioned coupling at the MEJ in the conducted response (12, 110). In particular, Siegl et al. (110) demonstrated that microinjection of carboxyfluorescein dye in either ECs or SMCs failed to couple together the opposing cell type (110). It is possible that carboxyfluorescein may be impermeable to the gap junctions at the MEJ [due to dye selective permeability through connexin isoforms (27)], as they were also unable to see dye transfer among ECs, a highly coupled cell type (110). However, the authors were also unable to obtain similar membrane potentials in ECs and SMCs, again indicating that open gap-junction channels at the MEJ were not readily apparent (110). Because no coupling was found either in ECs, SMCs, or between ECs and SMCs in the vessels tested, and connexins are readily expressed in these particular vessels (77), it is very likely these gap-junction channels were present, but closed. This process can occur because connexin proteins are highly regulated to open or close a gap-junction channel [e.g., in response to phosphorylation (63)]; indeed, closed gap junction channels have been hypothesized to be an important part of regulating cellular communication (10). It is clear that more work on this concept is required.
Last, few data have focused on SMC-to-EC communication through the MEJ, although three different model systems (two in vivo and one in vitro) have demonstrated that an increase in SMC [Ca2+]i causes an increase in EC [Ca2+]i, which is mediated by gap junctions at the MEJ (25, 50, 62). Two of these models independently demonstrated that inositol 1,4,5-trisphosphate (IP3) moves from SMCs to ECs through gap junctions to induce the change in EC [Ca2+]i (50, 62), which subsequently induces NO release (25). This may prove to be an important mechanism in which resistance vessels are able to control vasoconstriction originating in the SMCs.
Problems in Connexin Research
Studies of the physiologic role of gap junctions in the vasculature have been difficult to obtain with confidence because the mechanism of action from almost all known pharmacologic gap-junction inhibitors is not known, and they have multiple nonspecific effects [e.g., see discussion in (30)]. Inhibitors like oleamide and halothane are very potent, but they have an inherent problem of closing large numbers of other membrane channels. However, even inhibitors that have been described to have, when used at low dose, minimal nonspecific effects, like heptanol (114), carbenoxolone (99), glycyrrhetinic acid (9), and connexin mimetic peptides (123), have had problems associated with their use. For example, after application of carbenoxolone to bovine aortic ECs, Cx43 mRNA expression was reported to be 2 to 3 times higher, corresponding with increased Cx43 protein expression at the plasma membrane, indicative of a negative-feedback loop with gap junctions (99). Pharmacologically then, it is clear that confirmation of results with multiple gap-junction inhibitors should always be used.
Because connexin mRNA and protein do not normally correlate (1), detection of protein expression with connexin antibodies becomes vital for experimental purposes. The specificity of connexin antibodies, immunocytochemistry in particular, continues to produce conflicting data. One way to solve this problem has been the use of cell lines that do normally not express connexin proteins in appreciable levels. For this reason, use of HeLa cells transfected with connexin plasmids has been considered a good method for testing antibody specificity; however, the plasmids generated are usually derived against a human sequence, rather than the mouse or rat sequence, which is generally used experimentally. Concurrent testing of the antibodies on tissue from connexin-knockout animals (48) should help alleviate some of these problems. Last, issues regarding antibody access or inaccessibility due to different protein confirmation states or interaction with other proteins have rarely been addressed but may be of particular importance (35, 128).
Information regarding the role of gap junctions in vascular physiology has evolved from studies on knockout mice, demonstrating the importance of these animals (29, 69). However, connexin-knockout mice have also been reported to have important drawbacks resulting from compensatory effects. In particular, the Cx40−/− mouse has been demonstrated to have decreased expression of Cx37 as well as cellular reorganization of Cx43 (48, 111), making physiological interpretation from experiments in these animals very difficult. Therefore, it becomes increasingly clear that we must further develop other methods whereby gap-junction physiology could be studied (e.g., antisense or lentiviral shRNA delivery) that may have more minimal nonspecific effects.
Gap Junctions in Hypertension
Elevated blood pressure is a common health problem with widespread and sometimes devastating consequences. Although hypertension remains often asymptomatic until late in its course, it is one of the most important risk factors for coronary heart disease and cerebrovascular accidents. Finally, it may lead to cardiac hypertrophy with heart failure, aortic dissection, and renal failure. The detrimental effects of elevated blood pressure increase continuously as the pressure increases (57).
The magnitude of arterial pressure depends on two hemodynamic variables: cardiac output and total peripheral resistance. Cardiac output is influenced by blood volume; consequently body sodium homeostasis is central to blood-pressure regulation. Total peripheral resistance is predominantly determined at the level of the arterioles and depends effectively on its lumen size. Primary hypertension accounts for ∼90–95% of the cases; this essential hypertension is thought to result from an interaction of genetic and environmental factors that affect cardiac output, peripheral resistance, or both (57). The remaining 5–10% of hypertension cases are mostly secondary to renal disease or to atherosclerotic narrowing of the renal artery, both affecting the renin–angiotensin system and sodium homeostasis.
Hypertension and connexin expression
A large number of studies report changes in Cx expression in spontaneous hypertensive rats (SHRs) or after induction of hypertension in animal models of the disease. Unfortunately, these studies appear not always consistent, possibly due to intrinsic differences in the experimental models used, and sometimes different results have been obtained by using the same animal model. For example, observations on changes in connexins in SHRs are mixed: Cx40 and Cx37 are consistently reduced in ECs, but both increased and decreased arterial Cx43 has been reported in these rats (42, 53, 97, 96, 136). Moreover, in certain arteries (mesenteric), no differences in Cx43 mRNA could be observed between SHRs and their control Wistar–Kyoto rats, questioning the correlation between gap junctions and hypertension (117). Interestingly and possibly more relevant, normalization of blood pressure in the SHRs by using an angiotensin-converting enzyme inhibitor or candesartan restores endothelial connexin expression to normal in parallel with the normalization of blood pressure (53, 97).
In general, the increased expression of Cx40 and Cx43 is observed on induction of renal hypertension, by using the rat DOCA-salt, and the two-kidney, one-clip Goldblatt (2K1C) procedure (38, 39, 127). With an elegant combination of different models, it was hypothesized that Cx43 expression might be sensitive to hemodynamic changes such as an increase in intravascular pressure, rather than reacting to renin, because Cx43 is elevated in the SMCs of the aortas in both high-salt and 2K1C models of hypertension, models that display opposite changes in plasma renin (41). In contrast to the renovascular models, hypertension induced by inhibition of NO synthase is associated with a decreased Cx43 expression (40, 42, 127, 136). In addition, endothelial Cx37, but not Cx40, was reduced in these models (136). Interestingly, the changes in endothelial Cx43 and Cx37 expression in N(ω)-nitro-l-arginine methyl ester–induced hypertension were reversed by treatment with carvedilol, an adrenergic blocker (136).
Cx40-deficient mice and hypertension
Recent studies indicate that Cx40 plays an important role in blood-pressure regulation. Deletion of the Cx40 gene in mice results in a marked, sustained hypertension (21, 22). This deletion was associated with segmental constrictions and irregular vasomotion in small arterioles, suggesting a direct link between connexins, peripheral resistance, and blood pressure (23, 22). However, a series of recent reports suggest a strong association between renin secretion and Cx40 expression (56, 58, 122). In Cx40-deficient mice, both synthesis and plasma levels of renin secretion are increased. Moreover, the investigators observed increased number of renin-secreting cells as well as altered distribution of the renin-secreting cells in the afferent arterioles. Importantly, they showed that the major dysfunction in Cx40-deficient mice appeared to depend on local blood flow–induced signalling in the afferent arteriole, a concept that was elegantly confirmed in perfused kidney by using a gap-junction blocker (122).
Cx40 genetic polymorphism is associated with hypertension
Two closely linked polymorphisms in the promoter region of the Cx40 gene have been associated with atria-specific arrhythmias (31, 36). Recently, the same research group has investigated whether (a) these polymorphic variants are associated with hypertension, and (b) they interact with blood pressure in healthy individuals. They found a significant contribution of the minor Cx40 allele or genotype (S44AA/R71GG) to the risk of hypertension in men. In addition, they observed in the healthy control population a significant effect of Cx40 genotype and sex on systolic blood pressure (32). These findings not only confirm a strong association between Cx40 and hypertension but also identify these polymorphisms as a risk factor for the disease.
Gap Junctions in Atherosclerosis
Cardiovascular diseases represent the leading cause of death in developed countries and are increasing worryingly in developing countries as the access to food becomes easier. Atherosclerosis is the most important cardiovascular disease, with life-threatening complications such as myocardial infarction, cerebral infarction, and aortic aneurysm. It develops silently over years and usually becomes symptomatic after the fourth decade. Hypercholesterolemia, obesity, smoking, hypertension, diabetes, and infection by microorganisms are classic risk factors that promote atherosclerosis by triggering inflammation or endothelial injury or both. This chronic immunoinflammatory disease progressively narrows the lumen of medium and large arteries by accumulation of lipids, monocyte-derived macrophages, which recruit T lymphocytes, and migration and proliferation of SMCs in the vascular wall (34, 43, 57, 71, 95).
From endothelial injury to atherosclerotic plaque rupture
Traditional risk factors of atherosclerosis have been shown to trigger endothelial activation. By cumulating these deleterious signals, activated endothelium becomes dysfunctional, as indicated by the loss of its antiadhesive properties toward leukocytes, and increased permeability to circulating lipids. Antiadhesive and antiinflammatory genes are shut down, and many proinflammatory genes are upregulated in activated endothelium. In particular, new expression of adhesion molecules and concomitant release of chemoattractants and cytokines favor the rolling and transmigration of monocytes and T lymphocytes, which accumulate in the subendothelial compartment. Monocytes proliferate and progressively mature into macrophages, which express scavenger receptors to take up lipids. This process becomes unremitting when they turn into foam cells, unable to clear oxidized low-density lipoproteins (LDLs) yielded by free radicals and accumulated in their cytoplasm. Oxidized LDLs are highly antigenic, potent chemoattractants, thus enhancing the inflammatory response. This initial step of atherosclerotic process, which is called “fatty streak,” occurs more frequently in areas of altered shear stress [i.e., branch points of arteries (Fig. 3)], where inappropriate flow decreases genes associated with differentiated ECs and SMCs (19).
FIG. 3.
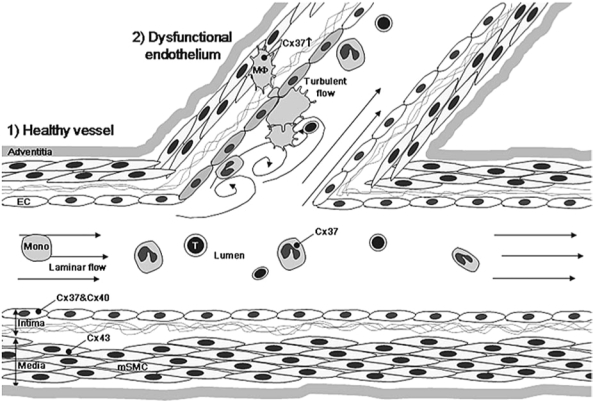
Expression of connexins at the arterial bifurcation. In healthy arteries, under a laminar flow, ECs express both Cx37 and Cx40. Medial SMCs (mSMC) display Cx43. Circulating monocytes express low levels of Cx37. Under low or oscillatory shear stress, ECs become dysfunctional and express Cx43. Monocytes and T lymphocytes are recruited by the dysfunctional endothelium and migrate over the EC barrier into the subendothelium. Macrophages (indicated as MO) start to express higher levels of Cx37. The dysfunctional endothelium slowly progresses over years to a fatty streak characterized by accumulation of lipids in the macrophages, which hence transform to macrophages foam cells with continued expression of Cx37.
As the inflammatory response progresses, growth factors and cytokines produced by the dysfunctional endothelium and monocytes/macrophages induce the migration of SMCs from the media to the intima. Once they have reached the inner part of the vessel, they proliferate, undergo phenotypic modulation, and produce ECM, thus constituting to the so-called “fibrous cap” that surrounds the atherosclerotic plaque (Fig. 4). Both macrophage foam cells and SMCs are subjected to apoptosis and therefore release their lipidic content in the necrotic core of advanced atherosclerotic plaques (Fig. 5). Decreased numbers of SMCs and further breakdown of the ECM by metalloproteinases, mostly released from macrophage foam cells, weakens the fibrous cap and might induce plaque rupture (121). This will then bring the highly thrombogenic plaque content in direct contact with the bloodstream and will trigger thrombosis at the site of the lesion, resulting in occlusion of the vessel, impairing blood flow, and causing ischemia of the tissue.
FIG. 4.
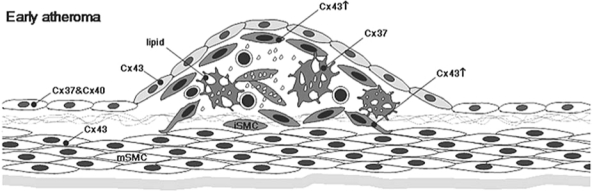
Expression of connexins in early atheroma. Under the influence of cytokines and growth factors secreted by the dysfunctional endothelium and infiltrated inflammatory cells, medial SMCs migrate to the intima, where they proliferate. Lipids start to accumulate outside the cells and within ECM secreted by intimal SMCs. Cx43 expression is upregulated in intimal SMC (ISMC), especially in the developing fibrous cap. ECs continue to express both Cx37 and Cx40, and macrophage foam cells still express Cx37.
FIG. 5.
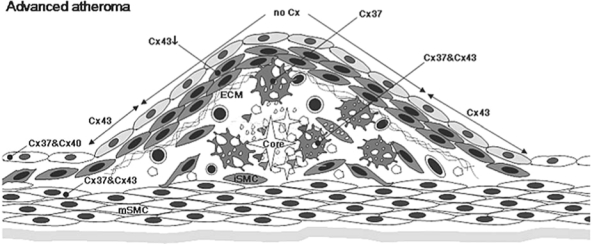
Expression of connexins in advanced atheroma. The fibrous cap completely covering the lesion area is composed of intimal SMCs and ECM. Extracellular lipids including cholesterol crystals (easily recognized in histology) are accumulated within the necrotic core composed of debris of apoptotic cells and degraded ECM. Macrophage foam cells close to the necrotic core express both Cx37 and Cx43. ECs still express Cx37 and Cx40 with two exceptions: ECs covering the lesion stop to express connexins, and those of shoulder regions start to express Cx43. In the fibrous cap, expression of Cx43 in intimal SMCs decreases as the lesion becomes more complicated. Medial SMCs beneath the lesion start to express Cx37.
Connexin expression and atherogenesis
The presence of newly recruited inflammatory cells in the vicinity of ECs and SMCs modify the normal vascular environment; this cellular interplay is tightly influenced by paracrine release of cytokines, chemokines, and growth factors. During the past 15 years, evidence has been seen of the contribution of direct cell–cell communication involving connexins in the development of atherosclerosis.
Important changes in the pattern of vascular Cx expression in human vessels and in animal models of atherosclerosis have been reported. The expression of Cx evolves as the disease progresses (Figs. 3–5) from a “healthy” vessel, to a dysfunctional endothelium, with early fatty streak to a “prone-to-rupture” vessel. As the cellularity of vulnerable plaque is very poor, the expression of Cx is reduced, and one can speculate that GJIC will be decreased, with the remaining cells being very sparse. Alterations of Cx expression in the different vascular cells might have several consequences on the molecular messages that are exchanged between these cells. The loss of one Cx between two adjacent cells would then hamper the passage of a particular type of signaling molecules, crucial to cell function.
Cx43 expression is normally restricted to medial SMCs of healthy vessels. Polacek and Davies (89) were the first to observe a concomitant expression of Cx43 mRNA in macrophage foam cells from human carotid arteries, even if Cx43 was not expressed by their precursor peripheral blood monocytes and the loss of Cx43 expression in intimal SMCs in atherosclerotic plaques (90). Later, a coordinated scenario was demonstrated, with a first upregulation of Cx43, followed by the previously described loss of Cx43 by medial SMCs underlying atherosclerotic plaques (6). These dynamic changes were further confirmed in a mouse model of atherosclerosis (59). Cx43 appears in ECs of the shoulder regions of the atherosclerotic lesion (59).
In a healthy vessel, Cx37 is expressed by ECs and circulating monocytes (59, 130). Once monocytes start to infiltrate into the vessel wall over a dysfunctional endothelium and progressively transform to macrophages, they exhibit increased levels of Cx37, which is maintained in fatty streaks. In advanced atheromatous plaques, Cx37 is downregulated in ECs and starts to be expressed by medial SMCs beneath the atherosclerotic lesion.
Cx40 expression is restricted to ECs of healthy vessels. Its pattern of expression is not affected until advanced atherosclerotic plaque forms; it is then strongly downregulated. So far, no Cx expression has been found in ECs covering advanced atherosclerotic lesions. As Cx has been implicated in tissue repair, the understanding of vascular lesions and arterial remodeling might benefit from studies on the expression of Cx in adventitia, fibroblasts, and perivascular tissues.
Local factors in favor of atherosclerotic development modify Cx expression
Disturbed hemodynamic forces at particular sites of the vascular tree that are of crucial importance in atherogenesis are known to affect the expression of vascular Cx or GJIC between vascular cells (17, 19, 24, 52, 61). Low shear stress or complex flow disturbances affect both EC and SMC behavior, directly by modifications of pressures exerted on cells and mechanical forces between cells, or indirectly by affecting the local soluble factors. In other words, while stimulation of mechanical sensors is disturbed, antiatherogenic transcription factors and antiadhesive substances such as NO are shut down. In contrast, proatherogenic transcription factors, adhesive proteins, and proinflammatory cytokines are enhanced (45). As a result, inflammatory cells more easily infiltrate these regions. Both mechanical strain and fluid shear stress have been shown to upregulate Cx43 expression in ECs and SMCs (17, 61). Moreover, disturbed flows induce the disruption of GJIC between ECs and upregulation and disorganization of Cx43 (24). Recent data demonstrate that flow-induced GJIC between human ECs is primarily mediated by Cx40, with a lesser contribution of Cx37 and Cx43 (26).
In addition, in vitro studies have shown that cytokines, growth factors, or substances expressed in the vicinity of vascular cells during development of atherosclerotic lesions modify Cx expression. Thus, TNF-α altered the Cx expression pattern and reduced GJIC in human ECs (120). Treatment with thrombin led to upregulation of Cx43 associated with increased synthetic activity, yet also enhanced contractile differentiation of aortic SMCs, and only growth arrest by contact inhibition led to progressive reduction in Cx43 expression (80). Surprisingly, PDGF-BB, known to modulate the SMC phenotype in vitro (44), did not affect Cx43 expression in this study. Cx43 expression was reversibly increased in hypoxic cultures of rat aortic SMCs, which exhibited enhanced intercellular communication in fluorescence recovery after photobleaching (FRAP) experiments (18). Interestingly, high glucose levels as observed in diabetes (a pathology associated with high risk of cardiovascular diseases) reduce Cx43-mediated microvascular endothelial cell communication (102). Moreover, as it releases high amounts of growth factors and cytokines, perivascular adipose tissue, which expresses Cx43, might contribute to the development and/or progression of atherosclerosis through multiple mechanisms, such as proliferative, proinflammatory, prothrombotic, and oxidant effects [for a review, see (55)]. Whether Cx43 is involved in the atherogenic effect of perivascular adipose tissue remains to be determined.
Accumulation and oxidative modification of LDLs is a key component to the development and progression of atherosclerosis (5, 68, 112). The oxidation of LDL gives rise to minimally oxidized LDL (78). Some of these oxidized phospholipids have been demonstrated to have extensive biologic activity, in particular the phospholipid derivatives of oxidized 1-palmitoyl-2-arachidonoyl-sn-3-glycero-phosphorylcholine (OxPAPC). For example, one of the components of OxPAPC, 1-palmitoyl-2-oxovaleroyl-sn-glycero-3-phosphorylcholine (POVPC) can activate protein kinase A (PKA), whereas 1-palmitoyl-2-glutaroyl-sn-glycero-3-phosphorylcholine (PGPC) activates PKC (67, 125, 126). This presents a fascinating possibility for differential regulation of proteins (e.g., connexins), especially in terms of phosphorylation. Thus far, studies have focused exclusively on inflammatory effects of oxidized lipids on ECs, SMCs, and leukocyte function (78), with few studies focusing on the effects of oxidized phospholipids on GJIC.
The functional consequences of changes in connexin protein expression during atherosclerosis, coupled with the extensive evidence for OxPAPC involvement in the development of atherosclerosis, gives rise to the possibility of an interaction between OxPAPC and connexins. Recent work demonstrated that all of the vascular connexins in large vessels are altered in response to direct application of OxPAPC to mouse carotid vessels, or cultures of ECs and SMCs (49), mimicking very similar although not exact patterns of connexin expression demonstrated in atherosclerotic plaques (59). The key difference in connexin expression is that Cx43 was upregulated in SMCs in response to the OxPAPC, whereas in advanced mouse atherosclerosis, Cx43 is largely absent from the intimal SMCs. Because OxPAPC is a mixture of oxidized phospholipids, it is possible that the multiple components with biologic activity may be having differential effects, and so experiments using OxPAPC species will be necessary to dissect which is capable of inducing changes in a particular connexin subtype, in either ECs or SMCs. Functionally, it was noted that after OxPAPC application, biocytin dye transfer was no longer capable of passing between cells, indicating that the gap junctions were closed by OxPAPC, a function attributed to Cx43 phosphorylation (49). Application of OxPAPC and its individual species has recently been shown to induce SMC differentiation (86). This fits closely to the observation made by several laboratories that a correlation exists between connexins and SMC differentiation in atherosclerosis (80, 91). Taken together, these data are strong evidence for a direct interaction between OxPAPC and connexin in regulation not only of connexin protein expression, but of gap-junction function as well.
Lessons from animal models
Two well-characterized mouse models are used to study the development of atherosclerosis (13): the low-density lipoprotein–receptor knockout mice (LDLR−/−) and the apolipoprotein E (ApoE−/−) knockout mice, which have in common the deletion of genes participating in the transport of lipoproteins. Mutations in the corresponding genes in humans result in familial hypercholesterolemia, a risk factor for atherosclerotic development, which is frequently associated with early myocardial infarction.
Deletion of the LDLR impairs the hepatic uptake of LDL and recirculation of cholesterol. ApoE is also necessary for the uptake of lipoproteins. Thus, both LDLR−/− and ApoE−/− mice have high cholesterol levels, even more pronounced in ApoE−/− mice. It is, however, necessary to feed LDLR−/− mice a high-cholesterol diet to induce significant hypercholesterolemia and consecutive atherosclerotic lesions (51), whereas ApoE−/− mice spontaneously develop atherosclerotic lesions, which become even more important with a high-cholesterol diet (88, 138, 137). The time course and distribution of plaques in the arterial tree are reproducible and present a great similarity to the human atherosclerotic lesions. The main limitation of these mouse models is that they lack the spontaneous rupture of plaques. Other animal models are therefore being used.
The Watanabe heritable hyperlipidemic (WHHL) rabbit produces a mutant receptor for plasma LDL that is not transported to the cell surface at a normal rate. As it partly mimics some human atherosclerotic plaque ruptures, it is also a frequently used animal model (2, 109). More recently, atherosclerosis and restenosis have been studied in pigs (44), which are closest to humans in terms of size and anatomy, and spontaneously develop atherosclerotic lesions in the abdominal aorta. Interestingly, natural pig strains produce mutants for lipoproteins.
As they are genetically well defined, mice can be easily interbred with other knockout mice lacking genes of the Cx family. We have demonstrated that ApoE−/− mice lacking Cx37 and fed a high-cholesterol diet develop accelerated atherosclerotic lesions in both the thoracic–abdominal aortas and aortic sinuses (130). We further showed that Cx37 hemichannels borne by monocytes/macrophages limit their adhesion and recruitment to the subendothelium by an autocrine release of ATP, thus explaining the protection this protein confers against atherosclerosis (130).
Cx43 is important in cardiac development and function. Cx43 plays a role in the looping of the ascending limb of the heart tube and the development of the right ventricle and the outflow tract, and Cx43-knockout mice die at birth of severe cardiac malformations (92). Heterozygous Cx43-deficient mice, expressing 50% of the Cx43 normal level, have thus been crossbred with LDLR−/− mice. Cx43+/+LDLR−/− and Cx43+/−LDLR−/− mice have comparable elevated serum cholesterol and triglyceride profiles, leukocyte counts, body weight, and respond similarly to a high-cholesterol diet. Comparison between these two groups of mice fed a high-cholesterol diet allowed us to observe that atherosclerosis progression was reduced by 50% in both the aortic roots and the thoracic–abdominal aorta of Cx43+/−LDLR−/− mice. Atherosclerotic lesions were less complex, as attested by a reduction in the number of inflammatory cells and a thicker fibrous cap with a high-collagen content and large numbers of SMCs, which resulted in a more stable plaque phenotype [i.e., less susceptible to rupture (60, 129)]. These data suggested that, in contrast to Cx37, Cx43 has an atherogenic effect.
As described earlier, Cx40-knockout mice have also been generated, but present characteristics of hypertension (21, 22, 122), an independent risk factor for atherosclerosis. Therefore, we recently established a mouse model with selective deletion of Cx40 in endothelium by using the Cre-LoxP system under the control of the Tie2 promotor. The endothelium-specific deletion of Cx40 did not affect mean arterial pressure or heart rate in mice (14). Preliminary data also indicate that the surface of atherosclerotic lesions in ApoE−/− mice lacking endothelial Cx40 is larger as compared with that in control animals fed a high-cholesterol diet, suggesting that Cx40 has atheroprotective potential (14). The loss of Cx40 in the endothelium covering early atherosclerotic lesions might thus further facilitate the development of atherosclerosis. The mechanism by which Cx40 is atheroprotective should be explored.
Cx37 genetic polymorphism influences the risk of cardiovascular disease
Recently, research demonstrated an association of a polymorphism in the Cx37 gene, a protein normally expressed in ECs but also found in monocytes and macrophages, with arterial stenosis and myocardial infarction in humans (7, 72, 130, 131, 134). The Cx37 C1019T polymorphism results in a nonconservative amino acid shift from a proline to a serine at codon 319, in the regulatory intracellular carboxy terminus of Cx37 protein. The human Cx37 genotype was then also shown to predict survival after an acute coronary syndrome (65). In this context, the observation that expression of Cx37-319S or Cx37-319P by transfection of a human macrophage cell line revealed differential adhesiveness to substrates is of particular importance (130). This may be caused by increased permeability of the Cx37-319P hemichannels for ATP, thus providing a potential mechanism by which the Cx37-1019T variant protects against atherosclerosis.
Drugs influencing the development of atherosclerotic plaques
The 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, the so-called statins, have been proven to be highly effective in the management of hypercholesterolemia and in the prevention of atherosclerotic vascular disease. As inhibitors of HMG-CoA reductase, the primary pharmacodynamic effect of statins is to inhibit the synthesis of cholesterol by the liver. The reduction of cholesterol in hepatocytes leads to the compensatory increase of hepatic LDLRs, followed by an increase in cholesterol uptake by the liver and a reduction of circulating lipoproteins.
The beneficial effects of statins were considered to depend initially on the reduction of LDL cholesterol, followed by the subsequent regression of atherosclerotic lesions. However, the observation that lipid lowering by means other than statins, such as ileal bypass surgery, required markedly more time to manifest clinical benefits pointed to additional effects from this class of drugs. Many data support more direct cholesterol-independent beneficial effects of statins on atherosclerosis or plaque rupture (103). They include increased synthesis of NO, inhibition of free radical release, decreased synthesis of endothelin-1, inhibition of LDL cholesterol oxidation, increased availability of endothelial progenitor cells, reduced number and activity of inflammatory cells in atherosclerotic lesions, increased SMC proliferation and differentiation, reduced production of metalloproteinases, and inhibition of platelet adhesion/aggregation. As more of such pleiotropic effects are discovered, increasing understanding exists on how statins mediate their actions.
Chemically, statins competitively inhibit HMG-CoA reductase, thereby reducing the availability of l-mevalonate and consequently the biosynthesis of cholesterol as well as prenylated proteins (Fig. 6A). Prenylated proteins or isoprenoids, such as farnesylpyrophosphate (FPP) and geranylgeranylpyrophosphate (GGPP), are important for the posttranslational modification (prenylation) of proteins involved in normal cell functions. More recently, statins were also hypothesized to disrupt cholesterol and sphingolipid membrane microdomains, so-called lipid rafts, which are essential platforms for the initiation of signal transduction.
FIG. 6.
(A) Metabolic pathway of mevalonate and cholesterol. The enzymatic reactions and their pharmacologic inhibitors are indicated. (B, C) To investigate the metabolic pathway involved in statin-induced modulation of Cx43 expression in primary human saphenous vein SMCs (C), cells were stimulated for 24 h with simvastatin (S) alone or in combination with chemical compounds affecting the downstream signalling pathway of HMG-CoA, such as mevalonate (M; 400 μM), farnesyl transferase inhibitor (FTI-277; 3 μM), or geranylgeranyl transferase inhibitor type I (GGTI-286; 10 μM), or with the isoprenoid intermediates FPP or GGPP (at 10 μM each). A typical example of a Western blot for Cx43 is shown in (C). Bar chart illustrates quantification of Cx43 expression (normalized to β-actin) in five independent experiments.
Only a few studies have examined the effects of statins on connexins. The expression of Cx43 was examined in vitro in primary human vascular cells isolated from saphenous veins in the presence of three different statins, the lipophilic simvastatin and atorvastatin and the hydrophilic pravastatin. Each of the statins effectively reduced the expression of Cx43 in ECs and in SMCs in a dose-dependent manner, and this effect was abolished in the presence of l-mevalonate (60). Cx43 was typically immunolocalized along the cell membranes of contacting SMCs. Simvastatin treatment strongly reduced the amount of Cx43 immunolabeling; however, the subcellular localization of Cx43 was not affected by the statin. Mechanistically, the statin-induced reduction in Cx43 in SMCs seems to involve geranylgeranylated proteins. As shown in Fig. 6B, the statin-induced reduction in Cx43 expression was reversed by 400 μM mevalonate and the isoprenoid intermediate GGPP (10 μM), but not by 10 μM FPP. Furthermore, 10 μM geranylgeranyl transferase inhibitor type I (GGTI-286) reduced Cx43 expression, an effect similar to the one observed with statins, whereas the farnesyl transferase inhibitor FTI-277 (3 μM) was without effect (Fig. 6B).
In vivo studies on LDLR−/− mice treated orally with pravastatin displayed a more stable plaque phenotype [i.e., atheromas contained fewer inflammatory cells and displayed thicker fibrous caps, which had more interstitial collagen and concentric layers of SMCs (60)]. As described before, a similar plaque phenotype was observed in atheromas of Cx43+/−LDLR−/− mice. Interestingly, immunostaining on atheromas in pravastatin-treated mice showed reduced Cx43 expression throughout the entire atherosclerotic lesion. This decreased expression of Cx43 in the statin-treated mice, as compared with control animals, was confirmed by Western blotting of their aorta protein extracts. In another study, it was shown that mouse aortic endothelial connexins and gap junctions were downregulated during long-term hyperlipidemia. Short-term treatment with simvastatin led to recovery of Cx37 expression but not Cx40 expression (135). Based on these two studies, the general hypothesis at present is that hyperlipidemia, a major risk factor of atherosclerosis, reduces Cx37 and Cx40 in ECs but induces Cx43 expression in ECs and SMCs. Interestingly, these connexin expression profiles are reversed by statins (e.g., increased Cx37 and Cx40 expression in ECs but decreased Cx43 in ECs and SMCs). Besides hyperlipidemia, smoking is another conventional risk factor of atherosclerosis, thus providing a rationale for studying the effect of nicotine and/or statins on Cx43 expression and GJIC in human ECs (116). Expression of Cx43 was dose-dependently reduced by nicotine. This effect was due to posttranscriptional modification, involving enhancement of Cx43 proteolysis, and was mediated via activation of acetylcholine receptors sensitive to nicotine (nAChRs). The effect of nicotine was attenuated by various statins, even in the presence of mevalonate, so thus through mechanisms outside the prenylation pathway. Interestingly, Cx43 has been found in lipid rafts (104). Whether the statin-induced nicotine effects on Cx43 involve lipid rafts remains to be investigated.
Gap Junctions in Restenosis
Development of atherosclerosis in coronary arteries results in ischemic heart disease. In addition to coronary bypass surgery, two common and effective mechanical treatments exist for coronary atherosclerosis: percutaneous transluminal coronary angioplasty (PTCA), which consists of disruption of the developing atherosclerotic plaque by means of balloon-catheter distention, and the implantation of a stent after PTCA. However, renarrowing of the vessels at the site of intervention hampers their long-term efficacy and the so-called “restenosis” occurs in 30–60% of the patients after angioplasty (118), and 5–30% of the patients after stent implantation (84). Restenosis mechanisms are not fully understood and are the subject of many investigations. Both intimal hyperplasia and arterial remodeling contribute to the reduction of arterial caliber. The parietal traumatism induced by the balloon distention triggers platelet aggregation and the formation of a mural thrombus. In addition to the mechanical injury, the balloon-catheter also stretches the artery, thus inducing an exaggerated and accelerated response to injury, involving the recruitment and rapid infiltration of inflammatory cells (57). Endothelialization repairs the lining of the damaged vasculature and is crucial to prevent thrombosis and restenosis. Both GJIC and purinergic signaling contribute to re-endothelialization of human EC by regulating both intra- and extracellular Ca2+ stores (139). The release of growth factors and proinflammatory cytokines by platelets, leukocytes, and SMCs, in addition to the loss of endothelial antiproliferating agents such as NO, modulates the phenotype of SMCs, shifting them from a quiescent and “contractile” phenotype to a “synthetic,” proliferating, and less differentiated one. SMCs start to proliferate 24–48 h after angioplasty and migrate toward the intima on the fourth day. They synthesize abundant ECM, which further contributes to intimal hyperplasia. The maximal intimal thickening is reached after 2–3 months, after which SMCs return to a “contractile” phenotype, the wall is remodeled, and intimal thickening stops.
Stenting has become the treatment of choice because it provides a mechanical support to the vessel and extends its internal diameter to avoid reocclusion of the vessel after angioplasty. However, the combined use of systemic antithrombotic drugs or the use of drug-eluting stents does not completely circumvent restenosis. Most important functions that should be fulfilled by “the ideal stent” are to decrease SMC proliferation to prevent in stent restenosis while increasing EC migration and proliferation to cover the stent, thus allowing the restoration of endothelial antiadhesive properties.
After ballooning in the rat carotid artery, Cx43 was upregulated in intimal and medial SMCs concomitant with their activation and phenotypic modulation (133). Enhanced Cx43 expression in SMCs and macrophages of restenotic lesions has been confirmed in different species (15, 87, 90, 124). Cx43+/−LDLR−/− mice, expressing reduced levels of Cx43, displayed restricted intimal thickening after balloon-distention injury in the carotid artery (Fig. 7), despite marked endothelial denudation and SMC activation, as compared with control littermate Cx43+/+LDLR−/− mice. The reduction of Cx43 resulted in decreased macrophage infiltration and SMC migration and proliferation, associated with accelerated reendothelialization, suggesting that Cx43 targeting could be a novel therapeutic strategy to prevent restenosis after PTCA or stent implantation.
FIG. 7.
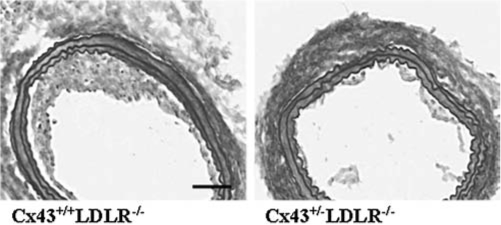
Balloon-injury in mice with reduced Cx43 expression. Representative Van Gieson-Miller staining of carotid cryosections 14 days after balloon distention injury in Cx43+/+LDLR−/− mouse (left) and in a Cx43+/−LDLR−/− mouse (right). Neointimal hyperplasia is reduced in the Cx43+/−LDLR−/− mouse as compared with its Cx43+/+LDLR−/− mouse littermate control. Bar is 100 μm.
Concluding Remarks
The previous sections have provided support for an important role of connexins in vascular physiology and a largely multifaceted role in the development of disease. So far, research has mostly concentrated on the roles of the three major vascular connexins; however, this does not preclude interesting expression patterns and regulatory functions for other gap-junction proteins (for example, Cx45 or Cx31.9) in blood vessels. After many years of research on the relation between gap junctions and longitudinal conduction along arterioles, we only recently have begun to understand the composition of the MEJ. This has begun to open up an increasing understanding of the signaling from ECs to SMCs, and vice versa. Disruption or alteration of this intercellular signaling pathway may have considerable implications for development of disease as well. After a decade of investigations of changes in connexin expression in diseased vessels, we have been helped by the availability of connexin-deficient mice to recognize the roles of these proteins in disease development. Thus, Cx40 appeared critical in blood-pressure regulation at the level of the control of renin secretion. Cx37 has a protective role against atherosclerosis by controlling ATP-dependent monocyte adhesion. Monocyte adhesion is critical for many other immunoinflammatory disorders as well. In contrast to Cx37, Cx43 seems to possess atherogenic properties. Interestingly, the upregulation of this protein observed in most vascular disease states can be downregulated by drugs commonly used in clinics, such as statins and adrenergic blockers. Last, increasing attention is seen in cardiology clinics to a person's genetic makeup with respect to risk stratification and disease prevention. Polymorphisms in connexin genes are therefore of particular interest.
Acknowledgments
We thank Cindy Wong, Christos Chadjichristos, Marc Chanson, Francois Mach, Angela Best, Brian Duling, and Richard K. Henley for helpful discussions. Our work has been supported by grants from the Swiss National Science Foundation (PPOOA-68883 and PPOOA-116897 to B.R.K.), the American Heart Association (0735167N to B.E.I.), and the United States National Institutes of Health (HL88554 to B.E.I.).
Abbreviations
ApoE, apolipoprotein E; Cx, connexin; ECM, extracellular matrix; ECs, endothelial cells; EDHF, endothelium-derived hyperpolarizing factor; FPP, farnesylpyrophosphate; FRAP, fluorescence recovery after photobleaching; FTI, farnesyl transferase inhibitor; GGPP, geranylgeranylpyrophosphate; GGTI, geranylgeranyl transferase inhibitor; GJIC, gap-junctional intercellular communication; HMG-CoA, 3-hydroxy-3-methylglutaryl-coenzyme A; H2O2, hydrogen peroxide; IP3, inositol triphosphate; LDL, low-density lipoprotein; LDLR, low-density lipoprotein receptor; MEJ, myoendothelial junctions; nAChRs, acetylcholine receptors sensitive to nicotine; NO, nitric oxide; OxPAPC, oxidized 1-palmitoyl-2-arachidonoyl-sn-3-glycero-phosphorylcholine; PGPC, 1-palmitoyl-2-glutaroyl-sn-glycero-3-phosphorylcholine; PKA, protein kinase A; PKC, protein kinase C; POVPC, 1-palmitoyl-2-oxovaleroyl-sn-glycero-3-phosphorylcholine; PTCA, percutaneous transluminal coronary angioplasty; SEM, scanning electron microscopy; SHR, spontaneously hypertensive rat; SMCs, smooth muscle cells; TEM, transmission electron microscopy; WHHL, Watanabe heritable hyperlipidemic.
References
- 1. Abraham V. Chou ML. DeBolt KM. Koval M. Phenotypic control of gap junctional communication by cultured alveolar epithelial cells. Am J Physiol. 1999;276:L825–L834. doi: 10.1152/ajplung.1999.276.5.L825. [DOI] [PubMed] [Google Scholar]
- 2. Aliev G. Castellani RJ. Petersen RB. Burnstock G. Perry G. Smith MA. Pathobiology of familial hypercholesterolemic atherosclerosis. J Submicrosc Cytol Pathol. 2004;36:225–240. [PubMed] [Google Scholar]
- 3. Beny J. Electrical coupling between smooth muscle cells and endothelial cells in pig coronary arteries. Pflugers Arch. 1997;433:364–367. doi: 10.1007/s004240050289. [DOI] [PubMed] [Google Scholar]
- 4. Beny JL. Information networks in the arterial wall. News Physiol Sci. 1999;14:68–73. doi: 10.1152/physiologyonline.1999.14.2.68. [DOI] [PubMed] [Google Scholar]
- 5. Berliner JA. Watson AD. A role for oxidized phospholipids in atherosclerosis. N Engl J Med. 2005;353:9–11. doi: 10.1056/NEJMp058118. [DOI] [PubMed] [Google Scholar]
- 6. Blackburn JP. Peters NS. Yeh HI. Rothery S. Green CR. Severs NJ. Upregulation of connexin43 gap junctions during early stages of human coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 1995;15:1219–1228. doi: 10.1161/01.atv.15.8.1219. [DOI] [PubMed] [Google Scholar]
- 7. Boerma M. Forsberg L. Van Zeijl L. Morgenstern R. De Faire U. Lemne C. Erlinge D. Thulin T. Hong Y. Cotgreave IA. A genetic polymorphism in connexin 37 as a prognostic marker for atherosclerotic plaque development. J Intern Med. 1999;246:211–218. doi: 10.1046/j.1365-2796.1999.00564.x. [DOI] [PubMed] [Google Scholar]
- 8. Boitano S. Dirksen ER. Sanderson MJ. Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science. 1992;258:292–295. doi: 10.1126/science.1411526. [DOI] [PubMed] [Google Scholar]
- 9. Boitano S. Evans WH. Connexin mimetic peptides reversibly inhibit Ca(2+) signaling through gap junctions in airway cells. Am J Physiol Lung Cell Mol Physiol. 2000;279:L623–L630. doi: 10.1152/ajplung.2000.279.4.L623. [DOI] [PubMed] [Google Scholar]
- 10. Brink PR. Gap junctions in vascular smooth muscle. Acta Physiol Scand. 1998;164:349–356. doi: 10.1046/j.1365-201X.1998.00439.x. [DOI] [PubMed] [Google Scholar]
- 11. Bruzzone R. Haefliger JA. Gimlich RL. Paul DL. Connexin40, a component of gap junctions in vascular endothelium, is restricted in its ability to interact with other connexins. Mol Biol Cell. 1993;4:7–20. doi: 10.1091/mbc.4.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Budel S. Bartlett IS. Segal SS. Homocellular conduction along endothelium and smooth muscle of arterioles in hamster cheek pouch: unmasking an NO wave. Circ Res. 2003;93:61–68. doi: 10.1161/01.RES.0000080318.81205.FD. [DOI] [PubMed] [Google Scholar]
- 13. Carmeliet P. Moons L. Collen D. Mouse models of angiogenesis, arterial stenosis, atherosclerosis and hemostasis. Cardiovasc Res. 1998;39:33. doi: 10.1016/s0008-6363(98)00108-4. [DOI] [PubMed] [Google Scholar]
- 14. Chadjichristos CE. Roth I. Hoepfl B. van Veen TA. Deutsch U. Van Kempen MJ. De Wit C. Kwak BR. Increased development of atherosclerosis in mice with endothelial-specific deletion of connexin40. Circulation. 2005;112:1–142. doi: 10.1161/CIRCULATIONAHA.109.867176. [DOI] [PubMed] [Google Scholar]
- 15. Chadjichristos CE. Matter CM. Roth I. Sutter E. Pelli G. Luscher TF. Chanson M. Kwak BR. Reduced connexin43 expression limits neointima formation after balloon distension injury in hypercholesterolemic mice. Circulation. 2006;113:2835–2843. doi: 10.1161/CIRCULATIONAHA.106.627703. [DOI] [PubMed] [Google Scholar]
- 16. Chaytor AT. Bakker LM. Edwards DH. Griffith TM. Connexin-mimetic peptides dissociate electrotonic EDHF-type signalling via myoendothelial and smooth muscle gap junctions in the rabbit iliac artery. Br J Pharmacol. 2005;144:108–114. doi: 10.1038/sj.bjp.0706046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cowan DB. Lye SJ. Langille BL. Regulation of vascular connexin43 gene expression by mechanical loads. Circ Res. 1998;82:786–793. doi: 10.1161/01.res.82.7.786. [DOI] [PubMed] [Google Scholar]
- 18. Cowan DB. Jones M. Garcia LM. Noria S. del Nido PJ. McGowan FX., Jr Hypoxia and stretch regulate intercellular communication in vascular smooth muscle cells through reactive oxygen species formation. Arterioscler Thromb Vasc Biol. 2003;23:1754–1760. doi: 10.1161/01.ATV.0000093546.10162.B2. [DOI] [PubMed] [Google Scholar]
- 19. Davies PF. Shi C. Depaola N. Helmke BP. Polacek DC. Hemodynamics and the focal origin of atherosclerosis: a spatial approach to endothelial structure, gene expression, and function. Ann N Y Acad Sci. 2001;947:16. discussion 16–17. [PubMed] [Google Scholar]
- 20. De Vriese AS. Van de Voorde J. Lameire NH. Effects of connexin-mimetic peptides on nitric oxide synthase- and cyclooxygenase-independent renal vasodilation. Kidney Int. 2002;61:177–185. doi: 10.1046/j.1523-1755.2002.00122.x. [DOI] [PubMed] [Google Scholar]
- 21. de Wit C. Roos F. Bolz SS. Kirchhoff S. Kruger O. Willecke K. Pohl U. Impaired conduction of vasodilation along arterioles in connexin40-deficient mice. Circ Res. 2000;86:649–655. doi: 10.1161/01.res.86.6.649. [DOI] [PubMed] [Google Scholar]
- 22. de Wit C. Roos F. Bolz SS. Pohl U. Lack of vascular connexin 40 is associated with hypertension and irregular arteriolar vasomotion. Physiol Genomics. 2003;13:169–177. doi: 10.1152/physiolgenomics.00169.2002. [DOI] [PubMed] [Google Scholar]
- 23. de Wit C. Hoepfl B. Wolfle SE. Endothelial mediators and communication through vascular gap junctions. Biol Chem. 2006;387:3–9. doi: 10.1515/BC.2006.002. [DOI] [PubMed] [Google Scholar]
- 24. DePaola N. Davies PF. Pritchard WF., Jr Florez L. Harbeck N. Polacek DC. Spatial and temporal regulation of gap junction connexin43 in vascular endothelial cells exposed to controlled disturbed flows in vitro. Proc Natl Acad Sci U S A. 1999;96:3154–3159. doi: 10.1073/pnas.96.6.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dora KA. Doyle MP. Duling BR. Elevation of intracellular calcium in smooth muscle causes endothelial cell generation of NO in arterioles. Proc Natl Acad Sci U S A. 1997;94:6529–6534. doi: 10.1073/pnas.94.12.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ebong EE. Kim S. DePaola N. Flow regulates intercellular communication in HAEC by assembling functional Cx40 and Cx37 gap junctional channels. Am J Physiol Heart Circ Physiol. 2006;290:H2015–H2023. doi: 10.1152/ajpheart.00204.2005. [DOI] [PubMed] [Google Scholar]
- 27. Elfgang C. Eckert R. Lichtenberg-Frate H. Butterweck A. Traub O. Klein RA. Hulser DF. Willecke K. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J Cell Biol. 1995;129:805–817. doi: 10.1083/jcb.129.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fanchaouy M. Serir K. Meister JJ. Beny JL. Bychkov R. Intercellular communication: role of gap junctions in establishing the pattern of ATP-elicited Ca2+ oscillations and Ca2+-dependent currents in freshly isolated aortic smooth muscle cells. Cell Calcium. 2005;37:25–34. doi: 10.1016/j.ceca.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 29. Figueroa XF. Paul DL. Simon AM. Goodenough DA. Day KH. Damon DN. Duling BR. Central role of connexin40 in the propagation of electrically activated vasodilation in mouse cremasteric arterioles in vivo. Circ Res. 2003;92:793–800. doi: 10.1161/01.RES.0000065918.90271.9A. [DOI] [PubMed] [Google Scholar]
- 30. Figueroa XF. Isakson BE. Duling BR. Vascular gap junctions in hypertension. Hypertension. 2006;48:804–811. doi: 10.1161/01.HYP.0000242483.03361.da. [DOI] [PubMed] [Google Scholar]
- 31. Firouzi M. Ramanna H. Kok B. Jongsma HJ. Koeleman BP. Doevendans PA. Groenewegen WA. Hauer RN. Association of human connexin40 gene polymorphisms with atrial vulnerability as a risk factor for idiopathic atrial fibrillation. Circ Res. 2004;95:e29–e33. doi: 10.1161/01.RES.0000141134.64811.0a. [DOI] [PubMed] [Google Scholar]
- 32. Firouzi M. Kok B. Spiering W. Busjahn A. Bezzina CR. Ruijter JM. Koeleman BP. Schipper M. Groenewegen WA. Jongsma HJ. de Leeuw PW. Polymorphisms in human connexin40 gene promoter are associated with increased risk of hypertension in men. J Hypertens. 2006;24:325–330. doi: 10.1097/01.hjh.0000200512.40818.47. [DOI] [PubMed] [Google Scholar]
- 33. Gabriels JE. Paul DL. Connexin43 is highly localized to sites of disturbed flow in rat aortic endothelium but connexin37 and connexin40 are more uniformly distributed. Circ Res. 1998;83:636–643. doi: 10.1161/01.res.83.6.636. [DOI] [PubMed] [Google Scholar]
- 34. Glass CK. Witztum JL. Atherosclerosis: the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 35. Gown AM. Unmasking the mysteries of antigen or epitope retrieval and formalin fixation. Am J Clin Pathol. 2004;121:172–174. doi: 10.1309/9G5F-Y3U3-QB4R-15DR. [DOI] [PubMed] [Google Scholar]
- 36. Groenewegen WA. Firouzi M. Bezzina CR. Vliex S. van Langen IM. Sandkuijl L. Smits JP. Hulsbeek M. Rook MB. Jongsma HJ. Wilde AA. A cardiac sodium channel mutation cosegregates with a rare connexin40 genotype in familial atrial standstill. Circ Res. 2003;92:14–22. doi: 10.1161/01.res.0000050585.07097.d7. [DOI] [PubMed] [Google Scholar]
- 37. Haddock RE. Grayson TH. Brackenbury TD. Meaney KR. Neylon CB. Sandow SL. Hill CE. Endothelial coordination of cerebral vasomotion via myoendothelial gap junctions containing connexins 37 and 40. Am J Physiol Heart Circ Physiol. 2006;291:H2047–H2056. doi: 10.1152/ajpheart.00484.2006. [DOI] [PubMed] [Google Scholar]
- 38. Haefliger JA. Castillo E. Waeber G. Aubert JF. Nicod P. Waeber B. Meda P. Hypertension differentially affects the expression of the gap junction protein connexin43 in cardiac myocytes and aortic smooth muscle cells. Adv Exp Med Biol. 1997;432:71–82. doi: 10.1007/978-1-4615-5385-4_8. [DOI] [PubMed] [Google Scholar]
- 39. Haefliger JA. Castillo E. Waeber G. Bergonzelli GE. Aubert JF. Sutter E. Nicod P. Waeber B. Meda P. Hypertension increases connexin43 in a tissue-specific manner. Circulation. 1997;95:1007–1014. doi: 10.1161/01.cir.95.4.1007. [DOI] [PubMed] [Google Scholar]
- 40. Haefliger JA. Meda P. Formenton A. Wiesel P. Zanchi A. Brunner HR. Nicod P. Hayoz D. Aortic connexin43 is decreased during hypertension induced by inhibition of nitric oxide synthase. Arterioscler Thromb Vasc Biol. 1999;19:1615–1622. doi: 10.1161/01.atv.19.7.1615. [DOI] [PubMed] [Google Scholar]
- 41. Haefliger JA. Meda P. Chronic hypertension alters the expression of Cx43 in cardiovascular muscle cells. Braz J Med Biol Res. 2000;33:431–438. doi: 10.1590/s0100-879x2000000400009. [DOI] [PubMed] [Google Scholar]
- 42. Haefliger JA. Polikar R. Schnyder G. Burdet M. Sutter E. Pexieder T. Nicod P. Meda P. Connexin37 in normal and pathological development of mouse heart and great arteries. Dev Dyn. 2000;218:331–344. doi: 10.1002/(SICI)1097-0177(200006)218:2<331::AID-DVDY7>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 43. Hansson GK. Libby P. Schonbeck U. Yan ZQ. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res. 2002;91:281–291. doi: 10.1161/01.res.0000029784.15893.10. [DOI] [PubMed] [Google Scholar]
- 44. Hao H. Ropraz P. Verin V. Camenzind E. Geinoz A. Pepper MS. Gabbiani G. Bochaton-Piallat ML. Heterogeneity of smooth muscle cell populations cultured from pig coronary artery. Arterioscler Thromb Vasc Biol. 2002;22:1093–1099. doi: 10.1161/01.atv.0000022407.91111.e4. [DOI] [PubMed] [Google Scholar]
- 45. Helderman F. Segers D. de Crom R. Hierck BP. Poelmann RE. Evans PC. Krams R. Effect of shear stress on vascular inflammation and plaque development. Curr Opin Lipidol. 2007;18:527–533. doi: 10.1097/MOL.0b013e3282ef7716. [DOI] [PubMed] [Google Scholar]
- 46. Hill CE. Phillips JK. Sandow SL. Heterogeneous control of blood flow amongst different vascular beds. Med Res Rev. 2001;21:1–60. doi: 10.1002/1098-1128(200101)21:1<1::aid-med1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 47. Isakson BE. Duling BR. Heterocellular contact at the myoendothelial junction influences gap junction organization. Circ Res. 2005;97:44–51. doi: 10.1161/01.RES.0000173461.36221.2e. [DOI] [PubMed] [Google Scholar]
- 48. Isakson BE. Damon DN. Day KH. Liao Y. Duling BR. Connexin40 and connexin43 in mouse aortic endothelium: evidence for coordinated regulation. Am J Physiol Heart Circ Physiol. 2006;290:H1199–H1205. doi: 10.1152/ajpheart.00945.2005. [DOI] [PubMed] [Google Scholar]
- 49. Isakson BE. Kronke G. Kadl A. Leitinger N. Duling BR. Oxidized phospholipids alter vascular connexin expression, phosphorylation, and heterocellular communication. Arterioscler Thromb Vasc Biol. 2006;26:2216–2221. doi: 10.1161/01.ATV.0000237608.19055.53. [DOI] [PubMed] [Google Scholar]
- 50. Isakson BE. Ramos SI. Duling BR. Ca2+ and inositol 1,4,5-trisphosphate-mediated signaling across the myoendothelial junction. Circ Res. 2007;100:246–254. doi: 10.1161/01.RES.0000257744.23795.93. [DOI] [PubMed] [Google Scholar]
- 51. Ishibashi S. Goldstein JL. Brown MS. Herz J. Burns DK. Massive xanthomatosis and atherosclerosis in cholesterol-fed low density lipoprotein receptor-negative mice. J Clin Invest. 1994;93:1885–1893. doi: 10.1172/JCI117179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Johnson TL. Nerem RM. Endothelial connexin 37, connexin 40, and connexin 43 respond uniquely to substrate and shear stress. Endothelium. 2007;14:215–226. doi: 10.1080/10623320701617233. [DOI] [PubMed] [Google Scholar]
- 53. Kansui Y. Fujii K. Nakamura K. Goto K. Oniki H. Abe I. Shibata Y. Iida M. Angiotensin II receptor blockade corrects altered expression of gap junctions in vascular endothelial cells from hypertensive rats. Am J Physiol Heart Circ Physiol. 2004;287:H216–H224. doi: 10.1152/ajpheart.00915.2003. [DOI] [PubMed] [Google Scholar]
- 54. Karagiannis J. Rand M. Li CG. Role of gap junctions in endothelium-derived hyperpolarizing factor-mediated vasodilatation in rat renal artery. Acta Pharmacol Sin. 2004;25:1031–1037. [PubMed] [Google Scholar]
- 55. Katagiri H. Yamada T. Oka Y. Adiposity and cardiovascular disorders: disturbance of the regulatory system consisting of humoral and neuronal signals. Circ Res. 2007;101:27–39. doi: 10.1161/CIRCRESAHA.107.151621. [DOI] [PubMed] [Google Scholar]
- 56. Krattinger N. Capponi A. Mazzolai L. Aubert JF. Caille D. Nicod P. Waeber G. Meda P. Haefliger JA. Connexin40 regulates renin production and blood pressure. Kidney Int. 2007;72:814–822. doi: 10.1038/sj.ki.5002423. [DOI] [PubMed] [Google Scholar]
- 57.Kumar V.Abbas AK.Fausto N. Robbins and Cotran pathologic basis of disease. Philadelphia: Saunders; 2004. [Google Scholar]
- 58. Kurtz L. Schweda F. de Wit C. Kriz W. Witzgall R. Warth R. Sauter A. Kurtz A. Wagner C. Lack of connexin 40 causes displacement of renin-producing cells from afferent arterioles to the extraglomerular mesangium. J Am Soc Nephrol. 2007;18:1103–1111. doi: 10.1681/ASN.2006090953. [DOI] [PubMed] [Google Scholar]
- 59. Kwak BR. Mulhaupt F. Veillard N. Gros DB. Mach F. Altered pattern of vascular connexin expression in atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2002;22:225–230. doi: 10.1161/hq0102.104125. [DOI] [PubMed] [Google Scholar]
- 60. Kwak BR. Veillard N. Pelli G. Mulhaupt F. James RW. Chanson M. Mach F. Reduced connexin43 expression inhibits atherosclerotic lesion formation in low-density lipoprotein receptor-deficient mice. Circulation. 2003;107:1033–1039. doi: 10.1161/01.cir.0000051364.70064.d1. [DOI] [PubMed] [Google Scholar]
- 61. Kwak BR. Silacci P. Stergiopulos N. Hayoz D. Meda P. Shear stress and cyclic circumferential stretch, but not pressure, alter connexin43 expression in endothelial cells. Cell Commun Adhes. 2005;12:261–270. doi: 10.1080/15419060500514119. [DOI] [PubMed] [Google Scholar]
- 62. Lamboley M. Pittet P. Koenigsberger M. Sauser R. Beny JL. Meister JJ. Evidence for signaling via gap junctions from smooth muscle to endothelial cells in rat mesenteric arteries: possible implication of a second messenger. Cell Calcium. 2005;37:311–320. doi: 10.1016/j.ceca.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 63. Lampe PD. Lau AF. Regulation of gap junctions by phosphorylation of connexins. Arch Biochem Biophys. 2000;384:205–215. doi: 10.1006/abbi.2000.2131. [DOI] [PubMed] [Google Scholar]
- 64. Lampe PD. Lau AF. The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell Biol. 2004;36:1171–1186. doi: 10.1016/S1357-2725(03)00264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lanfear DE. Jones PG. Marsh S. Cresci S. Spertus JA. McLeod HL. Connexin37 (GJA4) genotype predicts survival after an acute coronary syndrome. Am Heart J. 2007;154:561–566. doi: 10.1016/j.ahj.2007.04.059. [DOI] [PubMed] [Google Scholar]
- 66. Lang NN. Luksha L. Newby DE. Kublickiene K. Connexin 43 mediates endothelium-derived hyperpolarizing factor-induced vasodilatation in subcutaneous resistance arteries from healthy pregnant women. Am J Physiol Heart Circ Physiol. 2007;292:H1026–H1032. doi: 10.1152/ajpheart.00797.2006. [DOI] [PubMed] [Google Scholar]
- 67. Leitinger N. Tyner TR. Oslund L. Rizza C. Subbanagounder G. Lee H. Shih PT. Mackman N. Tigyi G. Territo MC. Berliner JA. Vora DK. Structurally similar oxidized phospholipids differentially regulate endothelial binding of monocytes and neutrophils. Proc Natl Acad Sci U S A. 1999;96:12010–12015. doi: 10.1073/pnas.96.21.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Leitinger N. Oxidized phospholipids as modulators of inflammation in atherosclerosis. Curr Opin Lipidol. 2003;14:421–430. doi: 10.1097/00041433-200310000-00002. [DOI] [PubMed] [Google Scholar]
- 69. Liao Y. Day KH. Damon DN. Duling BR. Endothelial cell-specific knockout of connexin 43 causes hypotension and bradycardia in mice. Proc Natl Acad Sci U S A. 2001;98:9989–9994. doi: 10.1073/pnas.171305298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Liao Y. Regan CP. Manabe I. Owens GK. Day KH. Damon DN. Duling BR. Smooth muscle-targeted knockout of connexin43 enhances neointimal formation in response to vascular injury. Arterioscler Thromb Vasc Biol. 2007;27:1037–1042. doi: 10.1161/ATVBAHA.106.137182. [DOI] [PubMed] [Google Scholar]
- 71. Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 72. Listi F. Candore G. Lio D. Russo M. Colonna-Romano G. Caruso M. Hoffmann E. Caruso C. Association between C1019T polymorphism of connexin37 and acute myocardial infarction: a study in patients from Sicily. Int J Cardiol. 2005;102:269–271. doi: 10.1016/j.ijcard.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 73. Little TL. Beyer EC. Duling BR. Connexin 43 and connexin 40 gap junctional proteins are present in arteriolar smooth muscle and endothelium in vivo. Am J Physiol. 1995;268:H729–H739. doi: 10.1152/ajpheart.1995.268.2.H729. [DOI] [PubMed] [Google Scholar]
- 74. Little TL. Xia J. Duling BR. Dye tracers define differential endothelial and smooth muscle coupling patterns within the arteriolar wall. Circ Res. 1995;76:498–504. doi: 10.1161/01.res.76.3.498. [DOI] [PubMed] [Google Scholar]
- 75. Liu C. Ngai CY. Huang Y. Ko WH. Wu M. He GW. Garland CJ. Dora KA. Yao X. Depletion of intracellular Ca2+ stores enhances flow-induced vascular dilatation in rat small mesenteric artery. Br J Pharmacol. 2006;147:506–515. doi: 10.1038/sj.bjp.0706639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lo CW. Waldo KL. Kirby ML. Gap junction communication and the modulation of cardiac neural crest cells. Trends Cardiovasc Med. 1999;9:63–69. doi: 10.1016/s1050-1738(99)00015-8. [DOI] [PubMed] [Google Scholar]
- 77. Looft-Wilson RC. Payne GW. Segal SS. Connexin expression and conducted vasodilation along arteriolar endothelium in mouse skeletal muscle. J Appl Physiol. 2004;97:1152–1158. doi: 10.1152/japplphysiol.00133.2004. [DOI] [PubMed] [Google Scholar]
- 78. Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mather S. Dora KA. Sandow SL. Winter P. Garland CJ. Rapid endothelial cell-selective loading of connexin 40 antibody blocks endothelium-derived hyperpolarizing factor dilation in rat small mesenteric arteries. Circ Res. 2005;97:399–407. doi: 10.1161/01.RES.0000178008.46759.d0. [DOI] [PubMed] [Google Scholar]
- 80. Matsushita T. Rama A. Charolidi N. Dupont E. Severs NJ. Relationship of connexin43 expression to phenotypic modulation in cultured human aortic smooth muscle cells. Eur J Cell Biol. 2007;86:617–628. doi: 10.1016/j.ejcb.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 81. McSherry IN. Spitaler MM. Takano H. Dora KA. Endothelial cell Ca2+ increases are independent of membrane potential in pressurized rat mesenteric arteries. Cell Calcium. 2005;38:23–33. doi: 10.1016/j.ceca.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 82. McSherry IN. Sandow SL. Campbell WB. Falck JR. Hill MA. Dora KA. A role for heterocellular coupling and EETs in dilation of rat cremaster arteries. Microcirculation. 2006;13:119–130. doi: 10.1080/10739680500466400. [DOI] [PubMed] [Google Scholar]
- 83. Nelson MT. Cheng H. Rubart M. Santana LF. Bonev AD. Knot HJ. Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- 84. Neumann FJ. Desmet W. Grube E. Brachmann J. Presbitero P. Rubartelli P. Mugge A. Di Pede F. Fullgraf D. Aengevaeren W. Spedicato L. Popma JJ. Effectiveness and safety of sirolimus-eluting stents in the treatment of restenosis after coronary stent placement. Circulation. 2005;111:2107–2111. doi: 10.1161/01.CIR.0000162467.53001.6B. [DOI] [PubMed] [Google Scholar]
- 85. Parthasarathi K. Ichimura H. Monma E. Lindert J. Quadri S. Issekutz A. Bhattacharya J. Connexin 43 mediates spread of Ca2+-dependent proinflammatory responses in lung capillaries. J Clin Invest. 2006;116:2193–2200. doi: 10.1172/JCI26605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Pidkovka NA. Cherepanova OA. Yoshida T. Alexander MR. Deaton RA. Thomas JA. Leitinger N. Owens GK. Oxidized phospholipids induce phenotypic switching of vascular smooth muscle cells in vivo and in vitro. Circ Res. 2007;101:792–801. doi: 10.1161/CIRCRESAHA.107.152736. [DOI] [PubMed] [Google Scholar]
- 87. Plenz G. Ko YS. Yeh HI. Eschert H. Sindermann JR. Dorszewski A. Hofnagel O. Robenek H. Breithardt G. Severs NJ. Upregulation of connexin43 gap junctions between neointimal smooth muscle cells. Eur J Cell Biol. 2004;83:521–530. doi: 10.1078/0171-9335-00417. [DOI] [PubMed] [Google Scholar]
- 88. Plump AS. Smith JD. Hayek T. Aalto-Setala K. Walsh A. Verstuyft JG. Rubin EM. Breslow JL. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 89. Polacek D. Lal R. Volin MV. Davies PF. Gap junctional communication between vascular cells: induction of connexin43 messenger RNA in macrophage foam cells of atherosclerotic lesions. Am J Pathol. 1993;142:593–606. [PMC free article] [PubMed] [Google Scholar]
- 90. Polacek D. Bech F. McKinsey JF. Davies PF. Connexin43 gene expression in the rabbit arterial wall: effects of hypercholesterolemia, balloon injury and their combination. J Vasc Res. 1997;34:19–30. doi: 10.1159/000159198. [DOI] [PubMed] [Google Scholar]
- 91. Rama A. Matsushita T. Charolidi N. Rothery S. Dupont E. Severs NJ. Up-regulation of connexin43 correlates with increased synthetic activity and enhanced contractile differentiation in TGF-beta-treated human aortic smooth muscle cells. Eur J Cell Biol. 2006;85:375–386. doi: 10.1016/j.ejcb.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 92. Reaume AG. de Sousa PA. Kulkarni S. Langille BL. Zhu D. Davies TC. Juneja SC. Kidder GM. Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- 93. Reidy MA. Proliferation of smooth muscle cells at sites distant from vascular injury. Arteriosclerosis. 1990;10:298–305. doi: 10.1161/01.atv.10.2.298. [DOI] [PubMed] [Google Scholar]
- 94. Rhodin JA. The ultrastructure of mammalian arterioles and precapillary sphincters. J Ultrastruct Res. 1967;18:181–223. doi: 10.1016/s0022-5320(67)80239-9. [DOI] [PubMed] [Google Scholar]
- 95. Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 96. Rummery NM. McKenzie KU. Whitworth JA. Hill CE. Decreased endothelial size and connexin expression in rat caudal arteries during hypertension. J Hypertens. 2002;20:247–253. doi: 10.1097/00004872-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 97. Rummery NM. Grayson TH. Hill CE. Angiotensin-converting enzyme inhibition restores endothelial but not medial connexin expression in hypertensive rats. J Hypertens. 2005;23:317–328. doi: 10.1097/00004872-200502000-00014. [DOI] [PubMed] [Google Scholar]
- 98. Saez JC. Berthoud VM. Branes MC. Martinez AD. Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- 99. Sagar GD. Larson DM. Carbenoxolone inhibits junctional transfer and upregulates connexin43 expression by a protein kinase A-dependent pathway. J Cell Biochem. 2006;98:1543–1551. doi: 10.1002/jcb.20870. [DOI] [PubMed] [Google Scholar]
- 100. Sandow SL. Hill CE. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ Res. 2000;86:341–346. doi: 10.1161/01.res.86.3.341. [DOI] [PubMed] [Google Scholar]
- 101. Sandow SL. Neylon CB. Chen MX. Garland CJ. Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (K(Ca)) and connexins: possible relationship to vasodilator function? J Anat. 2006;209:689–698. doi: 10.1111/j.1469-7580.2006.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sato T. Haimovici R. Kao R. Li AF. Roy S. Downregulation of connexin 43 expression by high glucose reduces gap junction activity in microvascular endothelial cells. Diabetes. 2002;51:1565–1571. doi: 10.2337/diabetes.51.5.1565. [DOI] [PubMed] [Google Scholar]
- 103. Schînbeck U. Libby P. Inflammation, immunity, and HMG-CoA reductase inhibitors: statins as antiinflammatory agents? Circulation. 2004;109:II18–II26. doi: 10.1161/01.CIR.0000129505.34151.23. [DOI] [PubMed] [Google Scholar]
- 104. Schubert AL. Schubert W. Spray DC. Isanti MP. Connexin family members target to lipid raft domains and interact with caveolin-1. Biochemistry. 2002;41:5754–5764. doi: 10.1021/bi0121656. [DOI] [PubMed] [Google Scholar]
- 105. Segal SS. Beny JL. Intracellular recording and dye transfer in arterioles during blood flow control. Am J Physiol. 1992;263:H1–H7. doi: 10.1152/ajpheart.1992.263.1.H1. [DOI] [PubMed] [Google Scholar]
- 106. Severs NJ. Cardiovascular disease. Novartis Found Symp. 1999;219:188–206. doi: 10.1002/9780470515587.ch12. [DOI] [PubMed] [Google Scholar]
- 107. Severs NJ. Rothery S. Dupont E. Coppen SR. Yeh HI. Ko YS. Matsushita T. Kaba R. Halliday D. Immunocytochemical analysis of connexin expression in the healthy and diseased cardiovascular system. Microsc Res Tech. 2001;52:301–322. doi: 10.1002/1097-0029(20010201)52:3<301::AID-JEMT1015>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 108. Shimokawa H. Yasutake H. Fujii K. Owada MK. Nakaike R. Fukumoto Y. Takayanagi T. Nagao T. Egashira K. Fujishima M. Takeshita A. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J Cardiovasc Pharmacol. 1996;28:703–711. doi: 10.1097/00005344-199611000-00014. [DOI] [PubMed] [Google Scholar]
- 109. Shiomi M. Ito T. Yamada S. Kawashima S. Fan J. Correlation of vulnerable coronary plaques to sudden cardiac events: lessons from a myocardial infarction-prone animal model (the WHHLMI rabbit) J Atheroscler Thromb. 2004;11:184–189. doi: 10.5551/jat.11.184. [DOI] [PubMed] [Google Scholar]
- 110. Siegl D. Koeppen M. Wolfle SE. Pohl U. de Wit C. Myoendothelial coupling is not prominent in arterioles within the mouse cremaster microcirculation in vivo. Circ Res. 2005;97:781–788. doi: 10.1161/01.RES.0000186193.22438.6c. [DOI] [PubMed] [Google Scholar]
- 111. Simon AM. McWhorter AR. Decreased intercellular dye-transfer and downregulation of non-ablated connexins in aortic endothelium deficient in connexin37 or connexin40. J Cell Sci. 2003;116:2223–2236. doi: 10.1242/jcs.00429. [DOI] [PubMed] [Google Scholar]
- 112. Skalen K. Gustafsson M. Rydberg EK. Hulten LM. Wiklund O. Innerarity TL. Boren J. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature. 2002;417:750–754. doi: 10.1038/nature00804. [DOI] [PubMed] [Google Scholar]
- 113. Sohl G. Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res. 2004;62:228–232. doi: 10.1016/j.cardiores.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 114. Takens-Kwak BR. Jongsma HJ. Rook MB. Van Ginneken AC. Mechanism of heptanol-induced uncoupling of cardiac gap junctions: a perforated patch-clamp study. Am J Physiol. 1992;262:C1531–C1538. doi: 10.1152/ajpcell.1992.262.6.C1531. [DOI] [PubMed] [Google Scholar]
- 115. Taugner R. Kirchheim H. Forssmann WG. Myoendothelial contacts in glomerular arterioles and in renal interlobular arteries of rat, mouse and Tupaia belangeri. Cell Tissue Res. 1984;235:319–325. doi: 10.1007/BF00217856. [DOI] [PubMed] [Google Scholar]
- 116. Tsai CH. Yeh HI. Tian TY. Lee YN. Lu CS. Ko YS. Down-regulating effect of nicotine on connexin43 gap junctions in human umbilical vein endothelial cells is attenuated by statins. Eur J Cell Biol. 2004;82:589–595. doi: 10.1078/0171-9335-00348. [DOI] [PubMed] [Google Scholar]
- 117. Tsai ML. Watts SW. Loch-Caruso R. Webb RC. The role of gap junctional communication in contractile oscillations in arteries from normotensive and hypertensive rats. J Hypertens. 1995;13:1123–1133. doi: 10.1097/00004872-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 118. van der Hoeven BL. Pires NM. Warda HM. Oemrawsingh PV. van Vlijmen BJ. Quax PH. Schalij MJ. van der Wall EE. Jukema JW. Drug-eluting stents: results, promises and problems. Int J Cardiol. 2005;99:9–17. doi: 10.1016/j.ijcard.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 119. van Kempen MJ. Jongsma HJ. Distribution of connexin37, connexin40 and connexin43 in the aorta and coronary artery of several mammals. Histochem Cell Biol. 1999;112:479–486. doi: 10.1007/s004180050432. [DOI] [PubMed] [Google Scholar]
- 120. van Rijen HV. van Kempen MJ. Postma S. Jongsma HJ. Tumour necrosis factor alpha alters the expression of connexin43, connexin40, and connexin37 in human umbilical vein endothelial cells. Cytokine. 1998;10:258–264. doi: 10.1006/cyto.1997.0287. [DOI] [PubMed] [Google Scholar]
- 121. Virmani R. Burke AP. Farb A. Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–C18. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 122. Wagner C. de Wit C. Kurtz L. Grunberger C. Kurtz A. Schweda F. Connexin40 is essential for the pressure control of renin synthesis and secretion. Circ Res. 2007;100:556–563. doi: 10.1161/01.RES.0000258856.19922.45. [DOI] [PubMed] [Google Scholar]
- 123. Wang J. Ma M. Locovei S. Keane RW. Dahl G. Modulation of membrane channel currents by gap junction protein mimetic peptides: size matters. Am J Physiol Cell Physiol. 2007;293:C1112–C1119. doi: 10.1152/ajpcell.00097.2007. [DOI] [PubMed] [Google Scholar]
- 124. Wang L. Chen J. Sun Y. Zhang F. Zhu J. Hu S. Wang DH. Regulation of connexin expression after balloon injury: possible mechanisms for antiproliferative effect of statins. Am J Hypertens. 2005;18:1146–1153. doi: 10.1016/j.amjhyper.2005.03.746. [DOI] [PubMed] [Google Scholar]
- 125. Watson AD. Leitinger N. Navab M. Faull KF. Horkko S. Witztum JL. Palinski W. Schwenke D. Salomon RG. Sha W. Subbanagounder G. Fogelman AM. Berliner JA. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J Biol Chem. 1997;272:13597–13607. doi: 10.1074/jbc.272.21.13597. [DOI] [PubMed] [Google Scholar]
- 126. Watson AD. Subbanagounder G. Welsbie DS. Faull KF. Navab M. Jung ME. Fogelman AM. Berliner JA. Structural identification of a novel pro-inflammatory epoxyisoprostane phospholipid in mildly oxidized low density lipoprotein. J Biol Chem. 1999;274:24787–24798. doi: 10.1074/jbc.274.35.24787. [DOI] [PubMed] [Google Scholar]
- 127. Watts SW. Webb RC. Vascular gap junctional communication is increased in mineralocorticoid-salt hypertension. Hypertension. 1996;28:888–893. doi: 10.1161/01.hyp.28.5.888. [DOI] [PubMed] [Google Scholar]
- 128. Werner M. Von Wasielewski R. Komminoth P. Antigen retrieval, signal amplification and intensification in immunohistochemistry. Histochem Cell Biol. 1996;105:253–260. doi: 10.1007/BF01463928. [DOI] [PubMed] [Google Scholar]
- 129. Wong CW. Burger F. Pelli G. Mach F. Kwak BR. Dual benefit of reduced Cx43 on atherosclerosis in LDL receptor-deficient mice. Cell Commun Adhes. 2003;10:395–400. doi: 10.1080/cac.10.4-6.395.400. [DOI] [PubMed] [Google Scholar]
- 130. Wong CW. Christen T. Roth I. Chadjichristos CE. Derouette JP. Foglia BF. Chanson M. Goodenough DA. Kwak BR. Connexin37 protects against atherosclerosis by regulating monocyte adhesion. Nat Med. 2006;12:950–954. doi: 10.1038/nm1441. [DOI] [PubMed] [Google Scholar]
- 131. Yamada Y. Izawa H. Ichihara S. Takatsu F. Ishihara H. Hirayama H. Sone T. Tanaka M. Yokota M. Prediction of the risk of myocardial infarction from polymorphisms in candidate genes. N Engl J Med. 2002;347:1916–1923. doi: 10.1056/NEJMoa021445. [DOI] [PubMed] [Google Scholar]
- 132. Yeh HI. Dupont E. Coppen S. Rothery S. Severs NJ. Gap junction localization and connexin expression in cytochemically identified endothelial cells of arterial tissue. J Histochem Cytochem. 1997;45:539–550. doi: 10.1177/002215549704500406. [DOI] [PubMed] [Google Scholar]
- 133. Yeh HI. Lupu F. Dupont E. Severs NJ. Upregulation of connexin43 gap junctions between smooth muscle cells after balloon catheter injury in the rat carotid artery. Arterioscler Thromb Vasc Biol. 1997;17:3174–3184. doi: 10.1161/01.atv.17.11.3174. [DOI] [PubMed] [Google Scholar]
- 134. Yeh HI. Chou Y. Liu HF. Chang SC. Tsai CH. Connexin37 gene polymorphism and coronary artery disease in Taiwan. Int J Cardiol. 2001;81:251–255. doi: 10.1016/s0167-5273(01)00574-5. [DOI] [PubMed] [Google Scholar]
- 135. Yeh HI. Lu CS. Wu YJ. Chen CC. Hong RC. Ko YS. Shiao MS. Severs NJ. Tsai CH. Reduced expression of endothelial connexin37 and connexin40 in hyperlipidemic mice: recovery of connexin37 after 7-day simvastatin treatment. Arterioscler Thromb Vasc Biol. 2003;23:1391–1397. doi: 10.1161/01.ATV.0000083508.21989.15. [DOI] [PubMed] [Google Scholar]
- 136. Yeh HI. Lee PY. Su CH. Tian TY. Ko YS. Tsai CH. Reduced expression of endothelial connexins 43 and 37 in hypertensive rats is rectified after 7-day carvedilol treatment. Am J Hypertens. 2006;19:129–135. doi: 10.1016/j.amjhyper.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 137. Zhang SH. Reddick RL. Piedrahita JA. Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 138. Zhang SH. Reddick RL. Burkey B. Maeda N. Diet-induced atherosclerosis in mice heterozygous and homozygous for apolipoprotein E gene disruption. J Clin Invest. 1994;94:937–945. doi: 10.1172/JCI117460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Zhao Z. Walczysko P. Zhao M. Intracellular Ca(2+) stores are essential for injury induced Ca(2+) signaling and re-endothelialization. J Cell Physiol. 2007 doi: 10.1002/jcp.21248. [DOI] [PubMed] [Google Scholar]



