Catalytic reactions of basic heteroaromatic reagents can be challenging because the ligating ability of the heteroatom can lead to catalyst deactivation and because the electronic properties of certain ring positions can be unfavorable for the elementary reactions required for the catalytic processes. These factors have particularly complicated the palladium-catalyzed coupling of amines1 with heteroaryl halides.2 We report a strategy to address these challenges. We show that the coordination of Lewis acids to the nitrogen of pyridylpalladium complexes leads to an acceleration of the rate of reductive elimination by more than 3 orders of magnitude and that this coordination can facilitate palladium-catalyzed coupling of amides with unactivated heteroaryl halides.3
We and others have observed that the coupling of heteroaryl halides with zinc and alkali metal reagents can be different.4 This observation led us to probe the potential effect of Lewis acids on the reactivity of isolated heteroarylpalladium amido complexes and on the catalytic coupling of heteroaryl halides with nitrogen nucleophiles. To do so, we compared the rates and yields of reductive elimination from pyridylpalladium amido complexes in the presence and absence of added Lewis acids.
Equation 1 summarizes the synthesis of pyridylpalladium complexes in this study. Addition of KN(p-t-BuC6H4)2 to DPPBz-ligated pyridyl- and p-tolylpalladium complexes 1–5 formed the diarylamido complexes 6–9.5 Likewise, addition of KNMe(SO2-Ar) formed the sulfonamidates 10 and 11. Complexes 6–11 were stable at room temperature and were characterized by conventional spectroscopic and microanalytical methods.
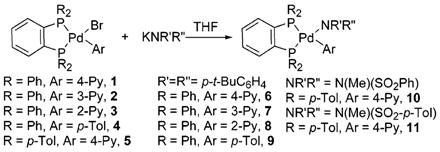 |
(1) |
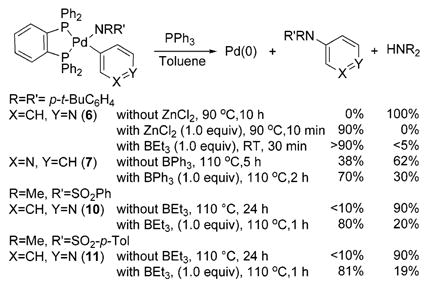
Reductive eliminations of the pyridyl- and arylpalladium amido complexes are summarized in eq 2. In the absence of Lewis acids, reductive elimination from 3-pyridyl- and 4-pyridylpalladium diarylamido complexes occurred in lower yields than from arylpalladium diarylamido complexes. Warming 4-pyridyl complex 6 at 90°C in toluene for 10 h with added PPh3 did not form the pyridyl diarylamine. Instead, free amine and biaryl products derived from the palladium-bound pyridyl and the phosphine phenyl groups formed. Likewise, reaction of the 3-pyridyl complex 7 required heating in toluene at 110 °C for 5 h and formed the triarylamine in a low 38% yield. Although reductive elimination from the DPPBz-ligated arylpalladium amido complexes is less favorable than that from complexes containing other phosphines,6,7 heating of p-tolylpalladium complex 9 in the presence of PPh3 did form triarylamine in 50% yield after 8 h at 90 °C.
In contrast to the slow rates and low yields for formation of heteroarylamines from reactions of 6 and 7 with phosphine alone, heating of 6 with PPh3 and suspended ZnCl2 in toluene solvent formed over 90% yield of 4-N,N-diarylaminopyridine after 10 min at 90°C (eq 2). Because of the lack of solubility of ZnCl2, we could not study the effect of this Lewis acid quantitatively. Thus, to obtain more quantitative data, we studied the reductive elimination of 4-pyridyl complex 6 in the presence of soluble boron Lewis acids.
 |
(2) |
Like ZnCl2, BEt3 accelerated the reductive elimination of heteroarylamines from 4-pyridyl complex 6 (eq 2). Mixing of 6 with 1 equiv of BEt3, followed by addition of PPh3, led to reductive elimination at room temperature within 30 min to give the pyridyl di(tert-butylphenyl)amine in >90% yield. A similar effect of the Lewis acid on reductive elimination from the pyridylpalladium amidate complexes 10 and 11 was observed. Heating of 10 or 11 with BEt3 at 110 °C for 30–60 min formed the N-pyridyl sulfonamide in 80 and 81% yields. In the absence of Lewis acid, little reaction occurred after 1 h; after 24 h at 110 °C, the complex was consumed but <10% yield of coupled product was observed.
Boron Lewis acids also accelerated reductive elimination from 3-pyridylpalladium amide 7, but the magnitude of this effect was smaller than that for reductive elimination from 4-pyridyl complex 6. Heating a toluene solution of 7 with 1 equiv of BPh3 and excess PPh3 at 90 °C for 2 h formed the corresponding triarylamine in 70% yield instead of the 38% yield that was observed after 5 h in the absence of Lewis acid (eq 2).
To define the origin of the effect of the Lewis acid more explicitly, the product from interaction of BEt3 with a heteroarylpalladium complex was isolated. Because the amide complexes are unstable after coordination of the Lewis acid, we were unable to isolate the BEt3 adduct of the 4-pyridylpalladium amide complex. However, addition of BEt3 to a THF solution of sulfonamidate 10 formed a stable complex 12 containing borane coordinated at the nitrogen of the pyridyl group (eq 3). Complex 12 was characterized by 1H NMR, 31P NMR, and 11B NMR spectroscopy. The 11B NMR resonance at δ 1.0, which lies in the range of chemical shifts that are characteristic of R3B·Py complexes,8 indicated that the boron was bound to nitrogen. This assignment was confirmed by X-ray diffraction, although the quality of the data was not high enough to obtain detailed structural information. Heating of this adduct in toluene for 2 h at 90 °C formed the N-methyl-N-4-pyridyl sulfonamide in 80% yield.
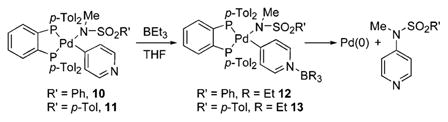 |
(3) |
Although the most stable adduct contains the Lewis acid at the pyridyl nitrogen, reductive elimination need not occur through this species. To distinguish an effect of the Lewis acid by coordination to the pyridyl nitrogen from an effect from coordination to the amido nitrogen or the metal center, we heated the p-tolylpalladium complex 9 in the presence of the Lewis acid. Acceleration of C–N reductive elimination by the Lewis acid was not observed. Warming of 9 with 1 equiv of BEt3 in the presence of PPh3 at 90 °C for 2 h formed 4-ethyltoluene in 72% yield from exchange of the amido and Lewis acid groups (eq 4). Warming of 9 with 1 equiv of BEt3 in the absence of PPh3 at 110 °C for 2 h formed 4-ethyltoluene in 57% yield. Thus, the accelerating effect of the Lewis acid on the reductive eliminations from the 4-pyridyl complex 6 appears to result from coordination of the Lewis acid to the pyridyl nitrogen.
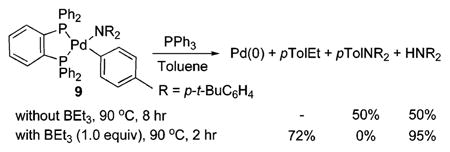 |
(4) |
The Lewis acids did not affect the rate or yield of reductive elimination of 2-pyridylpalladium amides. Reductive elimination from the 2-pyridylpalladium complex 8 is relatively fast in the absence of Lewis acids (heating of 8 at 70 °C for 5 h gave the 2-pyridyl diarylamine in 95% yield), and the nitrogen is sterically hindered. Further, the Pd–C–N bond angle in the solid-state structure of 8 is only 115.3°, which suggests the presence of a weak interaction of the pyridyl nitrogen with the palladium.
The rate of reductive elimination increased with increasing strength of the Lewis acids. Table 1 summarizes rates of reaction of the heteroarylpalladium amidate 11 in the presence of BEt3, BPh3, and B(C6F5)3. The complex containing BPh3 reacted nearly 12 times faster than the complex containing BEt3, and the complex containing B(C6F5)3 reacted even faster.
Table 1.
Rate Constants for Reductive Eliminations from Pyridylpalladium Sulfonamidate 11 in the Presence of Different Lewis Acids
| entry | Lewis acid | temp (°C) | k (s−1 × 10−4) | t1/2 (min) |
|---|---|---|---|---|
| 1 | BEt3 | 90 | 7.7 (0.8) | 15 |
| 2 | BEt3 | 70 | ~170 | |
| 3 | BPh3 | 70 | 8.3 (0.2) | 14 |
| 4 | B(C6F5)3 | 40 | 6.8 (0.5) | 17 |
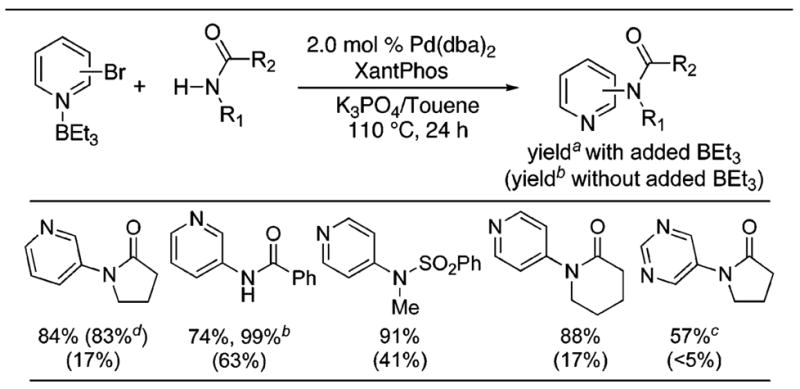
To probe whether this acceleration of reductive elimination could affect catalytic processes, we studied the effect of Lewis acids on the amidation of aryl halides. In principle, this effect could be used to improve these reactions conducted with any ligand. Data on couplings in the presence and absence of added BEt3 for reactions conducted with the Xantphos ligand commonly used for the amidation of haloarenes9 are shown in Table 2. The reactions of heteroaryl bromides with amides in the presence of K3PO4 as base and Pd(dba)2/Xantphos as catalyst in toluene solvent occurred to full conversion. The reactions formed the coupled product in 57–91% yields in the presence of 1.0 equiv of BEt3 and in similar yields with substoichiometric BEt3 in the example tested. Reactions under the same conditions but without Lewis acid occurred to lower conversions and formed the products in 5–63% yield.10 Products from ethyl group transfer were formed in less than 10% yield when BEt3 was used. Further investigation of the scope of C–N coupling in the presence of Lewis acids will be part of future studies.
Table 2.
Palladium-Catalyzed Amidation of Heteroaryl Halides in the Presence of Lewis Acids
 |
Isolated yield from an average of at least two runs; the borane is removed during chromatography.
Yield by GC with an internal standard.
48 h.
BEt3 (20 mol %) was used.
In summary, we report the promotion of reductive elimination from several heteroarylpalladium amido complexes by Lewis acids. This effect is most similar to the Lewis acid acceleration of the reductive elimination of nitriles during catalytic hydrocyanation by binding to the cyanide ligand.11 The origin of the effect of Lewis acids on the C–N reductive elimination is likely to result from the creation of an electron-poor aryl group by Lewis acid binding; reductive elimination of arylamines is known to be faster from arylpalladium complexes containing more electron-poor aryl groups.6 Studies to define the origin and scope of this effect are ongoing.
Supplementary Material
Acknowledgments
We thank the NIH (GM-55382) for support of this work, and Johnson Matthey for a gift of palladium catalysts.
Footnotes
Supporting Information Available: All experimental procedures and spectroscopic data of new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Hartwig JF. In: In Handbook of Organopalladium Chemistry for Organic Synthesis. Negishi EI, editor. Vol. 1. Wiley-Interscience; New York: 2002. p. 1051. [Google Scholar]; (b) Hartwig JF. In: In Modern Arene Chemistry. Astruc C, editor. Wiley-VCH; Weinheim, Germany: 2002. p. 107. [Google Scholar]; (c) Hartwig JF. In: In Modern Amination Methods. Ricci A, editor. Wiley-VCH; Weinheim, Germany: 2000. p. 195. [Google Scholar]; (d) Yang BH, Buchwald SL. J Organomet Chem. 1999;576:125. [Google Scholar]; (e) Muci AR, Buchwald SL. Top Curr Chem. 2002;219:131. [Google Scholar]
- 2.(a) Shen Q, Shekhar S, Stambuli JP, Hartwig JF. Angew Chem, Int Ed. 2004;44:1371. doi: 10.1002/anie.200462629. [DOI] [PubMed] [Google Scholar]; (b) Anderson KW, Tundel RE, Ikawa T, Altman RA, Buchwald SL. Angew Chem, Int Ed. 2006;45:6523. doi: 10.1002/anie.200601612. [DOI] [PubMed] [Google Scholar]
- 3.(a) Burton G, Cao P, Li G, Rivero R. Org Lett. 2003;5:4373. doi: 10.1021/ol035655u. [DOI] [PubMed] [Google Scholar]; (b) Alcaraz L, Bennion C, Morris J, Meghani P, Thom SM. Org Lett. 2004;6:2705. doi: 10.1021/ol049091l. [DOI] [PubMed] [Google Scholar]; (c) Ji J, Li T, Bunnelle WH. Org Lett. 2003;5:4611. doi: 10.1021/ol0357696. [DOI] [PubMed] [Google Scholar]; (d) Klapars A, Huang X, Buchwald SL. J Am Chem Soc. 2002;124:7421. doi: 10.1021/ja0260465. [DOI] [PubMed] [Google Scholar]
- 4.(a) Hama T, Culkin DA, Hartwig JF. J Am Chem Soc. 2006;128:4976. doi: 10.1021/ja056076i. [DOI] [PubMed] [Google Scholar]; (b) Liebeskind LS, Srogl J. Org Lett. 2002;4:979. doi: 10.1021/ol0200091. [DOI] [PubMed] [Google Scholar]; (c) Ghosh I, Jacobi PA. J Org Chem. 2002;67:9304. doi: 10.1021/jo026510o. [DOI] [PubMed] [Google Scholar]
- 5.(a) Hartwig JF. Acc Chem Res. 1998;31:852. [Google Scholar]; (b) Hooper MW, Hartwig JF. Organometallics. 2003;22:3394. [Google Scholar]
- 6.Driver MS, Hartwig JF. J Am Chem Soc. 1997;119:8232. [Google Scholar]
- 7.Yamashita M, Hartwig JF. J Am Chem Soc. 2004;126:5344. doi: 10.1021/ja0315107. [DOI] [PubMed] [Google Scholar]
- 8.(a) Noth H, Wrackmeyer B. Chem Ber. 1974;107:3070. [Google Scholar]; (b) Weisman A, Gozin M, Kraatz H, Milstein D. Inorg Chem. 1996;35:1792. doi: 10.1021/ic960285+. [DOI] [PubMed] [Google Scholar]
- 9.(a) Yin J, Buchwald SL. J Am Chem Soc. 2002;124:6043. doi: 10.1021/ja012610k. [DOI] [PubMed] [Google Scholar]; (b) Yin J, Buchwald SL. Org Lett. 2000;2:1101. doi: 10.1021/ol005654r. [DOI] [PubMed] [Google Scholar]; (c) Yin J, Zhao MM, Huffman MA, McNamara JM. Org Lett. 2002;4:3481. doi: 10.1021/ol0265923. [DOI] [PubMed] [Google Scholar]
- 10.The rate of the catalytic reactions was affected by whether the catalyst or reagents were added first. Further study is needed to optimize conditions, but the Lewis acid accelerated reactions conducted with either order.
- 11.(a) Tolman CA, Seidel WC, Druliner JD, Domaille PJ. Organometallics. 1984;3:33. [Google Scholar]; (b) Huang JK, Haar CM, Nolan SP, Marcone JE, Moloy KG. Organometallics. 1999;18:297. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


