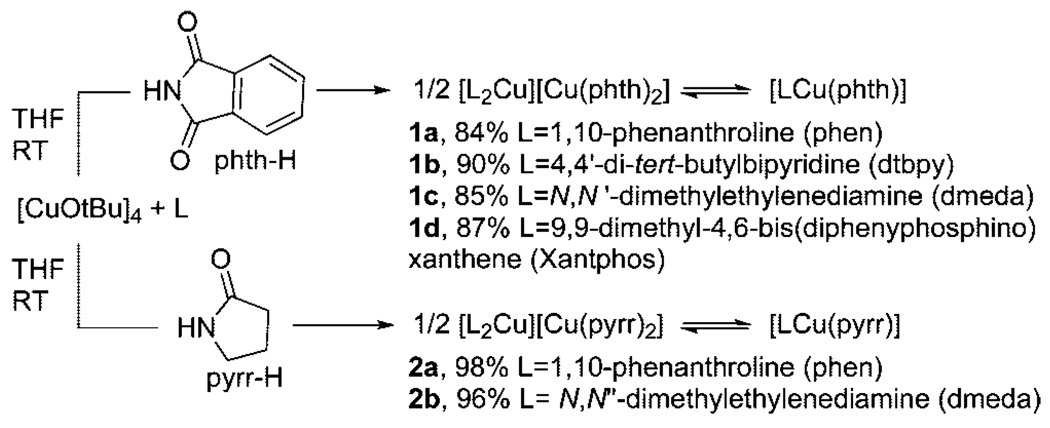
Abstract
Copper(I) imidate and amidate complexes of chelating N,N-donor ligands, which are proposed intermediates in copper-catalyzed amidations of aryl halides, have been synthesized and characterized by X-ray diffraction and detailed solution-phase methods. In some cases, the complexes adopt neutral, three-coordinate trigonal planar structures in the solid state, but in other cases they adopt an ionic form consisting of an L2Cu+ cation and a CuX2− anion. A tetraalkylammonium salt of the CuX2− anion in which X = phthalimidate was also isolated. Conductivity measurements and 1H NMR spectra of mixtures of two complexes all indicate that the complexes exist predominantly in the ionic form in DMSO and DMF solutions. One complex was sufficiently soluble for conductance measurements in less polar solvents and was shown to adopt some degree of the ionic form in THF and predominantly the neutral form in benzene. The complexes containing dative nitrogen ligands reacted with iodoarenes and bromoarenes to form products from C–N coupling, but the ammonium salt of [Cu(phth)2]− did not. Similar selectivities for stoichiometric and catalytic reactions with two different iodoarenes and faster rates for the stoichiometric reactions implied that the isolated amidate and imidate complexes are intermediates in the reactions of amides and imides with haloarenes catalyzed by copper complexes containing dative N,N ligands. These amidates and imidates reacted much more slowly with chloroarenes, including chloroarenes that possess more favorable reduction potentials than some bromoarenes and that are known to undergo fast dissociation of chloride from the chloroarene radical anion. The reaction of o-(allyloxy)iodobenzene with [(phen)2Cu][Cu(pyrr)2] results in formation of the C-N coupled product in high yield and no detectable amount of the 3-methyl-2,3-dihydrobenzofuran or 3-methylene-2,3-dihydrobenzofuran products that would be expected from a reaction that generated free radicals. These data and computed reaction barriers argue against mechanisms in which the haloarene reacts with a two-coordinate anionic copper species and mechanisms that start with electron transfer to generate a free iodoarene radical anion. Instead, these data are more consistent with mechanisms involving cleavage of the carbon–halogen bond within the coordination sphere of the metal.
Introduction
The catalytic amidation of aryl halides has become an important synthetic process.1 Much of this chemistry has been developed with palladium catalysts,2,3 but the oldest examples of this process were reported with copper catalysts.4 Much effort has been spent recently by many groups to improve upon the original copper catalysts for the coupling of aryl halides with nitrogen nucleophiles by conducting the reactions with added ligands (eq 1).5,6 Ligands used for this chemistry have included bipyridines and phenanthrolines,7–10 1,2-diamines,11,12 α-amino acids,13 bis-pyridylimines,14 1,3-diketones,15,16 and others.17–19
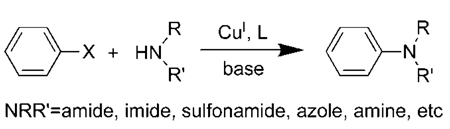 |
(1) |
Although much progress has been made toward improving the reaction scope and developing milder reaction conditions, a mechanistic foundation that explains the relative reactivities of different catalysts for various C–N, C–O, and C–S coupling processes has not been established. The effect of the dative and ancillary ligand on the identity and facility of the turnover-limiting step of the catalytic process and the effect of these ligands on the rates of the C–N bond-forming process have not been evaluated.
Studies to reveal the oxidation state of the starting copper were published many years ago,20 but an understanding of the relationships between reactivity and ligand structure requires the synthesis and characterization of copper amide, amidate, and imidate complexes containing a series of ancillary ligands. To date, few such complexes have been isolated in pure form and evaluated for their reactions with haloarenes. Examples of copper amidate and imidate complexes ligated by triphenylphosphine were reported by Yamamoto et al.21 However, most copper complexes with anionic nitrogen ligands that have been characterized22–25 have lacked dative ligands. Thus, the body of literature on the synthesis, isolation, and structures of ligated copper amide, amidate, and imidate complexes has been too limited to understand the relationships between the identity of the ligands and the patterns of reactivity.
In addition, few studies on the reactions of isolated, ligated copper amide, amidate, or imidate complexes with iodoarenes have been reported. In one of the few cases of such a study, PPh3-ligated amidates and imidates were shown to react slowly and in only modest yield with haloarenes.21 More recently, Strieter et al. generated a copper amidate from a 1:1:1 ratio of mesityl copper, pyrrolidinone, and N,N′-dimethylcyclohexanediamine.26 Although they were unable to isolate this complex in pure form, they showed the species generated in situ reacted with 3,5-dimethylphenyl iodide to form an N-arylpyrrolidinone with a rate that was faster than the rate of the related copper-catalyzed coupling. Finally, a computational study of the C–N bond-forming step of the coupling between acetamide and bromobenzene, catalyzed by ethylenediamine-ligated copper, was published recently, but experimental mechanistic data to compare and validate this computational work are limited.27
To begin to define the relationships between the structures and reactivity of proposed intermediates in the copper catalyzed coupling of aryl halides, we have sought to isolate, fully characterize, and study the reactivity of copper complexes containing anionic nitrogen ligands and the types of dative ligands that have been used to generate catalysts for copper-
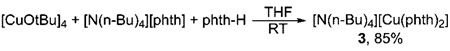 |
(2) |
catalyzed coupling processes. We report the synthesis of copper amidate and imidate complexes containing nitrogen and phosphorus ancillary ligands, the structure determinations of these species in the solid state and solution phase, the reactions of these species with a series of haloarenes, comparisons of the selectivities and rates of stoichiometric and catalytic reactions, quantitative kinetic data on the stoichiometric reactions of these species with haloarenes, the use of probes for aryl radical intermediates, and calculations of the free energies of activation for nonradical pathways by DFT methods. These studies provide rare data on the effect of ancillary and reactive ligands on the individual steps of this type of coupling, as well as mechanistic data that begin to distinguish between a series of proposed pathways for the reactions of haloarenes with ligated copper complexes containing anionic nitrogen ligands.
Results and Discussion
1. Synthesis of Ligated Amidate and Imidate Complexes
A series of copper amidate and imidate complexes ligated by phenanthroline, bipyridine, diamine, and diphosphine ligands were prepared by the methods shown in Scheme 1. The reaction of copper tert-butoxide28,29 with the dative ligand, followed by addition of phthalimide (phth-H), led to the formation of complexes 1a–d containing 1,10-phenanthroline (phen), 4,4′-di-tert-butylbipyridine (dtbpy), N,N′-dimethylethylenediamine (dmeda), and 9,9-dimethyl-4,6-bis(diphenylphosphino)xanthene (Xantphos) as the ancillary dative ligand and phthalimidate as the anionic nitrogen ligand. Reaction of [Cu(OtBu)]4 with 1,10-phenanthroline or N,N′-dimethylethylenediamine and pyrrolidinone (pyrr-H) led to complexes 2a or 2b containing phen or dmeda as the dative ligand and pyrrolidinonate as the anionic ligand. A stable two-coordinate, anionic copper imidate lacking dative ligands was also prepared. This complex, [N(n-Bu)4]+[Cu(phth)2]− (3), was prepared by combining [N(n-Bu)4][phth], [Cu(OtBu)]4, and phthalimide in THF (eq 2).
Scheme 1.
Complexes 1a–d and 2a were isolated in 84–98% yield by precipitation of the product or evaporation of solvent, followed by recrystallization. Attempts to isolate 2b led to decomposition. Therefore, studies on 2b were always conducted on material that was freshly generated in situ from a 1:1:1 mixture of [Cu(OtBu)]4, pyrrolidinone, and dmeda.30 Complex 3 precipitated from the reaction solution over the course of 15 min and was isolated in 85% yield. This material was judged to be pure by 1H NMR spectroscopy but required recrystallization from CH2Cl2 to yield material suitable for elemental analysis. Crystalline samples of these complexes displayed a range of colors. Complexes 1a and 1b were isolated as orange-red crystalline solids, and complex 1d as a light-yellow powder. Complex 1c was obtained as a green oil at room temperature that solidified when cooled to −35 °C. Complex 2a was isolated as an orange-red crystalline solid, while complex 3 was colorless. Solutions of complexes 1a–d and 2a,b decomposed in air within hours, but a solution of complex 3 was stable in air for days to weeks.
2. Structures of the Amidate and Imidate Complexes
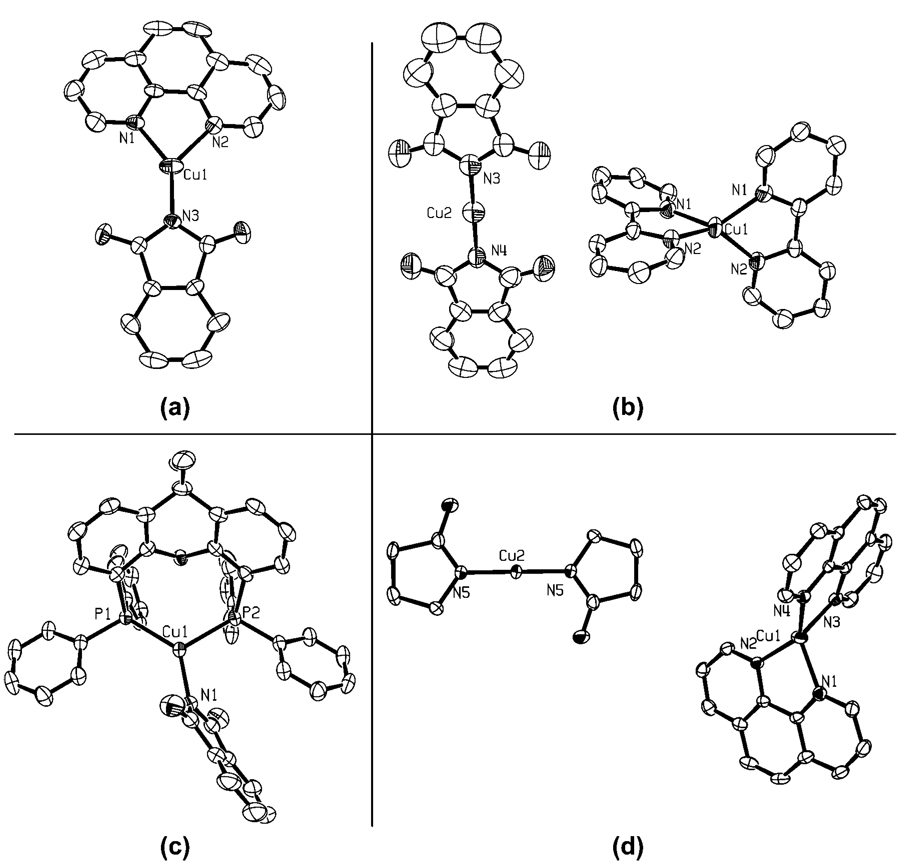
The identities of the imidate and amidate products were not straightforward to determine. A variety of methods were necessary, and the structures depended on the phase and the solvent. The 1H NMR spectra of each complex revealed a 1:1 ratio of the dative ligand to the imidate or amidate ligand. Moreover, all analytical data were consistent with this 1:1 ratio. However, the solid-phase structures of only complexes 1a and 1d reflected a simple LCu(imidate) structure, and solution data of 1a–d and 2a implied that the identity of these complexes depends on the solvent.
2.1. Solid-State Structures of the Amidate and Imidate Complexes
Figure 1 shows the solid-state structures of 1a,b,d and 2a determined by X-ray diffraction. The structure of 1c was also determined by X-ray diffraction. The connectivity of 1c was clearly defined, but disorder in the backbone of the dmeda ligand prevented a high-resolution structure in this case. The solid-state structures of complexes 1a and 1d consist of three-coordinate, trigonal planar geometries in which the copper(I) center is bound by one neutral bidentate ligand and one anionic imidate ligand. In contrast, the structures of 1b, 1c, and 2a consisted of ionic species containing one cationic tetrahedral copper center ligated by two of the dative N,N-donor ligands and one anionic, two-coordinate linear copper center ligated by two anionic nitrogen ligands. Such ionic structures have rarely been included in previous mechanistic proposals for reactions of ligated copper complexes, even though such ionic forms have been observed for copper halides. For example, Ouali et al. recently showed that the tetradentate bis-pyridylimine used in catalytic couplings forms dimeric complexes of the form [L2Cu2]X2 upon reaction with CuI or [Cu(CH3CN)4]PF6 in acetonitrile.31 Related structures of Cu(I) halides can be found in the Cambridge Crystallographic Database.32–35 For example, the reaction of copper(I) halides and various substituted phenanthrolines in a 1:1 ratio has formed monomeric copper complexes of the form (phen′)CuX, ionic complexes of the form [(phen′)2Cu][CuX2], and halide-bridged dimeric complexes of the form [(phen′)CuX]2, in which X = Cl, Br, or I and phen′ is either a substituted or unsubstituted 1,10-phenanthroline.
Figure 1.
Crystal structures of 1a (a), 1b (b), 1d (c), and 2a (d) with 50% ellipsoids. The tert-butyl groups of the dtbpy ligand of 1b have been omitted for clarity.
Phen-ligated and Xantphos-ligated imidates 1a and 1d are both three-coordinate monomers in which the copper center is trigonal planar. In 1a, the C–N distances to the dative ligand (2.01 and 2.07 Å) are longer than that to the formally anionic phthalimidate (phth) ligand (1.88 Å). The phth ligand is bound symmetrically between the two nitrogens of the dative ligand in complex 1a, giving nearly perfect C2v symmetry. The Cu–N distance to the phth ligand in 1d is longer (1.95 Å) than that in 1a, and, in contrast to that in 1a, the imidate ligand of 1d is canted toward one of the phosphorus donors, making one P–Cu–N angle larger than the other.
The X-ray data for complexes 1b, 1c, and 2a revealed an ionic structure of the general formula [L2Cu]+[CuX2]− (L = dtbpy, dmeda, or phen, X = phth or pyrr). The Cu–N distances in these species are similar to those in the neutral compound, despite the different coordination number and charge. The Cu–N distance in the [(dtbipy)2Cu]+ portion of 1b is 2.01 Å, which is similar to the Cu–N distances in neutral 1a. The Cu–N distance in the anionic [Cu(phth)2]− portion of 1b is 1.86 Å, which is also similar to the C–N(phth) distance in neutral 1a. Even the [Cu(pyrr)2]− portion of 2a has the same Cu–N distance of 1.86 Å. The anionic portions of 1b and 2a are linear. The N–Cu–N angle in 1a is 179.3°, while that in 2a is a rigorously linear 180.0° because the copper lies on a crystallographic inversion center.
2.2. Solution-Phase Structures of the Amidate and Imidate Complexes
To determine which structure was present in solution, techniques other than simple 1H NMR spectroscopy were required. A simple 1H NMR spectrum does not distinguish the neutral form from the ionic form because both contain a 1:1 ratio of dative to anionic nitrogen ligands. Instead, conductivity of solutions containing the complexes and spectra of mixtures of compounds were used to clarify the relationship between the solid-state and solution structures.
The conductivities of solutions of complexes 1a–d, 2a, and 3 in DMSO solvent were measured.36 The values of 1.0 mM solutions of these complexes in DMSO were 15.9 Ω−1 cm2 mol−1 for 1a, 14.5 Ω−1 cm2 mol−1 for 1b, 18.4 Ω−1 cm2 mol−1 for 1c, 23.1 Ω−1 cm2 mol−1 for 1d, 20.1 Ω−1 cm2 mol−1 for 2a, and 44.5 Ω−1 cm2 mol−1 for 3. The size and thus mobility of ions in solution affects the conductivity of the solution containing these ions.36 Thus, it is difficult to use these values to quantify the concentrations of ions in solution. Nevertheless, a comparison of these values to the 0.3 Ω−1 cm2 mol−1 value for ferrocene and the 23.5 Ω−1 cm2 mol−1 value for [NBu4][BPh4] implies that all the complexes contain substantial, if not exclusive, amounts of the ionic form in polar solvents. Measurements of conductivity in much less polar solvents is unconventional due to the small values and the resulting need for highly concentrated samples to obtain substantial readings, along with the lack of solubility of most ionic species in these media. Nevertheless, we were able to compare the conductivity of complex 1b with that of [(n-octyl)4N][Br] in THF and benzene. The conductivity of a 65.5 mM concentration of 1b in THF was 22.1 μΩ cm−1 (0.34 Ω−1 cm2 mol−1). For comparison, the conductivity of the same concentration of a THF solution of [(n-octyl)4N][Br] was 65.1 μΩ cm−1 (0.99 Ω−1 cm2 mol−1), and the value for a 65.5 mM solution of ferrocene was 0.0 μΩ cm−1 (0.0 Ω−1 cm2 mol−1). Thus, complex 1b appears to adopt at least some of the ionic structure in THF. However, the conductivity of a 65.5 mM solution of 1b in benzene was <0.1 μΩ cm−1 (<0.002 Ω cm2 mol−1), whereas the value for the same concentration of [(n-octyl)4N][Br] was 1.2 μΩ cm−1 (0.018 Ω−1 cm2 mol−1), and the value for a 65.5 mM solution of ferrocene was 0.0 μΩ cm−1 (0.0 Ω−1 cm2 mol−1). These data imply that complex 1b exists as the neutral, three-coordinate structure in an arene solvent.
To gain further data on the identity of these species in polar solvents, we compared the 1H and 15N spectral data for complexes 1a–c to those of [N(n-Bu)4][Cu(phthalimidate)2] (3). The 1H NMR resonances of the phthalimidate ligand in 3 and 1a–c in DMSO-d6 were indistinguishable. The 1H NMR spectrum of all four complexes contained a multiplet or broad singlet at ~7.6 ppm. Although one might think that the 15N chemical shifts of 15N-labeled 1a–c (−196.9 ppm vs neat MeNO2) would be more diagnostic, the signals were broad. The 15N chemical shifts of 15N-labeled 1a–c were identical to that of 15N-labeled 3. Although these values are distinct from those measured for H-phth (−152.6 ppm) and K-phth (−221.7 ppm), the 15N NMR spectra of a THF solution of 15N-enriched 1c contained a single resonance at −196 ppm, and the spectrum of labeled 1c in toluene consisted of a single resonance at −195 ppm with a linewidth of 400 Hz. Thus, all the copper complexes in all media that we examined have relatively similar 15N NMR chemical shifts.
More informative were some of the 1H NMR spectra of mixtures of two complexes containing the same dative ligand but a different anionic ligand. These studies proved most informative for phen complexes 1a and 2a. If complexes 1a and 2a are simple neutral monomers, then the 1H NMR spectrum of the mixture of the two complexes would contain one set of 1H NMR resonances for each of the anionic ligands and two sets of resonances for the dative ligand. However, if they are ionic species, then this spectrum would contain two sets of resonances for the anionic ligands (one set for the homoleptic anion and one set for the mixed anion) and one set for the dative ligand.
To obtain data for this analysis, the phen-ligated phthalimidate complex 1a was mixed with the phen-ligated amidate complex 2a in DMF-d7 solvent. Consistent with each complex existing largely in the ionic form in polar solvents, the 1H NMR spectrum of 1a and 2a at −25 °C in DMF-d7 contained two sets of resonances for the pyrrolidinone ligand. The 1H NMR resonances of the phth and phen ligands overlapped with each other, but the pyrr resonances were resolved. This mixture of 1a and 2a at −25 °C contained two distinct 1H NMR resonances at 3.25 and 3.18 ppm, corresponding to the methylene protons of the pyrr ligands alpha to the carbonyl group.37 Unfortunately, the 1H NMR spectra of dmeda-ligated phthalimidate complex 1c and dmeda-ligated pyrrolidinonate complex 2b in toluene were temperature dependent, and a temperature at which signals due to both compounds were sharp could not be obtained.
3. Reactivity of the Amidate and Imidate Complexes with Haloarenes
3.1. Reactions of Complexes Containing Dative Ligands
Having established the solution structures of 1a–c and 2a, we sought to determine the effect of dative ligand, anionic ligand, and solvent on the rates of the reaction of these complexes with haloarenes. Qualitative data on the relative reactivity of these complexes was obtained by heating them with iodoarene or bromoarene in DMSO, THF, or toluene in sealed NMR sample tubes. Yields of product were determined by 1H NMR spectroscopy with an internal standard.
The reactions of phthalimidates 1a–c with iodoarenes are summarized in eq 3. The reaction of phen-ligated 1a with 25 equiv of p-tolyl iodide in DMSO formed the coupled product in 98% yield after 3 h at 120 °C, and the reaction of dtbpy-ligated 1b occurred in 93% yield after 1.5 h. (Reactions for these times at lower temperatures occurred to lower conversions.) In contrast, reactions of these complexes with 25 equiv
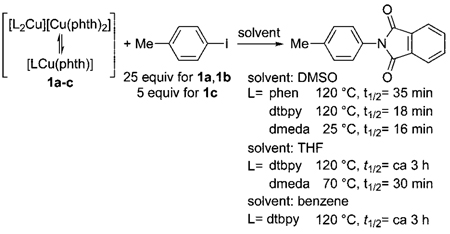 |
(3) |
of p-tolyl iodide in the less polar THF and benzene solvents occurred with half-lives of about 3 h at 120 °C. Complex 1c containing the diamine ligand was more reactive but showed a similar effect of the rate on the polarity of the solvent. The reaction of this species with 5 equiv of p-tolyl iodide in DMSO formed N-(p-tolyl)phthalimide in 95% yield after 2 h at 25 °C, but the same reaction in THF at 70 °C (in a sealed NMR tube) for 2 h formed N-(p-tolyl)phthalimide in 78% yield and for 4 h formed this product in 99% yield. Little reaction occurred over 2 h at 25 °C. Although the yields in THF solvent were lower than those in DMSO, neither biaryl nor hydrodehalogenated byproducts were observed in substantial amounts. For example, reaction of 4-iodo-tert-butylbenzene with 1c in THF formed 75% of the N-arylphthalimide and <2% tert-butylbenzene by reduction of the halogen, as determined by GC and GC/MS. Complex 1d containing a diphosphine ligand was much less reactive than any of the complexes containing dative nitrogen ligands; its reaction with 4-iodotoluene proceeded to only 50% conversion in DMSO after 17.5 h at 120 °C and formed no coupled product.
The reactions of amidates 2a and 2b with iodoarenes are summarized in eq 4. The reaction of phen-ligated 2a with 5 equiv of p-tolyl iodide in DMSO occurred in 98% yield after 3.3 h at 80 °C or 99% yield after only 20 min at 120 °C. This reaction is much faster than that of p-tolyl iodide with phen-ligated 1a containing a phthalimidate, which required 3 h at 120 °C in DMSO to form the coupled product in 98% yield. The amidate complex 2b ligated by dmeda was more reactive
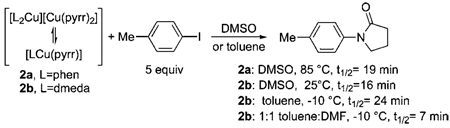 |
(4) |
than the amidate complex 2a ligated by phen. Reaction of 2b with p-iodotoluene in toluene for 1 h at −10 °C formed N-(p-tolyl) pyrrolidinone in 93% yield. Reaction of 2b with p-iodotoluene in a 1:1 mixture of toluene and DMF was only slightly faster than that in pure toluene (vide infra). Thus, the reactions of the pyrrolidinonate complexes were less affected by solvent polarity than were the reactions of the phthalimidate complexes. It is possible that this difference in solvent effect results from a change in rate-determining step due to the different electronic properties of these two anionic nitrogen ligands.
Bromoarenes reacted much more slowly than iodoarenes. The reaction of diamine-ligated imidate 1c with p-tolyl bromide in toluene required 5 h at 25 °C to produce p-tolylphthalimide in 50% yield, whereas the reaction of 1c with p-tolyl iodide in toluene produced p-tolylphthalimide in 95% yield in 2 h at 25 °C. In contrast to the reaction of 1c with p-tolyl iodide and bromide, no reaction occurred between 1c and p-tolyl chloride or p-tolyl triflate (DMSO, 120 °C, 18 h). Amidate complexes 2a and 2b were also less reactive toward bromoarenes than iodoarenes. In contrast to the high yield of N-aryl pyrrolidinone from reaction of phen-ligated 2a with 4-iodotoluene, heating of 2a with p-bromotoluene in DMSO solvent at 110 °C for 3 h led to 59% conversion of 2a, but no N-(p-tolyl)pyrrolidinone was formed; in contrast to the reaction of dmeda-ligated 2b with 4-iodotoluene at room temperature, the reaction of 2b with p-bromotoluene required 6.5 h at 110 °C to form the N-tolyl product in 94% yield.
3.2. Reactions of a Ligandless, Anionic Cu(I) Phthalimidate
One might expect that the anionic portions of the ionic complexes containing the nitrogen ligands would be the species that react with the haloarene because they are unsaturated and electron rich. Many conflicting conclusions have been drawn on the relative reactivity of ligandless anionic and neutral copper alkoxides in the copper-catalyzed coupling of phenoxides and alkoxides with haloarenes (see section 11 for more details on these previous studies). To help resolve the issue of the relative reactivity of anionic and neutral copper(I) complexes with haloarenes, and to address whether the iodoarene reacts with the anionic CuX2− portions of the ionic complexes containing the nitrogen ligands or with the neutral LCuX complexes resulting from comproportionation of the ions, we studied reactions of p-iodotoluene with the tetraalkylammonium salt of the anionic copper bis-imidate 3 lacking any dative nitrogen ligand. In contrast to complexes 1a–c and 2a,b, this ligandless anion did not react with haloarenes. Despite the unsaturation and anionic charge on complex 3, this complex by itself was stable toward 25 equiv of p-iodotoluene in DMSO after 23 h at 120 °C (eq 5).
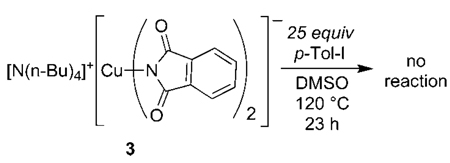 |
(5) |
We probed for potential cooperative effects between Lewis acidic cations and cuprate anions38 to cleave the carbon–halogen bond in the iodoarene by conducting reactions of anionic 3 with 25 equiv of iodotoluene in the presence and in the absence of alkali metal cations. The reaction of anionic complex 3 with p-iodotoluene in DMSO at 120 °C in the presence of added LiBF4 slowly formed N-(p-tolyl)phthalimide, but this reaction (t1/2 > 5.5 h) was much slower than the reaction of any of the ligated complexes with this iodoarene. Likewise, addition of KOTf to the reaction of complex 3 with p-iodotoluene in DMSO at 120 °C led to formation of the N-arylphthalimide with t1/2 > 9 h. Although the reactions were faster in the presence of alkali metal cations than in the absence of the cations, these reactions were much slower than reactions of the ligated complexes, which occurred with half-lives of minutes at 120 °C for bpy and phen complexes 1a,b or room temperature for dmeda complex 1c. As a side note, the rate and yield of the reactions of the phen-ligated phthalimidate 1a with iodotoluene were indistinguishable in the presence and in the absence of added LiBF4.
4. Identity of the Copper Products from Reactions with Haloarenes
The copper product of the reaction of 1c with p-tolyl iodide was determined by X-ray diffraction (see Supporting Information) and independent synthesis. From the reaction of p-tolyl iodide with 1c, the copper(II) species [Cu(dmeda)2]I2 (4) was formed in 43% isolated yield. The addition of 1 equiv of the dmeda ligand to this reaction had no effect on the identity and yield of this complex. We presume that disproportionation of [Cu(dmeda)I] to form [Cu(dmeda)2]I2 and Cu(0) accounts for the roughly 50% yield of the Cu(II) species from this reaction. Clearly, the efficiency of the recycling of the copper product to Cu(I) imidate and amidate can impact the amount of copper lying within the catalytic cycle and the observed kinetic behavior of the catalytic system.
5. Comparison of Selectivities of Stoichiometric and Catalytic Reactions
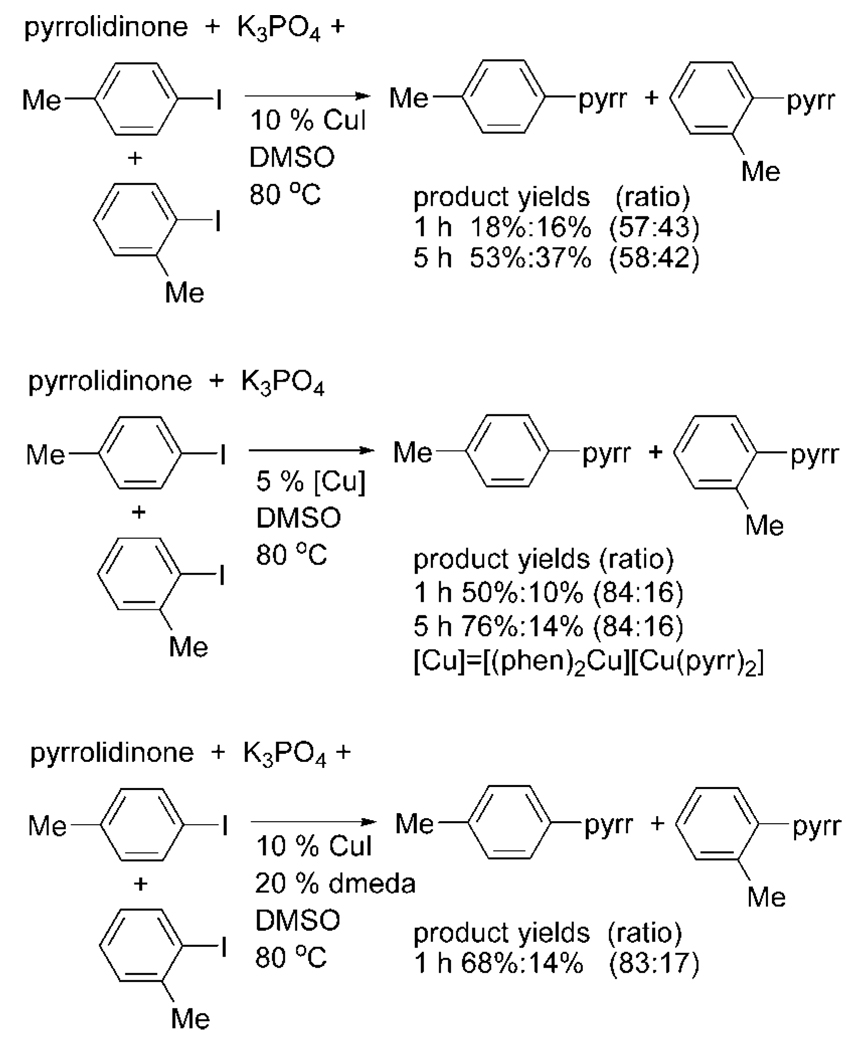
To evaluate the relevance of these isolated complexes to the amidation and imidation of aryl halides catalyzed by copper complexes, we compared the relative rates and selectivities of the catalytic and stoichiometric reactions. More specifically, we compared the relative reactivity of two iodoarenes toward the isolated copper complexes to the relative reactivity of two iodoarenes in catalytic processes. We compared these relative reactivities to those of pyrrolidinone catalyzed by CuI alone and the combination of CuI and dmeda or phen ligands. Most diagnostic were reactions with two sterically distinct iodoarenes, p-tolyl iodide and o-tolyl iodide.
The relative amounts of the two N-aryl products for three catalytic systems are shown in Scheme 2. These data show that the product ratios are distinct for reactions catalyzed by CuI and for reactions catalyzed by complexes of the two dative nitrogen ligands. In the absence of added dative ligand, the reaction conducted with 10 mol % CuI occurred at 80 °C to yield an approximately 1:1 ratio of N-(p-tolyl)pyrrolidinone and N-(o-tolyl)pyrrolidinone. The same product ratio was observed after 1 h and after 5 h. The coupling of pyrrolidinone with p-tolyl iodide and o-tolyl iodide at 80 °C in DMSO catalyzed by 5 mol % [(phen)2Cu][Cu(pyrr)2] (10 mol % copper) formed N-(p-tolyl) pyrrolidinone and N-(o-tolyl)pyrrolidinone in an 84:16 ratio in an overall yield <90% after 5 h. The same reaction catalyzed by 10 mol % CuI and 20 mol % dmeda gave a 83:17 ratio of the two products in <80% overall yield. Data obtained after 1 h clearly showed that the reaction catalyzed by CuI without added ligand was slower than the two reactions conducted with added ligand.
Scheme 2.
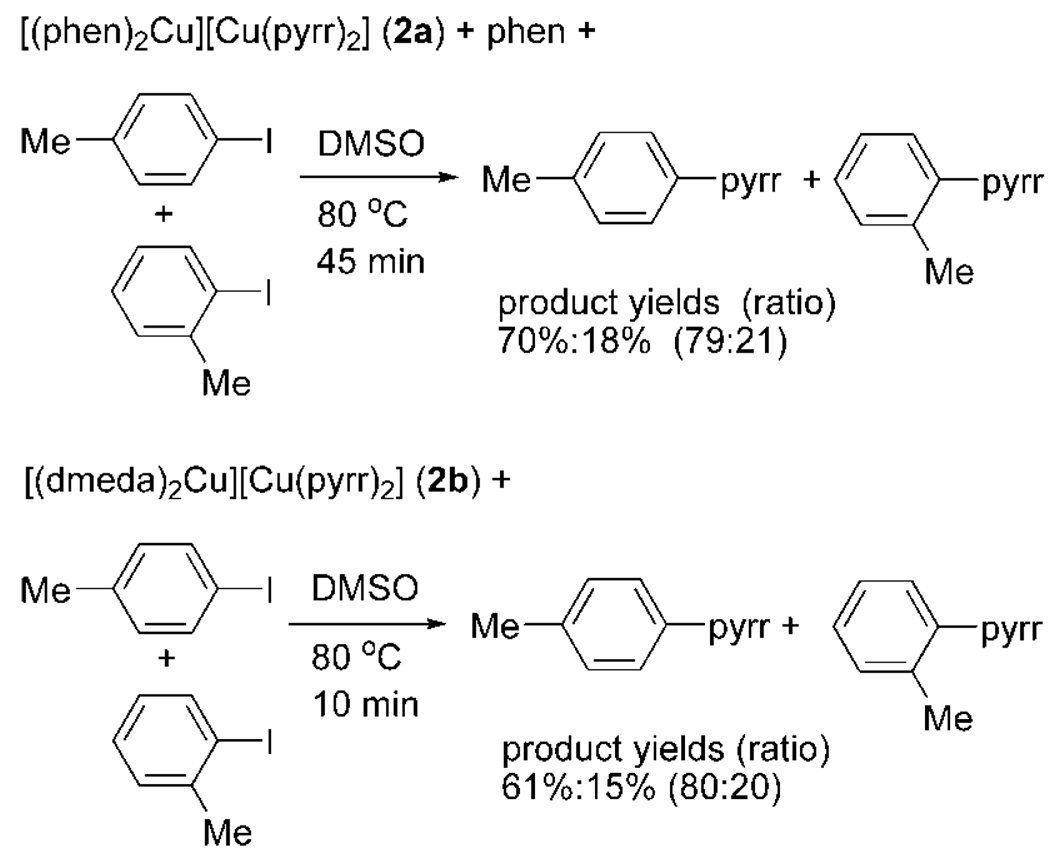
Data on reactions of the isolated complexes 2a and 2b with the two haloarenes are shown in Scheme 3. The reaction of complex 2a at 80 °C with a mixture of p-tolyl iodide and o-tolyl iodide with 1 equiv of added phen gave N-(p-tolyl)pyrrolidinone and N-(o-tolyl)pyrrolidinone in a 79:21 ratio and roughly 90% total yield after 45 min. The 84:16 product ratio observed for the reactions catalyzed by 5 mol % [(phen)2Cu][Cu(pyrr)2] does not match precisely the 79:21 ratio observed for the stoichiometric reaction, but it is close to this ratio and is far from the 1:1 ratio of products obtained from the reaction catalyzed by CuI alone.39
Scheme 3.
The reaction of in situ-generated complex 2b with a mixture of p-tolyl iodide and o-tolyl iodide at 80 °C formed N-(p-tolyl)pyrrolidinone and N-(o-anisyl)pyrrolidinone in 60.9 and 15.4% yields after 10 min (ratio of N-(p-tolyl)pyrrolidinone to N-(o-tolyl)pyrrolidinone is 80:20) (Scheme 3). This value matches well with the 83:17 selectivity observed for the catalytic reaction employing 10 mol % CuI and 20 mol % dmeda.
Several sets of conclusions can be drawn from these experiments. First, the rates of the stoichiometric reactions are greater than those of the catalytic reactions. These data demonstrate the kinetic competence of each of the isolated species to be intermediates in the corresponding catalytic process. Second, the selectivities of the stoichiometric reactions are similar to those of the catalytic processes. The congruence of these selectivities provides additional evidence that the copper complexes characterized in solution are intermediates or lead to intermediates in the catalytic process. Third, the steric properties of the phen and dmeda ligands lead to a greater discrimination of the steric properties of the aryl iodide than was observed from reactions catalyzed by CuI alone.
6. Effect of Ligand Properties on Reaction Rates
Comparisons of the relative rates of reactions of 1a–d and 2a,b allow one to draw some initial conclusions about the effect of ligand electronic properties on the rates of the reactions of these complexes with haloarenes. For reference, Table 1 provides νCO values for nickel carbonyl complexes containing phen, bpy, and two PPh3 ligands. These data imply that phenanthroline and bpy ligands are stronger electron donors than triarylphosphines. Although (diamine)Ni(CO)2 complexes are not known, we presume that the alkylamine ligand is a stronger electron donor than the phen and bpy ligands.
Table 1.
νCO Values for Nickel Carbonyl Complexes with Ligands Related to Those in This Work
| complex | νCO | ref |
|---|---|---|
| (PPh3)2Ni(CO)2 | 1990–2010, 1925–1955 | a, b, c |
| (phen)Ni(CO)2 | 1977–1980, 1897–1915 | a, d |
| 2,4-Me2bipyNi(CO)2 | 1982, 1883 | e |
| 4,4′-Me2bipyNi(CO)2 | 1973, 1893 | d |
Plankey, B. J.; Rund, J. V. Inorg. Chem. 1979, 18, 957.
Meriwether, L. S.; Fiene, M. L. J. Am. Chem. Soc. 1959, 81, 4200.
Tolman, C. A. J. Am. Chem. Soc. 1970, 92, 2956.
Christensen, P. A.; Hamnett, A.; Higgins, S. J.; Timney, J. A. J. Electroanal. Chem. 1995, 395, 195.
Sieler, J.; Than, N.-N.; Benedix, R.; Dinjus, E.; Walther, D. Z. Anorg. Allg. Chem. 1985, 522, 131.
Among the imidate complexes 1a–d, the complexes containing more-electron-donating dative ligands reacted faster than those containing less-electron-donating dative ligands. Consistent with the higher νCO values of the PPh3 complex and the previous slow rates for reactions of PPh3-ligated imidates,21 no reaction of the copper amidate 1d containing the Xantphos ligand was observed up to 120 °C for 17.5 h. In addition, a comparison of the reactivity of complexes 1a and 2a, which contain the same dative phenanthroline ligand but different anionic ligands, shows that the complex containing the more-electron-donating anionic ligand reacts faster.
7. Quantitative Kinetic Studies
Kinetic studies of the stoichiometric reaction of haloarenes with the copper imidates and amidates were conducted by 1H NMR spectroscopy to help determine the identity of the species that reacts with the haloarene and to provide free energies of activation that can be compared to the values determined by DFT methods (vide infra). Table 2 summarizes the observed rate constants, reaction temperatures, and free energies of activation. Reactions of 1a and 1b were conducted with 5–25 equiv of p-iodotoluene in DMSO in flame-sealed NMR tubes heated at 120 °C. 1H NMR spectra were obtained at various time points during the course of the reaction. Reactions of the copper imidate 1c and copper amidates 2a,b were conducted with 5 equiv of p-iodotoluene and p-bromotoluene in both toluene and DMSO solvents. Complex 2b was freshly generated in situ for each experiment. The rate constants for reactions of 1c, 2a, and 2b with these haloarenes at −20 to 85 °C were measured in the NMR spectrometer probe.
Table 2.
Rate Constants and Free Energies of Activation for the Reactions of L2CuNRR′ Complexes with 4-Iodotoluene and 4-Bromotoluene
| entry | complex | L | NRR′ | X in p-TolX | kobs (s−1) | ΔG‡(exp) (kcal/mol) | T (°C) | ΔG‡(calc) (kcal/mol)a | solvent |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1a | phen | phth | iodide | 5.4 × 10−4 | 29 | 120 | DMSO | |
| 2 | 1b | dtbpy | phth | iodide | 7.4 × 10−4 | 29 | 120 | DMSO | |
| 3 | 1c | dmeda | phth | iodide | 5.8 × 10−4 | 21 | 25 | DMSO | |
| 4 | 1c | dmeda | phth | bromide | 4.1 × 10−4 | 26 | 80 | DMSO | |
| 5 | 2a | phen | pyrr | iodide | 6.2 × 10−4 | 26 | 85 | DMSO | |
| 6 | 2b | dmeda | pyrr | iodide | 1.3 × 10−3 | 18 | −20 | 25 | toluene |
| 7 | 2b | dmeda | pyrr | iodide | 2.8 × 10−3 | 18 | −20 | 1:1 DMF:tol | |
| 8 | 2b | dmeda | pyrr | bromide | 1.6 × 10−4 | 29 | 120 | 38 | toluene |
| 9 | eda | HNAc | bromide | 29c | toluene (calc) | ||||
| 10 | [Cu(phth)2]− | iodide | b | b | 120 | 45 | toluene (calc) | ||
| 11 | [Cu(pyrr)2]− | iodide | 42 | toluene (calc) | |||||
| 12 | [Cu(HNAc)2]− | bromide | 44c | toluene (calc) | |||||
Computed values for the reaction of respective three coordinate copper complex and phenyl iodide or bromide and incorporate solvent effects using the CPCM method.
No reaction was observed when [Bu4N][Cu(phth)2] was heated to 120 °C for 24 h in DMSO solvent.
Reference 27.
All reactions occurred with a smooth first-order exponential appearance of the organic product. Because this product is directly related by stoichiometry to the copper reagent in the presence of excess haloarene, these data indicate that the reactions are first-order in the starting copper complexes. Similar first-order rate constants from reactions initiated with much different concentrations of copper confirmed this first-order behavior. The order in iodoarene was measured for the reactions of 1a–c by conducting reactions with varied excess of iodoarene. Each reaction was first-order in the iodoarene. This order in the two reagents might seem unremarkable; however, the first-order behavior in copper was observed regardless of whether the reaction was initiated with a complex that was ionic or neutral in the solid state and whether the reaction was conducted in a polar solvent in which the complexes contain a large degree of the ionic structure or in a nonpolar solvent in which the complexes contain a larger degree of the neutral structure.
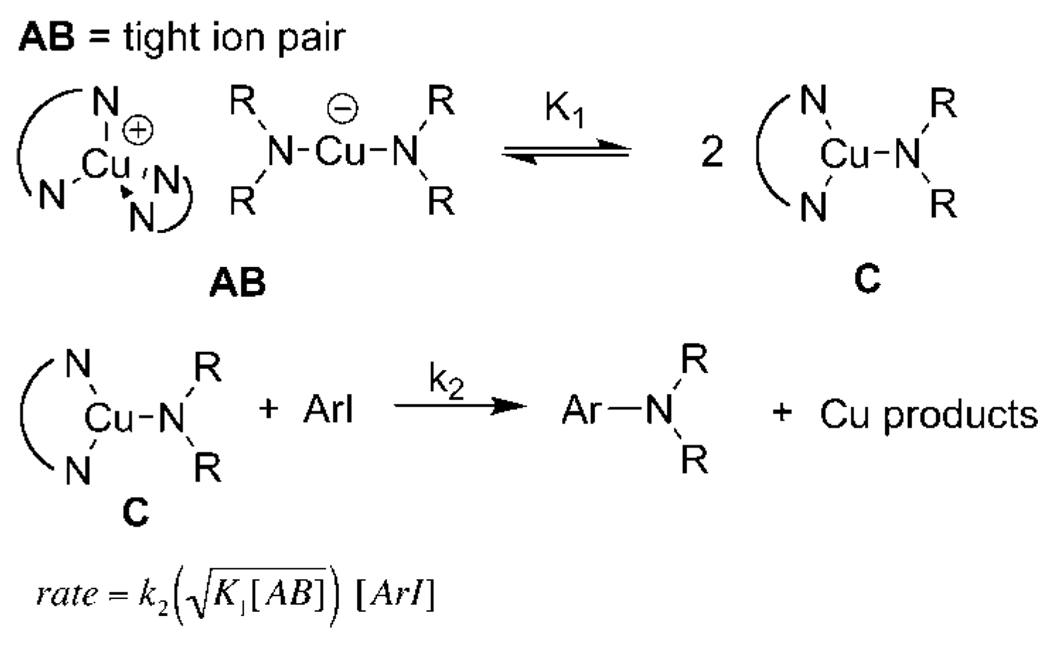
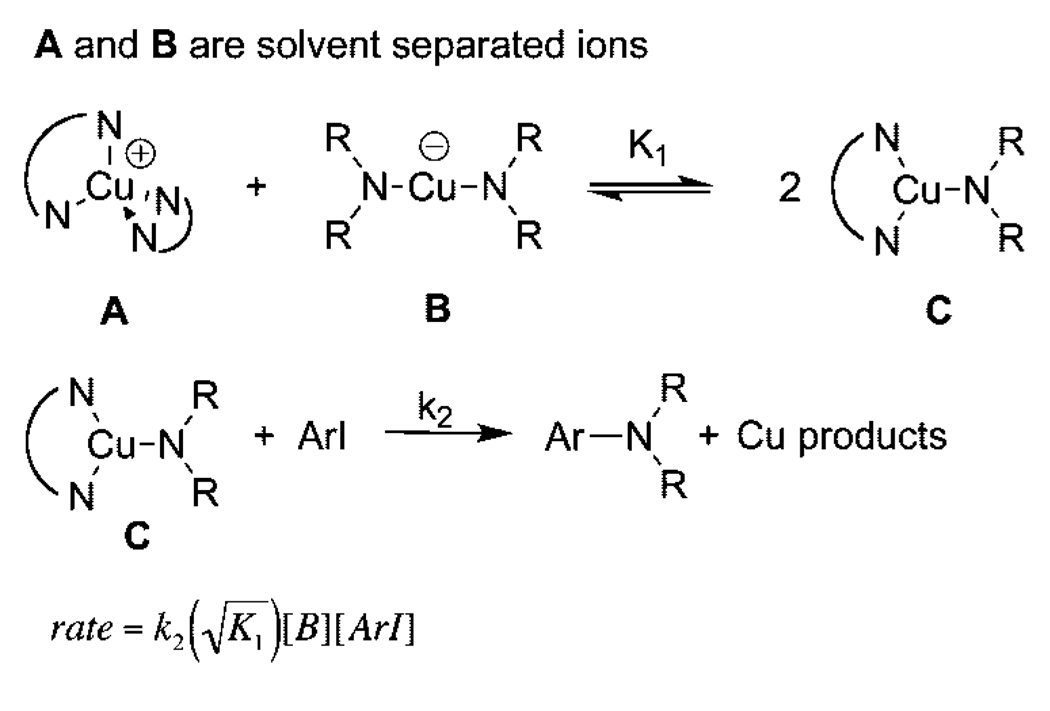
The rate laws for reactions of the ionic compounds through the neutral species initiated as tight ion pairs and as solvent-separated ions are shown in Scheme 4 and Scheme 5, respectively. In both cases, the reaction is assumed to occur from the neutral three-coordinate structure because of the lack of reactivity of [N(n-Bu)4][Cu(phth)2] with iodoarenes. Derivations of these expressions are provided as Supporting Information. One might expect that the first-order behavior for reactions starting with the ionic species in polar solvents requires that the irreversible step involve the ionic form of the complexes. However, these rate equations show that the reaction will be first-order in copper when the two ions are separate species and related in a 1:1 fashion by stoichiometry. Reactions in which a starting tight ion pair generates two neutral species give rise to a rate equation that is half-order in copper. Thus, the observation of first-order behavior in copper implies that the reactions in DMSO, after dissolution of the added copper species, start from solvent-separated ion pairs and generate small equilibrium amounts of the reactive neutral complex.
Scheme 4.
Scheme 5.
8. Comparative Reactivities To Probe an Electron-Transfer Mechanism
The mechanism of carbon–heteroatom bond formation from copper alkoxides and amides has been proposed in some cases to occur by radical20,44–46 and in other cases by nonradical27,47–53 pathways.5,54 The proposed radical mechanism is triggered by electron transfer from copper to the aryl halide to form a haloarene radical anion, which eliminates the halide to form a neutral aryl radical. This aryl radical could then combine with the amidate or imidate ligand to form the organic product.
To probe for this radical pathway, we conducted experiments with two aryl halides possessing different electronic properties and with aryl halides containing radical clocks. In the first set of experiments, we conducted reactions of copper complexes with a chloroarene and two bromoarenes chosen to have relative reduction potentials and reactivities such that the chloroarene is easier to reduce than the two bromoarenes and has a rate of dissociation of halide from the radical anion that is similar to that for dissociation of halide from the two bromoarene radical anions. If the reactions are initiated by an outer-sphere electron transfer, then one would expect this chloroarene to react with similar or faster rates than the bromoarene because of the larger driving force for reduction of the chloroarene and the connection between driving force and rates of outer-sphere electron transfer.55,56 Although less rigorous, one could also expect these two haloarenes to react with similar rates by an inner sphere electron-transfer process that forms a free aryl halide radical anion, particularly if the haloarene interacts through the arene π-system. Computational studies described later in this paper imply that coordination of the haloarene to the metal through the halide and that through the π-system are nearly isoenergetic.
The chloroarene and bromoarenes chosen for this study are 4-chlorobenzonitrile and 1- and 2-bromonaphthalene. The reduction potentials and rates for halide dissociation of the corresponding haloarene radical anions, along with values for bromobenzene as comparison, are shown in Table 3. This table also shows the potential for reduction of 1-naphthyl triflate, which forms aryl oxide upon reduction by S–O bond cleavage.42 The rate constants for halide dissociation have been measured several times,40,41,43 and a range of values are provided in the table. However, the rate constants measured for chloride dissociation from the radical anion of 4-chlorobenzonitrile and for bromide dissociation from 1- and 2-bromonaphthalene are within a factor of 3 of each other when measured by the same method.40,41,57
Table 3.
Reduction Potentials (E0) of Four Haloarenes and 1-Naphthyl Triflate and Rates for Halide Dissociation (kdissoc) from the Corresponding Radical Anions
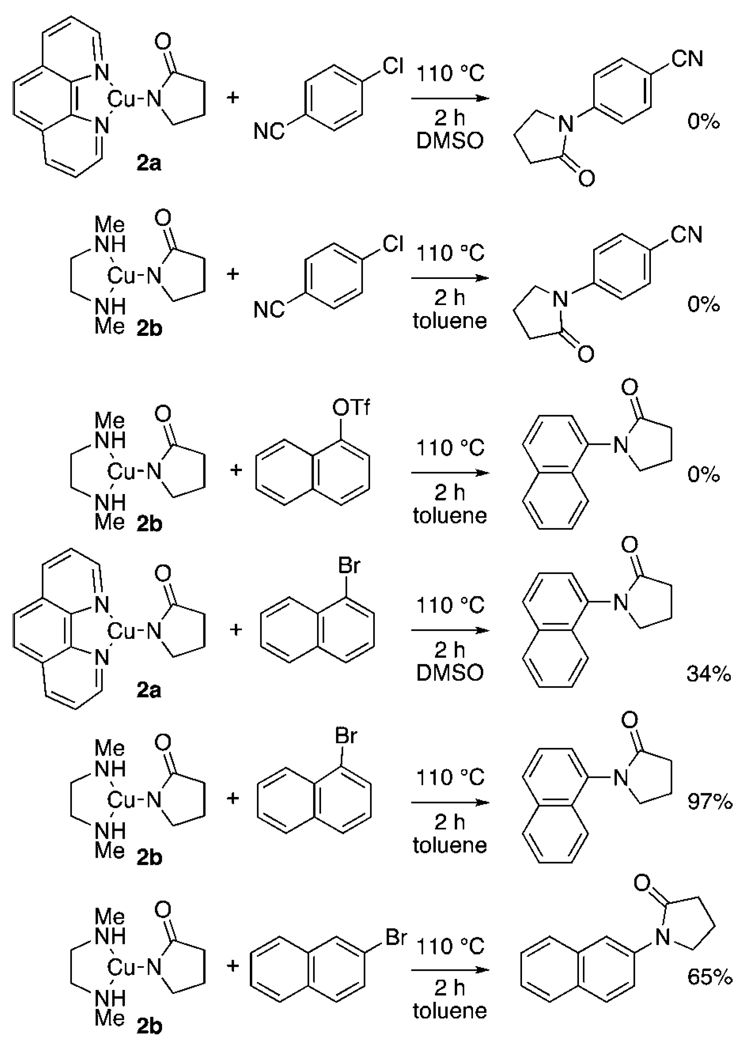
Reactions of 4-chlorobenzonitrile, 1-naphthyl triflate, and the two bromonaphthalenes with complexes 2a and 2b in DMSO and toluene, respectively, are shown in Scheme 6. Despite the more favorable reduction potential of 4-chlorobenzonitrile and 1-naphthyl triflate and the known fast chloride dissociation from the reduced chloroarene, these reagents reacted with 2a and 2b much more slowly than did 1- or 2-bromonaphthalene. Heating of these complexes with the chloroarene or 1-naphthyl triflate at 110 °C for 2 h led to decomposition identical to that seen in the absence of the haloarene; no observable N-arylpyrrolidinone was formed. No naphthol was detected from the reaction of in situ-generated 2b with the aryl triflate. In contrast, the bromonaphthalenes reacted with these complexes in each case tested. Phen-ligated 2a reacted with 1-bromonaphthalene in DMSO at 110 °C after 2 h to form the N-aryl product in 34% yield.58 The dmeda-ligated 2b reacted with 1-bromonaphthalene in toluene at 110 °C after 2 h to form the N-aryl product in 97% yield and with 2-bromonaphthalene under the same conditions to form the N-aryl product in 65% yield. These data argue against a reaction mechanism that would be initiated by outer-sphere electron transfer.
Scheme 6.
In a second set of experiments, the intermediacy of an aryl radical was probed by conducting reactions with an aryl halide linked to an olefin that serves as a radical clock. The aryl radical that would be generated from o-(allyloxy)iodobenzene is known to undergo cyclization to yield the 2,3-dihydrobenzofuranyl-methyl radical with a rate constant of 9.6 × 109 s−1 in DMSO and 7.87 × 107 s−1 in hexane.59,60 The resulting methyl radical can then abstract hydrogen from the solvent, can dimerize, or could combine with the amidate or imidate ligand to form a C–N bond.
To use this substrate to probe for aryl radicals in the copper-catalyzed couplings, [(phen)2Cu][Cu(pyrr)2] was allowed to react with 1 equiv of o-(allyloxy)iodobenzene in DMSO-d6. After 31 h at 110 °C, the product from C–N coupling at the aryl group formed in 95.5% yield (eq 6). GC and GC/MS analysis
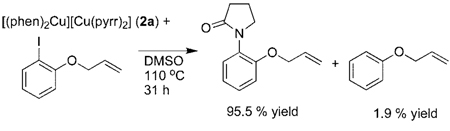 |
(6) |
of the reaction mixture revealed a 1.9% yield of phenyl allyl ether by hydrodehalogenation of the iodoarene. Comparison of the GC and GC/MS of authentic samples of phenyl allyl ether and 3-methyl-2,3-dihydrobenzofuran confirmed that no 3-methyl-2,3-dihydrobenzofuran product was formed. The reaction of pyrrolidinone with o-(allyloxy)iodobenzene, catalyzed by 10 mol % CuI and 20 mol % dmeda or 20 mol % phen, was also conducted in DMSO solvent at 110 °C (eq 7). These reactions formed the product from C–N coupling at the aryl group in >90% yield and phenyl allyl ether by hydrodeiodination in <2% yield. Again, no products from cyclization were detected. Thus, neither the stoichiometric or catalytic reactions yielded the products that one would expect from reaction through a free aryl radical. The absence of this product with detection limits of >100:1 implies that the rate constant for recombination of the aryl radical with the amidate or imidate ligand from within the solvent cage would have to be >1012 s−1.
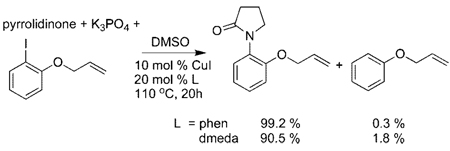 |
(7) |
9. Computed Barriers for Oxidative Addition of Iodobenzene and Bromobenzene to 1c and 2b
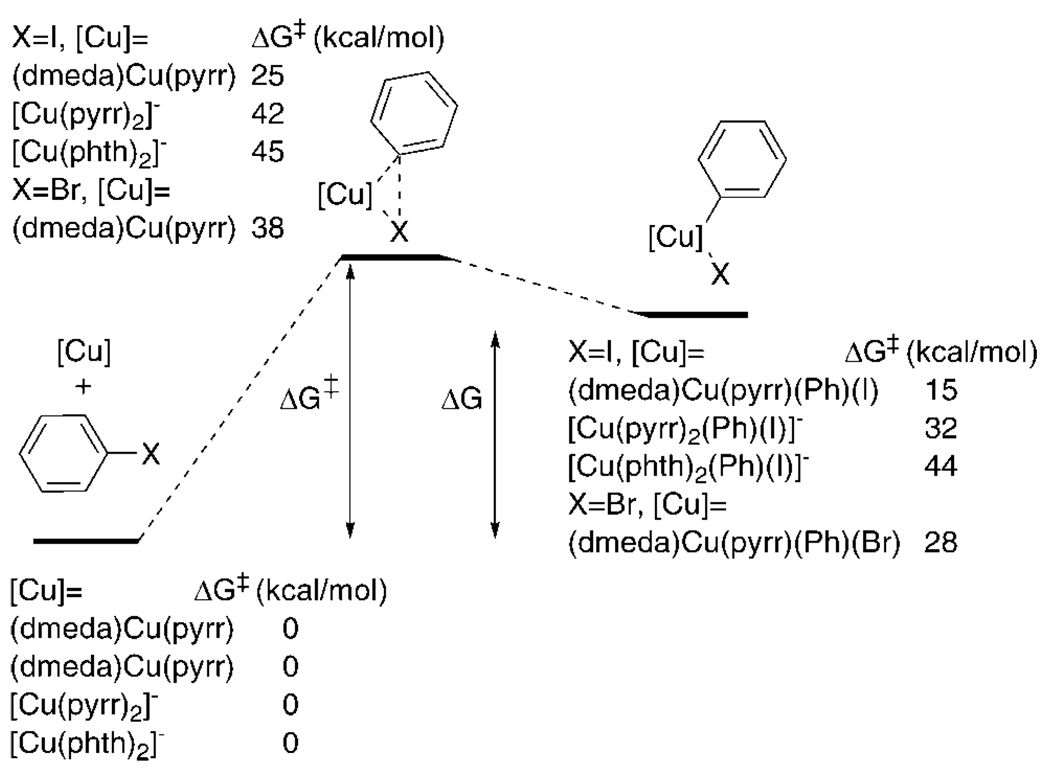
The free energies of activation for the oxidative addition of bromobenzene and iodobenzene to a series of neutral, three-coordinate and monoanionic, two-coordinate copper complexes by two-electron processes have been computed, and these results are summarized in Table 2. We have employed the B3LYP functional as implemented in Gaussian 0361 and the conductor-like polarizable contiuum model (CPCM) to model the toluene solvent. The free energies of activation are determined from the free energy of the geometry-optimized transition-state structure for C–X cleavage versus the sum of the free energies of the starting copper complex and haloarene, as shown in Figure 2. For computational efficiency, the p-tolyl group of the haloarene was abbreviated to a phenyl group. We computed the barriers for addition of iodobenzene to the neutral, ligated [(dmeda)Cu(pyrr)] for which we have kinetic data, the [Cu(phth)2]− anion that did not react with iodotoluene, and the analogous bis-pyrrolidinonate complex ([Cu(pyrr)2])−. Zhang et al. recently reported calculations of the free energy barriers for the reaction of [(eda)Cu-(NHAc)] (eda = ethylenediamine) and [Cu(NHAc)2]− with bromobenzene by DFT methods.27
Figure 2.
DFT-computed free energies in toluene solvent using the CPCM solvent model. Energies are relative to separate copper complex and halobenzene and are given in kcal/mol.
The computed barriers for reactions of iodobenzene with [Cu(phth)2]− (anionic portion of 3) are much higher than the barriers for reaction with the three-coordinate neutral species (vide infra). The computed free energy barrier for the oxidative addition of PhI to [Cu(phth)2]− to give [Cu(phth)2(Ph)(I)]− in toluene solvent was 45 kcal/mol. Much of the high barrier is due to the unfavorable thermodynamics for binding of the arene to the anionic, two-coordinate species and for addition of the iodoarene to form the anionic four-coordinate intermediate. For example, an η2-arene complex is computed to lie 41 kcal/mol uphill, and the initial, four-coordinate, anionic addition product [Cu(phth)2(Ph)(I)]− is computed to lie 44 kcal/mol uphill in free energy compared to the separate reactants. Zhang et al. computed a free energy barrier of 44 kcal/mol for oxidative addition of PhBr to [Cu(NHAc)2]− to give [Cu(NHAc)2-(Ph)(Br)]− in toluene solvent.27 In this case, the oxidative addition product [Cu(NHAc)2(Ph)(Br)]− was calculated to be 37 kcal/mol less stable than the separate reactants.
Calculations of the energies for oxidative addition of bromobenzene and iodobenzene to the neutral forms of the isolated copper complexes provide insight into the potential intermediacy of arylcopper(III) intermediates. The enthalpy for oxidative addition of iodobenzene to [(dmeda)Cu(pyrr)], a reaction we studied experimentally, was calculated to be only 2 kcal/mol, and the free energy for oxidative addition was calculated to be 15 kcal/mol. The analogous values for oxidative addition of bromobenzene were calculated to be 10 and 28 kcal/mol. These data imply that the Cu(III) intermediates lie uphill of the Cu(I) complex and free aryl halide, but that the Cu(III) species lie at energies that are accessible under mild conditions.
We also calculated the barriers for these two oxidative addition processes. The enthalpy of activation for oxidative addition of iodobenzene to [(dmeda)Cu(pyrr)] was calculated to be 6 kcal/mol, and the free energy of activation was calculated to be 25 kcal/mol at 25 °C. The activation energies for the analogous reaction of [(dmeda)Cu(pyrr)] with bromobenzene in toluene were calculated to be 9 and 38 kcal/mol at the 120 °C temperature of the experiment. Although these barriers are 7–9 kcal/mol higher than the 18 and 29 and kcal/mol barriers determined from the experimental kinetic data, these values agree reasonably when considering the uncertainties of the continuum solvent models and computed entropies.62
The barriers calculated by Zhang et al.27 for oxidative addition of bromobenzene using PCM to model the toluene solvent fit well with our calculations, although the modeled system is somewhat different from the actual system for which free energy barriers have been measured experimentally. The reaction of (eda)Cu(NHAc) (eda = ethlyenediamine) with PhBr to yield (eda)Cu(NHAc)(Ph)(Br) was calculated to have a free energy barrier of 29 kcal/mol (with free energy difference of 23 kcal/mol between the reactants and five-coordinate neutral product) at 25 °C, and the reaction of (dmeda)Cu(NHAc) with PhBr to yield (dmeda)Cu(NHAc)(Ph)(Br) was calculated to have a free energy barrier of 30 kcal/mol in toluene at 25 °C. This barrier is essentially identical to the free energy of activation we calculate for the reaction of bromobenzene with [(dmeda)Cu-(pyrr)], which is the same compound studied experimentally, at 25 °C using our computed activation parameters.
10. Mechanism of the C–N Bond Formation
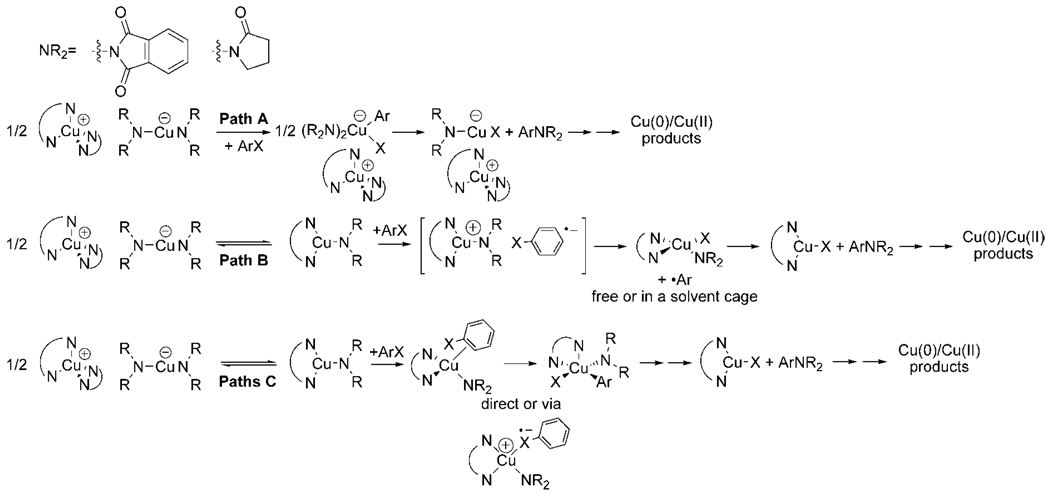
Three classes of potential mechanisms for the reactions of these copper amidate and imidate complexes with iodoarenes are shown in Scheme 7. In pathway A, the ionic complex reacts directly with the haloarene to form a four-coordinate, anionic oxidative addition product. This pathway was proposed for reactions of haloarenes with copper alkoxides,44,48 and it draws analogies to the proposed pathway for oxidative addition of haloarenes to anionic Pd(0).63,64
Scheme 7.
Pathways B and C involve reaction of the haloarene with the three-coordinate, neutral Cu(I) species. Path B involves initial electron transfer, followed by extrusion of halide. In the sequence shown, recombination of the resulting aryl radical with the Cu(II) center occurs to generate a cationic arylcopper(III) imidate or amidate complex that eliminates coupled product. Comproportionation of the copper(II) species [LCuI(NR2)] with copper(0) could then generate an LCuI product and regenerate one of the two equivalents of the LCu(NR2) reactant.65
Pathway C involves coordination of the haloarene to the copper amidate or imidate complex, followed by oxidative addition to form a Cu(III) intermediate. This oxidative addition could occur by several pathways, including direct expulsion of the halide to generate a four-coordinate cationic species, innersphere electron transfer followed by collapse of the resulting intermediate to form a neutral, five-coordinate Cu(III) species without dissociation of an aryl radical, or concerted insertion of copper into the carbon–halogen bond.
The absence of any reactivity of iodotoluene with the isolated anionic copper bis-imidate complex [N(n-Bu)4][Cu(phth)2] (described in section 3.1) and the high computed barrier for addition of iodobenzene to these two-coordinate, anionic copper amidates argue against reaction by pathway A. If the reaction occurred by pathway A, then one would expect [N(n-Bu)4][Cu(phth)2] to react with the haloarene under mild conditions. Instead, this anionic complex was stable toward an excess of p-iodotoluene in DMSO, even after hours at 120 °C (eq 5). Consistent with this observation, the barrier we computed for reaction of this anion with iodobenzene was 39 kcal/mol in the gas phase and 45–47 kcal/mol in toluene or DMSO solvent.
Apparently, the linear, two-coordinate anion alone is not capable of reacting with the haloarene, even though it is unsaturated and electron rich. This observation is counterintuitive, but it is consistent with several pieces of prior data. One set of results showed that the coupling of potassium phthalimide with iodoarenes or bromoarenes, catalyzed by CuBr, occurred faster when a 1:1 ratio of potassium phthalimide to copper was used, although the actual copper species in these systems were not identified.66 More recently, Karlin has shown that three-coordinate Cu(I) complexes undergo air oxidation much faster than linear two-coordinate Cu(I) complexes.67
Computational studies on the reactions of dialkyl cuprates with alkyl halides have suggested that alkali metal cations capable of binding halide lower the reaction barrier for carbon–halogen bond cleavage.68 These studies could imply that the absence of a Lewis acidic cation in [Bu4N][Cu(phth)2] creates the high barrier for reactions of this species with aryl halides. However, such a proposal is not consistent with relative rate data for reactions of haloarenes with the isolated ligated species and with the anion 3 in the presence of added alkali metal cations, and it is not consistent with the high computed energy of binding of a haloarene to the anionic Cu(phth)2−. First, we showed that the reactions of the ligated complexes were faster than those of the Bu4N+ salt of cuprate 3 in the presence of added alkali metal cation, even though the saturated L2Cu+ cation (L = bpy, phen, dmeda) is less likely than an alkali metal cation to bind halide. Second, we showed that added LiBF4 does not affect the reaction of iodotoluene with 1a. Third, the computed energy for binding of the aryl halide to the anionic species that would precede C–X bond cleavage is higher than the overall barrier for the reaction of the ligated species 1a–d and 2a,b measured experimentally. Thus, mechanisms for cleavage of the aryl halide by cooperative reactivity of the anion and cation of the ionic form of the ligated complexes should be considered but are not consistent with our current data.
Several lines of kinetic data argue against reaction by the family of steps initiated by electron transfer and followed by dissociation of halide from the haloarene radical anion (pathway B). First, the absence of biaryl products or substantial amounts of arenes from hydrodehalogenation formed in solvents possessing relatively weak C–H bonds argues against the intermediacy of free or caged phenyl radicals. The strengths of the weakest C–H bonds of the toluene, DMSO, and THF solvents used in these studies are between 88 and 94 kcal/mol,69 and the rate of hydrogen atom abstraction of aryl radicals from DMSO is on the order of 106–107 s−1.70
Second, an aryl chloride and an aryl triflate possessing a more positive reduction potential and a fast rate for chloride dissociation or X–O bond cleavage from the radical anion were shown to react with the copper amidate and imidate complexes much more slowly than bromoarenes possessing more negative reduction potentials and similar rates for halide dissociation.
Third, a substrate containing an ortho allyloxy group did not undergo cyclization, and the lack of cyclized products indicates that any aryl radical must combine with a copper amide with a rate constant of >1012 s−1. In other words, the recombination must occur with a half-life close to the time scale of a vibration, and this fast rate seems unlikely to occur in a system requiring such reorganization. Altogether, these data argue strongly against the intermediacy of free aryl radical intermediates. Thus, we conclude that the carbon–halogen bond is cleaved within the coordination sphere of the metal.
11. Comparison of the Mechanistic Data and Conclusions to Those of Previous Studies
The data and conclusions from this work can be compared and contrasted to those of several previous studies of the mechanism of copper-mediated coupling processes. Much of this early work led to different mechanistic proposals about the coordination number and charge of the copper species that reacts with the haloarene, the presence or absence of an arylcopper intermediate, the oxidation state of an arylcopper intermediate, the potential intermediacy of aryl radicals during the C–X activation step, and the possibility that electron transfer initiates the C–X bond cleavage.54 The relationship between our data and previous data on these issues is discussed in this section.
The coordination number and charge of the reactive species in ligandless copper-catalyzed reactions have been ambiguous. Several studies have drawn different conclusions about the active species in couplings to form the C–O bond in aryl ethers. Weingarten proposed that the reaction of bromobenzene with sodium phenoxide occurred through the two-coordinate, anionic [Cu(OPh)2]−.48 A few years later, van Koten and co-workers proposed that the reaction of copper(I) alkoxides with PhBr occurred through a similar [Cu(OMe)2]− intermediate.44 However, Whitesides and co-workers generated a series of copper(I) alkoxides that were shown by protonolysis to contain a 1:1 ratio of copper to alkoxide, and these species reacted under mild conditions with haloarenes to yield C–O coupled products.71 Thus, it is unclear from these studies if the active species in these reactions contain a Cu:OR ratio of 1:1 or 1:2.
Fewer data were available on the relative reactivity of haloarenes with anionic versus neutral copper complexes containing anionic nitrogen ligands. Most recently, a mechanism in which the haloarene reacts through a diamine-ligated copper amidate complex containing a Cu:NR2 ratio of 1:1 was deduced from kinetic data,26 but isolated compounds were not studied, and the anionic species was not generated independently. Moreover, Yamamoto’s isolated neutral, PPh3-ligated copper amidate and imidate complexes reacted only at high temperatures.21 During studies of related ligandless copper-catalyzed imidation of bromoarenes, Bacon and Karim showed that a 1:1 ratio of copper iodide to phthalimide led to the fastest formation of N-arylphthalimide product, but no copper complexes in this reaction were identified.66 We have now shown clearly by experiments with fully characterized compounds that two-coordinate imidates alone react with haloarenes more slowly than neutral, three-coordinate imidates.
Some of the studies that take into consideration the presence or absence of radical intermediates are summarized in a recent review on copper-catalyzed couplings.5 Van Koten and coworkers stated that addition of the radical scavenger 1,3-dinitrobenzene completely inhibited CuBr-catalyzed C–O bond formation between NaOMe and bromobenzene.44 Moreover, Arai et al. observed a stable quinone radical from reaction of 2-aminoethanol with 1-bromoanthraquinone, catalyzed by CuBr, and used these data to support reaction by an electron-transfer pathway.46 However, the reduction potentials of dinitrobenzene and of a haloquinone are much less negative than those of the more electron-rich arenes in our study, and the stability of the aryl radical resulting from reduction of the haloquinone is much greater than that of the more electron-rich haloarenes. Thus, the data of van Koten and Arai are not directly relevant to the mechanism of the reactions reported here. Moreover, Bethell et al. more recently did not observe radical intermediates in the reaction of primary alkyl amines, benzyl amine, and aniline with 1-halogenoanthraquinones, and they observed little hydrodehalogenated product.47
Other proposals involve formation of π-bound aryl halide radical anions. In the mid 1980s, Paine reported mechanistic data on C–N coupling reactions catalyzed by “ligandless” copper(I).20,45 This author suggested that the C–X cleavage of aryl halides by copper(I) catalysts proceeds by binding of Cu to the π system of the haloarene to form an η2-arene complex, followed by electron transfer to the arene and dissociation of iodide. Van Koten and co-workers also proposed that the reaction of potassium methoxide with bromoarenes occurs through a π-bound aryl radical. They concluded that this reaction proceeds by binding of the halide atom of the aryl halide to [Cu(OMe)2]−, followed by transfer of electron density to the haloarene to form an aryl halide radical anion that remains directly bound to the copper center.
Others have proposed the intermediacy of Cu(III) complexes. Cohen and co-workers have many times proposed mechanisms forcopper-catalyzedcouplingthrougharylcopper(III)complexes.49–53 Most recently, alkylcopper(III) species have been observed directly during the reactions of cuprates with alkyl iodides.72
We have provided strong evidence against the intermediacy of free aryl radicals during reaction of haloarenes with the copper amidate and imidate complexes of this study. The absence of significant quantities of biaryl or arene products from reactions of the copper amidate and imidate complexes in solvents that can act as hydrogen atom donors provides some evidence against the intermediacy of aryl radicals. The relative rates for reactions of different haloarenes further argued against mechanisms involving outer-sphere electron transfer to form haloarene radical anions, and the clean formation of aromatic C–N bonds by reactions of o-(allyloxy)iodobenzene provided particularly strong evidence against the generation of free aryl radicals.
It is challenging to rigorously distinguish between direct oxidative addition without formation of copper(II) intermediates and stepwise oxidative addition initiated by intimate electron transfer. However, the greater dependence of the reaction rates on the identity of the halogen than on the reduction potential of the haloarene provides some evidence against a mechanism initiated by electron transfer to the type of π-arene complex proposed in previous work.20,45
Summary
In summary, we have isolated copper imidate and amidate complexes that are kinetically and chemically competent to be intermediates in the copper-catalyzed amidation and imidation of aryl halides and that appear to exist in an unexpected ionic form in solvents more polar than hydrocarbons. A set of complexes with varied dative and neutral ligands has allowed us to probe the effect of the reactive and ancillary ligands on the rates of these reactions. The complexes with the more electron-rich anionic and dative ligands react faster. Reactions conducted in different media show that the rate of formation of the C–N coupled product from the phthalimidate complexes is much faster in more polar solvents, while the rate of formation of the C–N coupled product from the pyrrolidinonate complexes is only slightly faster in more polar solvents. Studies on the effect of the electronic properties of the haloarene, in particular the contrast between the lack of reaction of electron-poor chloroarenes and the high-yield reactions of more electron-rich bromoarenes, argue against pathways that involve electron transfer and halide dissociation from the resulting radical anion, and reactions of aryl iodides containing a radical clock argue against the generation of free aryl radicals. Arylcopper(III) intermediates containing dative nitrogen ligands are calculated to be kinetically accessible under mild conditions, and such species could be formed by a concerted oxidative addition or by internal electron transfer and formation of an arylcopper species within the coordination sphere of the metal. Overall, the isolation and full characterization of the solid-state and solution-phase structures of ligated copper amidates and imidates, in combination with quantitative studies on the reactivity of these species with a variety of haloarenes, has begun to provide kinetic benchmarks and structural data that should help to clarify some of the mechanistic questions on the reactions of aromatic electrophiles with copper compounds containing anionic nitrogen and oxygen ligands.
Supplementary Material
Acknowledgment
We thank the NIH NIGMS (GM-55382) for support of this work.
Footnotes
Supporting Information Available: Experimental procedures, characterization of products, computational details, and full citation for the Gaussian 03 program (ref 61). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Hartwig JF, Shekhar S, Shen Q, Barrios-Landeros F. In: Chemistry of Anilines. Rappoport Z, editor. Vol. 1. New York: Wiley-Interscience; 2007. p. 455. [Google Scholar]
- 2.Hartwig JF. In: Modern Amination Methods. Ricci A, editor. Weinheim, Germany: Wiley-VCH; 2000. [Google Scholar]
- 3.Jiang L, Buchwald SL. In: Metal-Catalyzed Cross-Coupling Reactions. De Meijere A, Diederich F, editors. Vol. 2. Weinheim: Wiley-VCH; 2004. p. 699. [Google Scholar]
- 4.Goldberg I. Chem. Ber. 1906;39:1691. [Google Scholar]
- 5.Ley SV, Thomas AW. Angew. Chem., Int. Ed. 2003;42:5400. doi: 10.1002/anie.200300594. [DOI] [PubMed] [Google Scholar]
- 6.Beletskaya IP, Cheprakov AV. Coord. Chem. Rev. 2004;248:2337. [Google Scholar]
- 7.Gujadhur R, Venkataraman D, Kintigh JT. Tetrahedron Lett. 2001;42:4791. [Google Scholar]
- 8.Gujadhur RK, Bates CG, Venkataraman D. Org. Lett. 2001;3:4315. doi: 10.1021/ol0170105. [DOI] [PubMed] [Google Scholar]
- 9.Goodbrand HB, Hu NX. J. Org. Chem. 1999;64:670. [Google Scholar]
- 10.Kiyomori A, Marcoux J-F, Buchwald SL. Tetrahedron Lett. 1999;40:2657. [Google Scholar]
- 11.Klapars A, Huang X, Buchwald SL. J. Am. Chem. Soc. 2002;124:7421. doi: 10.1021/ja0260465. [DOI] [PubMed] [Google Scholar]
- 12.Antilla JC, Klapars A, Buchwald SL. J. Am. Chem. Soc. 2002;124:11684. doi: 10.1021/ja027433h. [DOI] [PubMed] [Google Scholar]
- 13.Ma D, Zhang Y, Yao J, Wu S, Tao F. J. Am. Chem. Soc. 1998;120:12459. [Google Scholar]
- 14.Cristau H-J, Cellier PP, Spindler J-F, Taillefer M. Chem.-Eur. J. 2004;10:5607. doi: 10.1002/chem.200400582. [DOI] [PubMed] [Google Scholar]
- 15.de Lange B, Lambers-Verstappen MH, Schmieder-van de Vondervoort L, Sereinig N, de Rijk R, de Vries AHM, de Vries JG. Synlett. 2006:3105. [Google Scholar]
- 16.Shafir A, Buchwald SL. J. Am. Chem. Soc. 2006;128:8742. doi: 10.1021/ja063063b. [DOI] [PubMed] [Google Scholar]
- 17.Chen YJ, Chen HH. Org. Lett. 2006;8:5609. doi: 10.1021/ol062339h. [DOI] [PubMed] [Google Scholar]
- 18.Shen R, Porco JA. Org. Lett. 2000;2:1333. doi: 10.1021/ol005800t. [DOI] [PubMed] [Google Scholar]
- 19.Fagan PJ, Hauptman E, Shapiro R, Casalnuovo A. J. Am. Chem. Soc. 2000;122:5043. [Google Scholar]
- 20.Paine AJ. J. Am. Chem. Soc. 1987;109:1496. [Google Scholar]
- 21.Yamamoto T, Ehara Y, Kubota M, Yamamoto A. Bull. Chem. Soc. Jpn. 1980;53:1299. [Google Scholar]
- 22.Gambarotta S, Bracci M, Floriani C, Chiesivilla A, Guastini C. Dalton Trans. 1987:1883. [Google Scholar]
- 23.Hope H, Power PP. Inorg. Chem. 1984;23:936. [Google Scholar]
- 24.Tsuda T, Watanabe K, Miyata K, Yamamoto H, Saegusa T. Inorg. Chem. 1981;20:2728. [Google Scholar]
- 25.Tsuda T, Yazawa T, Watanabe K, Fujii T, Saegusa T. J. Org. Chem. 1981;46:192. [Google Scholar]
- 26.Strieter ER, Blackmond DG, Buchwald SL. J. Am. Chem. Soc. 2005;127:4120. doi: 10.1021/ja050120c. [DOI] [PubMed] [Google Scholar]
- 27.Zhang SL, Liu L, Fu Y, Guo QX. Organometallics. 2007;26:4546. [Google Scholar]
- 28.Lemmen TH, Goeden GV, Huffman JC, Geerts RL, Caulton KG. Inorg. Chem. 1990;29:3680. [Google Scholar]
- 29.Tsuda T, Hashimoto T, Saegusa T. J. Am. Chem. Soc. 1972;94:658. [Google Scholar]
- 30.For a related synthesis, see ref 26.
- 31.Ouali A, Taillefer M, Spindler JF, Jutand A. Organometallics. 2007;26:65. [Google Scholar]
- 32.Healy PC, Pakawatchai C, White AH. Dalton Trans. 1985:2531. [Google Scholar]
- 33.Klemens FK, Palmer CEA, Rolland SM, Fanwick PE, McMillin DR, Sauvage JP. New J. Chem. 1990;14:129. [Google Scholar]
- 34.Pallenberg AJ, Koenig KS, Barnhart DM. Inorg. Chem. 1995;34:2833. [Google Scholar]
- 35.tom Dieck H, Stamp L. Z. Naturforsch., B: Chem. Sci. 1990;45:1369. [Google Scholar]
- 36.Geary WJ. Coord. Chem. Rev. 1971;7:81. [Google Scholar]
- 37.The 1H NMR spectrum of the phen- and pyrr-ligated 2a alone in DMF-d7 at−25 °C contained resonances at 3.19 and 1.84 ppm in a 2:4 ratio for the six protons of the pyrr ligand.
- 38.Nakamura E, Mori S. Angew. Chem., Int. Ed. 2000;39:3751. doi: 10.1002/1521-3773(20001103)39:21<3750::aid-anie3750>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 39.Reactions with other reagents that would be present in the catalytic reaction were also investigated. The addition of 9 equiv of pyrrolidinone or 19 equiv of K3PO4 formed the two products in ratios of 72:28 and 77:23, respectively.
- 40.Enemaerke RJ, Christensen TB, Jensen H, Daasbjerg K. J. Chem. Soc., Perkin Trans. 2001;2:1620. [Google Scholar]
- 41.Andrieux CP, Saveant JM, Zann D. New J. Chem. 1984;8:107. [Google Scholar]
- 42.Jutand A, Negri S. Eur. J. Org. Chem. 1998:1811. [Google Scholar]
- 43.Takeda N, Poliakov PV, Cook AR, Miller JR. J. Am. Chem. Soc. 2004;126:4301. doi: 10.1021/ja0389671. [DOI] [PubMed] [Google Scholar]
- 44.Aalten HL, van Koten G, Grove DM, Kuilman T, Piekstra OG, Hulshof LA, Sheldon RA. Tetrahedron. 1989;45:5565. [Google Scholar]
- 45.Couture C, Paine AJ. Can. J. Chem. 1985;63:111. [Google Scholar]
- 46.Arai S, Hida M, Yamagishi T. Bull. Chem. Soc. Jpn. 1978;51:277. [Google Scholar]
- 47.Bethell D, Jenkins IL, Quan PM. J. Chem. Soc., Perkin Trans. 1985;2:1789. [Google Scholar]
- 48.Weingarten H. J. Org. Chem. 1964;29:3624. [Google Scholar]
- 49.Cohen T, Cristea I. J. Org. Chem. 1975;40:3649. [Google Scholar]
- 50.Cohen T, Cristea I. J. Am. Chem. Soc. 1976;98:748. [Google Scholar]
- 51.Cohen T, Tirpak JG. Tetrahedron Lett. 1975:143. [Google Scholar]
- 52.Cohen T, Wood J, Dietz AG., Jr Tetrahedron Lett. 1974:3555. [Google Scholar]
- 53.Lewin AH, Zovko MJ, Rosewater WH, Cohen T. Chem. Commun. 1967:80. [Google Scholar]
- 54.Lindley J. Tetrahedron. 1984;40:1433. [Google Scholar]
- 55.Douglas BE, McDaniel DH, Alexander JJ. Concepts and Models in Inorganic Chemistry. 3rd ed. New York: Wiley; 1993. [Google Scholar]
- 56.Marcus RA, Eyring H. Annu. Rev. Phys. Chem. 1964;15:155. [Google Scholar]
- 57.More recent measurements of the bromide dissociation from bro-monaphthalene radical anions by pulse radiolysis showed the absolute rate constants to be larger than those reported in previous studies (see ref 43). However, the value for dissociation of chloride was not remeasured, and it is likely to also increase in value because the assessment of smaller values resulted from technique, not the chemistry of these haloarene radical anions.
- 58.It was difficult to monitor the conversion of the copper complex due to the accumulation of paramagnetic materials during the reaction. However, this reaction formed 38% of the N-aryl product after 7 h. Thus, the yield after 2 h reflects nearly full conversion of the copper.
- 59.Abeywickrema AN, Beckwith ALJ. Chem. Commun. 1986:464. [Google Scholar]
- 60.Annunziata A, Galli C, Marinelli M, Pau T. Eur. J. Org. Chem. 2001:1323. [Google Scholar]
- 61.All calculations were carried out with the Gaussian 03 program: Frisch MJ, et al. Gaussian 03. Wallingford, CT: Gaussian Inc; 2004. revision C.02. See Supporting Information for the full reference.
- 62.Alternatively, a lower energy pathway to the Cu(III) species could involve an intimate electron-transfer process prior to formation of the Cu(III) species without generation of a free aryl halide radical anion or aryl radical. Calculations of mechanisms involving diradical intermediates are challenging, and we have not been able to optimize either singlet or triplet structures in which an unpaired electron is located on the iodoarene.
- 63.Goossen LJ, Koley D, Hermann HL, Thiel W. Organometallics. 2005;24:2398. [Google Scholar]
- 64.Senn HM, Ziegler T. Organometallics. 2004;23:2980. [Google Scholar]
- 65.As noted by one reviewer, there are many possible pathways related to this one, such as the reaction of a second copper(I) amidate complex with the aryl radical to generate a copper(II) complex of the form (L)CuII(NR2)(Ar) that reductively eliminates ArNR2 to generate copper(0).
- 66.Bacon RGR, Karim A. J. Chem. Soc., Perkin Trans. 1973;1:272. [Google Scholar]
- 67.Himes RA, Park GY, Barry AN, Blackburn NJ, Karlin KD. J. Am. Chem. Soc. 2007;129:5352. doi: 10.1021/ja0708013. [DOI] [PubMed] [Google Scholar]
- 68.(a) Mori S, Nakamura E, Morokuma K. J. Am. Chem. Soc. 2000;122:7294. [Google Scholar]; (b) Nakamura E, Mori S, Morokuma K. J. Am. Chem. Soc. 1998;120:8273. [Google Scholar]
- 69.Luo YR. Handbook of Dissociation Energies in Organic Compounds. Boca Raton, FL: CRC Press; 2003. [Google Scholar]
- 70.(a) M’Halla F, Pinson J, Saveant JM. J. Am. Chem. Soc. 1980;102:4120. [Google Scholar]; (b) Abeywickrema AN, Beckwith ALJ, Gerba S. J. Org. Chem. 1987;52:4072. [Google Scholar]; (c) Branchi B, Galli C, Gentili P. Eur. J. Org. Chem. 2002:2844. [Google Scholar]
- 71.Whitesides GM, Sadowski JS, Lilburn J. J. Am. Chem. Soc. 1974;96:2829. [Google Scholar]
- 72.Bertz SH, Cope S, Murphy M, Ogle CA, Taylor BJ. J. Am. Chem. Soc. 2007;129:7208. doi: 10.1021/ja067533d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.