Abstract
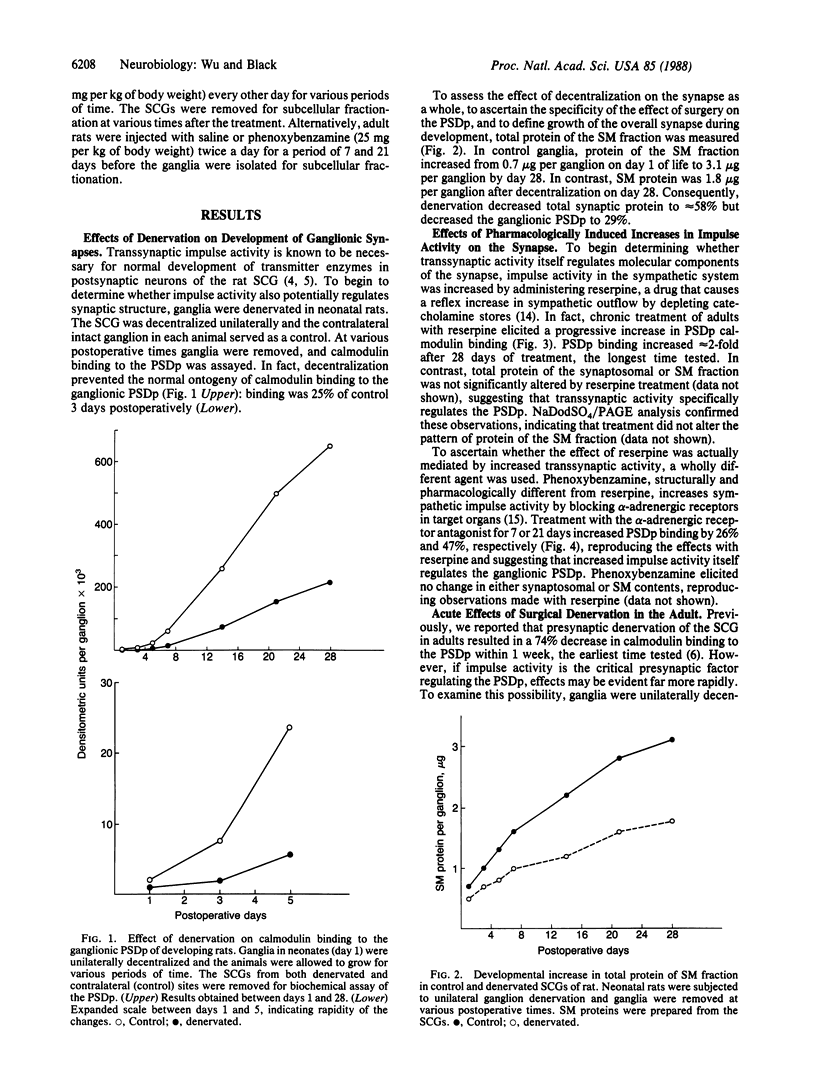
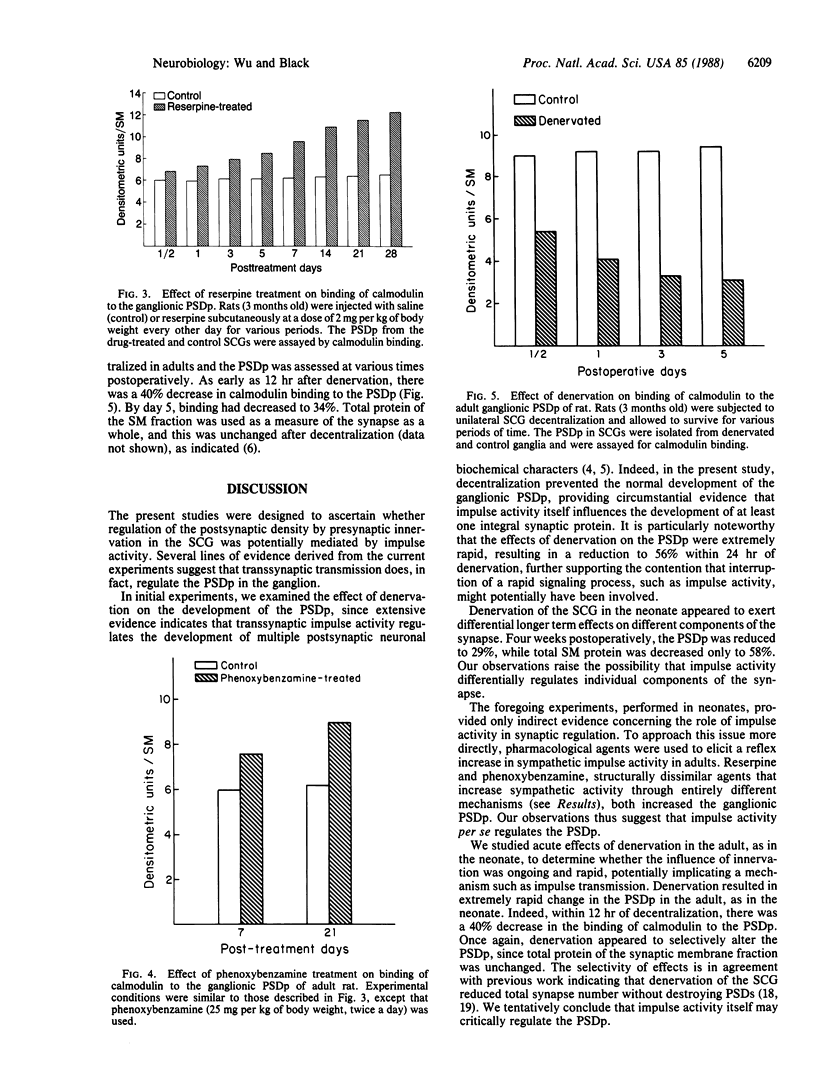
Ganglionic postsynaptic density protein (PSDp) was used to monitor the influence of transsynaptic impulse activity on synaptic structure in the developing and adult rat superior cervical sympathetic ganglion (SCG). Since transsynaptic activity is known to regulate ontogeny of postsynaptic transmitter enzymes, we initially studied the developing ganglion. Denervation in neonates prevented normal development, decreasing calmodulin binding to the ganglionic PSDp by 71% after 4 weeks. During this period, denervation elicited only a 42% decrease in total protein of the synaptic membrane fraction, suggesting that innervation regulates development of various synaptic components differentially. Effects of denervation were extremely rapid, resulting in a 44% decrease in calmodulin binding within 1 day, consistent with regulation by a signaling process such as impulse activity. The effect of impulse activity was examined more directly in adults by treatment with the agents reserpine or phenoxybenzamine, which elicit reflex increases in sympathetic transmission. Administration of reserpine resulted in a progressive 90% increase in calmodulin binding to the PSDp over 4 weeks. Phenoxybenzamine also elicited an increase, mimicking the effects of reserpine. Neither agent altered total protein of the synaptic membrane fraction, suggesting that impulse activity regulates specific synaptic components. Finally, ganglionic denervation in adults decreased PSDp binding within 12 hr, consistent with acute effects of impulse reduction. Our results suggest that transsynaptic impulse activity plays an important role in regulation of specific molecular components of the synapse.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALPER M. H., FLACKE W., KRAYER O. PHARMACOLOGY OF RESERPINE AND ITS IMPLICATIONS FOR ANESTHESIA. Anesthesiology. 1963 Jul-Aug;24:524–542. doi: 10.1097/00000542-196307000-00014. [DOI] [PubMed] [Google Scholar]
- Banker G., Churchill L., Cotman C. W. Proteins of the postsynaptic density. J Cell Biol. 1974 Nov;63(2 Pt 1):456–465. doi: 10.1083/jcb.63.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezamahouta C., Zanetta J. P., Revel M. O., Zwiller J., Meyer A., Malviya A. N., Vincendon G. Nature and immunochemical characteristics of a Ca2+/calmodulin kinase activity endowed in a highly insoluble protein purified from adult rat brain. J Neurochem. 1987 Aug;49(2):584–591. doi: 10.1111/j.1471-4159.1987.tb02903.x. [DOI] [PubMed] [Google Scholar]
- Black I. B., Chikaraishi D. M., Lewis E. J. Trans-synaptic increase in RNA coding for tyrosine hydroxylase in a rat sympathetic ganglion. Brain Res. 1985 Jul 22;339(1):151–153. doi: 10.1016/0006-8993(85)90635-3. [DOI] [PubMed] [Google Scholar]
- Black I. B., Hendry I. A., Iversen L. L. Effects of surgical decentralization and nerve growth factor on the maturation of adrenergic neurons in a mouse sympathetic ganglion. J Neurochem. 1972 May;19(5):1367–1377. doi: 10.1111/j.1471-4159.1972.tb01461.x. [DOI] [PubMed] [Google Scholar]
- Black I. B., Hendry I. A., Iversen L. L. Trans-synaptic regulation of growth and development of adrenergic neurones in a mouse sympathetic ganglion. Brain Res. 1971 Nov;34(2):229–240. doi: 10.1016/0006-8993(71)90278-2. [DOI] [PubMed] [Google Scholar]
- Carlin R. K., Grab D. J., Cohen R. S., Siekevitz P. Isolation and characterization of postsynaptic densities from various brain regions: enrichment of different types of postsynaptic densities. J Cell Biol. 1980 Sep;86(3):831–845. doi: 10.1083/jcb.86.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin R. K., Grab D. J., Siekevitz P. Function of a calmodulin in postsynaptic densities. III. Calmodulin-binding proteins of the postsynaptic density. J Cell Biol. 1981 Jun;89(3):449–455. doi: 10.1083/jcb.89.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R. S., Blomberg F., Berzins K., Siekevitz P. The structure of postsynaptic densities isolated from dog cerebral cortex. I. Overall morphology and protein composition. J Cell Biol. 1977 Jul;74(1):181–203. doi: 10.1083/jcb.74.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman C. W., Banker G., Churchill L., Taylor D. Isolation of postsynaptic densities from rat brain. J Cell Biol. 1974 Nov;63(2 Pt 1):441–455. doi: 10.1083/jcb.63.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinoff P. B., Brimijoin S., Axelrod J. Induction of dopamine- -hydroxylase and tyrosine hydroxylase in rat hearts and sympathetic ganglia. J Pharmacol Exp Ther. 1972 Jul;182(1):116–129. [PubMed] [Google Scholar]
- Ostberg A. J., Raisman G., Field P. M., Iversen L. L., Zigmond R. E. A quantitative comparison of the formation of synapses in the rat superior cervical sympathetic ganglion by its own and by foreign nerve fibres. Brain Res. 1976 May 14;107(3):445–470. doi: 10.1016/0006-8993(76)90137-2. [DOI] [PubMed] [Google Scholar]
- Peters A., Kaiserman-Abramof I. R. The small pyramidal neuron of the rat cerebral cortex. The synapses upon dendritic spines. Z Zellforsch Mikrosk Anat. 1969 Sep 22;100(4):487–506. doi: 10.1007/BF00344370. [DOI] [PubMed] [Google Scholar]
- Raisman G., Field P. M., Ostberg A. J., Iversen L. L., Zigmond R. E. A quantitative ultrastructural and biochemical analysis of the process of reinnervation of the superior cervical ganglion in the adult rat. Brain Res. 1974 May 10;71(1):1–16. doi: 10.1016/0006-8993(74)90187-5. [DOI] [PubMed] [Google Scholar]
- Siekevitz P. The postsynaptic density: a possible role in long-lasting effects in the central nervous system. Proc Natl Acad Sci U S A. 1985 May;82(10):3494–3498. doi: 10.1073/pnas.82.10.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K., Black I. B. Regulation of molecular components of the synapse in the developing and adult rat superior cervical ganglion. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8687–8691. doi: 10.1073/pnas.84.23.8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K., Sachs L., Carlin R. K., Siekevitz P. Characteristics of a Ca2+/calmodulin-dependent binding of the Ca2+ channel antagonist, nitrendipine, to a postsynaptic density fraction isolated from canine cerebral cortex. Brain Res. 1986 Nov;387(2):167–184. doi: 10.1016/0169-328x(86)90008-2. [DOI] [PubMed] [Google Scholar]



