Abstract
At one time the synthetic chemist’s last resort, reactions catalysed by transition metals are now the preferred method for synthesizing many types of organic molecule. A recent success in this type of catalysis is the discovery of reactions that form bonds between carbon and heteroatoms (such as nitrogen, oxygen, sulphur, silicon and boron) via complexes of transition metals with amides, alkoxides, thiolates, silyl groups or boryl groups. The development of these catalytic processes has been supported by the discovery of new elementary reactions that occur at metal–heteroatom bonds and by the identification of factors that control these reactions. Together, these findings have led to new synthetic processes that are in daily use and have formed a foundation for the development of processes that are likely to be central to synthetic chemistry in the future.
Organometallic complexes contain organic groups attached to a central metal through metal–carbon (M–C) bonds. These complexes undergo a set of elementary reactions, many of which release products containing new C–C or C–H bonds. The linking of these reactions to generate catalytic processes that form C–C bonds has been a recent focus of synthetic chemistry research. Such organometallic catalysts are now used to form C–C bonds in commodity chemicals (cheap chemicals sold in bulk) and polymers that are produced on the scale of millions of tonnes per year. They are also used in tailor-made polymers that are produced in small quantities and in highly intricate pharmaceuticals and biologically active natural products (see page 323), which are typically produced in milligram quantities for biological testing.
The backbone of many organic compounds is composed of C–C bonds, but the function of these molecules is often derived from the presence of heteroatoms, such as nitrogen, oxygen and sulphur, which are held in these molecules by C–heteroatom bonds. For example, pharmaceuticals and conductive polymers (plastics that conduct electricity) often contain amine C–N bonds, and almost all natural products contain ether, ketone or ester C–O bonds. Heterocyclic compounds in which C–N, C–O or C–S bonds are present in the ring structure are found in all applications of chemistry. Moreover, useful intermediates in synthesis often contain C–B or C–Si bonds that are later converted into C–C, C–O or C–N bonds in the final products.
Amines, alkoxides, thiolates and related reagents are common nucleophiles in reactions that occur without a catalyst. However, these reagents do not react with the weak electrophiles that are useful for synthesis, such as aromatic halides, and they do not react with common electron-rich reagents, such as alkenes. Thus, the ability to carry out catalytic reactions that form these C–heteroatom bonds would substantially affect the synthesis of molecules with important functions. Such catalytic reactions would involve reactions at M–heteroatom bonds that resemble classic organometallic reactions at M–C bonds. Compounds with M–heteroatom bonds that undergo these types of reaction are referred to here as hetero-organometallic complexes. To tap the potential of these compounds as intermediates in emerging catalytic processes, the principles that control their reactivity need to be understood, and these are now beginning to be delineated.
In this review, I describe carbon–heteroatom bond formation catalysed by organometallic complexes and the underlying hetero-organometallic chemistry. I focus on several examples: cross-coupling to form C–N, C–O and C–S bonds1–3; C–H bond functionalization to form C–O (ref. 4), C–X (where X is a halide) (refs 5, 6) and C–B (ref. 7) bonds; and olefin oxidations8 and aminations9,10 to form C–O and C–N bonds (where olefins are compounds that contain at least one C–C double bond, and alkenes are aliphatic olefins with the general formula CnH2n). In addition, the principles of metal–ligand bonding are used as a framework to explain the differences in reactivity between organometallic intermediates and hetero-organometallic intermediates.
Classic processes catalysed by organometallic systems
Several classic catalytic reactions of organometallic systems have been used for decades. Three of these processes — cross-coupling, hydrogenations of olefins and metathesis of olefins (Fig. 1a–c) — are commonly used by synthetic chemists. A fourth process — dehydrogenation of alkanes (Fig. 1d) — has been highly sought and is being developed.
Figure 1. Catalytic C–C and C–H bond-forming processes.
Cross-coupling (a), hydrogenation of olefins (b) and metathesis of olefins (c) are commonly used reactions in organic synthesis. Alkane dehydrogenation (d) has been studied as a means to modify typically unreactive alkanes and has been combined with alkene metathesis to develop a new alkane metathesis. Ln, ancillary ligand; M, metal.
Palladium-catalysed cross-coupling to form C–C bonds is one of the most frequently used catalytic processes for synthesizing medicinally active compounds. Examples of products prepared by this method are the antihypertensive drugs known as sartans11, the anti-asthma drug Singulair (montelukast sodium)12, products such as sunscreen12 and the polymers used for light-emitting diodes and for sensing the explosive trinitrotoluene (TNT)13–16. The intermediates that form the new C–C bond in the final product are classic organometallic species.
Hydrogenations of olefins are carried out on a large scale for the synthesis of commodity chemicals and on a small scale for the synthesis of fine chemicals and advanced pharmaceutical intermediates. Materials ranging from margarine (partially hydrogenated vegetable oil)17 to the most structurally and stereochemically intricate natural products are prepared by hydrogenation, and in 2001 the Nobel Prize in Chemistry was awarded in part for work on enantioselective hydrogenation18.
Another classic catalytic reaction of olefins — the cleavage and re-formation of C=C bonds, known as olefin metathesis — occurs through complexes that contain M=C bonds, and the chemists behind this work were awarded the Nobel Prize in Chemistry in 2005 (see ref. 19 for a recent review).
The conversion of typically unreactive C–H bonds to C=C and C–X bonds (where X is O, N, B or Si)20,21 holds promise for reducing the reliance on existing functional groups. The cleavage of two C–H bonds in an alkane to form the C=C π-bonds in alkenes (Fig. 1d) has been studied intensively. Such dehydrogenation has recently been used, in tandem with olefin metathesis, to develop a catalytic process termed alkane metathesis22, which cleaves and reforms the C–C bonds in alkanes.
Classic elementary reactions of organometallic complexes
Catalytic processes consist of a series of elementary, stoichiometric reactions. Advances in the synthesis of transition-metal complexes, the discovery of new elementary, stoichiometric reactions of these complexes, and the development of methods to link these steps together into catalytic cycles have led to the development of many of the catalytic organometallic processes used today. Typical elementary reactions of organometallic complexes in such catalytic cycles include oxidative addition, reductive elimination, migratory insertion, β-hydrogen elimination and [2 + 2] cycloadditions23,24 (Fig. 2). Oxidative addition adds an organic reagent to a transition metal through insertion of the metal into one of the bonds of the incoming reagent. Reductive elimination extrudes an organic product by coupling two ligands on the metal. Migratory insertion leads to the incorporation of a bound, neutral (dative) ligand into a metal–ligand covalent bond, and β-elimination extrudes such a ligand.
Figure 2. Mechanisms of three common catalytic organometallic processes.
a, Cross-coupling is initiated by oxidative addition of an organic halide, continues through transmetallation and terminates by reductive elimination. b, Hydrogenation begins with oxidative addition of the H–H bond in dihydrogen, migratory insertion of an olefin to form a C–H bond and reductive elimination to form a second C–H bond. c, The metathesis of olefins occurs through a series of [2 + 2] cycloadditions and retro [2 + 2] cycloadditions. Ar, aryl.
These elementary reactions (and several others) have been used to create hundreds of catalytic processes. Cross-coupling (Fig. 2a) occurs by a sequence of steps initiated by the oxidative addition of an organic halide and finished by reductive elimination. Hydrogenation (Fig. 2b) often occurs by a combination of oxidative addition of the H–H bond in dihydrogen, olefin insertion to form the first C–H bond and reductive elimination to form the second C–H bond. The closely related and industrially important addition of silanes to alkenes occurs by a similar sequence that is initiated by oxidative addition of an H–Si bond25, and many reactions that lead to functionalization of C–H bonds begin with oxidative addition of a C–H bond. The metathesis of olefins occurs by a series of [2 + 2] and retro [2 + 2] cycloadditions (Fig. 2c).
Elementary reactions of hetero-organometallic complexes
Although the classic organometallic reactions, which form C–H bonds and C–C bonds, have been known for decades, few examples of reactions that form or cleave M–heteroatom bonds (shown generically in Fig. 3) have been recorded until fairly recently. The absence of this reaction chemistry hampered efforts to develop catalytic cross-couplings, additions to olefins, and C–H bond functionalizations that form C–N, C–O or C–S bonds. Until recently, there were no isolated transition-metal complexes that underwent reductive elimination to form C–N, C–O and C–S bonds in amines, ethers and sulphides26,27, and few compounds reacted with the N–H bond in ammonia to form a monomeric product28,29. Isolated complexes that inserted simple alkenes into the M–N or M–O bonds of M–amido or M–alkoxo complexes were also unknown30–32. And M–imido or M–oxo complexes that undergo [2 + 2] cycloadditions with alkenes (in which the two atoms of the M=O or M=N π-bond combine to make a saturated four-membered ring) were not known until the past decade33. The limited precedent for these processes raised the question of whether it was possible to develop organometallic processes to form amines, ethers and sulphides, or whether the metal–ligand combinations necessary to trigger this reactivity had not yet been identified. Recent progress clearly indicates that the latter was true.
Figure 3. Recently discovered organometallic reactions of transition-M–heteroatom bonds.

a, Reductive elimination to form C–N, C–O and C–S bonds in amines, ethers and thioethers. b, Oxidative addition of amine N–H bonds. c, Migratory insertions of olefins into metal amides and metal alkoxides. d, [2 + 2] Cycloadditions between olefins and M–imido or M–oxo complexes. These reactions are analogues of classic reactions occurring at M–C bonds and have only recently been discovered.
Much of the literature on M–alkoxo or M–amido complexes can be divided into two sets. In one set, high-valent early-transition M–alkoxo and M–amido complexes were commonly used, but the amides and alkoxides were typically ancillary ligands because the M–O and M–N bonds in these complexes were too strong to display extensive reactivity. In the second set, methods to prepare late-transition-metal–amido and –alkoxo complexes were beginning to be developed, and the properties of these complexes were beginning to be explored34. These complexes, however, were often too unstable towards β-hydrogen elimination to observe reactions that would form C–N or C–O bonds.
In recent years, our understanding of the reactions of M–amido, M–alkoxo and M–thiolato complexes has changed markedly. Many researchers are beginning to develop synthetically valuable processes relying on hetero-organometallic intermediates. One current fundamental issue is how the differences in properties between carbon and a heteroatom change the course of these reactions. For example, does an increase in the electronegativity of the atom bound to the metal increase or decrease the rate of addition, insertion, elimination or cycloaddition? How does the presence of an electron pair on the heteroatom affect the rates of these reactions? Does the presence of an unoccupied valence orbital on the heteroatom affect the rates of these reactions? The examples described here show that the set of guidelines needed to explain the patterns of reactivity is more complex than matching or mismatching hard or soft ligands with hard or soft metals35. A few such guidelines are presented in the context of the emerging catalytic processes that form C–heteroatom bonds through the reactions of hetero-organo metallic species: namely cross-coupling to form C–N, C–O and C–S bonds; C–H bond functionalization to form C–O, C–X (where X is a halide) and C–B bonds; and olefin oxidations and aminations to form C–O and C–N bonds.
Catalysis by carbon–heteroatom reductive elimination
Cross-coupling reactions to form the C–N, C–O and C–S bonds in amines, ethers and sulphides (Fig. 4a) are widely used for the synthesis of pharmaceutical candidates, fine chemicals, polymers, components of organic devices and even ligands for other catalysts1,36. The C–N coupling process evolved from an initially37 promising, but relatively impractical, coupling of tin amides with aryl halides in the presence of a palladium catalyst containing a sterically hindered, monodentate (bonded through a single atom) aromatic phosphine38 into a general process that has been made practical by several successive generations of catalysts.
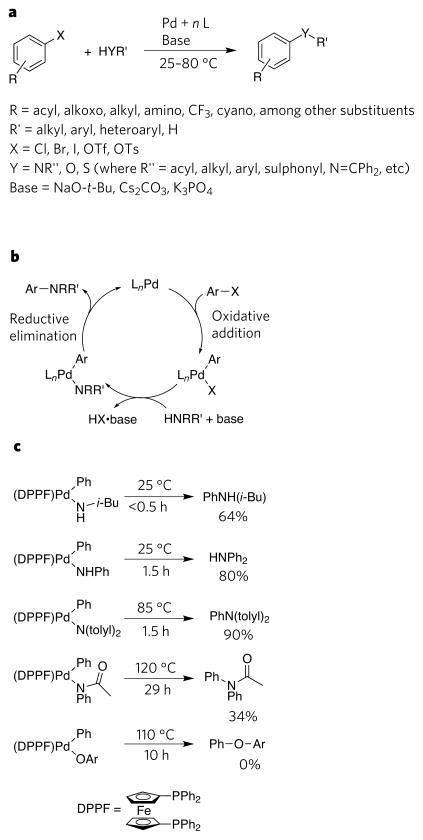
Figure 4. Aspects of palladium-catalysed amination of aryl halides and related reactions.
a, The overall transformation in the presence of a palladium (Pd) catalyst. Four generations of ligands (L) have been developed for the catalytic process. The first-generation catalyst contained P(o-tolyl)3. The second-generation catalysts contained chelating aromatic phosphines as ligand: DPPF (1,1′-bis(diphenylphosphino)ferrocene), BINAP (2,2′-bis(diphenylphosphino)-1,1′-binaphthyl) and xantphos (4,5-bis(diphenyl-phosphino)-9,9-dimethylxanthene). The third-generation catalysts contain hindered alkylphosphines and carbenes: P(t-Bu)3, Ph5FcP(t-Bu)2, N-heterocyclic carbenes, (biaryl)PR2, (heterobiaryl)PR2 and caged P(NRR′)3. The fourth (most recent) generation of catalysts contain hindered ferrocenyl alkyl bisphosphines. b, The catalytic cycle involving oxidative addition of an aryl halide, formation of an arylpalladium amide and reductive elimination of an amine. c, Reactions revealing the electronic effects on reductive elimination to form C–N bonds in amines and amides, and C–O bonds in ethers. The rates of reductive elimination from Pd(II) are faster when the heteroatom bound to the metal is more electron rich. Fc, ferrocenyl; i-Bu, isobutyl; Me, methyl; OTf, trifluoromethanesulphonate; OTs, 4-toluenesulphonate; Ph, phenyl; t-Bu, tert-butyl; tolyl, C6H4-4-Me.
The initial process involving tin amides was replaced by reactions involving amines and a base39,40. The scope of the new process became broader with the use of bidentate aromatic phosphines that inhibited competing β-hydrogen elimination41,42, and later with the use of sterically hindered alkyl monophosphines1,43–48 that allowed the activation of less reactive haloarenes and the coupling of weaker oxygen nucleophiles. Recent ‘fourth-generation’ catalysts containing sterically hindered alkyl bisphosphines further improved catalyst efficiency49,50. Overall, the development of these processes began to demonstrate the capability of late-transition-metal–amido, –alkoxo and –thiolato complexes to participate productively in catalytic cycles.
The basic steps of the mechanism of the amination process are shown in Fig. 4b. Analogous steps lead to catalytic cycles to form ethers and thioethers. Like cross-coupling to form C–C bonds, this process is initiated by oxidative addition of a haloarene. An arylpalladium–amido, –alkoxo or –thiolate complex is then formed from the oxidative addition product by reaction of an amine, alcohol, or thiol and base. The catalytic cycle is then completed by reductive elimination to form the C–N, C–O or C–S bond in the product amine, ether or thioether.
The scope of the final reductive elimination reaction in this catalytic cycle was striking. Although reductive eliminations to form the C–N, C–O or C–S bond in an amine, ether or thioether were unknown when the coupling reactions were first being developed, the broad scope of catalytic couplings of aryl halides with amines, alcohols and thiols now implies that these types of reductive elimination can occur from aryl-palladium(II) complexes containing a diverse set of aryl and heteroaryl groups, as well as a diverse set of amido, alkoxo and thiolato groups. In fact, with the right ancillary ligands on the metal, these reactions are typically faster than competing processes, such as β-hydrogen eliminations, that lead to undesired side products.
Electronic effects on carbon–heteroatom reductive elimination
Although amido and alkoxo ligands have now been shown to participate in the type of reductive elimination reactions that alkyl ligands undergo, the electronic properties of amido and alkoxo ligands cause the rates and scope of these reactions to differ in synthetically important ways27.
The reactions51,52 in Fig. 4c show that the rate of reductive elimination from a series of compounds containing the same ancillary ligand is faster when the covalent heteroatom ligand has stronger electron-donating properties. Comparisons of the rates of reactions of arylamido complexes and phenoxo complexes53, and the rates of reaction of alkylamido versus alkoxo complexes54, also show that the rate of reductive elimination from the amido complexes is faster than from complexes containing less electron-donating phenoxo and alkoxo ligands. But a comparison of the rates of these reactions to those of thiolate complexes shows that basicity alone does not control the rate. Complexes containing the less basic, but more polarizable and nucleophilic, thiolato ligand undergo reductive elimination much faster than do alkoxo complexes and at rates that are similar to, or faster than, those of the amido complexes55.
These relative rates clearly argue against a trend in relative reactivity based on hard–soft matches and mismatches. The compounds containing the largest hard–soft mismatch are the least reactive. (An amidate ligand is harder than an amide ligand, and a phenoxide is harder than an arylamide.) Two possible alternative explanations could be based on the participation of the electron pair on the heteroatom. In one case, the compounds containing the most basic electron pairs would be the most reactive because these electron pairs weaken the metal–ligand bond through filled–filled dπ-pπ-orbital interactions56; in a second case, the reaction would occur by attack of the electron pair on the palladium-bound aryl group, and the most nucleophilic electron pair would lead to the fastest rate. If either of these theories is correct, then substantially smaller electronic effects would be observed for analogous reactions of alkyl complexes because the alkyl complexes lack the basic electron pair on the atom bound to the metal.
Studies on reductive elimination from related arylpalladium–alkyl complexes in which the alkyl groups have varied electronic properties57 showed that the electronic effects on reductive elimination from the alkyl complexes were similar to those on reductive elimination from the amido complexes. Thus, the relative rates for reductive elimination from amido complexes are unlikely to result from the properties of the lone pair on nitrogen. Instead, the dominant electronic effect on reductive elimination seems to result from differences in the electronegativity of the atom bound to the metal58. Apparently, the more covalent and less ionic the M–heteroatom bond, and the more polarizable the heteroatom, the faster the rate of concerted reductive elimination from the intermediates in the catalytic coupling to form C–heteroatom bonds59,60.
Recent studies on catalytic coupling and stoichiometric reductive elimination from platinum(IV) have shown that the metal and the reaction mechanism can change the electronic effects on reductive elimination. Reductive eliminations of alkyl aryl ethers, alkyl acetates and N-alkyl sulphonamides from platinum(IV) that are part of the mechanism of alkane functionalizations (discussed in the next section) occur by a non-concerted mechanism, and these reactions occur faster from complexes containing ligands that can better support anionic charge at the heteroatom than from those whose ligands are less able to support such charge59,60. Reductive elimination from arylpalladium(IV) complexes has also been observed, but it is too soon to draw conclusions about the electronic effects on these reductive eliminations61. In addition, catalytic couplings that form C–N and C–O bonds in the presence of iron62,63 and copper64 catalysts are being developed, and future studies on the intermediates in these processes should show whether the electronic effects on reductive elimination from these transition metals are more like those on reductive elimination from palladium(II) or platinum(IV).
Catalytic C–H bond functionalization
In addition to cross-couplings that occur by reductive elimination to form C–heteroatom bonds, catalytic C–H bond functionalizations that occur through this elementary reaction have been developed. Two classes of such reactions are shown in Figs 5 and 6. In one case (Fig. 5), the hydrogen in a C–H bond of an arene or alkyl chain is converted to a halide or acetate with regioselectivity controlled by a ligating functionality. This chemistry draws parallels with previous functionalization of alkyl C–H bonds catalysed by platinum complexes, originally discovered by Alexander Shilov and studied intensely in recent years with the goal of developing selective oxidation of methane65–68. In a second case (Fig. 6), a terminal C–H bond in an alkane is functionalized by the use of a rhodium catalyst and boron reagents.
Figure 5. Organometallic oxidative C–O and C–halogen bond-forming functionalization of C–H bonds.
a, Two representative directed functionalizations. In the upper example, the metal catalyst binds to the nitrogen of the oxazoline substituent, and this binding positions the catalyst for cleavage and functionalization of the ortho C–H bond on the phenyl ring. The combination of the PhI(OAc)2 oxidant and iodine is thought to lead to an intermediate arylpalladium(IV)–iodide complex, which forms the aryl C–I bond in the product. In the lower example, a related process occurs. In this case, binding of the catalyst to the oxime nitrogen positions it for cleavage of the C–H bond shown. The PhI(OAc)2 oxidant is then thought to lead to an alkylpalladium(IV) acetate intermediate, which forms the C–O bond in the product. b, General catalytic cycle for palladium-catalysed oxidation of C–H bonds involving cleavage of a C–H bond, oxidation to a high-valent metal centre and reductive elimination to form a C–heteroatom bond. c, Example of the C–X bond-forming step in a well-characterized system. This reaction is thought to model the C–heteroatom bond-forming step of directed C–H-bond functionalizations. Reductive elimination to form the C–O bond on the organic product is followed by coordination of deuterated pyridine solvent. D, deuterium. equiv., equivalents; OAc, acetate.
Figure 6. Summary of the functionalization of alkanes with M–B intermediates.
a, The overall transformation. In this process, the rhodium catalyst selectively cleaves the terminal C–H bond of an alkane or of the alkyl group of an acetal, ether, amine or alkyl fluoride, and it delivers a boryl group from the diboron (pinB–Bpin) reagent to the resultant alkyl intermediate to form the functionalized product. b, The catalytic cycle for alkane borylation involves C–H bond cleavage by an M–B intermediate followed by reductive elimination to form a B–C bond. c, The energetics of the reaction coordinate for C–H bond functionalization by M–B complexes deduced by using a density-functional-theory method. This reaction coordinate shows that the p orbital on boron assists in the C–H bond-cleavage step. Cp*, pentamethylcyclo pentadienyl; pin, pinacolate.
These processes require transition-metal complexes that can both cleave a C–H bond and form a C–heteroatom bond. Some catalysts, such as iron–oxo complexes, do so without formation of an intermediate containing an M–C bond69. These complexes often abstract C–H bonds and deliver the resultant hydroxo group to an alkyl radical. These ‘outer-sphere’ reactions typically favour cleavage of the weaker of the available C–H bonds. In other cases, a transition-metal complex cleaves a C–H bond to form an organometallic intermediate containing a metal–carbon bond. These ‘inner-sphere’ processes preferentially lead to cleavage of primary alkyl C–H bonds over weaker secondary alkyl C–H bonds to form n-alkyl intermediates70–72, and preferentially add even stronger aromatic C–H bonds over alkyl C–H bonds to form M–aryl intermediates71. Thus, a method to couple the resultant organometallic ligand to an alkoxide, amide or halide ligand would create methods for inner-sphere, organometallic C–H bond ‘functionalization’ that complement the regioselectivity of methods based on outer-sphere C–H activation.
The reactions shown in Figs 5 and 6 demonstrate two approaches to C–H bond functionalization by C–heteroatom bond-forming reductive elimination. In the first approach, the barrier to reductive elimination was reduced by generating high-valent intermediates. Although reductive eliminations to form C–O and C–halogen bonds from palladium(II) intermediates are slow and require particular ligands54, reductive eliminations to form C–O and C–halogen bonds from palladium(IV) could be more rapid and more general. Thus, oxidation of an alkylpalladium(II) product of C–H activation could generate a high-valent intermediate that would result in functionalization of the alkyl intermediate by reductive elimination to form C–O or C–halogen bonds61,73. This approach, in combination with the incorporation of a functional group in the substrate that can ligate the metal, has been used to develop directed functionalization of both aromatic74 and aliphatic C–H bonds4,75 (Fig. 5a).
The mechanism of the palladium-catalysed oxidations (Fig. 5b) is initiated by cleavage of the C–H bond by palladium(II) complexes. This cleavage has been shown by several studies to occur by coordination of the C–H bond to the metal, followed by deprotonation76. Oxidation of the palladium(II) intermediate then forms a palladium(IV) intermediate that undergoes reductive elimination to form the C–heteroatom bond. Reductive elimination to form a C–O bond from an isolated palladium(IV) complex has now been observed directly61 (Fig. 5c).
In the second approach, the functionalization of terminal alkyl C–H bonds and sterically accessible aryl C–H bonds77 was developed by exploiting the electronic properties of intermediates containing M–B bonds7 (Fig. 6a). The catalytic cycle for the alkane functionalization (Fig. 6b) involves the reaction of rhodium–boryl intermediates with primary alkyl C–H bonds to form organometallic intermediates that undergo B–C bond-forming reductive elimination. Currently available mechanistic data imply that the C–H bond cleavage and the B–C bond formation occur with low barriers, owing to participation of the unoccupied orbital on boron.
A detailed mechanism of the C–H bond cleavage step of this process78, as deduced by calculations using density functional theory (DFT), is shown in Fig. 6c. These calculations imply that the hydrogen atom is passed from the coordinated alkane to the unoccupied p orbital at boron to cleave the C–H bond. These modes of C–H bond cleavage are new and result from the presence of an unoccupied p orbital on boron. The current computational data on this process do not address the issue of regioselectivity, but previous studies on the stoichiometric cleavage of C–H bonds to form M–alkyl products also selectively cleave terminal C–H bonds, in part owing to steric effects72.
This new pathway for C–H bond cleavage would not be productive without accompanying B–C bond formation. Fortunately, this B–C bond formation occurs with a low barrier, presumably because of a favourable match between the electrophilic boryl group and a nucleophilic alkyl group. Thus, the electronic properties of boron are intimately involved in both the activation and functionalization stages of this catalytic, organometallic alkane functionalization.
Catalysis by olefin insertions into M–O and M–N bonds
Addition reactions to olefins constitute some of the most desirable, and when successfully developed, most used catalytic reactions in the chemical industry. Thus, much recent effort has been focused on reactions that form C–heteroatom bonds using olefins as reagents. Several types of catalyst for the oxidations and oxidative aminations of olefins through organometallic intermediates have been shown recently to react through yet another set of elementary reactions of alkoxo and amido complexes, paralleling the reactions of alkyl complexes. These reactions are olefin insertions into M–N and M–O bonds, and catalytic reactions that involve this class of elementary reaction include the following: palladium-catalysed oxidations of olefins to form aldehydes, vinyl ethers and vinyl acetates; related oxidative aminations to form enamides; and hydroaminations of alkenes to form alkylamines. Other catalysts for the hydroamination of alkenes have been shown to react through organometallic amido intermediates generated by [2 + 2] cycloadditions between imido complexes and alkenes.
Organometallic olefin oxidation and oxidative amination
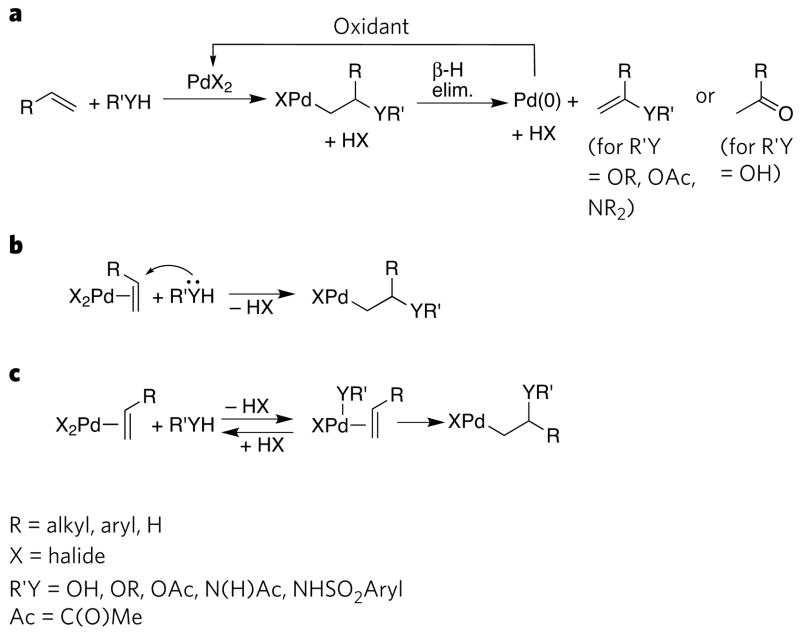
A generic scheme for palladium-catalysed olefin oxidation is shown in Fig. 7. Extensive mechanistic studies on this process have led researchers to agree on the intermediacy of hydroxyalkyl, alkoxyalkyl, acetoxyalkyl and aminoalkyl complexes (Fig. 7a). However, there has been a longstanding debate about the mechanism for formation of these intermediates79. By one mechanism (Fig. 7b), the β-functionalized alkyl intermediate is formed by coordination of an olefin and subsequent nucleophilic attack onto the coordinated olefin. By an alternative mechanism (Fig. 7c), the olefin coordinates and then inserts into an accompanying hydroxo, alkoxo, carboxylate or amidate ligand. Several recent experiments have shown that insertion of the olefin into an M–O or M–N bond leads to hydroxyalkyl, alkoxyalkyl or aminoalkyl intermediates under some of the commonly used reaction conditions.
Figure 7. Organometallic oxidation and oxidative amination of olefins.
a, The overall mechanism for the palladium-catalysed oxidation of olefins. b, c, Mechanisms for the C–heteroatom bond-forming step of the catalytic process. This step can occur by nucleophilic attack on a coordinated olefin (b) or migratory insertion into an M–heteroatom bond (c). β-H elim., β-hydrogen elimination.
The stereochemistry of certain oxidation products differentiates these two paths. External nucleophilic attack leads to an anti addition of the metal and the nucleophile across the C=C bond, whereas insertion leads to a syn addition of the two. Oxidations of internal olefins or deuterium-labelled terminal olefins in the presence of a source of electrophilic chlorine (such as CuCl2) would lead to different stereo isomeric halohydrin products from the two mechanisms. The identity of products from cyclic substrates can also reveal the mechanism of formation of the hydroxyalkyl, alkoxyalkyl or aminoalkyl intermediate.
These studies80–84 showed that reactions conducted in the absence of chloride form products through a syn addition across the C=C bond, whereas analogous reactions conducted in the presence of chloride form products through an anti addition across the C=C bond. Most probably, at high concentrations of added chloride, coordination of chloride occurs and discourages the generation of a hydroxo or alkoxo ligand positioned cis to the olefin, whereas in the absence of chloride, hydroxo or alkoxo ligands are generated cis to the olefin, and subsequent migratory insertion occurs. Recent related experiments on the oxidative amination of olefins provide similar stereochemical evidence for migratory insertion of an olefin into a palladium amidate bond9.
Hydroaminations of olefins through metal–amido complexes
Some of the catalysts for olefin hydroamination have also been shown to occur by insertion of an olefin into an M–amide linkage. Complexes that contain two substituted cyclopentadienyl ligands on lanthanide metals were some of the first catalysts for intramolecular hydroaminations of alkenes to form nitrogen heterocycles that occurred with substantial turnover numbers85. These reactions have been recently extended to the formation of long-chain amines by the combination of ethylene and a dialkylamine86. The initial work has led to a family of lanthanide complexes that catalyse intramolecular alkene hydroamination87–89.
The discovery of these lanthanide-catalysed hydroaminations dispelled the idea that M–N bonds involving high-valent electrophilic metals are too strong to undergo processes that would parallel the reactions of high-valent organometallic complexes, such as olefin insertion. Measurements of lanthanide–amide and lanthanide–alkyl bond strengths led to the prediction that insertions of alkenes into lanthanide–amide bonds would be close to thermoneutral90, and Tobin Marks first showed that lanthanide-catalysed hydroaminations of aminoalkenes occur by intramolecular insertion of olefins into lanthanide amides91 (Fig. 8a). The catalytic reactions occur over the course of hours at room temperature by rate-limiting insertion of the olefin into the lanthanide–amide bond.
Figure 8. Two mechanisms for catalytic hydroaminations of C–C multiple bonds through M–amido intermediates.
a, Hydroamination of olefins catalysed by lanthanide–amides occurring by intramolecular insertions of alkenes into an M–amide bond. b, Hydroamination of olefins catalysed by group IV complexes occurring by [2 + 2] cycloaddition to form a metallocyclic amido intermediate. Cp, cyclopentadienyl; La, lanthanide.
An alternative approach to the hydroamination of C–C multiple bonds exploits the ability of zirconium(IV) and titanium(IV) complexes containing M–N multiple bonds (imido complexes) to undergo [2 + 2] cycloadditions with substrates containing C–C multiple bonds92. The catalytic cycle for the hydroamination of alkenes by neutral titanium and zirconium complexes (Fig. 8b) occurs by a [2 + 2] cycloaddition between an alkene, alkyne or allene and an M–N multiple bond to form a four-membered metallocycle containing one M–N and one M–C bond. This step is similar to that of the [2 + 2] cycloadditions between alkenes and complexes containing M=C bonds (carbene complexes) that occur during olefin metathesis. Protonation of the M–C bond in the resultant metallocycle by an amine, followed by elimination of the organic addition product regenerates the M–imido complex and releases the hydroamination product. In the past few years, ancillary ligands on the metal centre have been identified that extend these original hydroaminations of alkynes92 to the intramolecular hydroamination of aminoalkenes to form pyrrolidines and piperidines93,94.
Insertions of olefins into metal–amide and metal–alkyl bonds
The conclusion that some oxidative aminations and hydroaminations of olefins occur by insertion of an olefin into an M–amide linkage begins to allow a comparison of the rates of insertions of alkenes into various M–alkyl and M–amido complexes and an understanding of the factors that control these relative rates. These data provide qualitative comparisons that can be used to formulate preliminary theories about the effect of the non-bonded electron pair on rates of olefin insertions into high-valent and low-valent amido complexes.
A comparison of lanthanide-catalysed hydroamination and olefin polymerization reveals the relative rates of insertions of olefins into lanthanide-alkyl and -amide bonds. The polymerizations of alkenes by lanthanide catalysts involves intermolecular, rate-limiting insertions of ethylene into lanthanocene alkyl intermediates95, whereas alkene hydroaminations by lanthanide catalysts have been largely limited to reactions occurring by intramolecular insertions90,91. Because lanthanide-catalysed olefin hydroamination is slower than lanthanide-catalysed olefin polymerization, the rate of olefin insertions into lanthanide amides seems to be slower than that of olefin insertions into lanthanide alkyls. This slower rate of insertion into lanthanide amides can be understood by the reaction’s requirement to break an M–N bond that is stronger than the M–C bond. The M–N bond is stronger in this case because of a favourable match of a hard metal with a hard ligand and donation of the non-bonded electrons from the amido group into unoccupied orbitals on the metal.
If the relative rates of insertions of olefins into lanthanide–amide and lanthanide–alkyl complexes are affected by the hardness of the lanthanide metal and the ability of this metal to accept electron density from the electron pair on nitrogen, then the relative rates for insertions of olefins into amide and alkyl complexes of softer, more electron-rich late transition metals are likely to be different. Although insertions of olefins into cationic or electrophilic neutral palladium–alkyl and nickel–alkyl complexes can be fast96,97, two recent studies suggest that the insertions of olefins into more electron-rich, late-transition-metal amides and alkoxides can be faster than into the analogous alkyl complexes.
Certain catalytic amidoarylations and alkoxyarylations of olefins reveal the relative rates of insertion into M–amido and M–aryl linkages98,99. These reactions occur through arylpalladium amido and arylpalladium alkoxo complexes, and in each case the product from insertion of the olefin into the M–amido or M–alkoxo bond is formed. In one particularly revealing experiment (Fig. 9a), the selectivity from a catalytic alkoxyarylation process involving a palladium centre bound by one alkoxo and one aryl ligand showed that insertion into the palladium–alkoxo bond was faster than insertion into the palladium–aryl bond99.
Figure 9. Data and basis for relative rates of olefin insertion into alkyl, amide and alkoxo complexes.
a, b, Examples of insertions into late-transition-metal–alkoxo and –amido complexes. c, Rationalization for why the rates of olefin insertion into late-transition-metal alkoxides and amides are faster than into late-transition-metal alkyls, based on the destabilization of the alkoxo and amide reactants and the stabilization of the products of insertion into the alkoxo and amido complexes by an M–Y dative bond. Et, ethyl.
A second set of data (Fig. 9b) reveals the relative rates of olefin insertions into discrete rhodium–alkoxo and rhodium–amido complexes31,32. In one study, intermolecular insertion of an olefin into a rhodium–amido complex forms a rhodium hydride and an enamine as the final products after β-hydrogen elimination from the initially formed aminoalkyl species31. In a closely related study, intramolecular insertion of a coordinated alkene into a rhodium alkoxide forms a cyclic vinyl ether after β-hydrogen elimination from the resultant alkoxyalkyl intermediate32. The corresponding rhodium methyl complex does not insert alkenes. Thus, the rates for insertions of olefins into these rhodium alkoxides and amides seem to be faster than for insertions into the analogous alkyls, and these early data imply that this trend applies to the reactions of many other late-transition-metal–alkyl, –alkoxo and –amido complexes. These rates could be controlled by the electron pair of the ligand as summarized in Fig. 9c. First, the M–heteroatom bonds in the starting, low-valent alkoxide or amide would probably be destabilized, relative to the analogous alkyl complexes, by repulsion between the filled metal d orbital and the filled amide or alkoxide p orbital. Second, the M–heteroatom bond need not be fully cleaved during insertions into metal alkoxides and amides because the insertions can form metallocyclic products in which the covalent M–Y bond of the starting amido or alkoxo complex has been converted into an M–Y dative bond (where Y is NH or O). Because the M–N and M–O bonds have not been fully cleaved in this process, the barrier for insertion can be envisaged to be lower than for insertion into the related alkyl complex. Thus, several differences in the properties of organometallic and hetero-organometallic complexes can make amido and alkoxo complexes more reactive than M–alkyl complexes towards insertions of alkenes.
Conclusions
Catalytic reactions that are widely used in organic synthesis occur with a broad scope of substrates, a high tolerance of the reaction for auxiliary functional groups, high turnover numbers, fast rates, inexpensive reagents and minimal side products. Although great progress has been made in the coupling of amines with aryl halides, the additions of amines to olefins, the oxidation of olefins and the functionalization of C–H bonds, further progress is needed before all of these reactions will become everyday tools of the synthetic chemist.
The additions of amines to aryl halides occur with sufficient scope and efficiency that it is now a commonly used synthetic method in both academic and industrial settings. However, advances in the other catalytic reactions discussed in this review are needed for them to become a common synthetic tool. For example, coupling of aryl halides with weaker heteroatom nucleophiles, such as alcohols, does not yet occur with an acceptable scope and efficiency. Likewise, intramolecular hydroamination of alkenes needs to occur with more hindered alkenes, with control of stereochemistry and with much lower amounts of catalyst, and it needs be developed into an intermolecular process. The functionalization of C–H bonds needs to be developed into a process that occurs with milder and less expensive reagents, and it needs to occur with much lower amounts of catalyst and additives. It is anticipated that an increased understanding of the factors that control the elementary reactions of these catalytic cycles will greatly accelerate the discovery of new catalysts that overcome these current limitations.
The establishment of these principles and their use in generating improved reactions will bring the field of homogeneous catalysis closer to a stage at which new catalysts and catalytic reactions can be designed. Although a framework has been established to plan synthetic sequences100, a similar conceptual framework to design catalysts and catalytic reactions is far from established. As additional reactions to form C–C and C–heteroatom bonds are discovered and further refined, and as our understanding of the factors that control the rates and selectivities of these processes improves, the field of homogeneous catalysis will move closer to the point at which researchers can make rational choices about how to modify existing catalysts and can begin to design new catalysts for discovering the next set of dream reactions.
Acknowledgments
I thank the National Institutes of Health, the US Department of Energy and the National Science Foundation for funding my research related to the catalytic formation of carbon–heteroatom bonds. I also thank my co-workers who helped to formulate the concepts included in the Review, and E. Alexanian for suggestions and thorough editing of the manuscript.
Footnotes
Author Information Reprints and permissions information is available at www.nature.com/reprints. The author declares no competing financial interests.
References
- 1.Muci AR, Buchwald SL. Practical palladium catalysts for C–N and C–O bond formation. Top Curr Chem. 2002;219:131–209. This paper reviews the primary literature on cross-coupling to form C–N and C–O bonds. [Google Scholar]
- 2.Hartwig JF. In: Handbook of Organopalladium Chemistry for Organic Synthesis. Negishi EI, editor. Vol. 1. Wiley-Interscience; 2002. pp. 1051–1096. [Google Scholar]
- 3.Hartwig JF. In: Handbook of Organopalladium Chemistry for Organic Synthesis. Negishi EI, editor. Vol. 1. Wiley-Interscience; 2002. pp. 1097–1106. [Google Scholar]
- 4.Dick AR, Sanford MS. Transition metal catalyzed oxidative functionalization of carbon–hydrogen bonds. Tetrahedron. 2006;62:2439–2463. This paper reviews various modern approaches to C–H bond functionalization to form C–heteroatom bonds. [Google Scholar]
- 5.Hull KL, Anani WQ, Sanford MS. Palladium-catalyzed fluorination of carbon–hydrogen bonds. J Am Chem Soc. 2006;128:7134–7135. doi: 10.1021/ja061943k. [DOI] [PubMed] [Google Scholar]
- 6.Giri R, Chen X, Yu JQ. Palladium-catalyzed asymmetric iodination of unactivated C–H bonds under mild conditions. Angew Chem Int Edn Engl. 2005;44:2112–2115. doi: 10.1002/anie.200462884. [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Schlecht S, Semple TC, Hartwig JF. Thermal, catalytic, regiospecific functionalization of alkanes. Science. 2000;287:1995–1997. doi: 10.1126/science.287.5460.1995. This paper reports the only catalytic process to functionalize terminal alkyl C–H bonds selectively. [DOI] [PubMed] [Google Scholar]
- 8.Jira R. In: Applied Homogeneous Catalysis with Organometallic Compounds: A Comprehensive Handbook in Two Volumes. Cornils B, Herrmann WA, editors. Wiley-VCH; 2002. pp. 386–405. [Google Scholar]
- 9.Liu G, Stahl SS. Two-faced reactivity of alkenes: cis- versus trans-aminopalladation in aerobic Pd-catalyzed intramolecular aza-Wacker reactions. J Am Chem Soc. 2007;129:6328–6335. doi: 10.1021/ja070424u. [DOI] [PubMed] [Google Scholar]
- 10.Muller TE. In: Encyclopedia of Catalysis. Horváth IT, editor. Vol. 3. Wiley-Interscience; 2003. pp. 518–541. [Google Scholar]
- 11.King AO, Yasuda N. Palladium-catalyzed cross-coupling reactions in the synthesis of pharamaceuticals. Topics Organomet Chem. 2004;6:205–245. [Google Scholar]
- 12.de Vries JG. The Heck reaction in the production of fine chemicals. Can J Chem. 2001;79:1086–1092. [Google Scholar]
- 13.Aizawa M, Yamada T, Shinohara H, Akagi K, Shirakawa H. Electrochemical fabrication of a polypyrrole-polythiophene p-n-junction diode. J Chem Soc, Chem Commun. 1986;17:1315–1317. [Google Scholar]
- 14.Jen KY, Miller GG, Elsenbaumer RL. Highly conducting, soluble, and environmentally-stable poly(3-alkylthiophenes) J Chem Soc, Chem Commun. 1986;17:1346–1347. [Google Scholar]
- 15.Sato MA, Tanaka S, Kaeriyama K. Soluble conducting polythiophenes. J Chem Soc, Chem Commun. 1986;11:873–874. [Google Scholar]
- 16.McQuade DT, Pullen AE, Swager TM. Conjugated polymer-based chemical sensors. Chemical Reviews. 2000;100:2537–2574. doi: 10.1021/cr9801014. [DOI] [PubMed] [Google Scholar]
- 17.Hasenhuettl GL. Kirk-Othmer Encyclopedia of Chemical Technology. Wiley; 2005. [DOI] [Google Scholar]
- 18.Blaser HU, Schmidt E. Asymmetric Catalysis on Industrial Scale: Challenges, Approaches and Solutions. Wiley-VCH; 2004. [Google Scholar]
- 19.Hoveyda AH, Zhugralin AR. The remarkable metal-catalysed olefin metathesis reaction. Nature. 2007;450:243–251. doi: 10.1038/nature06351. [DOI] [PubMed] [Google Scholar]
- 20.Kakiuchi F, Chatani N. Catalytic methods for C–H bond functionalization: application in organic synthesis. Adv Synth Catal. 2003;345:1077–1101. [Google Scholar]
- 21.Labinger JA, Bercaw JE. Understanding and exploiting C–H bond activation. Nature. 2002;417:507–514. doi: 10.1038/417507a. [DOI] [PubMed] [Google Scholar]
- 22.Goldman AS, et al. Catalytic alkane metathesis by tandem alkane dehydrogenation olefin metathesis. Science. 2006;312:257–261. doi: 10.1126/science.1123787. [DOI] [PubMed] [Google Scholar]
- 23.Crabtree RH. The Organometallic Chemistry of the Transition Metals. 4. Wiley; 2005. [Google Scholar]
- 24.Collman JP, Hegedus LS, Norton JR, Finke RG. Principles and Applications of Organotransition Metal Chemistry. University Science Books; 1987. [Google Scholar]
- 25.Ojima I, Li Z, Zhu J. In: The Chemistry Of Organic Silicon Compounds. Rappoport Z, Apeloig Y, editors. Vol. 2. Wiley; 1998. pp. 1687–1792. [Google Scholar]
- 26.Hartwig JF. Carbon–heteroatom bond-forming reductive elimination of amines, ethers, and sulfides. Acc Chem Res. 1998;31:852–860. [Google Scholar]
- 27.Hartwig JF. Electronic effects on reductive elimination to form carbon–carbon and carbon–heteroatom bonds from palladium(II) complexes. Inorg Chem. 2007;46:1936–1947. doi: 10.1021/ic061926w. This paper reviews how the properties of a heteroatom affect the rates of reductive elimination to form C–heteroatom bonds. [DOI] [PubMed] [Google Scholar]
- 28.Hillhouse GL, Bercaw JE. Reactions of water and ammonia with bis(pentamethyl-cyclopentadienyl) complexes of zirconium and hafnium. J Am Chem Soc. 1984;106:5472–5478. [Google Scholar]
- 29.Zhao J, Goldman AS, Hartwig JF. Oxidative addition of ammonia to form a stable monomeric amido hydride complex. Science. 2005;307:1080–1082. doi: 10.1126/science.1109389. [DOI] [PubMed] [Google Scholar]
- 30.Casalnuovo AL, Calabrese JC, Milstein D. Rational design in homogeneous catalysis. Ir(I)-catalyzed addition of aniline to norbornylene via N–H activation. J Am Chem Soc. 1988;110:6738–6744. [Google Scholar]
- 31.Zhao PJ, Krug C, Hartwig JF. Transfer of amido groups from isolated rhodium(I) amides to alkenes and vinylarenes. J Am Chem Soc. 2005;127:12066–12073. doi: 10.1021/ja052473h. [DOI] [PubMed] [Google Scholar]
- 32.Zhao P, Incarvito CD, Hartwig JF. Carbon–oxygen bond formation between a terminal alkoxo ligand and a coordinated olefin. Evidence for olefin insertion into a rhodium alkoxide. J Am Chem Soc. 2006;128:9642–9643. doi: 10.1021/ja063347w. [DOI] [PubMed] [Google Scholar]
- 33.Polse JL, Andersen RA, Bergmann RG. Reactivity of a terminal Ti(IV) imido complex toward alkenes and alkynes: cycloaddition vs C–H activation. J Am Chem Soc. 1998;120:13405–13414. [Google Scholar]
- 34.Fulton JR, Holland AW, Fox DJ, Bergman RG. Formation, reactivity, and properties of nondative late transition metal–oxygen and –nitrogen bonds. Acc Chem Res. 2002;35:44–56. doi: 10.1021/ar000132x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bryndza H, Tam W. Monomeric metal hydroxides, alkoxides, and amides of the late transition metals: synthesis, reactions, and thermochemistry. Chem Rev. 1988;88:1163–1188. [Google Scholar]
- 36.Hartwig JF. In: Modern Arene Chemistry. Astruc C, editor. Wiley-VCH; 2002. pp. 107–168. [Google Scholar]
- 37.Boger DL, Panek JS. Palladium (0) mediated β-carboline synthesis: preparation of the CDE ring system of lavendamycin. Tetrahedron Lett. 1984;25:3175–3178. [Google Scholar]
- 38.Kosugi M, Kameyama M, Migita T. Palladium-catalyzed aromatic amination of aryl bromides with N,N-di-ethylamino-tributyltin. Chem Lett. 1983;12:927–928. [Google Scholar]
- 39.Guram AS, Rennels RA, Buchwald SL. A simple catalytic method for the conversion of aryl bromides to arylamines. Angew Chem Int Edn Engl. 1995;34:1348–1350. [Google Scholar]
- 40.Louie J, Hartwig JF. Palladium-catalyzed synthesis of arylamines from aryl halides. mechanistic studies lead to coupling in the absence of tin reagents. Tetrahedron Lett. 1995;36:3609–3612. [Google Scholar]
- 41.Wolfe JP, Wagaw S, Buchwald SL. An improved catalyst system for aromatic carbon–nitrogen bond formation: the possible involvement of bis (phosphine) palladium complexes as key intermediates. J Am Chem Soc. 1996;118:7215–7216. [Google Scholar]
- 42.Driver MS, Hartwig JF. A second generation catalyst for aryl halide amination: mixed secondary amines from aryl halides and primary amines catalyzed by (DPPF)PdCl2. J Am Chem Soc. 1996;118:7217–7218. [Google Scholar]
- 43.Nishiyama M, Yamamoto T, Koie Y. Synthesis of N-arylpiperazines from aryl halides and piperazine under a palladium tri-tert-butylphosphine catalyst. Tetrahedron Lett. 1998;39:617–620. [Google Scholar]
- 44.Hartwig JF, Kawatsura M, Hauck SI, Shaughnessy KH, Alcazar-Roman LM. Room-temperature palladium-catalyzed amination of aryl bromides and chlorides and extended scope of aromatic C–N bond formation with a commercial ligand. J Org Chem. 1999;64:5575–5580. doi: 10.1021/jo990408i. [DOI] [PubMed] [Google Scholar]
- 45.Stambuli JP, Kuwano R, Hartwig JF. Unparalleled rates for the activation of aryl chlorides. Coupling with amines and boronic acids in minutes at room temperature. Angew Chem Int Edn Engl. 2002;41:4746–4747. doi: 10.1002/anie.200290036. [DOI] [PubMed] [Google Scholar]
- 46.Zapf A, et al. Practical synthesis of new and highly efficient ligands for the Suzuki reaction of aryl chlorides. Chem Commun. 2004;10:38–39. doi: 10.1039/b311268n. [DOI] [PubMed] [Google Scholar]
- 47.Singer RA, Dore ML, Sieser JE, Berliner MA. Development of nonproprietary phosphine ligands for the Pd-catalyzed amination reaction. Tetrahedron Lett. 2006;47:3727–3731. [Google Scholar]
- 48.Kataoka N, Shelby Q, Stambuli JP, Hartwig JF. Air stable, sterically hindered ferrocenyl dialkylphosphines for palladium-catalyzed C–C, C–N and C–O bond-forming cross-couplings. J Org Chem. 2002;67:5553–5566. doi: 10.1021/jo025732j. [DOI] [PubMed] [Google Scholar]
- 49.Shen Q, Shekhar S, Stambuli JP, Hartwig JF. Highly reactive, general, and long-lived catalysts for coupling heteroaryl and aryl chlorides with primary nitrogen nucleophiles. Angew Chem Int Edn Engl. 2004;44:1371–1375. doi: 10.1002/anie.200462629. [DOI] [PubMed] [Google Scholar]
- 50.Shen Q, Hartwig JF. Palladium-catalyzed coupling of ammonia and lithium amide. J Am Chem Soc. 2006;128:10028–10029. doi: 10.1021/ja064005t. [DOI] [PubMed] [Google Scholar]
- 51.Driver MS, Hartwig JF. Carbon–nitrogen bond-forming reductive elimination of arylamines from Pd(II) J Am Chem Soc. 1997;119:8232–8245. [Google Scholar]
- 52.Fujita KI, et al. Organometallic chemistry from amidate complexes. Reductive elimination of N-aryl amidates from palladium(II) J Am Chem Soc. 2006;128:9044–9045. doi: 10.1021/ja062333n. [DOI] [PubMed] [Google Scholar]
- 53.Mann G, Incarvito C, Rheingold AL, Hartwig JF. Palladium-catalyzed C–O coupling involving unactivated aryl halides. Sterically induced reductive elimination to form the C–O bond in diaryl ethers. J Am Chem Soc. 1999;121:3224–3225. [Google Scholar]
- 54.Stambuli JR, Weng ZQ, Incarvito CD, Hartwig JF. Reductive elimination of ether from T-shaped, monomeric arylpalladium alkoxides. Angew Chem Int Edn Engl. 2007;46:7674–7677. doi: 10.1002/anie.200702809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mann G, Barañano D, Hartwig JF, Rheingold AL, Guzei IA. Carbon–sulfur bond-forming reductive elimination involving sp-, sp2, and sp3-hybridized carbon. Mechanism, steric effects, and electronic effects on sulfide formation. J Am Chem Soc. 1998;120:9205–9219. [Google Scholar]
- 56.Holland PL, Andersen RA, Bergman RG. Application of the E-C approach to understanding the bond energies thermodynamics of late-metal amido, aryloxo and alkoxo complexes: an alternative to p π/d π repulsion. Comments Inorg Chem. 1999;21:115–129. [Google Scholar]
- 57.Culkin DA, Hartwig JF. Carbon–carbon bond-forming reductive elimination from arylpalladium complexes containing functionalized alkyl groups. Influence of ligand steric and electronic properties on structure, stability, and reactivity. Organometallics. 2004;23:3398–3416. [Google Scholar]
- 58.Huheey JE, Keiter EA, Keiter RL. Inorganic Chemistry. 4. Harper Collins; 1993. [Google Scholar]
- 59.Williams BS, Goldberg KI. Studies of reductive elimination reactions to form carbon–oxygen bonds from Pt(IV) complexes. J Am Chem Soc. 2001;123:2576–2587. doi: 10.1021/ja003366k. [DOI] [PubMed] [Google Scholar]
- 60.Pawlikowski AV, Getty AD, Goldberg KI. Alkyl carbon–nitrogen reductive elimination from platinum(IV)–sulfonamide complexes. J Am Chem Soc. 2007;129:10382–10393. doi: 10.1021/ja069191h. [DOI] [PubMed] [Google Scholar]
- 61.Dick AR, Kampf JW, Sanford MS. Unusually stable palladium(IV) complexes: detailed mechanistic investigation of C–O bond-forming reductive elimination. J Am Chem Soc. 2005;127:12790–12791. doi: 10.1021/ja0541940. [DOI] [PubMed] [Google Scholar]
- 62.Bistri O, Correa A, Bolm C. Iron-catalyzed C–O cross-couplings of phenols with aryl iodides. Angew Chem Int Edn Engl. 2008;47:586–588. doi: 10.1002/anie.200704018. [DOI] [PubMed] [Google Scholar]
- 63.Correa A, Bolm C. Iron-catalyzed N-arylation of nitrogen nucleophiles. Angew Chem Int Edn Engl. 2007;46:8862–8865. doi: 10.1002/anie.200703299. [DOI] [PubMed] [Google Scholar]
- 64.Beletskaya IP, Cheprakov AV. Copper in cross-coupling reactions — the post-Ullmann chemistry. Coord Chem Rev. 2004;248:2337–2364. [Google Scholar]
- 65.Shilov AE, Shul’pin GB. Activation of C–H bonds by metal complexes. Chem Rev. 1997;97:2879–2932. doi: 10.1021/cr9411886. [DOI] [PubMed] [Google Scholar]
- 66.Periana RA, et al. Platinum catalysts for the high-yield oxidation of methane to a methanol derivative. Science. 1998;280:560–564. doi: 10.1126/science.280.5363.560. [DOI] [PubMed] [Google Scholar]
- 67.Sen A. Catalytic functionalization of carbon–hydrogen and carbon–carbon bonds in protic media. Acc Chem Res. 1998;31:550–557. [Google Scholar]
- 68.Stahl SS, Labinger JA, Bercaw JE. Homogeneous oxidation of alkanes by electrophilic late transition metals. Angew Chem Int Edn Engl. 1998;37:2181–2192. doi: 10.1002/(SICI)1521-3773(19980904)37:16<2180::AID-ANIE2180>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 69.Groves JT. High-valent iron in chemical and biological oxidations. J Inorg Biochem. 2006;100:434–447. doi: 10.1016/j.jinorgbio.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 70.Wenzel TT, Bergman RG. Inter- and intramolecular insertion of rhenium into carbon–hydrogen bonds. J Am Chem Soc. 1986;108:4856–4867. [Google Scholar]
- 71.Jones WD, Feher FJ. The mechanism and thermodynamics of alkane and arene carbon–hydrogen bond activation in (C5Me5)Rh(PMe3)(R)H. J Am Chem Soc. 1985;106:1650–1663. [Google Scholar]
- 72.Arndtsen BA, Bergman RG, Mobley TA, Peterson TH. Selective intermolecular carbon–hydrogen bond activation by synthetic metal complexes in homogeneous solution. Acc Chem Res. 1995;28:154. [Google Scholar]
- 73.Whitfield SR, Sanford MS. Reactivity of Pd(II) complexes with electrophilic chlorinating reagents: isolation of Pd(IV) products and observation of C–Cl bond-forming reductive elimination. J Am Chem Soc. 2007;129:15142–15143. doi: 10.1021/ja077866q. [DOI] [PubMed] [Google Scholar]
- 74.Giri R, Chen X, Yu JQ. Palladium-catalyzed asymmetric iodination of unactivated C–H bonds under mild conditions. Angew Chem Int Edn Engl. 2005;44:2112–2115. doi: 10.1002/anie.200462884. [DOI] [PubMed] [Google Scholar]
- 75.Desai LV, Hull KL, Sanford MS. Palladium-catalyzed oxygenation of unactivated sp3 C–H bonds. J Am Chem Soc. 2004;126:9542–9543. doi: 10.1021/ja046831c. [DOI] [PubMed] [Google Scholar]
- 76.Davies DL, Donald SMA, Macgregor SA. Computational study of the mechanism of cyclometalation by palladium acetate. J Am Chem Soc. 2005;127:13754–13755. doi: 10.1021/ja052047w. [DOI] [PubMed] [Google Scholar]
- 77.Ishiyama T, Miyaura N. Transition metal-catalyzed borylation of alkanes and arenes via C–H activation. J Organomet Chem. 2003;680:3–11. [Google Scholar]
- 78.Hartwig JF, et al. Rhodium–boryl complexes in the catalytic, terminal functionalization of alkanes. J Am Chem Soc. 2005;127:2538–2552. doi: 10.1021/ja045090c. [DOI] [PubMed] [Google Scholar]
- 79.Henry PM. In: Handbook of Organopalladium Chemistry for Organic Synthesis. Negishi EI, editor. Wiley-Interscience; 2002. [Google Scholar]
- 80.Francis JW, Henry PM. Palladium(II)-catalyzed exchange and isomerization reactions. 14 Kinetics and stereochemistry of the isomerization and water exchange of 2-(methyl-d3)-4-methyl-1,1,1,5,5,5-hexafluoro-3-penten-2-ol in aqueous solution catalyzed by PdCl42−: two new mechanistic probes for catalytic chemistry. Organometallics. 1991;10:3498–3503. [Google Scholar]
- 81.Francis JW, Henry PM. Palladium(II)-catalyzed exchange and isomerization reactions. 15 Kinetics and stereochemistry of the isomerization and water exchange of 2-(methyl-d3)-4-methyl-1,1,1,5,5,5-hexafluoro-3-penten-2-ol in aqueous solution catalyzed by PdCl42− at high chloride concentrations. Organometallics. 1992;11:2832–2836. [Google Scholar]
- 82.Hamed O, Thompson C, Henry PM. Stereochemistry of the Wacker reaction: modes of addition of hydroxide, methoxide, and phenyl at high and low Cl−. A study using chirality transfer. J Org Chem. 1997;62:7082–7083. doi: 10.1021/jo971051q. [DOI] [PubMed] [Google Scholar]
- 83.Hayashi T, Yamasaki K, Mimura M, Uozumi Y. Deuterium-labeling studies establishing stereochemistry at the oxypalladation step in Wacker-type oxidative cyclization of an O-allylphenol. J Am Chem Soc. 2004;126:3036–3037. doi: 10.1021/ja031946m. [DOI] [PubMed] [Google Scholar]
- 84.Trend RM, Ramtohul YK, Stoltz BM. Oxidative cyclizations in a nonpolar solvent using molecular oxygen and studies on the stereochemistry of oxypalladation. J Am Chem Soc. 2005;127:17778–17788. doi: 10.1021/ja055534k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hong S, Marks TJ. Organolanthanide-catalyzed hydroamination. Acc Chem Res. 2004;37:673–686. doi: 10.1021/ar040051r. This paper reviews the development of some of the most active catalysts for alkene hydroamination, which function by insertions of alkenes into M–amide bonds. [DOI] [PubMed] [Google Scholar]
- 86.Amin SB, Marks TJ. Organolanthanide-catalyzed synthesis of amine-capped polyethylenes. J Am Chem Soc. 2007;129:10102–10103. doi: 10.1021/ja0740465. [DOI] [PubMed] [Google Scholar]
- 87.Roesky PW, Muller TE. Enantioselective catalytic hydroamination of alkenes. Angew Chem Int Edn Engl. 2003;42:2708–2710. doi: 10.1002/anie.200301637. [DOI] [PubMed] [Google Scholar]
- 88.Hultzsch KC. Transition metal-catalyzed asymmetric hydroamination of alkenes (AHA) Adv Synth Catal. 2005;347:367–391. [Google Scholar]
- 89.Kim JY, Livinghouse T. Enantioselective intramolecular alkene hydroaminations catalyzed by yttrium complexes of axially chiral bis(thiolate) ligands. Org Lett. 2005;7:1737–1739. doi: 10.1021/ol050294z. [DOI] [PubMed] [Google Scholar]
- 90.Gagné MR, Marks TJ. Organolanthanide-catalyzed hydroamination. Facile regiospecific cyclization of unprotected amino olefins. J Am Chem Soc. 1989;111:4108–4109. [Google Scholar]
- 91.Gagne MR, Stern CL, Marks TJ. Organolanthanide-catalyzed hydroamination — a kinetic, mechanistic, and diastereoselectivity study of the cyclization of N-unprotected amino olefins. J Am Chem Soc. 1992;114:275–294. [Google Scholar]
- 92.Walsh PJ, Baranger AM, Bergman RG. Stoichiometric and catalytic hydroamination of alkynes and allene by zirconium bisamides Cp2Zr(NHR)2. J Am Chem Soc. 1992;114:1708–1719. [Google Scholar]
- 93.Wood MC, Leitch DC, Yeung CS, Kozak JA, Schafer LL. Chiral neutral zirconium amidate complexes for the asymmetric hydroamination of alkenes. Angew Chem Int Edn Engl. 2007;46:354–358. doi: 10.1002/anie.200603017. [DOI] [PubMed] [Google Scholar]
- 94.Gott AL, Clarke AJ, Clarkson GJ, Scott P. Catalytic alkene cyclohydroamination via an imido mechanism. Chem Commun. 2008;12:1422–1424. doi: 10.1039/b718373a. [DOI] [PubMed] [Google Scholar]
- 95.Jeske G, et al. Highly reactive organolanthanides — systematic routes to and olefin chemistry of early and late bis(pentamethylcyclopentadienyl) 4f hydrocarbyl and hydride complexes. J Am Chem Soc. 1985;107:8091–8103. [Google Scholar]
- 96.Ittel SD, Johnson LK, Brookhart M. Late-metal catalysts for ethylene homo- and copolymerization. Chem Rev. 2000;100:1169–1203. doi: 10.1021/cr9804644. [DOI] [PubMed] [Google Scholar]
- 97.Mecking S. Olefin polymerization by late transition metal complexes — a root of Ziegler catalysts gains new ground. Angew Chem Int Edn Engl. 2001;40:534–540. [PubMed] [Google Scholar]
- 98.Wolfe JP. Palladium-catalyzed carboetherification and carboamination reactions of γ-hydroxy- and γ-aminoalkenes for the synthesis of tetrahydrofurans and pyrrolidines. Eur J Org Chem. 2007;4:571–582. [PMC free article] [PubMed] [Google Scholar]
- 99.Nakhla JS, Kampf JW, Wolfe JP. Intramolecular Pd-catalyzed carboetherification and carboamination. Influence of catalyst structure on reaction mechanism and product stereochemistry. J Am Chem Soc. 2006;128:2893–2901. doi: 10.1021/ja057489m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Corey EJ, Cheng X-M. The Logic of Chemical Synthesis. Wiley-Interscience; 1995. [Google Scholar]