Abstract
The parameters controlling the generation of robust CD4+ T cell recall responses remain poorly defined. In this study we compare recall responses by CD4+ and CD8+ memory T cells following rechallenge. Homologous rechallenge of mice immune to either lymphocytic choriomeningitis virus (LCMV) or Listeria monocytogenes results in robust CD8+ T cell recall responses but poor boosting of CD4+ T cell recall responses in the same host. In contrast, heterologous rechallenge with a pathogen sharing only a CD4+ T cell epitope results in robust boosting of CD4+ T cell recall responses. The disparity in CD4+ and CD8+ T cell recall responses cannot be attributed to competition for growth factors or APCs, as robust CD4+ and CD8+ T cell recall responses can be simultaneously induced following rechallenge with peptide-pulsed dendritic cells. Instead, CD4+ T cell recall responses are dependant on the duration of the secondary challenge. Increasing the rechallenge dose results in more potent boosting of CD4+ T cell recall responses, and artificially limiting the duration of secondary infection following heterologous rechallenge adversely impacts the magnitude of CD4+ T cell, but not CD8+ T cell, recall responses. These findings suggest that rapid pathogen clearance by secondary CTL following homologous rechallenge prevents optimal boosting of CD4+ T cell responses and therefore have important practical implications in the design of vaccination and boosting strategies aimed at promoting CD4+ T cell-mediated protection.
Keywords: T cells, cell differentiation, memory, vaccination
Introduction
Following acute infection, antigen-specific CD8+ T cells undergo rapid expansion, dividing 15–20 times and developing CTL function. After clearance of the pathogen, the vast majority (90–95%) of antigen-specific T cells die, leaving behind a population of long-lived memory cells that provide protection from secondary challenges with the same or a related pathogen (1). A major research focus in recent years has been to delineate the nature and timing of the signals that promote the differentiation, function and longevity of memory T cell populations, as well as their ability to respond to secondary challenges.
While the activation and differentiation of CD4+ and CD8+ T cells is in many ways similar, important distinctions warrant further study, as they will impact vaccine strategies aimed at inducing and boosting either CD8+ T cell or CD4+ T cell responses. For example, while CD8+ T cells require only a short period (6–24 hours) of interaction with antigen in order to undergo an antigen-independent period of programmed expansion and differentiation (2–5), CD4+ T cells, may require several days interaction with antigen in vivo for optimal activation and expansion (6, 7). Furthermore, we have recently shown that low-avidity CD4+ T cell responders demonstrate reduced function and survival (8). These results suggest that while CD8+ T cells may rely on antigen for recruitment into the response, once these cells are programmed, differentiation and expansion can be antigen independent (9). CD4+ T cells, on the other hand, conform more to a model in which increasing stimulatory signals progressively drive proliferation, differentiation and survival (10, 11).
A further distinction between CD8+ and CD4+ T cell differentiation is the stability of their ensuing memory populations. While CD8+ memory T cells are maintained at stable levels throughout the life of the mouse, in some circumstances CD4+ memory T cells have been noted to decline (12–14). This decline has led some to question whether these cells can truly be called memory cells, as the population appears to lack the capacity for self-renewal (15). In contrast, CD4+ memory T cells in humans are detectable for up to 75 years post-immunization with a half-life similar to CD8+ memory T cells (16). CD8+ and CD4+ memory T cells both depend on the cytokines IL-7 and IL-15 for survival (17, 18), but the other factors that promote long-term CD4+ memory T cell maintenance need to be resolved.
It has also been suggested CD4+ T cells, whether intrinsically (19) or due to clonal competition (20, 21), are less capable of robust expansion than CD8+ T cells. Competition among CD4+ T cell responders for access to APCs or other resources has been shown to play an important role in regulating cell division. One recent study also found that memory CD4+ T cells displayed limited expansion following secondary challenge due to their production of growth-limiting cytokines even in environments in which antigen was plentiful and the frequency of responders was low (22). These findings suggest that memory CD4+ T cells may be intrinsically limited in the ability to expand upon secondary challenge.
We have repeatedly observed in settings of homologous rechallenge that stimuli sufficient to promote robust secondary expansion of CD8+ memory T cells only weakly promote secondary expansion of CD4+ memory T cells. As the parameters controlling the effectiveness of CD4+ T cell recall responses have not been well-characterized, it is unclear whether these differences are intrinsic, related to the impact of inter- or intra-clonal competition or due to insufficient access to secondary stimuli. In this study we find that following heterologous rechallenge with a pathogen sharing only a CD4+ T cell epitope with the primary pathogen, CD4+ memory T cells underwent robust secondary responses comparable to those seen for CD8+ T cells. CD4+ T cell recall responses did not compete with CD8+ T cells for space or resources but rather required a longer stimulatory period following rechallenge. Our findings suggest that homologous rechallenge targeting both CD8+ and CD4+ T cell recall responses results in a shortened infectious time course that, because the pathogen is rapidly cleared by secondary CTL, is sufficient to promote robust secondary CD8+ T cell responses but insufficient to induce optimal CD4+ T cell secondary responses. While our results also suggest that CD4+ memory T cells may have an intrinsically limited capacity for secondary expansion as compared to primary responders, in the context of a prime/boost strategy robust boosting of CD4+ T cell responses to levels comparable to those typically seen for CD8+ T cell responses is possible under appropriate stimulatory conditions. Therefore, these findings have important practical implications for the design of prime/boost vaccination strategies targeting CD4+ T cells.
Materials and Methods
Mice and Infections
6–8 week old C57BL/6 (B6) and B6.SJL-PtprcaPepcb/BoyJ (B6.SJL) mice were purchased from Jackson Laboratories (Bar Harbor, ME). All animal experiments were conducted with the approval of the IACUC committee at the University of Utah. LCMV Armstrong 53b was grown in BHK cells and titered in Vero cells (23). For primary challenges and heterologous rechallenges, mice were infected intraperitoneally (i.p.) with 2 × 105 plaque-forming units (PFU). For homologous rechallenges, mice were infected with 2 × 106 PFU intravenously (i.v.). Lm-gp61 (a gift from M. Kaja-Krishna, University of Washington, generated using described methods (8, 24, 25)), Lm-Ova (26) and wildtype Lm were propagated in BHI broth and agar plates. Prior to infection, the bacteria were grown to log phase and concentration determined by measuring the O.D. at 600 nm (O.D. of 1 = 1 × 109 CFU/ml). For Lm-gp61, mice were injected intravenously (i.v.) with 2 × 105 colony forming units (CFU). For Lm-Ova, mice were given a primary challenge of 2 × 103 CFU and secondary challenges as indicated in the text.
Dendritic Cell Immunizations
DCs were expanded in B6 mice with a Flt-3L–secreting B16 mouse melanoma cell line as previously described (27). DCs were enriched to 70–80% purity from spleens and lymph nodes by transient adherence overnight. They were then pulsed with either 0.1 μM of the LCMV GP33–41 Class I-restricted peptide (KAVYNFATC) or 1 μM of the LCMV GP61–80 Class II-restricted peptide (GLKGPDIYKGVYQFKSVEFD) for two hours in the presence of 1 μg/ml LPS. Mice were injected with 1 × 106 DCs i.v. on days 0, 2 and 4, along with a bystander Lm infection (2 × 103 CFU i.v.) on day 0.
Adoptive Transfers
Untouched CD4+ T cells were isolated from the spleens of Lm-gp61 B6 mice (CD45.2+) by incubation with a biotinylated antibody cocktail followed by anti-biotin magnetic beads and depletion on a magnetic column, per manufacturer’s recommendations (Miltenyi). 1 × 106 cells were injected i.v. into B6.SJL mice (CD45.1+), followed by infection with LCMV.
Peptide Re-stimulation and Intracellular Cytokine Staining
Splenocytes were re-suspended in RPMI 1640 containing 10% fetal bovine serum and supplemented with antibiotics and L-glutamine. Mice were re-stimulated with 0.1 μM Class I-restricted peptide (GP33–41) or 1 μM Class II-restricted peptide (GP61–80) as indicated, in the presence of Brefeldin A (1 μl/ml GolgiPlug). Cells were stained with cell surface antibodies, permeabilized and stained with cytokine antibodies using a kit per manufacturer’s instructions (BDBiosciences, Mountain View, CA).
Antibodies and Flow Cytometry
Fluorescent dye-conjugated antibodies were purchased from eBioscience (San Diego, CA) or BDBiosciences (Mountain View, CA) with the following specificities: CD4, CD8, IFNγ, IL-2, CD45.1, CD45.2, CD11c, I-Ab. Cell surface antibody staining was done in PBS containing 1% FBS, and intracellular cytokine staining was done as described above. Antibody-stained cells were analyzed on a FACSCanto flow cytometer (BDBiosciences, Mountain View, CA) and results analyzed using FlowJo software (TreeStar).
Results
Heterologous rechallenge results in potent boosting of CD4+ memory T cells
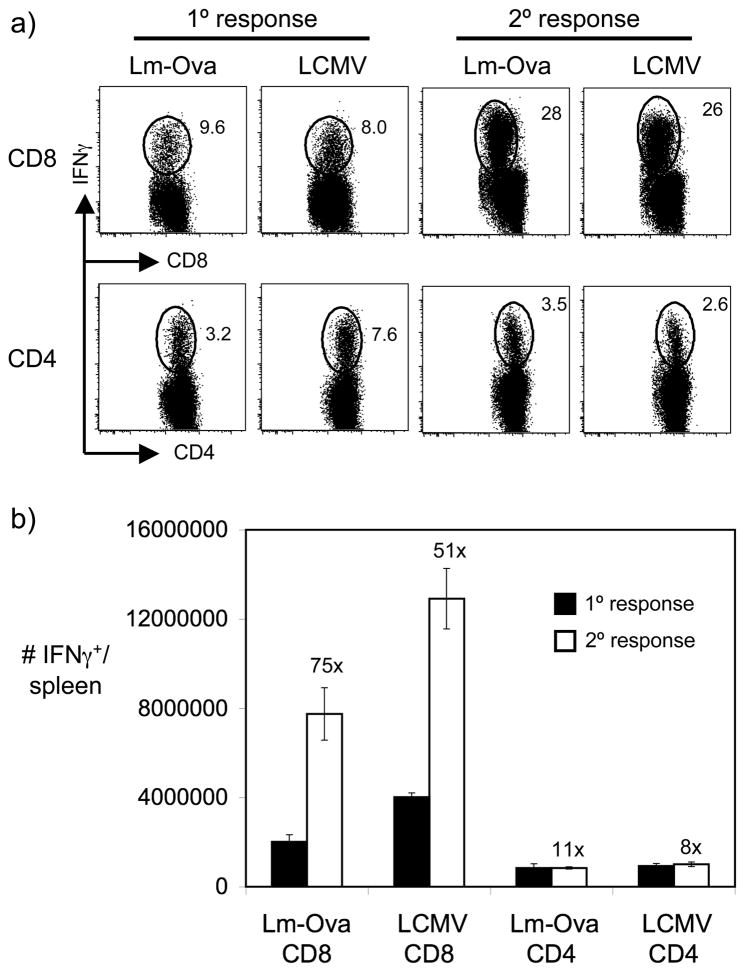
We directly compared the secondary responses of CD4+ and CD8+ memory T cells following homologous rechallenge. C57BL/6 (B6) mice were infected with lymphocytic choriomeningitis virus (LCMV)3 or a recombinant Listeria monocytogenes expressing secreted Ovalbumin (Lm-Ova). At days 8 (peak of the primary response) and 42 (establishment of memory) post-infection, immunodominant CD8+ T cell responses (as measured by the GP33–41-specific response for LCMV and the Ova257–264-specific response for Lm-Ova) and CD4+ T cell responses (as measured by the GP61–80-specific response for LCMV and the LLO190–201-specific response for Lm-Ova) in the spleen were measured by ex vivo peptide restimulation followed by intracellular cytokine staining (Fig. 1). Lm-Ova-immune and LCMV-immune mice were then given a high-dose homologous rechallenge, and T cell recall responses were analyzed at the peak of the recall response (day 5 post-rechallenge). At rechallenge doses sufficient to potently boost CD8+ T cell responses beyond the initial primary response, secondary CD4+ T cell responses were comparatively poor, with no boosting beyond the levels of the initial primary response (Fig. 1). The poor recall responses observed for CD4+ T cells cannot be simply attributed to delayed secondary responses, as analysis of later times points revealed that day 5 represented the peak of the secondary CD4+ T cell response (data not shown). These findings suggested that in these model systems CD4+ T cells were not boosted at all, as seen in the comparison of peak primary and secondary CD4+ T cell responses (Fig 1B). We sought to better understand the underlying factors promoting robust CD4+ T cell recall responses, as resolution of these factors could play an important role in the design of vaccination strategies targeting CD4+ T cells.
Figure 1.
Homologous rechallenge results in robust boosting of CD8+ memory T cells but poor boosting of CD4+ memory T cells. We infected B6 mice with Lm-Ova (2 × 103 CFU i.v.) or LCMV Armstrong (2 × 105 PFU i.p.) and analyzed their primary response 8 days post-infection. At > 6 weeks post-infection, Lm-Ova-immune mice were rechallenged with Lm-Ova (1 × 105 CFU i.v.) and LCMV-immune mice were rechallenged with LCMV Armstrong (2 × 106 PFU i.v.). Secondary responses were assessed 5 days post-rechallenge. A) Representative flow plots depict the frequency of IFNγ-producing CD8+ (specific for Ova257–264 (Lm-Ova CD8) or LCMV GP33–41 (LCMV CD8), respectively) or CD4+ (specific for LLO190–201 (Lm-Ova CD4) or LCMV GP61–80 (LCMV CD4), respectively) T cells in the spleen 8 days after primary challenge with either Lm-Ova or LCMV or 5 days after homologous secondary challenge. B) The graph displays the number of IFNγ-producing CD8+ or CD4+ T cells in the spleen specific for each of the above epitopes. Error bars are the SEM (n=3). Results are representative of three separate experiments.
We formulated several non-exclusive hypotheses to explain these differences. First, memory CD4+ T cell expansion may be intrinsically limited. Second, CD8+ T cells may out-compete CD4+ T cell for resources or access to APCs during secondary expansion. Third, secondary CD4+ T cell expansion may be limited at the population level by intra- or inter-clonal competition. Lastly, because secondary challenges are rapidly cleared by recall CTL responses, antigen levels or the shortened kinetics of antigen presentation may be insufficient to induce robust and prolonged secondary CD4+ T cell expansion.
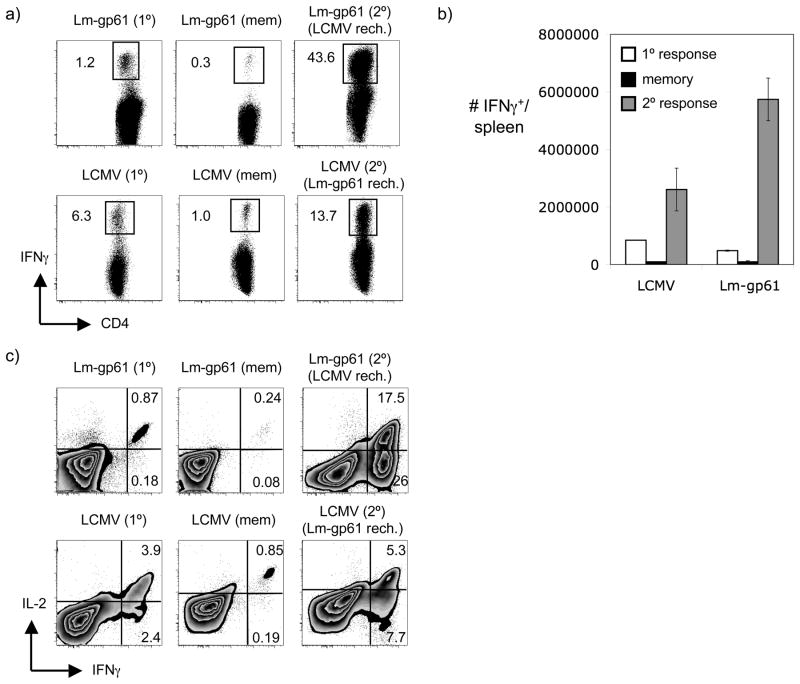
To determine whether CD4+ memory T cells were capable of secondary expansion following a more robust secondary challenge that was not rapidly cleared, we adopted a model of heterologous rechallenge. We immunized mice with either LCMV or a recombinant Listeria sharing a single CD4+ T cell epitope with LCMV (Lm-gp61). Following the establishment of memory (>42 days post-infection), mice were given a reciprocal rechallenge with either Lm-gp61 or LCMV and their CD4+ T cell recall responses were analyzed at their peak (day 7). Rechallenge of Lm-gp61-immune mice with LCMV resulted in massive expansion of CD4+ memory T cells (Fig. 2A–B) similar to that seen for CD8+ T cell recall responses following homologous rechallenge. Similarly, rechallenge of LCMV-immune mice with Lm-gp61 resulted in robust, though more modest, expansion of CD4+ memory T cells (Fig. 2A–B). Importantly, while homologous rechallenge failed to significantly boost CD4+ T cell responses beyond the levels seen after primary challenge, heterologous rechallenge resulted in a significant boosting of the CD4+ T cell response beyond that seen for the primary response. Heterologous rechallenges were characterized by the emergence of IFNγ-only producing effector cells, whereas, the primary response was characterized by the dominance of effector cells capable of producing both IFNγ and IL-2 upon antigen stimulation (Fig. 2C). These results demonstrate that under the appropriate rechallenge conditions CD4+ memory T cells are capable of equally robust secondary responses as CD8+ memory T cells. At least in the context of rechallenge of an already immune host, CD4+ memory T cells did not display any intrinsic limitation in their ability to divide and accumulate as compared to CD8+ memory T cells.
Figure 2.
Heterologous rechallenge results in potent boosting of CD4+ memory T cell responses. We infected B6 mice with Lm-gp61 (2 × 105 CFU i.v.) or LCMV Armstrong (2 × 105 PFU i.p.) and analyzed their LCMV GP61–80-specific CD4+ T cell response at the peak of the primary response (8 days post-infection) and following the establishment of memory (> 6 weeks post-infection). LCMV-immune mice received a heterologous rechallenge with Lm-gp61 (2 × 105 CFU i.v.) while Lm-gp61-immune mice received a heterologous rechallenge with LCMV (2 × 105 PFU i.p.). CD4+ T cell recall responses were analyzed at their peak (7 days post-rechallenge). A) Representative flow plots depict the frequency of IFNγ-producing CD4+ T cells in the spleen specific for LCMV GP61–80 following infection with either Lm-gp61, LCMV or after heterologous rechallenge. B) The bar graph depicts the number of GP61–80-specific IFNγ-producing CD4+ T cells in the spleen at the peak of the primary response, at the establishment of memory and following heterologous rechallenge. C) Representative flow plots depict the frequencies of IFNγ-producing and IL-2-producing CD4+ T cells specific for GP61–80 at the same time points. Error bars represent the SEM (n=3) and the results are representative of at least four independent experiments.
CD4+ and CD8+ T cells do not compete for resources following secondary challenge
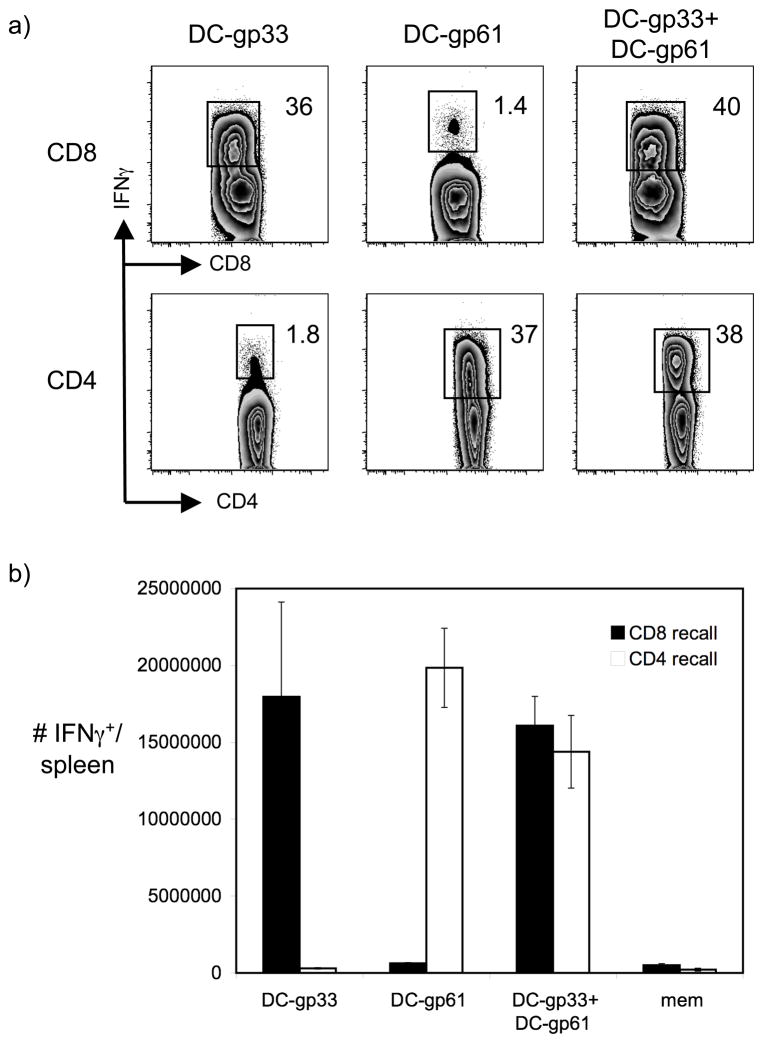
We next sought to determine why CD4+ memory T cells failed to respond well following homologous rechallenge but expanded robustly following heterologous rechallenge. Again we considered several possibilities. Because the heterologous rechallenges specifically and uniquely boosted a CD4+ T cell response without an accompanying CD8+ T cell recall response, we considered the possibility that robust boosting of CD8+ and CD4+ T cells would result in competition for resources such as growth or inflammatory factors such as IFNγ (28). In this setting, CD4+ T cell recall responses could be generally inhibited and out-competed in the presence of a CD8+ T cell recall response. To address this possibility, we generated a model system of DC immunization in which CD4+ and CD8+ T cells could be rechallenged simultaneously in the presence of a bystander acute infection. We injected mice with B16 cells secreting Flt3L and two weeks later isolated DCs via transient adherence. DCs were loaded with either the gp33–41 immunodominant LCMV Class I-restricted peptide or the gp61–80 immunodominant LCMV Class II-restricted peptide and injected into LCMV-immune mice along with a wildtype Listeria monocytogenes (Lm) infection. Injection of either DC subset selectively and potently induced CD8+ or CD4+ T cell recall responses, respectively, while injection of both DC subsets simultaneously induced both CD8+ and CD4+ T cell recall responses in the same host (Fig. 3). These findings confirm that even robust CD8+ T cell recall responses do not out-compete CD4+ T cell recall responses for resources and growth factors. However, they leave open the possibility that large numbers of responding CD8+ T cells could prevent sufficient access to APCs by CD4+ T cells.
Figure 3.
Simultaneous boosting of CD8+ and CD4+ memory T cells does not result in competition for APCs or resources. We rechallenged LCMV-immune mice with 1 × 106 DCs loaded with either LCMV GP33–41 or GP61–80 peptide, as indicated, on days 0, 2 and 4. Recipients also received a wildtype Lm infection on day 0 (2 × 103 CFU i.v.) to provide bystander inflammation. Recall responses were assessed on day 7. A) Representative flow plots display the frequency of IFNγ-producing cells specific for GP33–41 (CD8) or GP61–80 (CD4) following rechallenge with DCs loaded with GP33–41, DCs loaded with GP61–80 or both. B) The graph depicts the number of GP33–41-specific (CD8 recall) or GP61–80-specific (CD4 recall) IFNγ-producing T cells in the spleen following DC rechallenge. Error bars are the SEM (n=3–4) and the results are representative of two independent experiments.
Increasing the rechallenge stimulus results in enhanced CD4+ T cell recall responses
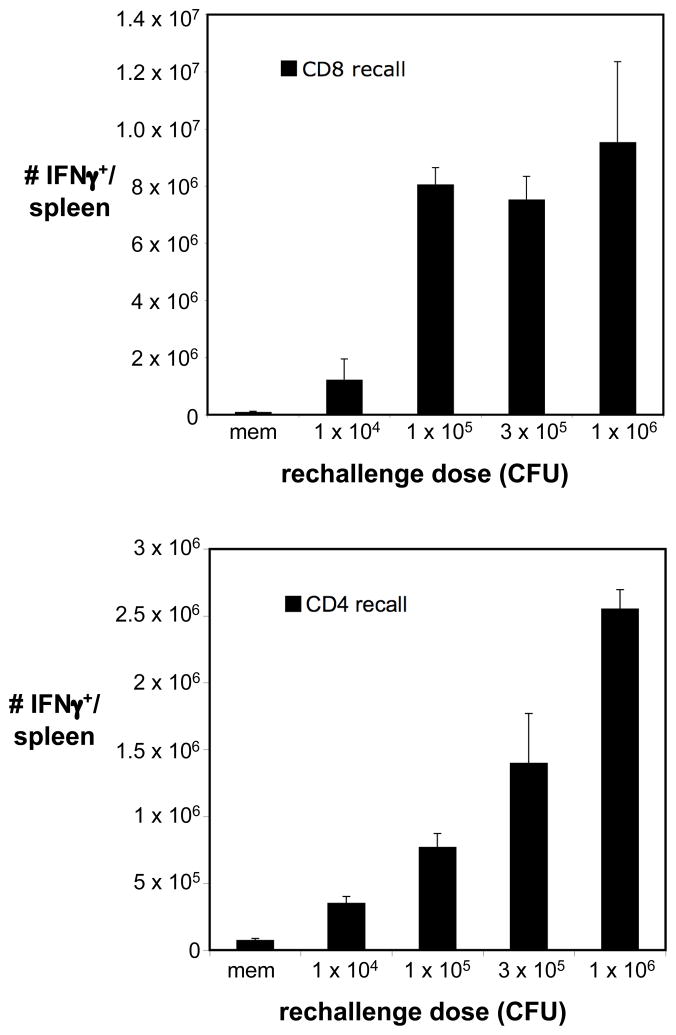
We next hypothesized that the discrepancies between CD4+ and CD8+ T cell recall responses following recall responses could be due to differential stimulatory requirements. Because CD8+ T cell recall responses rapidly cleared secondary challenges in these settings, one possible consequence was an insufficient duration or potency of stimulus to induce robust CD4+ T cell recall responses. This might involve an increased reliance on antigen, as has been demonstrated for primary CD4+ T cell responses, or an increased need for inflammatory stimuli that are limited by rapid clearance mediated by CD8+ T cell recall responses. As an initial approach to address this possibility, we gave Lm-Ova-immune mice a homologous rechallenge with increasing doses of Lm-Ova. CD8+ T cell recall responses directed toward Ova257–264 were potently induced following rechallenge with 1 × 105 CFU Lm-Ova, and increasing the rechallenge dose beyond these levels did not significantly enhance the magnitude of this response (Fig. 4A). In contrast, increasing the rechallenge dose progressively and significantly boosted CD4+ T cell recall responses directed towards LLO190–201. These results demonstrate that rechallenge doses sufficient to recruit a maximally potent CD8+ T cell recall response only weakly recruit CD4+ T cell recall responses and suggest that CD4+ memory T cells are tightly regulated by exposure to the secondary stimulus. CD8+ T cells are also likely to be tightly regulated by access to antigen, and these findings do not rule out the possibility that MHC Class I-restricted responses are more readily induced at lower infectious doses due to wider distribution of MHC Class I expression and antigen presentation. Conversely, dendritic cells are required for initiating robust recall responses (29), suggesting that at least during the initiation of secondary responses, antigen presentation to CD8+ and CD4+ memory T cells is similar. In either case, our findings suggest that in the context of an in vivo secondary challenge CD4+ memory T cell recall responses display a higher activation threshold as it relates to infectious dose than do CD8+ T cells.
Figure 4.
Increasing the homologous rechallenge dose selectively boosts CD4+ T cell recall responses. Lm-Ova immune mice (> 6 weeks post-infection) received a secondary Lm-Ova challenge over a range of doses, as indicated. Ova257–264-specific (CD8 recall) and LLO190–201-specific (CD4 recall) responses were assessed in the spleen five days later based on the frequencies of IFNγ-producing T cells. Error bars represent the SEM (n=3–4).
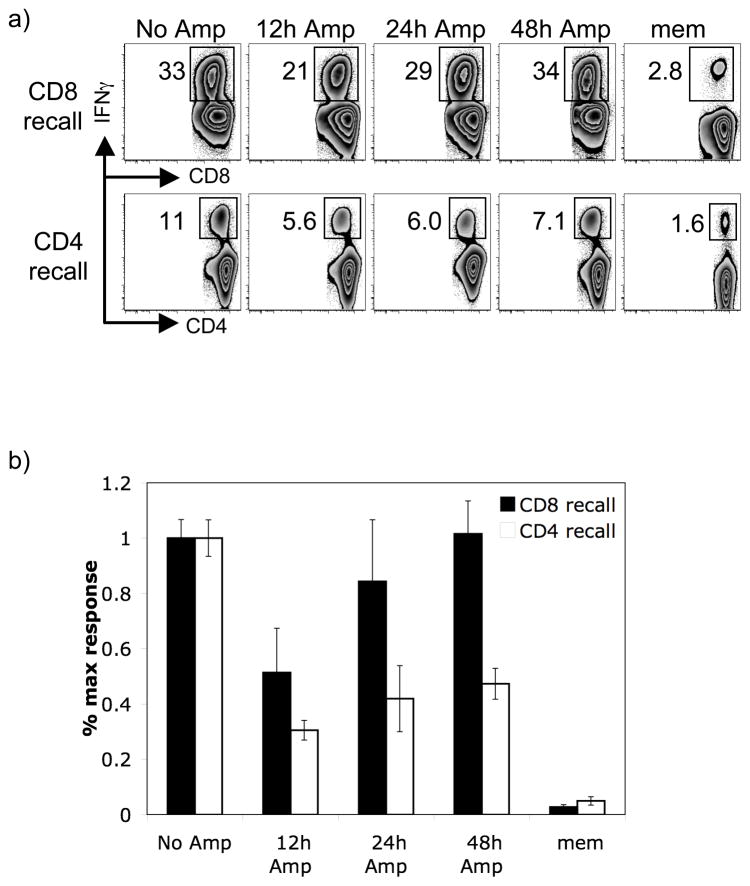
CD4 recall responses require a longer stimulatory period than CD8 recall responses
As a second approach to assessing the impact of the strength or duration of the secondary stimulus in controlling CD4+ T cell recall responses, we artificially limited the duration of the secondary infection following heterologous rechallenge of either CD4+ or CD8+ T cell recall responses. We rechallenged LCMV-immune mice with either Lm-gp61 to boost CD4+ T cell recall responses or a recombinant Listeria secreting the LCMV gp33–41 peptide (Lm-gp33) to boost CD8+ T cell recall responses. Mice were treated with ampicillin to limit the course of infection at 12, 24 or 48 hours post-infection. Ampicillin treatment in our hands resulted in bacterial clearance within 24 hours (data not shown). Furthermore, using previously described methods (7), we assessed antigen presentation by transferring LCMV glycoprotein-specific CFSE labeled TCR transgenic T cells (P14 T cells following Lm-gp33 infection or SMARTA T cells following Lm-gp61 infection) into ampicillin-treated mice at various time points post-treatment. TCR transgenic T cells failed to dilute CFSE when transferred 48 hours after ampicillin treatment, indicating minimal antigen presentation (data not shown).
While CD8+ T cell recall responses neared the maximal response with ampicillin treatment as early as 24 hours post-infection, CD4+ T cell responses were significantly lower even when ampicillin treatment began as late as 48 hours post-infection (Fig. 5). In comparison, primary responses analyzed at the same time demonstrated a highly similar pattern for both CD8+ (GP33–41-specific) and CD4+ (GP61–80-specific) T cells (data not shown), agreeing with a previously published report (7). These results are similar to previous studies of primary CD4+ and CD8+ T cell responses and again suggest that CD4+ T cell recall responses require an extended stimulatory period as compared to CD8+ T cell recall responses.
Figure 5.
Shortening the infectious period selectively blunts CD4+ T cell recall responses. We rechallenged LCMV-immune mice (> 6 weeks post-infection) with 2 × 105 CFU Lm-gp33 (to induce a CD8+ T cell recall response) or Lm-gp61 (to induce a CD4+ T cell recall response). Mice were treated with ampicillin at various time points post-rechallenge, as indicated, and recall responses were assessed at their peak (day 7 post-rechallenge) based on the frequency of IFNγ-producing responders. A) Representative flow plots depict the frequency of GP33–41-specific CD8+ T cells following Lm-gp33 rechallenge (CD8 recall) and the frequency of GP61–80-specific CD4+ T cells following Lm-gp61 rechallenge (CD4 recall), along with the indicated time at which ampicillin treatment began. B) The impact of ampicillin treatment on the CD8 and CD4 recall responses, respectively, are depicted as the percent maximal response, defined as the fraction of responders in the spleen at the peak of the recall response as compared to the recall response in the untreated control groups. Measurements are based on the absolute numbers of IFNγ-producing cells in the spleen for each epitope. Error bars are the SEM (n=3) and results are representative of two separate experiments.
Secondary proliferation of CD4+ memory T cells is intrinsically limited
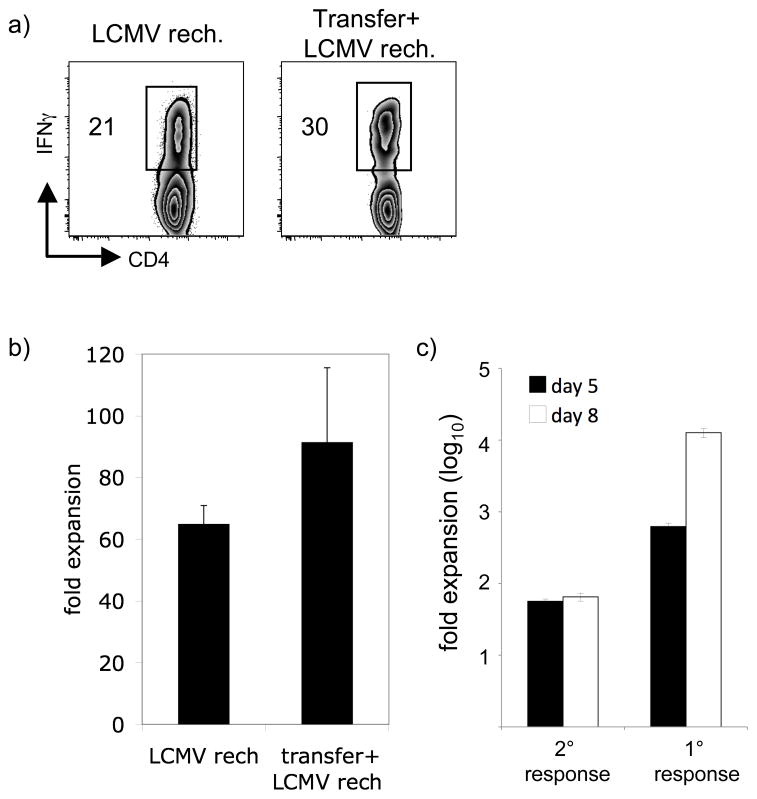
Lastly, we sought to determine the extent to which clonal competition could inhibit the generation of robust CD4+ T cell recall responses. Competition among activated monoclonal CD4+ T cells has been shown to prevent adequate access to antigen, resulting in blunted CD4+ T cell responses (20). While the CD4+ T cell recall responses analyzed in this study are polyclonal, we hypothesized that competition for access to the same antigen when dose is limiting (as during homologous rechallenge) could prevent optimal CD4+ T cell secondary expansion. Conversely, when dose is not limiting (as during heterologous rechallenge) secondary expansion could proceed unimpeded. However, even under the best stimulatory conditions antigen-specific recall responses represent only a 50–100-fold expansion over memory levels. In contrast, primary expansion of CD4+ T cells to LCMV infection has been estimated at >10,000-fold, as based on enumeration of naïve precursors (30, 31). The factors that limit secondary expansion are unclear, particularly given that the kinetics of infection following heterologous boosting of CD4+ T cell recall responses are similar to that of the primary infection.
We hypothesized that secondary expansion of CD4+ T cells was limited by competition for antigen or resources. To address this issue, we transferred CD4+ T cells from Lm-gp61 immune B6 (CD45.2+) mice into naïve B6.SJL (CD45.1+) congenic hosts, followed by challenge with LCMV. The size of the transfer was designed to generate precursor frequencies of gp61–80-specific CD4+ memory cells approaching endogenous levels of naïve precursor cells. Based on our analysis of the frequency of antigen-specific IFNγ-producing memory cells in the initial transfer, as well as the measurement of the “take” of the initial transfer one day later, we estimated a total of ~600–800 GP61–80-specific memory cells in the sleens of secondary hosts prior to challenge. The frerquency of naïve endogenous responders specific for this epitope has been estimeted at 100–200 (31). This system therefore allowed us to analyze primary and secondary responses in the same host following LCMV infection while mitigating the effects of competition for antigen among the two populations. We challenged mice with LCMV and analyzed secondary responses in the spleen eight days later. Following LCMV heterologous rechallenge of Lm-gp61-immune hosts, CD4+ T cell recall responses expanded robustly (60–70)-fold (Fig. 6A–B). While LCMV rechallenge of B6.SJL hosts that had received CD4+ memory T cells also resulted in secondary expansion (80–90-fold), it only marginally exceeded the expansion seen following rechallenge of Lm-gp61-immune hosts (Fig. 6A–B). These results suggested an intrinsic limit to the recall potential of CD4+ memory T cells even in the presence of an abundant secondary stimulus. To rule out the possibility that secondary responses were peaking earlier, we repeated the experiments and analyzed recall responses at day 5 and day 8 post-challenge. CD4+ memory T cells did not display any significant differences in their recall responses at day 5 or day 8, expanding to a maximum of ~60-fold. In contrast primary responders, based on an estimated precursor frequency of 200, expanded almost 1,000-fold by day 5 and >10,000-fold by day 8 (Fig. 6C). These findings suggest that secondary responses by CD4+ memory T cells are intrisically curtailed and parallel another recent study suggesting an intrinsic limit in secondary CD4+ T cell expansion (22). While our prior results indicate that enhancing the secondary stimulus can propel a CD4 recall response towards its upper limits, these findings clearly indicate that an upper limit does indeed exist. It will be critical in future studies to define the parameters that both promote and regulate secondary expansion of CD4+ memory T cells.
Figure 6.
CD4+ memory T cells display an intrinsic limit to their recall capacity. Lm-gp61-immune B6 mice (CD45.2+) were either rechallenged with LCMV as before, or their CD4+ T cells were harvested and transferred (1 × 106) into naïve B6.SJL congenic hosts (CD45.1+). Adoptive transfer recipients were infected with LCMV one day later. The GP61–80-specific CD4+ T cell recall response was then assessed. A) Representative flow plots indicate the frequency of IFN γ-producing CD4+ T cell specific for GP61–80. The plot on the left depicts the recall response of LM-gp61-immune mice rechallenged with LCMV, while the plot on the left depicts the recall response of donor CD4+ memory T cells following adoptive transfer (gated on CD45.2+ donor cells). B) The graph displays the relative fold expansion of CD4+ memory T cells following rechallenge with or without adoptive transfer. C) Fold expansion of secondary and primary responders in the same host is compared at days 5 and 8 post-challenge. Fold expansion is based on the measured frequency of memory cells prior to challenge and an estimated frequency of endogenous naïve cells of 200. Error bars are the SEM (n=3).
Discussion
Our findings highlight key differences between the stimulatory requirements for inducing secondary expansion of CD4+ and CD8+ T cells and parallel differences seen in the generation of primary responses. In those earlier studies, an extended infectious period (as compared to that needed for CD8+ T cells) was required for the full expansion of antigen-specific CD4+ T cells (7). Furthermore, expansion in the setting of primary stimulation was found to be dependent on the continued presences of antigen (6). Importantly, while the size of the response was altered, the differentiation of effector function appeared to be independent of the duration of infection (7). In the present study, we find that secondary CD4+ T cell responses are similarly dependent on the duration of infection for their secondary expansion. Therefore, in settings where antigen is cleared quickly, such as following a homologous rechallenge, CD8+ T cell recall responses are efficiently generated whereas CD4+ T cell recall responses are poor. In contrast, heterologous rechallenge with a pathogen sharing only a CD4+ T cell epitope results in an infectious time course similar to a primary challenge. We conclude, therefore, that the differing magnitude of the CD4+ T cell recall response induced following heterologous vs. homologous rechallenge reflects the pace of CD8+ T cell-mediated pathogen clearance.
One important unknown arising from these studies is the mechanism behind the differential requirements for robust secondary expansion. CD4+ memory T cells have a demonstrably lower activation threshold at the level of TCR signaling than their naïve counterparts (32). However, CD4+ T cells may have an intrinsically lower proliferation rate than CD8+ T cells (19), and their clonal expansion, at least during the primary response, is mildly delayed as compared to CD8+ T cells (31, 33). While it is difficult to directly compare antigen sensitivity of CD4+ and CD8+ T cells, one possibility is that CD4+ T cells in general have a higher activation threshold for activation and/or a continued dependence on antigen for clonal expansion. From the standpoint of preventing auto-reactive or immunopathologic responses, such tight regulation of CD4+ T cells play an important immunoregulatory function. On the other hand, the dependence by CD4+ memory T cells on an extended infectious period in order to mount robust recall responses may reflect a role for inflammatory or co-stimulatory factors in driving their continued expansion, independent of the continued presence of antigen. For example, Type I IFNs drive clonal expansion of Th1 cells in some settings (34), while OX-40 co-stimulation plays a role in promoting CD4+ T cell recall responses (35). Whether CD4 expansion is driven by antigen availability or non-specific access to inflammatory, growth or co-stimulatory factors, intra- and/or inter-clonal competition likely plays an important role in regulating the magnitude of the CD4+ T cell recall response. High frequencies of clonal populations of T cells can also outcompete CD8+ T cell responses to the same epitope. To a much lesser extent large frequencies CD8+ T cell responders may inhibit the response to other Class I-restricted epitopes (36–39). While there is no current evidence that large numbers of CD8+ T cells can efficiently out-compete Class II-restricted T cell responses, our findings do not rule out the possibility that in the presence of robust CD8+ recall responses, CD4+ memory T cells do not have sufficient access to APCs to induce optimal recall responses.
While our findings suggest an important role for the duration of secondary infection in promoting secondary CD4 responses, they do not rule out a role for the strength of the initial antigenic stimulus in driving secondary responses. Antigen dose is extremely important in the differentiation of CD4+ T cells, and we have recently found evidence for the role of antigen dose in CD4+ effector and memory T cell differentiation (8). We hypothesize that they both antigen dose and the duration of infection play important roles in primary and secondary CD4 differentation. Because disrupting the duration of antigen presentation during secondary responses impairs the secondary CD4+ T cell response, we have concluded that the duration of infection is one factor in the induction of robust CD4 recall responses. These findings correspond to the original homologous rechallenges in which the secondary infection is shortened due to rapid CTL-mediated clearance. But it is possible, even likely, that increasing the antigen dose during the early stages of infection could compensate for the shortened infectious period. Also, these results do not distinguish the relative roles of continued antigen presentation and the inflammatory environment induced by the infection.
A striking difference between the poor CD4+ T cell recall responses induced by homologous rechallenge and the robust recall responses induced by heterologous rechallenge is the cytokine-producing profile. In particular, heterologous rechallenge promotes the accumulation of CD4+ effector T cells capable of producing IFNγ but not IL-2. Recent studies have demonstrated that the CD4+ memory T cells capable of the most robust secondary protection maintain the ability to produce IFNγ, TNFα and IL-2, whereas the absence of IL-2- or TNFa-producing capability signals a reduced recall capacity (40, 41). An important question will be the extent to which the observed skewing towards IFNγ-only producers following heterologous challenge impacts the make-up and functionality of ensuing memory populations.
One recent study suggested that CD4+ memory cells are intrinsically less capable of clonal expansion than their naïve counterparts (22). We also find that CD4+ memory T cell are limited in their expansion even following heterologous challenge and when present at frequencies approaching naïve responders. While limited exposure to antigen during homologous rechallenge limits that ability of CD4+ T cells to reach the upper limit of their recall potential, we also suggest that there is an upper limit. These findings are not mutually exclusive but represent two aspects that regulate CD4 recall responses. While our data suggests that the duration of infection may impact the magnitude of secondary expansion, it does not suggest that there is no upper limit to that expansion. Rather, we find that secondary expansion by CD4+ T cells is intrinsically controlled. Naïve responders expanded 10,000–20,000-fold following infection with LCMV, while memory responders in the same host present at similar frequencies expanded 50–100-fold, a similar level of expansion seen following heterologous rechallenge but severely curtailed when compared to the primary response in the same host. These findings lead to important questions regarding the biology of CD4+ T cell recall responses. For instance, is there a combined limit of expansion following primary activation and subsequent boosts, or does the proliferative potential of the ensuing memory population “reset” after each challenge? How do successive boosts impact the protective function of CD4+ memory T cells? And lastly, in the absence of competition for antigen or resources, what are the mechanisms that control the magnitude of secondary CD4+ T cell responses?
Importantly, because our findings suggest that the optimal conditions for boosting CD4+ or CD8+ T cell responses differ substantially, they have relevance for the design of vaccination strategies aimed at specifically boosting CD4+ T cell responses. Vaccines that simultaneously attempt to boost CD4+ and CD8+ T cell responses may be inefficient in promoting optimal secondary expansion of CD4+ memory T cells due to rapid clearance of antigen by CTL recall responses.
Footnotes
Abbreviations used in this manuscript: C57BL/6, B6; B6.SJL-PtprcaPepcb/BoyJ, B6.SJL; lymphocytic choriomeningitis virus (LCMV); Listeria monocytogenes expressing secreted Ovalbumin, Lm-Ova; Listeria monocytogenes expressing LCMV GP61–80, Lm-gp61; Listeria monocytogenes expressing LCMV GP33–41, Lm-gp33; Listeria monocytogenes, Lm; plaque forming unit, PFU; colony forming unit, CFU; intraperitoneal, i.p.; intravenous, i.v.
This work was supported by the NIH (K22AI071112) and the Department of Pathology at the University of Utah.
References
- 1.Williams MA, Bevan MJ. Effector and Memory CTL Differentiation. Annual Review of Immunology. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 2.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mercado R, Vijh S, Allen SE, Kerksiek K, Pilip IM, Pamer EG. Early Programming of T Cell Populations Responding to Bacterial Infection. J Immunol. 2000;165:6833–6839. doi: 10.4049/jimmunol.165.12.6833. [DOI] [PubMed] [Google Scholar]
- 4.Prlic M, Hernandez-Hoyos G, Bevan MJ. Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response. J Exp Med. 2006;203:2135–2143. doi: 10.1084/jem.20060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Stipdonk MJB, Hardenberg G, Bijker MS, Lemmens EE, Droin NM, Green DR, Schoenberger SP. Dynamic programming of CD8+ T lymphocyte responses. Nat Immunol. 2003;4:361–365. doi: 10.1038/ni912. [DOI] [PubMed] [Google Scholar]
- 6.Obst R, van Santen HM, Mathis D, Benoist C. Antigen persistence is required throughout the expansion phase of a CD4+ T cell response. J Exp Med. 2005;201:1555–1565. doi: 10.1084/jem.20042521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams MA, Bevan MJ. Shortening the Infectious Period Does Not Alter Expansion of CD8 T Cells but Diminishes Their Capacity to Differentiate into Memory Cells. J Immunol. 2004;173:6694–6702. doi: 10.4049/jimmunol.173.11.6694. [DOI] [PubMed] [Google Scholar]
- 8.Williams MA, Ravkov EV, Bevan MJ. Rapid culling of the CD4+ T cell repertoire in the transition from effector to memory. Immunity. 2008;28:533–545. doi: 10.1016/j.immuni.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prlic M, Williams MA, Bevan MJ. Requirements for CD8 T-cell priming, memory generation and maintenance. Current Opinion in Immunology. 2007;19:315–319. doi: 10.1016/j.coi.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nat Immunol. 2003;4:355–360. doi: 10.1038/ni908. [DOI] [PubMed] [Google Scholar]
- 11.Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat Rev Immunol. 2002;2:982–987. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- 12.Homann D, Teyton L, Oldstone MBA. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat Med. 2001;7:913–919. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- 13.McKinstry KK, Strutt TM, Swain SL. The effector to memory transition of CD4 T cells. Immunol Res. 2008;40:114–127. doi: 10.1007/s12026-007-8004-y. [DOI] [PubMed] [Google Scholar]
- 14.Schiemann M, Busch V, Linkemann K, Huster KM, Busch DH. Differences in maintenance of CD8+ and CD4+ bacteria-specific effector-memory T cell populations. Eur J Immunol. 2003;33:2875–2885. doi: 10.1002/eji.200324224. [DOI] [PubMed] [Google Scholar]
- 15.Robertson JM, MacLeod M, Marsden VS, Kappler JW, Marrack P. Not all CD4+ memory T cells are long lived. Immunological Reviews. 2006;211:49–57. doi: 10.1111/j.0105-2896.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 16.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 17.Purton JF, Tan JT, Rubinstein MP, Kim DM, Sprent J, Surh CD. Antiviral CD4+ memory T cells are IL-15 dependent. J Exp Med. 2007;204:951–961. doi: 10.1084/jem.20061805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunological Reviews. 2006;211:154–163. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 19.Foulds KE, Zenewicz LA, Shedlock DJ, Jiang J, Troy AE, Shen H. Cutting Edge: CD4 and CD8 T Cells Are Intrinsically Different in Their Proliferative Responses. J Immunol. 2002;168:1528–1532. doi: 10.4049/jimmunol.168.4.1528. [DOI] [PubMed] [Google Scholar]
- 20.Foulds KE, Shen H. Clonal Competition Inhibits the Proliferation and Differentiation of Adoptively Transferred TCR Transgenic CD4 T Cells in Response to Infection. J Immunol. 2006;176:3037–3043. doi: 10.4049/jimmunol.176.5.3037. [DOI] [PubMed] [Google Scholar]
- 21.Troy AE, Shen H. Cutting Edge: Homeostatic Proliferation of Peripheral T Lymphocytes Is Regulated by Clonal Competition. J Immunol. 2003;170:672–676. doi: 10.4049/jimmunol.170.2.672. [DOI] [PubMed] [Google Scholar]
- 22.MacLeod MK, McKee A, Crawford F, White J, Kappler J, Marrack P. CD4 memory T cells divide poorly in response to antigen because of their cytokine profile. Proc Natl Acad Sci U S A. 2008;105:14521–14526. doi: 10.1073/pnas.0807449105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen H, Slifka MK, Matloubian M, Jensen ER, Ahmed R, Miller JF. Recombinant Listeria monocytogenes as a live vaccine vehicle for the induction of protective anti-viral cell-mediated immunity. Proc Natl Acad Sci U S A. 1995;92:3987–3991. doi: 10.1073/pnas.92.9.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slifka MK, Shen H, Matloubian M, Jensen ER, Miller JF, Ahmed R. Antiviral cytotoxic T-cell memory by vaccination with recombinant Listeria monocytogenes. J Virol. 1996;70:2902–2910. doi: 10.1128/jvi.70.5.2902-2910.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pope C, Kim SK, Marzo A, Masopust D, Williams K, Jiang J, Shen H, Lefrancois L. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J Immunol. 2001;166:3402–3409. doi: 10.4049/jimmunol.166.5.3402. [DOI] [PubMed] [Google Scholar]
- 27.Mempel TR, Henrickson SE, von Andrian UH. T-cell priming by dendriticcells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 28.Whitmire JK, Benning N, Eam B, Whitton JL. Increasing the CD4+ T cell precursor frequency leads to competition for IFN-gamma thereby degrading memory cell quantity and quality. J Immunol. 2008;180:6777–6785. doi: 10.4049/jimmunol.180.10.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zammit DJ, Cauley LS, Pham QM, Lefrancois L. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity. 2005;22:561–570. doi: 10.1016/j.immuni.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitmire JK, Benning N, Whitton JL. Precursor Frequency, Nonlinear Proliferation, and Functional Maturation of Virus-Specific CD4+ T Cells. J Immunol. 2006;176:3028–3036. doi: 10.4049/jimmunol.176.5.3028. [DOI] [PubMed] [Google Scholar]
- 32.Chandok MR, Okoye FI, Ndejembi MP, Farber DL. A biochemical signature for rapid recall of memory CD4 T cells. J Immunol. 2007;179:3689–3698. doi: 10.4049/jimmunol.179.6.3689. [DOI] [PubMed] [Google Scholar]
- 33.Jelley-Gibbs DM, Lepak NM, Yen M, Swain SL. Two Distinct Stages in the Transition from Naive CD4 T Cells to Effectors, Early Antigen-Dependent and Late Cytokine-Driven Expansion and Differentiation. J Immunol. 2000;165:5017–5026. doi: 10.4049/jimmunol.165.9.5017. [DOI] [PubMed] [Google Scholar]
- 34.Havenar-Daughton C, Kolumam GA, Murali-Krishna K. Cutting Edge: The Direct Action of Type I IFN on CD4 T Cells Is Critical for Sustaining Clonal Expansion in Response to a Viral but Not a Bacterial Infection. J Immunol. 2006;176:3315–3319. doi: 10.4049/jimmunol.176.6.3315. [DOI] [PubMed] [Google Scholar]
- 35.Dawicki W, Bertram EM, Sharpe AH, Watts TH. 4–1BB and OX40 Act Independently to Facilitate Robust CD8 and CD4 Recall Responses. J Immunol. 2004;173:5944–5951. doi: 10.4049/jimmunol.173.10.5944. [DOI] [PubMed] [Google Scholar]
- 36.Butz E, Bevan MJ. Dynamics of the CD8+ T cell response during acute LCMV infection. Adv Exp Med Biol. 1998;452:111–122. doi: 10.1007/978-1-4615-5355-7_13. [DOI] [PubMed] [Google Scholar]
- 37.Kedl RM, Schaefer BC, Kappler JW, Marrack P. T cells down-modulate peptide-MHC complexes on APCs in vivo. Nat Immunol. 2002;3:27–32. doi: 10.1038/ni742. [DOI] [PubMed] [Google Scholar]
- 38.Smith AL, Wikstrom ME, Fazekas de St Groth B. Visualizing T cell competition for peptide/MHC complexes: a specific mechanism to minimize the effect of precursor frequency. Immunity. 2000;13:783–794. doi: 10.1016/s1074-7613(00)00076-5. [DOI] [PubMed] [Google Scholar]
- 39.Willis RA, Kappler JW, Marrack PC. CD8 T cell competition for dendritic cells in vivo is an early event in activation. Proc Natl Acad Sci U S A. 2006;103:12063–12068. doi: 10.1073/pnas.0605130103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Darrah PA, Patel DT, De Luca PM, Lindsay RWB, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 41.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]