Abstract
Glycerol-3-phosphate acyltransferase (GPAT) initiates the synthesis of triacylglycerol and phospholipids and, in the process, regulates the formation of several lipid metabolites known to be intracellular signaling molecules. The recent identification of a new GPAT isoform (Cao et al., 2006) suggests a role for GPAT isoforms in nutrient-mediated signaling.
In the pathways of carbohydrate, amino acid, and protein synthesis, it is rare to find that more than a single enzyme catalyzes a specific reaction; with complex lipid synthesis, however, the reverse is true. For example, in the pathway of glycerolipid synthesis outlined in the 1950s and 60s by Eugene Kennedy and colleagues (Kennedy, 1957), every step is catalyzed by two or more isoforms, each encoded by a separate gene (Figure 1). Although the most prominent isoforms may differ across tissues, most tissues express at least two of these isoforms. With the exception of lipin, each enzyme in this pathway is an intrinsic membrane protein containing transmembrane domains and long stretches of hydrophobic amino acids that probably interact closely with both their hydrophobic substrates and the membrane surface. Despite the fact that the catalytic site of each enzyme involved in triacylglycerol (TAG) synthesis faces the cytosol, evidence suggests that the lipid intermediates lysophosphatidic acid (LPA), phosphatidic acid (PA), and diacylglycerol (DAG) are present in nonmingling pools.
Figure 1. Multiple Isoforms Catalyze Each Step in the Synthesis of TAG.
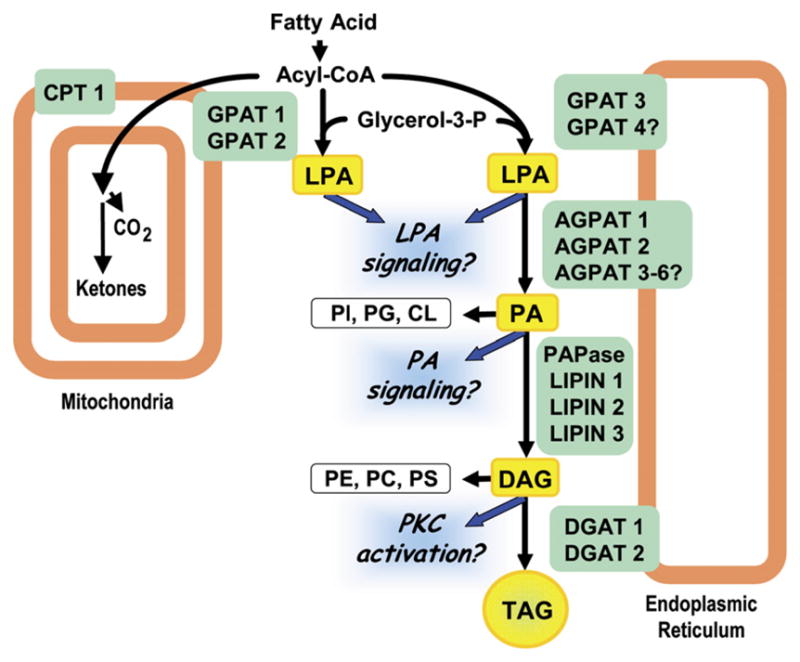
GPAT1 and 2 are located on the outer mitochondrial membrane, where they may compete with carnitine palmitoyltransferase (CPT1) for acyl-CoAs. GPAT3 (as well as other GPATs) is located on the endoplasmic reticulum. The lysophosphatidic acid (LPA) that these GPAT isoforms synthesize is acylated by lysophosphatidic acid acyltransferases (AGPATs) to form phosphatidic acid (PA), the precursor for phosphatidylinositol (PI), phosphatidylglycerol (PG), and cardiolipin (CL). Phosphatidic acid phosphohydrolase (PAPase or LIPIN) converts PA to diacylglycerol (DAG), the precursor of phosphatidylcholine (PC), phosphatidylethanolamine (PE), and phosphatidylserine (PS), and diacylglycerol acyltransferase (DGAT) acylates DAG to form triacylglycerol (TAG).
The committed step in the synthesis of TAG is glycerol-3-phosphate acyltransferase (GPAT), which has traditionally been believed to exist in every cell as two major isoforms, one in the endoplasmic reticulum (ER) and one on the mitochondrial outer membrane. In addition to their subcellular locations, these GPAT isoforms were differentiated by their varying susceptibility to inhibition by sulfhydryl reagents like N-ethylmaleimide and their different preferences for saturated and unsaturated long-chain fatty acyl-CoAs. This picture has now been modified by the recognition that at least three GPAT isoforms are present in most tissues. These include two mitochondrial GPATs (Gonzalez-Baró et al., 2007; Lewin et al., 2004) and a recently cloned ER GPAT (Cao et al., 2006). It has also been noted that the acyl-CoA:lysophosphatidic acid acyl-transferase 6 (AGPAT6) is closely related to GPAT3, implying that it, too, may have GPAT activity (Beigneux et al., 2006; Cao et al., 2006). Furthermore, the activities of other designated AGPAT isoforms have not been confirmed, and they may also express GPAT activity. How are we to understand this plethora of enzymes whose catalytic functions are identical?
GPAT1, the major mitochondrial isoform cloned by Sul and colleagues (Shin et al., 1991), contributes about 10% of total GPAT activity in most tissues and 30%–50% in liver. It is highly regulated by insulin and SREBP-1c (Coleman and Lee, 2004), suggesting an important role in TAG synthesis during dietary caloric excess. In fact, the reduced hepatic TAG content and increased plasma ketone body concentrations in Gpat1−/− mice suggest that GPAT1 normally diverts acyl-CoAs away from β-oxidation and toward TAG synthesis (Hammond et al., 2005).
The second mitochondrial isoform, GPAT2, is expressed at highest levels in testis, where its function remains unexplored. GPAT2 has no acyl-CoA preference, but, like GPAT1, overexpression of GPAT2 in COS-7 cells results in increased incorporation of oleate into TAG, but not phospholipid (Lewin et al., 2004; S. Wang and R.A.C. unpublished data).
In an important publication in the November 2006 issue of the Proceedings of the National Academy of Sciences of the United States of America, Cao et al. (2006) reported the first characterization of an ER GPAT. GPAT3 contains the four motifs common to glycerolipid acyltransferases (Coleman and Lee, 2004), and its mRNA increases dramatically when 3T3-L1 cells differentiate into adipocytes. GPAT3 is most highly expressed in epididymal fat and small intestine in mouse and in thyroid, muscle, heart, testis, and kidney in humans. In both species, GPAT3 expression is notably low in liver, suggesting the presence of yet another ER isoform in that tissue. Overexpression of GPAT3 in HEK293 cells results in an increase in the incorporation of [14C]oleate into TAG, even at very low oleate concentrations.
Thus, each of the GPAT isoforms reported so far increases the incorporation of long-chain fatty acid into TAG, but not phospholipid, and hepatic TAG stores are diminished in Gpat1−/− mice (Hammond et al., 2002). None of these GPAT isoforms appears to initiate the exclusive synthesis of phospholipids; instead, it is likely that the GPAT isoforms serve to control the flux of available LPA, PA, and DAG precursors and that phospholipid synthesis is regulated at the PA and DAG branchpoints.
In liver and certain adipose depots, all three GPAT isoforms are expressed and are presumably translated into functional proteins. Importantly, gain-and loss-of-function studies indicate that their primary role is to synthesize TAG. But if the synthesis of TAG is their only function, why, then, are multiple GPAT isoforms required? Is it possible that each isoform has an additional role in regulating the synthesis of the glycerolipid intermediates that have been well established in other contexts as components of intracellular signaling pathways? LPA, the immediate product of GPAT, interacts with extra-cellular LPA receptors that control cell growth and may act intracellularly to activate PPARγ. PA activates several phosphatases and protein kinases, and DAG activates the conventional and novel isoforms of PKC. The established model has been that these lipid intermediates are released from membrane phospholipids after hormones or cytokines activate phospholipases C, D, and/or A2. Recent studies, however, support the novel concept that signaling pathways involving LPA, PA, and DAG can also be initiated via the de novo pathway of TAG synthesis. It is well known, for example, that exposure to excess fatty acid induces IRS1 phosphorylation and increases insulin resistance (Petersen and Shulman, 2006). An intimate role for GPAT in this process can be inferred because mice lacking GPAT1 are protected against diet-induced hepatic insulin resistance and their livers contain less LPA and DAG and show diminished activation of PKCε (Neschen et al., 2005). In contrast, mice that overexpress hepatic GPAT1 activity show pronounced hepatic insulin resistance with elevated LPA and DAG content (unpublished data).
LPA, PA, and DAG synthesis initiated by each of the GPAT isoforms constitutes a set of independent signaling pathways that are likely to respond to the nutrient or caloric content of the diet and sense changes in total body and cellular fuel metabolism. A focus on the control of both expression and acute changes in enzyme activity of the GPAT isoforms will enhance our understanding of the complex metabolic roles played by nutrients and may identify the signaling links between excess tissue TAG content and insulin resistance.
Acknowledgments
This work was supported by National Institutes of Health grants DK56598 and DK59935.
References
- Beigneux AP, Vergnes L, Qiao X, Quatela S, Davis RG, Watkins SM, Coleman RA, Walzem RL, Philips M, Reue K, Young SG. J Lipid Res. 2006;47:734–744. doi: 10.1194/jlr.M500556-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Li JL, Li D, Tobin JF, Gimeno RE. Proc Natl Acad Sci USA. 2006;103:19695–19700. doi: 10.1073/pnas.0609140103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman RA, Lee DP. Prog Lipid Res. 2004;43:134–176. doi: 10.1016/s0163-7827(03)00051-1. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Baró MR, Lewin TM, Coleman RA. Am J Physiol Gastrointest Liver Physiol. 2007 doi: 10.1152/ajpgi.00553.02006. Published online December 7, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond LE, Gallagher PA, Wang S, Posey-Marcos E, Hiller S, Kluckman K, Maeda N, Coleman RA. Mol Cell Biol. 2002;22:8204–8214. doi: 10.1128/MCB.22.23.8204-8214.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond LE, Neschen S, Romanelli AJ, Cline GW, Ilkayeva OR, Shulman GI, Muoio DM, Coleman RA. J Biol Chem. 2005;280:25629–25636. doi: 10.1074/jbc.M503181200. [DOI] [PubMed] [Google Scholar]
- Kennedy EP. Annu Rev Biochem. 1957;26:119–148. doi: 10.1146/annurev.bi.26.070157.001003. [DOI] [PubMed] [Google Scholar]
- Lewin TM, Schwerbrock NMJ, Lee DP, Coleman RA. J Biol Chem. 2004;279:13488–13495. doi: 10.1074/jbc.M314032200. [DOI] [PubMed] [Google Scholar]
- Neschen S, Morino K, Hammond LE, Zhang D, Liu ZX, Romanelli AJ, Cline GW, Pongratz RL, Zhang XM, Choi CS, et al. Cell Metab. 2005;2:55–65. doi: 10.1016/j.cmet.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Shulman GI. Am J Med. 2006;119:S10–S16. doi: 10.1016/j.amjmed.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DH, Paulauskis JD, Moustaid N, Sul HS. J Biol Chem. 1991;266:23834–23839. [PubMed] [Google Scholar]


