Abstract
The B cell leukemia 11A protein (BCL11A/Evi9/CTIP1) has been implicated in hematopoietic cell development and malignancies. BCL11A is a transcriptional repressor that binds directly to a GC-rich motif and is also recruited to a promoter template via interaction with the orphan nuclear receptor, chicken ovalbumin upstream promoter transcription factor II. In both cases, BCL11A-mediated transcriptional repression is only minimally reversed by trichostatin A, suggesting the possible lack of involvement of class I or II histone deacetylases. Nonetheless, chromatin immunoprecipitation assays revealed that expression of BCL11A in mammalian cells resulted in deacetylation of histones H3 and/or H4 that were associated with the promoter region of a reporter gene. BCL11A-mediated transcriptional repression, as well as deacetylation of histone H3/H4 in BCL11A-transfected cells, was partially reversed by nicotinamide, an inhibitor of class III histone deacetylases such as SIRT1. SIRT1 was found to interact directly with BCL11A and was recruited to the promoter template in a BCL11A-dependent manner leading to transcriptional repression. These findings define a role for SIRT1 in transcriptional repression mediated by BCL11A in mammalian cells.
Keywords: SIRT1, Bcl11a, CTIP1, Evi9, Transcriptional repression, Histone deacetylase
BCL111A was originally identified as a protein that interacted with and stimulated the transcriptional repression activity of chicken ovalbumin transcription factor II (COUP-TFII), and was therefore named COUP-TF-interacting protein 1 (CTIP1 [1]). BCL11A was also independently identified by Copeland’s group as ecotropic viral integration site 9 (Evi9), the locus of which was demonstrated to be a site of proviral integration resulting in acute myeloid leukemia in BXH2 mice [2]. Subsequently, the human locus of BCL11A was shown to be involved in a translocation event, t(2; 14)(p13; q32.3), that may underlie some forms of chronic lymphocytic leukemia (CLL) and immunocytoma, linking the gene to human disease [3,4]. However, the mechanistic basis for the contribution of BCL11A to neoplastic processes in hematopoietic cells of murine or human origin remains unclear.
Although BCL11A has been shown to interact directly with COUP-TF II [1], as well as BCL6 [2], BCL11A also binds directly to a GC-rich motif and represses transcription of a downstream reporter gene in the absence of overexpressed COUP-TF family members or BCL6 [5]. This finding suggests that COUP-TF- and BCL6-independent mechanisms of BCL11A-mediated transcriptional repression may be operant on some cell types and/or promoter contexts.
The analysis of BCL11A-null mice has demonstrated the role for BCL11A in both hematopoiesis and postnatal development [6]. BCL11A and its paralog BCL11B/CTIP2 are similar in sequence, DNA binding specificity, and interacting partners (i.e., COUP-TF proteins). However, the lymphoidal defects resulting from disruption of each locus differ. BCL11A is essential for B cell development [6], whereas BCL11B is required for αβ T cell development [7].
Previous studies in transiently transfected cells revealed that both BCL11A and BCL11B mediated transcriptional repression of reporter gene in a manner that was only partially reversed by trichostatin A (TSA) [1,5,8]. More recently, BCL11B was demonstrated to interact with and recruit the class III HDAC, SIRT1, to a promoter template resulting in deacetylation of histones H3 and/or H4 and transcriptional repression in transiently transfected cells [8]. This finding implicated SIRT1 in the transcriptional activity of BCL11B in mammalian cells, and at least in part, may explain the TSA-insensitive nature of BCL11B-mediated transcriptional repression.
The structural and biochemical relatedness of BCL11A and BCL11B prompted us to speculate that the histone deacetylase SIRT1 may also underlie the mechanism of BCL11A-mediated transcriptional repression. Six lines of evidence, described herein, indicate that SIRT1 is involved in BCL11A-mediated transcriptional repression in transfected cells: (1) overexpression of BCL11A resulted in deacetylation of histones H3 and/or H4 that were associated with the promoter region of a target gene, (2) both the deacetylation of histone H3/H4 in BCL11A-transfected cells and BCL11A-mediated transcriptional repression were found to be partially reversed by nicotinamide, an inhibitor SIRT1, (3) endogenous SIRT1 was specifically recruited to the reporter gene template by overexpressed BCL11A, (4) SIRT1, but not a catalytically inactive mutant, stimulated transcriptional repression mediated by BCL11A, (5) endogenous BCL11A and SIRT1 were found to coimmunoprecipitate from nuclear extracts prepared from untransfected 70z/3 cells, and (6) BCL11A and SIRT1 were found to participate in a direct, physical interaction in vitro. Collectively, these findings implicate the histone deacetylase SIRT1 in the transcriptional repression activity of BCL11A in mammalian cells.
Materials and methods
Constructs
The (17-mer)4-tk-CAT reporter construct was a kind gift from Dr. Mingjer Tsai (Baylor College of Medicine). Flag-BCL11A was prepared by PCR amplification of the BCL11A open reading frame [1] with appropriate primers and insertion into pcDNA3(+) (Invitrogen). The Gal4 DBD-BCL11A construct was prepared by PCR amplification with appropriate primers followed by insertion into pM (Clontech). Myc-SIRT1, Myc-SIRT1 H363Y, and GST-SIRT1 constructs [9] were kind gifts from Dr. T. Kouzarides (University of Cambridge, Cambridge, UK). All vectors encoding GST fusion proteins were prepared by PCR amplification of appropriate templates followed by insertion into pGEX-2T (Amersham–Pharmacia Biotech). The constructs used for generating [35S]methionine-labeled proteins were prepared by PCR amplification with primers containing appropriate restriction sites for insertion into pcDNA3(+) or pcDNA3.1/His (Invitrogen). All constructs were verified by complete DNA sequence analysis.
Antibodies
Purified rabbit anti-Sir2α, mouse anti-SIRT1, and rabbit anti-acetylated-histone H3 and -histone H4 antibodies were obtained from Upstate. Mouse anti-Flag and -Myc monoclonal antibodies were purchased from Sigma and Oncogene, respectively. Mouse anti-Gal4 was obtained from Santa Cruz Biotechnology. The mouse anti-BCL11A monoclonal antibody was raised by Dr. Michael Marusich (Monoclonal Antibody Facility, Institute for Neuroscience, University of Oregon, Eugene, Oregon) against a cocktail of GST-BCL11A fusion proteins and recognizes an epitope located within the central region of the protein, amino acids 171–434 (data not shown).
Chromatin immunoprecipitation assays
Chromatin immunoprecipitation (ChIP) assays were performed on transfected cells essentially as described previously [8] with the slight modifications. HEK293 cells were co-transfected at 60% conFluency (10 cm plates) with 3 μg of the (17-mer)4-tk-CAT reporter, 5–20 μg Gal4-BCL11A, 0.5 μg Myc-SIRT1, and/or the corresponding parental vectors using the calcium-phosphate method. Cells were washed twice with phosphate buffered saline (PBS) after 48 h, and cross-linked with 1% formaldehyde in PBS at room temperature for 10 min. Cells were then washed twice with ice-cold PBS buffer and collected in harvesting buffer (100 mM Tris–HCl, pH 9.4, containing 10 mM DTT). The cells were lysed in lysis buffer (50 mM Tris–HCl, pH 8.1, containing 1% SDS, 10 mM EDTA, and a protease inhibitor cocktail). The sonicated lysates were then cleared by centrifugation and diluted 2.5- to 3.75-fold with ChIP dilution buffer (16.7 mM Tris–HCl, pH 8.1, containing 0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 167 mM NaCl, and a protease inhibitor cocktail). One-tenth of the diluted lysate was reserved as an input sample to determine total amount of reporter plasmid in transfected cells for subsequent normalization procedures. Two equal aliquots of the remaining lysate were used for immunoprecipitation with and without the addition of antibodies against K9-, K16-di-acetylated histone H3, and K5-, K8-, K12-, K16-tetra-acetylated histone H4 (Upstate; 5 μg of each antibody/immunoprecipitation reaction). Immune complexes were recovered with protein A or protein G–Sepharose (Amersham–Pharmacia) and washed under stringent conditions. Chromatin complexes were eluted with the freshly prepared elution buffer (0.1 M NaHCO3 containing 1% SDS). The eluates and the above input samples were subjected to an overnight reversal of cross-links at 65 °C, followed by a treatment with Proteinase K at 45 °C for 1–2 h. DNA was recovered by using QIAquick PCR Purification Kit (Qiagen) and amplified using a forward primer (5′-GGCATCAGAGCAGATTGTACT-3′) upstream of the multimerized 17-mer and a reverse primer (5′-CCT-TAGCTCCTGAAAATCTCG-3′) downstream of the tk promoter but upstream of the transcriptional start site. The resulting PCR product (327 bp) was analyzed by agarose gel electrophoresis and ethidium bromide staining. All experiments were performed three to five times.
Transfection and reporter gene studies
HEK293 cells were transfected and harvested as described above. Where indicated, TSA (100 ng/ml) or nicotinamide (15 mM) treatments were initiated 24 h after transfection, and cells were harvested 24 h later. A β-galactosidase expression vector (pCMV-Sport-βGal, Life Technologies) was co-transfected as an internal control, and β-galactosidase activity was used to normalize CAT activity as described [10].
Coimmunoprecipitation
HEK293 cells were transfected as described above with 15 μg each of expression vectors encoding Flag-BCL11A and/or Myc-SIRT1. Forty-eight hours after transfection the cells were lysed in NET-N buffer (20 mM Tris–HCl, pH 8, containing 150 mM NaCl, 0.5% NP-40, 10% glycerol, 1 mM EDTA, and a protease inhibitor cocktail) by agitation at 4 °C for 30 min. After a brief sonication, lysates were cleared by centrifugation, and immunoprecipitated as described previously [11] using the antibodies described above. Whole cell extracts from 70z/3 pre-B lymphocytes were prepared by using NET-N buffer as described above, and immunoprecipitated (10 mg of total protein per reaction) with purified anti-BCL11A (10–15 μg) or anti-Sir2α (0.5–2.5 μg) antibodies. All immunoprecipitates were analyzed by immunoblotting with appropriate antibodies.
GST pulldown experiments
GST pulldown experiments were conducted as described previously [12]. Briefly, equivalent amounts of GST or GST-SIRT1 fusion proteins were bound to glutathione–Sepharose (Pharmacia) and incubated with [35S]methionine-labeled proteins (BCL11A or BCL11A truncation mutants) prepared using the TNT transcription–translation system (Promega). The reactions were washed five times with binding buffer (10 mM Na-Hepes containing 10% glycerol, 1 mM EDTA, 1 mM DTT, 150 mM NaCl, and 0.05% NP-40) and bound proteins were eluted and resolved on denaturing SDS–PAGE gels for analysis by autoradiography.
Results
Transfection of BCL11A results in deacetylation of histone H3/H4 associated with the promoter region of a target gene
Previous studies indicated that BCL11A represses transcription in a manner that was only partially reversed by TSA suggesting the lack of involvement of class I or II HDACs [1,5,8]. However, these results do not preclude the possibility that TSA-insensitive HDACs, such as class III HDACs [13] or novel, TSA-insensitive HDAC(s), may be involved in BCL11A-mediated transcriptional repression. Thus, chromatin immunoprecipitation (ChIP) studies were conducted to assess possible alterations in the extent of histone H3/H4 acetylation on the template of a reporter gene in cells transiently transfected with an expression vector encoding a Gal4-BCL11A fusion protein. The amount of acetylated histones H3 and/or H4 at the promoter region of a target gene (a multimerized 17-mer in the context of the herpes simplex thymidine kinase promoter upstream of chloramphenicol acetyl-transferase) was found to be decreased in Gal4-BCL11A-transfected cells (upper panel of Fig. 1A, compare lane 4 with lane 6). Deacetylation of histone H3/H4 in BCL11A-transfected cells was unaffected by treatment with TSA (Fig. 1A, compare lanes 3–6 of the top and middle panels), consistent with previous studies that demonstrated TSA-insensitive, BCL11A-mediated transcriptional repression [1,5]. However, deacetylation of promoter-associated histone H3/H4 in BCL11A-transfected cells was inhibited by nicotinamide (NAM), at least partially (Fig. 1A, compare lanes 3–6 of the top and bottom panels), indicative of the possible involvement of a TSA-insensitive, NAD+ -sensitive, class III HDAC [8,9].
Fig. 1.
Deacetylation of promoter-associated histones H3 and/or H4 in BCL11A-transfected cells and BCL11A-mediated transcriptional repression are partially reversed by nicotinamide. (A) HEK293 cells were transfected with 3 μg (17-mer)4-tk-CAT reporter and 20 μg of an expression vector encoding Gal4-BCL11A. Twenty-four hours after transfection, cells were treated with histone deacetylase inhibitors, TSA (100 ng/ml; open bars), and nicotinamide (NAM; 15 mM; hatched bars) as indicated, for 24 h before collection. Transfection efficiency was normalized by total amount of the transfected (17-mer)4-tk-CAT reporter present as determined by PCR amplification using template-specific primers (input lanes 1 and 2; 5% of total). Lanes 3–6 represent template amplification reactions from samples immunoprecipitated with or without antibodies specific for acetylated histones H3 and H4 as indicated. Amplification reactions were separated on a 1% agarose gel that was stained with ethidium bromide to visualize DNA products. The indicated band is the expected, 327 bp amplification product from the reporter gene template. Results are representative of three independent experiments. (B) HEK293 cells were transiently transfected with 5 μg of the (17-mer)4-tk-CAT reporter along with 5 μg of expression vectors encoding either Gal4-BCL11A or Gal4 DBD as indicated. The treatments with vehicle (water; solid bars), TSA (open bars), and NAM (hatched bars) were carried out as described above. Transfection efficiency was normalized by use of a co-transfected β-galactosidase expression vector. CAT activity determined in the presence of Gal4 DBD and TSA (lane 2) was taken to be maximal and that against which all other determined CAT activities were compared. The results presented represent means (±SEM) of three independent experimental determinations.
BCL11A represses transcription in a nicotinamide-sensitive manner
Reporter gene assays were conducted to determine if BCL11A-mediated transcription was also sensitive to reversal by NAM. Transcriptional repression mediated by a Gal4-BCL11A fusion protein in HEK293 cells was partially reversed by NAM (Fig. 1B, compare lanes 4 and 6 with lane 1), in a manner similar to that of BCL11B/CTIP2 [8]. Consistent with previous studies [1,5], TSA, at 100 ng/ml, had a minimal effect on transcriptional repression mediated by Gal4-BCL11A (Fig. 1B, compare lanes 1 and 2 with lanes 4 and 5).
BCL11A interacts with and recruits SIRT1 to the promoter template
The reversibility of histone deacetylation in BCL11A-transfected cells and of BCL11A-mediated transcriptional repression by NAM implicated a NAM-sensitive HDAC(s) in both events. The simplest explanation for these findings would be that BCL11A interacts with and recruits a class III, NAM-sensitive HDAC to the template, at which the enzyme catalyzes histone deacetylation resulting in chromatin condensation and subsequent transcriptional repression. To investigate this possibility, we used a co-immunoprecipitation assay to determine if BCL11A associated with SIRT1, a class III HDACs, in mammalian cells. first, the interaction of co-overexpressed proteins was determined using Flag-BCL11A and Myc-SIRT1. Myc-SIRT1 was immunoprecipitated by anti-Flag antibody but only when Flag-BCL11A was co-expressed in HEK293 cells (Fig. 2A, compare lane 5 with lane 6). Similarly, overexpressed BCL11A associated with endogenous SIRT1 in HEK293 cells (Fig. 2B, compare lane 3 with lane 4). Finally, the interaction of endogenous SIRT1 and BCL11A in untransfected mouse pre-B lymphocytes, 70z/3 cells, was verified by co-immunoprecipitation experiments. Endogenous Sir2α (the mouse homolog of human SIRT1) was co-immunoprecipitated with BCL11A by an anti-BCL11A monoclonal antibody (Fig. 3C, lane 3), but not by an irrelevant antibody (mouse anti-Gal4, lane 4). Similar results were obtained when precipitating and detecting antibodies were reversed (data not shown). These findings suggest that endogenous BCL11A and Sir2α physically associate with similar complex(es) when expressed at physiological levels in untransfected mouse 70z/3 cells.
Fig. 2.

BCL11A interacts with and recruits SIRT1 to a promoter template in mammalian cells. (A) Myc-SIRT1 coimmunoprecipitates with Flag-BCL11A. Whole-cell extracts from HEK293 cells, untransfected and transiently transfected with the indicated expression vectors, were immunoprecipitated with anti-Flag monoclonal antibody. Immunoprecipitates were resolved by SDS–PAGE and analyzed by Western blotting with anti-Myc monoclonal antibody that detects Myc-SIRT1. The position of Myc-SIRT1 is indicated. (B) Endogenous (human) SIRT1 coimmunoprecipitates with transfected Flag-BCL11A from HEK293 cell lysates. Whole cell extracts from HEK293 cells, untransfected and transiently transfected with expression vectors encoding Flag-BCL11A, were immunoprecipitated with anti-Flag monoclonal antibody, and the immunocomplexes were analyzed by immunoblotting with anti-SIRT1 antibody, which detects the human protein. The position of endogenous SIRT1 is indicated. (C) Endogenous Sir2α, the mouse homolog of human SIRT1, and BCL11A interact in 70z/3 whole cell extracts. The cell extracts were immunoprecipitated with no antibody, anti-BCL11A or irrelevant (anti-Gal4) monoclonal antibodies. The immunocomplexes were then analyzed by Western blotting with an anti-Sir2α monoclonal antibody. The position of endogenous Sir2α is indicated, which corresponds to ~120 kDa as previously reported [29]. (D) Endogenous SIRT1 is recruited to promoter template of the reporter gene upon expression of Gal4-BCL11A. HEK293 cells were transfected with 5 μg of (17-mer)4-tk-CAT reporter and 20 μg of expression vectors encoding either Gal4-BCL11A or Gal4 DBD. Transfection efficiency was normalized as described in the legend of Fig. 1A (lanes 1 and 2; 5% of input). Lanes 3–6 represent template amplification reactions from samples immunoprecipitated with or without antibody directed against SIRT1. Results are representative of three independent experiments.
Fig. 3.
BCL11A interacts directly with SIRT1 in vitro. (A) In vitro translated and [35S]Met-labeled full-length BCL11A and truncation BCL11A mutants were incubated with equivalent amounts of bacterially expressed GST (lane 2) or GST-SIRT1 fusion protein (lane 3). After extensive washing, [presup35S]Met-labeled BCL11A associated with the affinity resin was determined by SDS–PAGE and autoradiography. Input [35S]Met-labeled proteins are shown in lane 1. BCL11A truncation mutants used in these studies (B–F) are schematically represented on the right with zinc finger motifs denoted by vertical bars. (B) A schematic representation of full-length SIRT1 and SIRT1 truncation mutants used to generate GST-SIRT1 fusion proteins for in vitro pulldown experiments (see below). (C) [35S]Met-labeled BCL111A 194–378 (the minimal SIRT1-interaction domain) was incubated with equivalent amounts of GST (lane 2) or GST-SIRT1 fusion proteins (lanes 3–8). The position of bound [35S]Met-labeled BCL11A 194–378 is indicated by an arrow on the left. Lane 1 corresponds to 10% of the [35S]Met-labeled BCL11A 194–378 that was incubated with GST or GST-SIRT1 fusion proteins. Shown in (A and C) are representative experiments that were replicated three to five times.
Based on the observed ability of BCL11A to coimmunoprecipitate with SIRT1, we next sought to determine if endogenous SIRT1 was recruited to the promoter template by BCL11A in HEK293 cells transfected with a Gal4-BCL11A expression vector. Endogenous SIRT1 was recruited to the promoter template in cells expressing the Gal4-BCL11A fusion protein (Fig. 2D, lane 6) but not in cells expressing the Gal4 DBD (Fig. 2D, lane 4). These results demonstrate BCL11A-dependent recruitment of endogenous SIRT1 to the promoter template in HEK293 cells, suggesting a role for this histone deacetylase in the transcriptional repression mechanism of BCL11A.
BCL11A interacts directly with SIRT1 in vitro
The above results demonstrated that BCL11A and SIRT1 associated with similar complex(es) in mammalian cells. However, co-immunoprecipitation experiments cannot distinguish if the two proteins interact directly or indirectly via intermediary protein(s). This possibility was tested directly in a series of GST pulldown assays. Full-length, in vitro translated BCL11A was found to interact with full-length SIRT1, which was fused to GST (Fig. 3A, lane 3), but not with GST alone (lane 2). This finding suggests that BCL11A and SIRT1 participate in a direct, physical interaction. The SIRT1 interaction interface of BCL11A was mapped to amino acids 194–378 (Fig. 3A, lane 3 of D), which is relatively rich (22%) in proline residues [1]. This proline-rich region is 61% identical to corresponding region of BCL11B/CTIP2, which was previously shown to be required for the interaction of this protein with SIRT1 [8]. All BCL11A deletion mutants containing this region strongly interacted with GST-SIRT1 (Figs. 3A, lane 3 of C and D) but mutants lacking it interacted weakly or not at all (Figs. 3A, lane 3 of B, E, and F). Thus, a region of BCL11A that is relatively rich in proline, amino acids 194–378, appears to be primarily responsible for direct interaction with SIRT1 in vitro.
The BCL11A interaction interface of SIRT1 was similarly mapped by use of a series of SIRT1 deletion mutants (see Fig. 3B) fused to GST. Both full-length BCL11A and BCL11A 194–378 interacted strongly with the sirtuin homology domain of SIRT1 (Fig. 3C, lane 5), very weakly with SIRT1 1–214 (lane 4), and not at all with SIRT1 541–747 (lane 6). Moreover, BCL11A was found to interact primarily with amino terminal region of sirtuin homology domain, SIRT1 214–441 (Fig. 3C, lane 7). The interaction of BCL11A with the carboxyl terminal part of the sirtuin homology domain, SIRT1 441–541, was not detectable (lane 8). Considered together, these findings suggest that BCL11A interacts directly with SIRT1 in vitro, and that the interaction requires residues within the amino terminal region of sirtuin homology domain of SIRT1 and the proline-rich region of BCL11A (amino acids 194–378). Because the BCL11A-interaction interface of SIRT1 is relatively conserved among other sirtuin family members [13], it is conceivable that BCL11A may interact with other members of this important class of regulatory proteins.
SIRT1 enhances BCL11A-mediated transcriptional repression
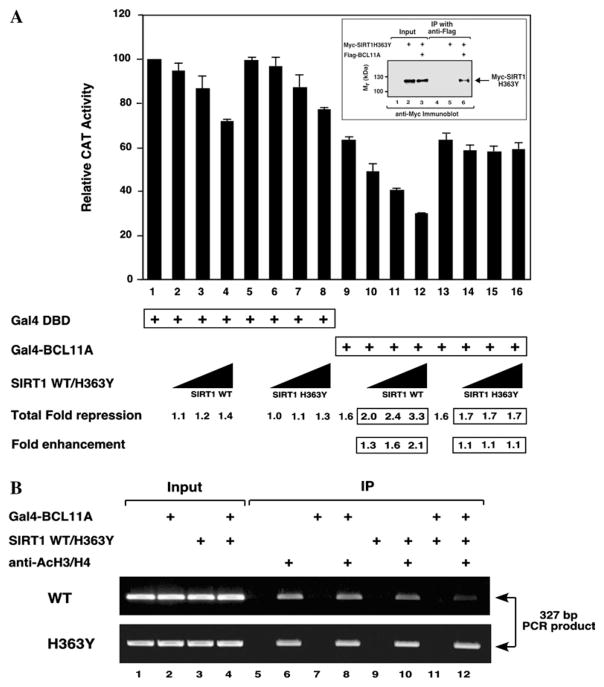
Based on the ability of BCL11A to interact with and recruit SIRT1 to the promoter template, we hypothesized that transcriptional repression by BCL11A is mediated through SIRT1, at least in part. To assess a potential role for SIRT1 in BCL11A-mediated transcriptional repression, reporter gene assays were carried out in transiently transfected HEK293 cells. In the absence of BCL11A, both wild-type SIRT1 and a catalytically inactive point mutant, SIRT1 H363Y, repressed reporter gene expression in a concentration-dependent manner (Fig. 4A, lanes 1–4 and 5–8, respectively), suggesting that the catalytic activity of SIRT1 is not required for basal transcriptional repression of the reporter gene. These findings are consistent with previous reports [8,9], however, the mechanism(s) for the observed repression of basal transcription by both wild-type SIRT1 and SIRT1 H363Y under these experimental conditions is unknown. In contrast, the catalytic activity of SIRT1 was required for enhancement of BCL11A-mediated transcriptional repression. Wild-type SIRT1 enhanced transcriptional repression mediated by BCL11A in a concentration-dependent manner (Fig. 4A, lanes 9–12). A catalytically inactive form, SIRT1 H363Y, did not enhance BCL11A-mediated repression (Fig. 4A, lanes 13–16), even though it was capable of interacting with BCL11A in HEK293 cells (Fig. 4A, lane 6, inset). These findings demonstrate that SIRT1 enhances the transcriptional repression activity of BCL11A and this effect requires the catalytic activity of the enzyme.
Fig. 4.
SIRT1 enhances BCL11A-mediated transcriptional repression. (A) Wild-type SIRT1, but not SIRT1 H363Y, stimulates BCL11A-mediated transcriptional repression. HEK293 cells were transiently transfected with 5 μg of the (17-mer)4-tk-CAT reporter along with 5 μg of expression vectors encoding either Gal4-BCL11A or Gal4 DBD, and increasing amounts (0.125, 0.25, and 0.5 μg) of expression vectors encoding either SIRT1 WT or SIRT1 H363Y, as indicated. Transfection efficiency was normalized as described in the legend of Fig. 1B. The activity of the CAT reporter in the presence of Gal4 DBD alone (lane 1) was taken to be maximal and that against which all other determined CAT activities were compared. The results presented represent means (±SEM) of three independent experimental determinations. (Inset) The catalytically inactive Myc-SIRT1 H363Y, coimmunoprecipitates with Flag-BCL11A in a manner indistinguishable from wild-type SIRT1 (see Fig. 2A). Transfections, immunoprecipitations, and immunoblotting were conducted as described in Fig. 2A. (B) SIRT1 stimulates deacetylation of template-associated histones H3 and/or H4 in BCL11A-transfected cells. HEK293 cells were transfected with 3 μg of the (17-mer)4-tk-CAT reporter along with expression vectors encoding Gal4- BCL11A (10 μg) and SIRT1 WT or SIRT1 H363Y (0.5 μg) as indicated. The level of acetylated histones H3 and H4 that were associated with the promoter region of the reporter gene template was determined by a ChIP assay as described in Materials and methods. Transfection efficiency was normalized as described in the legend of Fig. 1A. Input lanes (1–4) correspond to amplification reactions conducted using 3.75% (upper panel) and 5% (lower panel) of the lysates used for IP reactions. Lanes 5–12 represent template amplification reactions from samples immunoprecipitated with or without anti-acetylated histone H3/H4 antibodies as indicated. Results are representative of three independent experiments.
Based on the above results from reporter gene studies, ChIP assays were conducted to determine if SIRT1-mediated histone deacetylation may underlie the molecular basis for BCL11A-mediated transcriptional repression. Under the experimental conditions employed, co-transfection of Gal4-BCL11A and SIRT1 resulted in a decrease in the level of acetylated histones H3 and/or H4 associated at the promoter region of the target gene when compared with that observed by transfection of either expression vector individually (Fig. 4B, compare lanes 6, 8, 10, and 12 of the upper panel). Co-transfection of Gal4-BCL11A and SIRT1 H363Y resulted in no change in the level of acetylated histone H3/H4 that was associated with the reporter gene template (Fig. 4B, compare lanes 6, 8, 10, and 12 of the bottom panel). These findings are similar to that of previous report for BCL11B/CTIP2 [8], suggesting that SIRT1-catalyzed histone deacetylation may underlie, at least in part, the ability of the enzyme to stimulate both BCL11A- and BCL11B-mediated transcriptional repression.
Discussion
Regulation of eukaryotic gene expression in response to developmental or environmental signals is a complex, multi-step process that requires the sequential and combinatorial action of many cellular factors, which minimally includes proteins harboring histone-modifying activities, ATP-dependent nucleosome remodeling complexes, and components of the 26S proteosomal complex [14,15]. Of the histone-modifying activities, acetylation may represent the modification that is most readily reversible, and hence, perhaps the most dynamic in nature. Several families of histone acetyl transferases have been identified and many of these enzymes acetylate a wide variety of substrates in addition to histones (reviewed in [16,17]). Although histone acetylation has been universally associated with transcriptional activation, histone deacetylation appears to play a dual role in transcriptional regulation. First, histone deacetylation has been firmly implicated in transcriptional silencing, possibly by inducing chromatin condensation and/or facilitating formation of heterochromatin, both of which would favor the repressed state [18–20]. However, some HDACs, possibly in association with ATP-dependent remodeling complexes, are recruited to the template by transcriptional activators, such as activated estrogen receptor α, but this appears to occur relatively late in the cyclical process of transcriptional activation and may play a role in nucleosomal remodeling and/or “resetting” the basal state of the promoter [14]. In any event, a large number of HDACs (presently eighteen) from three different families have been identified, which are conserved across numerous species suggesting that each plays an important biological role(s).
We previously observed that BCL11A repressed transcription of a reporter gene in a TSA-insensitive manner [1,5]. This observation may be explained, at least in part, by our present finding demonstrating that BCL11A recruited SIRT1, a TSA-insensitive, class III HDAC to the promoter region of a reporter gene template. The results from ChIP studies presented herein suggested that SIRT1 catalyzed deacetylation of histones H3 and/or H4 on this template. Our present findings and past work [8], firmly implicate SIRT1 in the mechanistic basis of transcriptional repression mediated by both BCL11A and BCL11B in the context of a model system. The observation that both endogenous BCL11A (Fig. 2C) and BCL11B [8] exist within a nuclear complex(es) that contains SIRT1 provides further support for the physiological role of SIRT1 in the BCL11A/B signaling pathways. The possibility that SIRT1 may be directly or indirectly involved in the proliferative, hematopoietic cell disorders associated with dysregulated BCL11A [6] or BCL11B [7] expression remains an open question.
Histones represent one of at least three classes of acetylated substrates for SIRT1; two other non-histone, transcriptional regulatory proteins have been shown previously to serve as SIRT1 substrates. The groups of Guarente [21], Gu [21], and Kouzarides [9] have shown that SIRT1 deacetylates p53. Acetylation of p53 by the histone acetyltransferase/transcriptional coactivator p300 appears to represent an important step in the activation of this tumor suppressor protein [22]. Consistent with this, SIRT1-mediated deacetylation was found to result in inhibition of p53-mediated transcriptional activation [9] and attenuate p53-mediated, stress-induced cell death [9,21]. Similarly, SIRT1 was found to deacetylate Foxo3a and inhibit the ability of this forkhead transcription factor to activate transcription and regulate apoptosis [23]. Theoretically, SIRT1-mediated deacetylation of transcriptional regulatory proteins involved in tumor suppression and apoptosis, such as p53 and Foxo3a, may promote tumorigenesis. However, to our knowledge, this has not been demonstrated conclusively.
The findings that overexpression of BCL11A following proviral integration resulted in myeloid leukemia and that BCL11A induces anchorage-independent growth of NIH 3T3 cells [2] implicated BCL11A as a dominant oncogene. However, the mechanistic basis for the role of BCL11A in hematopoietic cell neoplastic processes remains unclear. Based on our findings that BCL11A interacts directly with and recruits SIRT1 to a promoter template to repress transcription, one may envision a role for SIRT1 in BCL11A-mediated leukemic transformation of hematopoietic cells. In this case, one may expect to observe a selective dysregulation/repression of BCL11A target genes that may be relieved by inhibitors of SIRT1, such as nicotinamide. Although, no such genes have been identified to date, data from null animals suggest that BCL11A may be upstream of Pax5 and Ebf1 in B cell development [6]. Additionally, BCL11A may be recruited to promoter templates by direct interaction with BCL6, suggesting that BCL11A may play a role in the BCL6 signaling pathway in B cells [2]. BCL6 is a transcriptional repressor required for the development of germinal centers (GCs) that has been implicated in the pathogenesis of GC-derived, B cell lymphoma [24,25]. If SIRT1 plays a role in transcriptional repression mediated by BCL11A/BCL6 complexes in B cell lymphomas, one may expect that this transcriptional repression could be at least partially relieved by inhibitors of SIRT1.
Our finding that treatment of BCL11A-transfected cells with nicotinamide, an inhibitor of TSA-insensitive class III HDACs, did not fully relieve BCL11A-mediated transcriptional repression suggests that an additional protein(s) may be required for the BCL11A-mediated transcriptional repression. One such family of proteins may be the heterochromatin-associated proteins (HP1), which have been shown to interact directly with CTIP2/BCL11B [26]. Recruitment of HP1 proteins to the promoter template results in transcriptional repression either by HP1 self-assembly into a supramolecular, heterochromatinized template [27], or by interaction of HP1 with a component of TFIID, TAFII130, precluding the nucleation of the preinitiation complex formation [28]. It is tempting to speculate that BCL11A, by virtue of its sequence-specific DNA binding activity and/or by tethering to promoter-bound BCL6 or COUP-TF family members, may recruit SIRT1 to specific genomic loci, at which the enzyme deacetylates H3-K9, the initial step in creation of a high-affinity HP1 binding site [18], on the path to heterochromatinization and transcriptional silencing. In this way, BCL11A may serve to nucleate a transcriptional repression complex that minimally includes a histone deacetylase (SIRT1) and HP1 protein(s), and target this complex to specific genomic loci.
The results described in this study and previous study [8] strongly suggest that the NAD+-dependent, nicotinamide-sensitive histone deacetylase SIRT1 contributes, at least in part, to the transcriptional repression activities of both BCL11A and BCL11B. However, it is also possible that both BCL11A and BCL11B could themselves be substrates of SIRT1, which could thereby modulate function(s) of these proteins. This feature may provide another level of control in the regulation of transcriptional networks by BCL11A and BCL11B in cells of the hematopoietic and central nervous systems.
Acknowledgments
We thank Drs. T. Kouzarides, M.-J. Tsai, and D. Avram for providing constructs, and Dr. M. Marusich for raising the anti-BCL11A monoclonal antibody. We are grateful to M. Beilstein for expert technical advice. The studies were supported by grants from the National Institutes of Health (GM60852) to M.L. and by a NIEHS Center grant (ES00210) to the Oregon State University Environmental Health Sciences Center. T.S. was supported by a graduate fellowship from the Royal Thai Government.
Footnotes
Abbreviations used: BCL, B cell leukemia; bp, base pair; CAT, chloramphenicol acetyltransferase; COUP-TF, chicken ovalbumin upstream promoter transcription factor; CTIP 1 and 2, COUP-TF-interacting proteins 1 and 2; GST, glutathione S-transferase; HA, hemagglutinin; HDAC, histone deacetylase; HEK293, human embryonic kidney 293 cells; IgG, immunoglobulin G; kDa, kilodalton; NAM, nicotinamide; Sir2, silent information regulator 2; SIRT1, Sir2-like protein 1 or sirtuin 1; TSA, trichostatin A; WT, wild type.
References
- 1.Avram D, Fields A, Pretty On Top K, Nevrivy DJ, Ishmael JE, Leid M. J Biol Chem. 2000;275:10315–10322. doi: 10.1074/jbc.275.14.10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakamura T, Yamazaki Y, Saiki Y, Moriyama M, Largaespada DA, Jenkins NA, Copeland NG. Mol Cell Biol. 2000;20:3178–3186. doi: 10.1128/mcb.20.9.3178-3186.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Satterwhite E, Sonoki T, Willis TG, Harder L, Nowak R, Arriola EL, Liu H, Price HP, Gesk S, Steinemann D, Schlegelberger B, Oscier DG, Siebert R, Tucker PW, Dyer MJ. Blood. 2001;98:3413–3420. doi: 10.1182/blood.v98.12.3413. [DOI] [PubMed] [Google Scholar]
- 4.Dyer MJ, Oscier DG. Leukemia. 2002;16:973–984. doi: 10.1038/sj.leu.2402528. [DOI] [PubMed] [Google Scholar]
- 5.Avram D, Fields A, Senawong T, Topark-Ngarm A, Leid M. Biochem J. 2002;368:555–563. doi: 10.1042/BJ20020496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu P, Keller JR, Ortiz M, Tessarollo L, Rachel RA, Nakamura T, Jenkins NA, Copeland NG. Nat Immunol. 2003;4:525–532. doi: 10.1038/ni925. [DOI] [PubMed] [Google Scholar]
- 7.Wakabayashi Y, Watanabe H, Inoue J, Takeda N, Sakata J, Mishima Y, Hitomi J, Yamamoto T, Utsuyama M, Niwa O, Aizawa S, Kominami R. Nat Immunol. 2003;4:533–539. doi: 10.1038/ni927. [DOI] [PubMed] [Google Scholar]
- 8.Senawong T, Peterson VJ, Avram D, Shepherd DM, Frye RA, Minucci S, Leid M. J Biol Chem. 2003;278:43041–43050. doi: 10.1074/jbc.M307477200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langley E, Pearson M, Faretta M, Bauer UM, Frye RA, Minucci S, Pelicci PG, Kouzarides T. Embo J. 2002;21:2383–2396. doi: 10.1093/emboj/21.10.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dowell P, Ishmael JE, Avram D, Peterson VJ, Nevrivy DJ, Leid M. J Biol Chem. 1997;272:33435–33443. doi: 10.1074/jbc.272.52.33435. [DOI] [PubMed] [Google Scholar]
- 11.Nevrivy DJ, Peterson VJ, Avram D, Ishmael JE, Hansen SG, Dowell P, Hruby DE, Dawson MI, Leid M. J Biol Chem. 2000;275:16827–16836. doi: 10.1074/jbc.275.22.16827. [DOI] [PubMed] [Google Scholar]
- 12.Dowell P, Peterson VJ, Zabriskie TM, Leid M. J Biol Chem. 1997;272:2013–2020. doi: 10.1074/jbc.272.3.2013. [DOI] [PubMed] [Google Scholar]
- 13.Frye RA. Biochem Biophys Res Commun. 1999;260:273–279. doi: 10.1006/bbrc.1999.0897. [DOI] [PubMed] [Google Scholar]
- 14.Metivier R, Penot G, Carmouche RP, Hubner MR, Reid G, Denger S, Manu D, Brand H, Kos M, Benes V, Gannon F. Embo J. 2004 doi: 10.1038/sj.emboj.7600377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu W, Cho H, Kadam S, Banayo EM, Anderson S, Yates JR, III, Emerson BM, Evans RM. Genes Dev. 2004;18:144–156. doi: 10.1101/gad.1141704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sterner DE, Berger SL. Microbiol Mol Biol Rev. 2000;64:435–459. doi: 10.1128/mmbr.64.2.435-459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang XJ. Nucleic Acids Res. 2004;32:959–976. doi: 10.1093/nar/gkh252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lachner M, O’Sullivan RJ, Jenuwein T. J Cell Sci. 2003;116:2117–2124. doi: 10.1242/jcs.00493. [DOI] [PubMed] [Google Scholar]
- 19.Lachner M, Jenuwein T. Curr Opin Cell Biol. 2002;14:286–298. doi: 10.1016/s0955-0674(02)00335-6. [DOI] [PubMed] [Google Scholar]
- 20.Jenuwein T, Allis CD. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 21.Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 22.Gu W, Roeder RG. Cell. 1997;90:595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 23.Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- 24.Dalla-Favera R, Ye BH, Lo Coco F, Gaidano G, Lista F, Knowles DM, Louie DC, Offit K, Chaganti RS. Ann Oncol. 1994;5(Suppl 1):55–60. doi: 10.1093/annonc/5.suppl_1.s55. [DOI] [PubMed] [Google Scholar]
- 25.Dent AL, Shaffer AL, Yu X, Allman D, Staudt LM. Science. 1997;276:589–592. doi: 10.1126/science.276.5312.589. [DOI] [PubMed] [Google Scholar]
- 26.Rohr O, Lecestre D, Chasserot-Golaz S, Marban C, Avram D, Aunis D, Leid M, Schaeffer E. J Virol. 2003;77:5415–5427. doi: 10.1128/JVI.77.9.5415-5427.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lachner M, O’Carroll D, Rea S, Mechtler K, Jenuwein T. Nature. 2001;410:116–120. doi: 10.1038/35065132. [DOI] [PubMed] [Google Scholar]
- 28.Vassallo MF, Tanese N. Proc Natl Acad Sci USA. 2002;99:5919–5924. doi: 10.1073/pnas.092025499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McBurney MW, Yang X, Jardine K, Hixon M, Boekelheide K, Webb JR, Lansdorp PM, Lemieux M. Mol Cell Biol. 2003;23:38–54. doi: 10.1128/MCB.23.1.38-54.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]