Abstract
Delayed myeloid engraftment following cord blood transplantation (CBT) is thought to result from inadequate numbers of progenitor cells in the graft and is associated with increased early transplant related morbidity and mortality. Novel culture strategies that increase the number of cord blood (CB) progenitors capable of rapid myeloid engraftment following CBT would allow more widespread use of this stem cell source for transplantation. Here we report development of a clinically relevant Notch-mediated ex vivo expansion system for human CD34+ CB progenitors that results in a >100 fold increase in the absolute number of stem/progenitor cells, including those capable of enhanced repopulation in the marrow of immunodeficient NOD/SCID mice. Furthermore, when Notch-mediated ex vivo expanded CB progenitors were infused in a clinical setting, rapid recovery of myeloid cells was achieved, demonstrating the first observation of rapid engraftment derived from ex vivo expanded stem/progenitor cells in humans.
Introduction
Cord blood is an effective and widely used source of stem cells for patients undergoing hematopoietic stem cell transplantation. Most patients now receive two units of CB derived from different donors to better ensure provision of adequate stem cell numbers for reliable donor engraftment. However, the time to donor engraftment remains relatively delayed, averaging more than 3 weeks to achieve adequate numbers of myeloid cells. This leaves patients susceptible to infection and associated morbidity and mortality. Previous efforts to improve the rate of engraftment using ex vivo cytokine-mediated expansion methodologies to generate increased numbers of cells have not shown significant clinical effects1–3. To address this, our laboratory has investigated the role of the Notch signaling pathway in regulating ex vivo expansion of hematopoietic stem/progenitor cells (HSPC) with the goal of generating increased numbers of progenitor cells capable of rapid repopulation in vivo to improve the kinetics of hematopoietic recovery following CBT.
A role for Notch in hematopoiesis was initially suggested by our detection of the human Notch1 gene in CD34+ or CD34+lin− human hematopoietic precursors, and enhanced self-renewal of repopulating cells due to retrovirus-mediated expression of a constitutively active form of Notch14,5. Subsequently, activation of endogenous Notch receptors using immobilized Notch ligand revealed profound effects on the growth and differentiation of mouse marrow precursors with a multi-log increase in the number of Sca-1+Gr-1− cells with short-term lymphoid and myeloid repopulating ability 6. For human cells, incubation of CB progenitors in the presence of immobilized ligand generated an approximate 100-fold increase in CD34+ cell numbers with enhanced repopulating ability in an immunodeficient mouse model7,8. Overall, these observations demonstrated that Notch signaling plays an important regulatory role in hematopoiesis and suggest that Notch ligands will be useful reagents for improving ex vivo culture of stem/progenitor cells.
We herein report development of an optimized, clinically feasible methodology for generating cord blood stem/progenitor cells for clinical evaluation. We demonstrate a multi-log increase in the ex vivo generation of CD34+ cells that repopulate immunodeficient mice with markedly enhanced kinetics and magnitude and, in a Phase I myeloablative CBT trial, provide more rapid myeloid engraftment.
Results
Preclinical optimization and validation
Our earlier published studies utilized enriched CD34+CD38− CB progenitors as the starting cell population for Notch-mediated expansion7. To limit cell separation procedures, we first determined whether isolation of the CD38− subset of CD34+ cells was required. We compared growth characteristics and generation of SCID repopulating cells (SRC) of CD34+ and CD34+CD38− CB cells. Cells were cultured for 17–21 days in the presence of fibronectin fragments and immobilized engineered Notch ligand (Delta1ext-IgG) or control human IgG in serum free conditions supplemented with cytokines (SCF 300 ng ml−1, Flt3L 300 ng ml−1, TPO 100 ng ml−1, IL–6 100 ng ml−1, and IL–3 10 ng ml−1, denoted as “5GF”). Delta1ext-IgG consists of the extracellular domain of Delta1 fused to the Fc domain of human IgG1. We observed no significant difference in absolute numbers of CD34+ cells generated, with a CD34+ cell fold expansion of 138 ±64 and 163±64, (mean±sem, p=0.1612, data not shown) for the CD34+ versus the CD34+CD38− starting cell population, respectively. Assessment of in vivo NOD/SCID repopulating ability at 3, 6 and 10 weeks post infusion revealed enhanced human engraftment in the marrow of recipient mice when a CD34+ compared to CD34+CD38− starting cell population was used (mean CD45% in CD34+ versus CD34+CD38− starting cell populations cultured in the presence of Delta1ext-IgG: 3 weeks; 6.7% versus 1.6%, p=0.02 and 10 weeks: 1.0% vs. 0.2%, p=0.1). We further determined that the 5GF combination was superior to use of fewer cytokines for in vitro generation of CD34+ cells and SRC frequency determined by limiting dilution analysis (Supplementary Fig. 1).
We evaluated multiple, closed system tissue culture bags, tissue culture and non-tissue culture treated flasks for ligand binding and growth of cells (see Methods). We also evaluated commercially available clinical grade serum-free media. Based on in vitro generation of CD34+ cells and NOD/SCID repopulating ability, StemSpan SFEM media and X-fold tissue culture bags (Baxter) and Nunc flasks were found to be superior (data not shown). Details regarding the methods for large scale production of cGMP engineered Delta1ext-IgG can be found in Supplementary Methods.
Reproducibility of this optimized culture system under cGMP conditions was validated in 19 runs using CB units previously cryopreserved as part of the NHLBI Cord Blood Transplantation (COBLT) Study (obtained via the NHLBI repository). We observed consistent growth, averaging greater than 150 fold expansion of CD34+ cells regardless of the starting purity which ranged from 19 to 95% (mean 64% ± 5.2%, sem), with the low 19% purity value being an outlier (Table 1). All validation run experiments resulted in detectable engraftment of human CD45+ cells in immunodeficient mice.
Table 1.
Preclinical Full-Scale Validation Runs (N=19)
| Mean (± sem) | Median | |
|---|---|---|
| CD34 Purity (%) | 64 ± 5 | 67 |
| CD34 Fold Increase | 184 ± 35 | 140 |
| Total Cell # Fold Increase | 789 ± 130 | 645 |
| *Marrow CD45% 3 weeks | 3.7 ± 1.3 | 1.7 |
| *Marrow CD45% 9 weeks | 7.9 ± 2.2 | 2.1 |
N=124 (total number of mice evaluated)
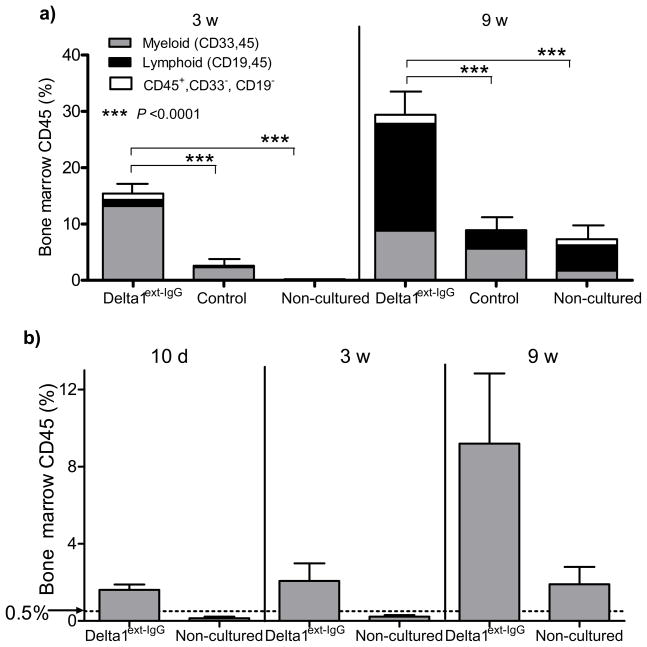
We further evaluated the functional properties of cells generated using CD34+ cells selected from pooled fresh CB and cryopreserved until use. We found a 222 ± 57 (mean ± sem, n=5) fold expansion of CD34+ cells cultured in the presence of Delta1ext-IgG versus a 68±30 fold expansion for cells cultured in the presence of control IgG (p=0.006). Repopulating ability and SRC frequency was significantly higher in mice receiving cells cultured with Delta1ext-IgG (p<0.0001) and included both myeloid and lymphoid cells (Fig. 1a). The observed increase in overall human hematopoietic reconstitution at 9 versus 3 weeks after culture with Delta1ext-IgG resulted from an increase in engrafted lymphoid cells. Myeloid engraftment decreased somewhat, suggesting at least a portion of the starting engrafting cells were short term in nature. However, the continued presence of at least some longer-term repopulating cells after ex vivo culture was suggested by secondary transplantation of whole marrow from NOD/SCID IL-2Rγnull; mice transplanted 17–22 weeks prior with expanded cells that revealed human engraftment (defined as ≥0.5% human CD45+ cells) in the marrow of 7/11 secondary recipient mice, also NOD/SCID IL-2Rγnull; mice (Supplementary Fig. 2). Moreover, SRC frequency was found to be markedly enhanced with limit dilution analysis demonstrating a 15.6 fold increase in SRC frequency in mice that received Delta1ext-IgG-cultured cells as compared to those receiving non-cultured cells at 3 weeks post transplant (p=0.0001) and a 6.2 fold increase in SRC frequency at 9 weeks (p=0.0001) (Table 2). To our knowledge, this is the highest reported expansion of human SRC frequency reported to date 9–11. Furthermore, the fold-increase in repopulating cells revealed in these limit dilution assays suggest that the observed increase in overall human engraftment in mice receiving Delta1-cultured cells resulted mainly or completely from the generation of repopulating cells as a direct result of culture with Notch ligand.
Figure 1. Culture of CD34+ CB progenitors with Notch ligand results in enhanced and more rapid human engraftment as well as increased SRC frequency.
CD34+ cord blood progenitors were cultured in the presence of Delta1ext-IgG or control human IgG. The progeny of 30,000 starting CD34+ cells depleted of CD14+ cells were infused into individual sublethally irradiated NOD/SCID mice via tail vein injection. The number of cultured cells infused per mouse on average was 2.2 × 107 following culture with Notch ligand and 1.8 ×107 following culture with control human IgG. A third group of mice received 30,000 CD34+ non-cultured cells plus 2×105 irradiated CD34neg carrier cells. \ (a) Bone marrow aspirates were performed at 3 weeks and both femurs and tibiae were harvested at 9 weeks when mice were sacrificed. Y axis represents the percent of human CD45 in the marrow of recipient mice. Results shown are the mean ± sem of mice from 5 independent experiments (total number of mice per group: Delta1ext-IgG, N=46; Control IgG, N=32; Non-cultured, N=44). Contribution to engraftment from the lymphoid and myeloid compartments are represented by the black (lymphoid, as measured by co-staining of human CD19 and CD45) and gray portions (myeloid, as measured by co-staining of human CD33 and CD45). The white portion are human cells that did not co-stain for CD33 or CD19. (b) Cultured and non-cultured cells were infused into sublethally irradiated mice and human engraftment measured at 10 days, 3 weeks and 9 weeks. The y axis represents the percent of human CD45% in the marrow of recipient mice. Results shown are the mean ± sem of mice from 3 independent experiments.
Table 2.
SRC Frequency Determined by Limiting Dilution Analysis
| 3 weeks post transplant | 9 weeks post transplant | |||||
|---|---|---|---|---|---|---|
| No. of CD34+ starting cells per mouse | No. of mice engrafted with ≥ 0.5 % CD45/total no. of mice | No. of mice engrafted with ≥ 0.5 % CD45/total no. of mice | ||||
| Delta 1 | Control | Non-cultured | Delta 1 | Control | Non-cultured | |
| 1200 | 3/46 | 0/38 | 5/45 | 1/36 | ||
| 6000 | 24/46 | 7/36 | 2/41 | 15/45 | 7/36 | 3/36 |
| 30000 | 46/46 | 23/32 | 6/44 | 43/43 | 27/30 | 17/42 |
| 150000 | 32/42 | 34/38 | ||||
| No. of SRC/106 starting cells | 125 | 38*** | 8*** | 99 | 56* | 16*** |
p=0.01,
p=0.0001; reference group Delta1ext-IgG, 3 and 9 weeks
Critical to future clinical efficacy of this approach was whether more rapid engraftment could be observed after Notch-mediated expansion. In three independent experiments, measurable human engraftment at 10 days post transplant (defined as ≥0.5% human CD45+ cells in the marrow of recipient mice) was seen in all mice receiving Delta1-cultured cells, whereas no mice receiving non-cultured CB cells showed human engraftment. This engraftment consisted of > 95% myeloid cells as measured by co-expression of the human antigens, CD33 and CD45 (Fig. 1c and data not shown), demonstrating the ability of Notch-expanded CB progenitors to provide markedly accelerated hematopoietic repopulation in this animal model system.
Proof of principal: preliminary results of phase I trial
A phase I trial involving transplantation of a non-manipulated unit along with CB progenitors from a second CB unit that have undergone Notch-mediated ex vivo expansion is ongoing. The primary objective is evaluation of safety of infusing the ex vivo expanded CB progenitors, while secondary objectives include evaluation of the kinetics and durability of hematopoietic reconstitution and the relative contribution to engraftment as provided by the expanded and non-manipulated CB units.
To date, we have enrolled ten patients with high risk acute leukemias in morphologic remission at the time of transplant (Supplementary Table 1) with a median age of 27.5 years (range, 3 to 43) and median weight of 61.5 kilograms (range, 16 to 79). Based on studies by Wagner and colleagues at the University of Minnesota demonstrating safety of double CB unit infusion, patients receive a myeloablative preparative regimen (1320 cGy TBI, 120mg/kg cytoxan and 75mg/m2 Fludarabine) followed by infusion of one non-manipulated and one ex vivo expanded CB graft. CB donors are chosen on the basis of cell dose and HLA, with all units being ≥ 4/6 matched to the recipient (intermediate resolution at HLA-A and B, and high resolution at DRB1) and at least 3/6 matched to each other (Supplementary Table 1). Additional criteria for donor CB selection include a minimum requirement of a total nucleated cell (TNC) dose in the non-manipulated graft of at least 2.5 × 107 TNC/kg (based on pre-cryopreservation numbers), independent of the match grade (HLA) of the unit to the recipient. All patients receive prophylaxis for graft-versus-host-disease (GVHD) consisting of cyclosporine and mycophenolate mofetil beginning on day –3.
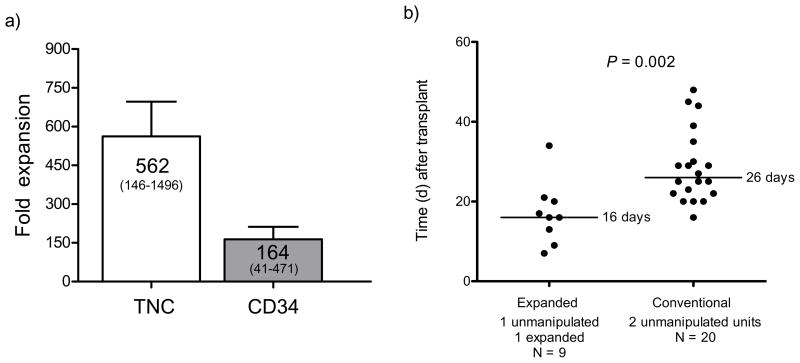
Sixteen days prior to the stem cell infusion date, we thawed the unit selected for ex vivo expansion, selected for CD34using the Isolex 300i and initiated cultures as described above. Cultures were expanded as required to maintain cell densities at ≤1×106 total cells ml−1. On the day of transplant, the cultures were harvested and infused 4 hours after infusion of a non-cultured, unrelated donor CB graft. At harvest, there was an average fold expansion of CD34+ cells of 164 (±48 s.e.m, range 41–471) and an average fold expansion of total cell numbers of 562 (±134 s.e.m, range 146–1496) (Fig. 2a). Additional details of the final harvested product can be found in the supplemental data (Supplementary Tables 2 and 3). Of note, there were no mature T cells infused with the expanded graft. Despite cell losses that occur with CD34 cell selection, in all cases the absolute number of CD34+ cells at the end of culture greatly exceeded the pre-cryopreservation CD34 cell number. The infused CD34+ cell dose derived from the expanded CB graft averaged 6 ×106 CD34/kg (range 0.93 to 13 ×106) versus 0.24 ×106 CD34/kg (range 0.06 to 0.54 ×106) (p = 0.0004) from the non-manipulated CB graft. There was no significant difference in the average TNC/kilogram dose infused between the non-manipulated and expanded cell grafts.
Figure 2. Clinical grade culture of CB progenitors with Delta1ext-IgG results in significant in vitro expansion of CD34+ cells and more rapid neutrophil recovery in a myeloablative double CBT setting.
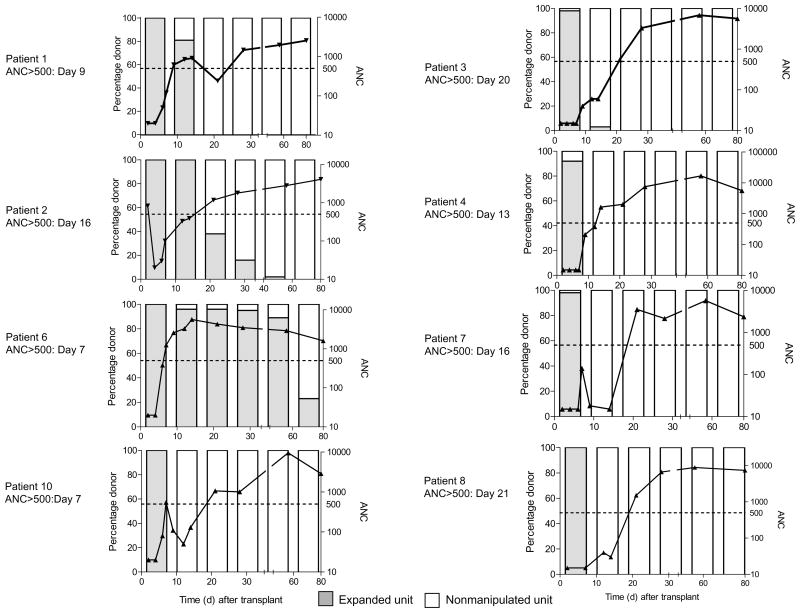
CD34+ cord blood progenitor cells were enriched and placed into culture with Delta1ext-IgG as described. (a) The mean fold expansion for both total nucleated cells (TNC) and CD34+ cells upon harvest is presented. Data shown in the bar graph are the mean ± sem for the ten patients enrolled to date (range in parentheses). (b) The individual and median times (solid line) to absolute neutrophil counts (ANC) of ≥500 μl−1 for patients receiving double unit CB transplants with two non-manipulated units (“conventional”) versus with one ex vivo expanded unit and one non-manipulated unit (“expanded”) is presented. (c) The absolute neutrophil count and specific donor CB unit contribution to myeloid engraftment over time (x axis, days 0 to 80) determined by ampFLP analysis are presented. The bars indicate percent expanded (hatched gray) or non-manipulated (white) unit contributing to myeloid (CD33+) engraftment (left axis) weekly through day 28 (day 7, 14, 21 and 28) post transplant and then at day 56 and 80. Eight of ten evaluable patients are presented (see results). The left column represents patients who achieved an ANC of ≥500 μl−1 when the expanded unit was the dominant donor while the right column represents patients who had converted to the non-manipulated donor when the ANC reached ≥500 μl−1. The solid line represents ANC over time (right Y axis). Individual time to ANC ≥500 μl−1 is shown to the left of each graph.
The kinetics of hematopoietic recovery and the relative contribution of the expanded and non-manipulated CB grafts to engraftment were determined beginning 7 days post transplant. Time to ANC ≥500 μl−1 was evaluable in nine out of ten patients in whom time to achieve an ANC of ≥500 μl−1 was shortened significantly, with a median time of 16 days (range, 7–34 days). This compares quite favorably with a median time of 26 days (range, 16–48 days; p= 0.002) in a concurrent cohort of 20 patients undergoing double CB transplantation at our institution with identical conditioning and post-transplant immunosuppressive regimen (Fig. 2b). This cohort also did not differ significantly in age, weight, diagnosis or infused cell doses as provided by the non-manipulated units (Supplemental Table 1). Furthermore, prior publications suggest that an ANC threshold of >100 μl−1 is strongly associated with a survival benefit post allogeneic stem cell transplant12. Among enrolled patients median time to achieve an ANC >100 μl−1 was 9 days versus 19 days in the conventional setting (as above) (p=0.006, data not shown). One patient experienced primary graft rejection. Of note, we have observed no infusional toxicities or other safety concerns. Average follow-up time for this set of 10 patients is currently 354 days (range 77–806), and 7 out of 10 patients remain alive with no evidence of disease, and sustained complete donor engraftment. Acute grade II GVHD has been observed in all evaluable patients, except for one who had overall grade III acute GVHD. All patients responded to therapy. No chronic extensive GVHD has been observed and three patients have been diagnosed with chronic limited GVHD.
Contribution to donor engraftment by the expanded or non-manipulated grafts was determined weekly in the first month beginning at day 7 post-transplant on peripheral blood sorted cell fractions. In eight of the nine engrafted patients there were sufficient numbers of peripheral blood sorted myeloid cells for evaluation and in each of these patients revealed a predominance of donor cell engraftment derived from the expanded cell graft in both the CD33 and CD14 cell fractions (Fig. 2c). The one patient that was not evaluable at day 7 due to an insufficient quantity of cells had concurrent reactivation of HHV6, a virus that has been shown to inhibit progenitor cell proliferation 13. This patient was also the only patient with neutrophil engraftment occurring after day 21 post transplant and the only engrafted patient with no contribution to engraftment observed from the expanded graft.
In four patients, an ANC of ≥500 μl−1 was attained at a time when the predominant contribution to myeloid engraftment was from the expanded cell graft (81, 96, 100 and 100% contribution to sorted CD33 and CD14 cell fractions (Fig. 2c, left column). Three of these four patients reached an ANC of ≥500 μl−1 exceptionally early (7, 7 and 9 days), with the fourth at day 16. However, in the remaining four patients who all engrafted early (≤21 days), ANC ≥500 occurred at a time when contribution to myeloid engraftment was derived from the non-manipulated graft. This may suggest a potential facilitating effect exerted by the ex vivo expanded cells on the non-manipulated CB graft (Fig. 2c, right column).
We observed longer-term in vivo persistence of the expanded cell graft in two patients. In one patient, analysis at day 240 post transplant revealed a portion (10–15%) of the donor CD14, CD56 and CD19 cells were derived from the expanded graft but was no longer present by one year. In the second patient at day 180 post transplant, contribution to engraftment from the expanded cell population at day 180 post transplant in CD33, CD14, CD56 and CD19 cells ranged from 25 to 66% of total donor engraftment (Table 3). However, the expanded graft did not contribute to CD3+ cell engraftment.
Table 3.
Long-term Persistence in Patients 2 and 6: Percent Peripheral Blood Cells Derived from the Expanded Cell Graft
| % Expanded Donor* | Patient 2 Day 240 | Patient 6 Day 180 |
|---|---|---|
| CD33 | 0 | 25 |
| CD14 | 12 | 31 |
| CD56 | 15 | 50 |
| CD19 | 5 | 66 |
| CD3 | 0 | 0 |
No host cells were observed (level of detection 1–5%).
Discussion
Our prior reports demonstrated that expansion of mouse and human HSPC can result from constitutive activation of Notch signaling, and subsequent studies used recombinant ligands to activate endogenous Notch receptors, with the goal of enhancing ex vivo generation of non-genetically altered hematopoietic repopulating cells for therapeutic intent 5–8. Here we have shown that Notch-mediated expansion of CB stem/progenitor cells results in a dramatic expansion of hematopoietic precursors capable of rapid multi-lineage in vivo NOD/SCID reconstitution, while possibly retaining longer-term repopulating ability. Moreover, preliminary results from our Phase I trial provide the first evidence that a key regulator of cell fate, the Notch pathway, can be used in a clinically compliant manner to generate a safe and potentially efficacious cell therapy product.
Significantly enhanced rates of myeloid engraftment have been seen, with an overall median time to neutrophil recovery (ANC 500 μl−1) of 16 days, with six of nine patients achieving neutrophil engraftment between 7 and 17 days, faster than would be expected using 2 non-manipulated units (median time 23 to 26 days or longer in the published literature) 14,15. Of note, early (day 7) myeloid engraftment was derived almost entirely from the expanded cell graft (Fig. 2). In four patients the expanded cell graft remained the dominant donor graft when an ANC ≥500 μl−1 was attained early (≤16 days) post-transplant. Furthermore, all but one of the remaining patients achieved an ANC of ≥500 μl−1 by day 21 post-transplant, despite loss of contribution to engraftment from the expanded cell graft. This is highly suggestive of a facilitating effect of the cultured cells in promoting engraftment from the non-manipulated CB unit, an hypothesis currently being explored.
The expanded cell population may also have retained a portion of longer-term repopulating stem/progenitor cells as suggested in the two patients with in vivo persistence of cultured donor cells. The lack of in vivo persistence in the remaining patients could either be due to loss of stem cell self-renewal capacity during culture, or to immune mediated rejection. It has been well documented that in most patients who receive two non-manipulated units for CBT, only one unit contributes to persistent long-term engraftment14,16,17. The mechanisms responsible for this single donor dominance remain ill-defined; however, one possibility is immune mediated rejection of the losing unit by the winning graft. If this is correct, even if longer-term repopulating cells were present after culture of CB progenitor cells in the presence of Notch ligand, immune cells present in the non-manipulated unit would be expected to reject the expanded cell graft, which itself does not contain either mature or newly generated T cells. This hypothesis is further supported by recent studies demonstrating an IFN-γ response by CD8+CD45RO+/− CCR7− T cells in the “winning” unit against the rejected one in patients undergoing double cord blood unit transplantation20. Included in these studies were four patients in the present trial which showed an IFN-γ response by cells derived from the non-cultured cord blood unit in response to cells derived from the cultured unit in three of the patients and in whom only cells from the non-cultured unit survived long-term. In the fourth patient, failure to detect an IFN-γ response was associated with persistence of the cultured as well as non-cultured unit. Consequently, persistence of long-term engrafting potential retained within the expanded progenitor cell population was not expected and the >180 day in vivo persistence of progeny derived from the cultured cells observed in the two CBT patients despite these barriers was surprising, but consistent with our pre-clinical data in immunodeficient mice in which ex vivo expanded CB progenitors proved capable of secondary transplantation.
To our knowledge, this is the first demonstration of rapid hematopoietic engraftment derived from ex vivo expanded hematopoietic progenitors. However, while rapid neutrophil engraftment has been demonstrated in the patients treated to date, larger phase II/III studies are required to test the hypothesis that enhanced kinetics of engraftment observed will improve patient outcomes, including effects on overall survival, incidence of clinically significant infections, and days in the hospital.
Methods
Cell processing
We obtained human CB samples for research from normal deliveries under institutional review board approval and after consent was obtained. Units were incubated in ammonium chloride red blood cell lysis buffer, washed, and suspended in phosphate-buffered saline (PBS) with 2% human type AB serum. We incubated cells with anti-CD34 antibody 12.8 (generated in our laboratory), followed by immunomagnetic bead-conjugated rat anti-mouse IgM (Miltenyi Biotec), and purified them using an Automacs (Miltenyi Biotec). We then froze the cells. In some cases, we enriched thawed cells for CD34+ or CD34+CD38− cells by flow cytometry.
For pre-clinical validation studies, frozen CB units obtained from the National Heart Lung and Blood Institute (NHLBI), or units obtained fresh, processed and cryopreserved, were thawed with Dextran-HSA 8 and CD34+cells selected using the Isolex 300i Magnetic Cell Selector (Baxter).
In vitro culture
We cultured cells for 17–21 days for preclinical studies and 16 days for clinical grade cultures in non-tissue culture treated plates or 75 cm2 tissue culture flasks (Falcon, Becton-Dickinson, or Nunc, Thermo Fisher Scientific), or Lifecell X-Fold cell culture bags (Baxter). We pre-coated culture vessels with Delta1ext-IgG or human control IgG at 2.5 μg ml−1 (a density previously been shown optimal for generation of NOD/SCID repopulating cells 7), together with 5 μg ml−1 of fibronectin fragment CH-296 (Takara Shuzo Co. LTD) overnight at 4°, washed them with PBS, and then blocked the vessels with PBS-2% BSA or HSA at 37°.
We cultured cells in serum-free medium (StemSpan SFEM, Stemcell Technologies) with recombinant human IL-3 (10 ng ml−1) IL-6 (100 ng ml−1), Thrombopoietin (TPO, 100 ng ml−1), Flt-3 Ligand (300 ng ml−1) and Stem Cell Factor (SCF 300 ng ml−1)(known as “5 GF”) (Invitrogen). Other media tested were QBSF-60 (Quality Biological Inc.), Stemspan H3000 (Stemcell Technologies), Stemline (Sigma-Aldrich), X-Vivo 10 and 20 (Cambrex), and Stempro 34 (Invitrogen). We obtained clinical grade cytokines from CellGenix and clinical grade ligand was prepared in the Fred Hutchinson Cancer Research Center Biologics Production Facility.
We initiated cultures with 6,000 CD34+ cells/well in 24 well plates, or 2.0–3.5×105 CD34+ cells/75 cm2 flask or 85 cm2 bag and expanded them after 7–10 days into one 390 cm2 tissue culture bag or four to six 75 cm2 tissue culture flasks. In some experiments, we further expanded cultures between days 10 and 14 into one or two additional 390 cm2 bags or up to six additional flasks. We added fresh medium with cytokines every 3 to 4 days.
Flow cytometric analysis
We performed immunofluorescence analysis as described previously using FITC-labeled antibodies against CD34 and mouse CD45.1 (Becton Dickinson Pharmingen,), or PE-labeled antibodies against CD14, CD19, CD33, CD34, CD38 (Becton Dickinson Pharmingen), CD7 (8H8.1), CD56 (Beckman Coulter), and PerCP-conjugated antibody against CD45 (Becton Dickinson Pharmingen)7, FITC, PE or PerCP-conjugated, isotype-matched antibodies served as controls.
In vivo repopulation studies
We infused sublethally irradiated (325 cGy) NOD/SCID or NOD/SCID IL-2Rγnull mice with the progeny of 30,000 starting CD34+ cells per mouse via tail vein. In some cases, the total derived progeny were depleted of CD14+ cells using immunomagnetic beads. CD34+ non-cultured cells (30,000) were combined with 2×105 irradiated (15 Gy) CD34− CB cells as carrier cells. We assessed repopulating ability at 10 days to 3 weeks post-transplant using marrow removed from the knee joint of anesthetized mice. At nine weeks post-transplant, we sacrificed mice and assessed both femurs and tibias for the number and types of human cells. We determined the frequency of SRC (SCID mouse repopulating cells) in limiting dilution assays by the method of maximum likelihood using the L-CALC™ software (StemCell Technologies) from the proportions of engrafted recipients (≥0.5% human CD45+ cell engraftment) measured in groups of mice transplanted with the progeny of different numbers of starting cells.
We performed secondary transplants following the method from Peled, et.al. 19. Marrow cells harvested from bilateral femurs and tibias were resuspended in Stemspan with IL6 and SCF at 50 ng ml−1 and incubated for 48 hours at 37° and then infused into secondary NOD/SCID IL-2Rγnull recipients at a ratio of one donor to one recipient.
Chimerism analysis
We performed chimerism testing on peripheral blood samples that were sorted by FACS into CD3+, CD33+, CD14+, CD56+ and CD19+ cell fractions beginning on day +7 post transplant, using a DNA-based assay for short tandem repeat (STR) loci.
Phase I Study
We have enrolled ten patients to date in a Fred Hutchinson Cancer Research Center Protocol (Protocol 2044) after IRB approval was obtained and an IND filed with the FDA (BB-IND 12657, Sponsor C Delaney).
Statistics
Data are presented as the mean +/−SEM. The significance of the differences between groups was determined using paired or unpaired t tests, two-tailed (GraphPad Software).
Supplementary Material
Acknowledgments
This work was supported by grants R24 HL74445, K23 HL077446, K12 CA076930, 5R01HL080245-02 (from the National Institutes of Health), CA15704 and DK56465. C.D. is a Damon Runyon Clinical Investigator supported in part by the Damon Runyon Cancer Research Foundation (CI# 35-07). I.D.B. is an American Cancer Society-F.M. Kirby Clinical Research Professor. The authors would like to thank Fred Appelbaum for his contribution to clinical trial design and clinical consultation, Jonathan Gutman for help in manuscript editing and clinical data coordination, and Doug Woodle for expert technical assistance. We also acknowledge the work of the clinical faculty and staff at the Seattle Cancer Care Alliance for the excellent clinical care provided to the patients.
Footnotes
Competing interests statement: The authors declare no competing financial interests.
Author Contributions: C.D. conceived of project, designed experiments, analyzed the data, wrote manuscript, performed in vitro and animal studies, Principal Investigator of clinical studies, preparation of figures; S.H. experimental design, data analysis, manuscript editing; C.B.S. and H.V. performed in vitro studies, data analysis, animal studies; R.M. methods for large scale production of cGMP engineered Delta1; I.D.B conceived of project, experimental design, manuscript editing.
References
- 1.Shpall E, et al. Transplantation of Ex Vivo Expanded Cord Blood. Biology of Blood and Marrow Transplantation. 2002;8:368–376. doi: 10.1053/bbmt.2002.v8.pm12171483. [DOI] [PubMed] [Google Scholar]
- 2.de Lima M, et al. Transplantation of ex vivo expanded cord blood cells using the copper chelator tetraethylenepentamine: a phase I/II clinical trial. Bone Marrow Transplantation. 2008;41:771–778. doi: 10.1038/sj.bmt.1705979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaroscak J, et al. Augmentation of umbilical cord blood (UCB) transplantation with ex vivo-expanded UCB cells: results of a phase 1 trial using the AastromReplicell System. Blood. 2003;101:5061–7. doi: 10.1182/blood-2001-12-0290. [DOI] [PubMed] [Google Scholar]
- 4.Milner LA, Kopan R, Martin DI, Bernstein ID. A human homologue of the Drosophila developmental gene, Notch, is expressed in CD34+ hematopoietic precursors. Blood. 1994;83:2057–62. [PubMed] [Google Scholar]
- 5.Varnum-Finney B, et al. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat Med. 2000;6:1278–81. doi: 10.1038/81390. [DOI] [PubMed] [Google Scholar]
- 6.Varnum-Finney B, Brashem-Stein C, Bernstein ID. Combined effects of Notch signaling and cytokines induce a multiple log increase in precursors with lymphoid and myeloid reconstituting ability. Blood. 2003;101:1784–9. doi: 10.1182/blood-2002-06-1862. [DOI] [PubMed] [Google Scholar]
- 7.Delaney C, Varnum-Finney B, Aoyama K, Brashem-Stein C, Bernstein ID. Dose-dependent effects of the Notch ligand Delta1 on ex vivo differentiation and in vivo marrow repopulating ability of cord blood cells. Blood. 2005;106:2693–9. doi: 10.1182/blood-2005-03-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohishi K, Varnum-Finney B, Bernstein ID. Delta-1 enhances marrow and thymus repopulating ability of human CD34(+)CD38(−) cord blood cells. J Clin Invest. 2002;110:1165–74. doi: 10.1172/JCI16167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatia M, et al. Quantitative analysis reveals expansion of human hematopoietic repopulating cells after short-term ex vivo culture. J Exp Med. 1997;186:619–24. doi: 10.1084/jem.186.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conneally E, Cashman J, Petzer A, Eaves C. Expansion in vitro of transplantable human cord blood stem cells demonstrated using a quantitative assay of their lympho-myeloid repopulating activity in nonobese diabetic-scid/scid mice. Proc Natl Acad Sci U S A. 1997;94:9836–41. doi: 10.1073/pnas.94.18.9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki T, et al. Highly efficient ex vivo expansion of human hematopoietic stem cells using Delta1-Fc chimeric protein. Stem Cells. 2006;24 doi: 10.1634/stemcells.2006-0258. [DOI] [PubMed] [Google Scholar]
- 12.Offner F, Schoch G, Fisher LD, Torok-Storb B, Martin PJ. Mortality hazard functions as related to neutropenia at different times after marrow transplantation. Blood. 1996;88:4058. [PubMed] [Google Scholar]
- 13.Nitsche A, et al. Inhibition of cord blood cell expansion by human herpesvirus 6 in vitro. Stem Cells Dev. 2004;13:197–203. doi: 10.1089/154732804323046800. [DOI] [PubMed] [Google Scholar]
- 14.Barker JN, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–7. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 15.Bradstock K, et al. Single versus double unrelated umbilical cord blood units for allogeneic transplantation in adults with advanced hematological malignancies: a retrospective comparison of outcomes. Internal Medicine Journal. 2008 November 3;9999 doi: 10.1111/j.1445-5994.2008.01825.x. (epub) [DOI] [PubMed] [Google Scholar]
- 16.Brunstein CG, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110:3064. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang HJ, et al. Early engraftment kinetics of two units cord blood transplantation. Bone Marrow Transplantation. 2006;38:197–201. doi: 10.1038/sj.bmt.1705423. [DOI] [PubMed] [Google Scholar]
- 18.Rubinstein P, et al. Processing and Cryopreservation of Placental/Umbilical Cord Blood for Unrelated Bone Marrow Reconstitution. Proceedings of the National Academy of Sciences. 1995;92:10119–10122. doi: 10.1073/pnas.92.22.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peled A, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–8. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 20.Gutman JA, et al. Single Unit Dominance Following Double Unit Umbilical Cord Blood Transplantation Coincides with a Specific CD8+ T Cell Response Against the Non-Engrafted Unit. Blood. 2009 September; doi: 10.1182/blood-2009-07-228999. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.