Abstract
Parkinson’s disease (PD) is a common neurodegenerative disease affecting up to one million individuals in the United States. Depression is found in 40 to 50% of these patients and is associated with a variety of poor outcomes for both patients and their families. Despite this, there are few evidence-based data to guide clinical care. This was an NIH-funded, randomized, controlled trial of paroxetine, nortriptyline, and placebo. It included an 8 week acute phase and a 16 week blind extension phase. This report details the impact of depression treatment on quality of life (QoL) and disability in the acute and extension phase of this study. Secondary outcomes included relapse, tolerability, safety, and the impact of depression treatment on PD physical functioning. Patients who had improvement in depression, compared with those who did not, had significant gains in measures of QoL and disability (PDQ-8, P = 0.0001; SF-36, P = 0.0001) at 8 weeks and maintained their gains in the extension phase. Patients who were on active drug were significantly less likely to relapse in the extension phase than those on placebo (P = 0.041). Though relatively modest in size, this trial provides the first controlled data on the impact of treatment of depression on QoL and disability in PD. It suggests that successfully treating depression in PD leads to important, sustained improvements in these outcomes and that patients who improve on antidepressants are less likely to relapse than are patients who initially improve on placebo.
Introduction
Parkinson’s disease (PD) is the second most prevalent neurodegenerative illness in the United States, affecting approximately 1 million individuals.1 Although the illness is characterized by tremor, rigidity and postural imbalance, nonmotor features are both common and functionally important. Depression is one of the most common nonmotor aspects of PD, with a reported prevalence of approximately 40 to 50%,2 and is associated with a variety of poor short- and longterm outcomes.3
Depression is of particular importance to these patients because, in addition to personal suffering, it has been associated with poorer quality of life (QoL), greater disability, faster progression of physical symptoms, greater decline in cognitive skills and ability to care for oneself, poorer treatment compliance, and greater caregiver distress.4–6 In fact, depression seems to be more predictive of QoL than motor disability.7
Despite the prevalence and importance of depression in PD, there are few well-designed studies that inform treatment.8 We have recently shown, in an 8-week, NIH-funded, randomized trial of nortriptyline, controlled release paroxetine and placebo, that nortriptyline was associated with a significantly larger change than placebo in the Hamilton Depression Rating Scale (HAM-D) and produced significantly more responders (50% change in HAM-D) than paroxetine; its effect size was also large.9 Both drugs were well tolerated and there were no apparent adverse cardiac effects of nortriptyline.
We now report on the results of the treatment of depression on QoL and disability in the acute (8 weeks) and extension phase (16 weeks) of this study. In particular, we hypothesized that patients who showed improvement in depression at the end of the acute phase, compared to those who did not, would have significant gains in measures of QoL and disability. In addition, we report on the comparative relapse rates for the three blinded treatments in the extension phase of the trial.
Patients and Methods
Study Design
Fifty-two patients were enrolled in this randomized, double-blind trial of nortriptyline, controlled release paroxetine or placebo. Those patients achieving at least a 3 (minimally improved) on the Clinical Global Impression-Change Scale (CGI-C)10 in the first 8 weeks of the study were given the opportunity to continue in blinded treatment for an additional 4 months. The primary outcomes for this analysis were: the change in QoL and disability for the acute phase in patients, who had improvement in depression, compared with those who did not, and whether patients who entered the extension phase maintained their gains.
Secondary outcomes included relapse, tolerability, safety, and the impact of depression treatment on PD physical functioning. All procedures used were approved by the University of Medicine and Dentistry of New Jersey’s Institutional Review Board and all patients signed an approved informed consent. Patients were evaluated between October 2003 and July 2007.
Patients
Inclusion and exclusion criteria for the study were previously published9 and included, in brief, a confirmed diagnosis of PD by research criteria,11 35 to 80 years of age and a primary diagnosis of major depression or dysthymia on the Structured Clinical Interview12 (SCID) for the Diagnostic and Statistical Manual of Mental Disorders 4th ed. (DSM-IV).13
Patients were excluded if they had cognitive impairment (MMSE14 < 26), were ‘‘off’’ for greater than 50% of the day or had any current DSM-IV Axis I diagnosis other than a depressive or anxiety disorder. Benign (insight retained) visual hallucinations were not an exclusion criterion. Patients were required to maintain a stable dose of the PD medication that they were on at the start of the trial and all evaluations were done in the ‘‘on’’ state.
Dosing
In the acute phase, patients were randomized, in variable length blocks, to equivalent-appearing nortriptyline, paroxetine, or placebo. Dosing was flexible with decisions on dose being made at each visit based on efficacy and tolerability. Paroxetine was started at 12.5 mg and could be increased up to 37.5 mg. Nortriptyline was started at 25 mg and could be increased up to 75 mg. Placebo was started at 1 pill and could be increased up to 3 pills. During the extension phase, dosing remained flexible and all patients and study personnel remained blind to drug assignment.
Assessments
Patients’ perception of improvement in QoL and disability was assessed using the Parkinson’s Disease Questionnaire (PDQ-8)15 and the Medical Outcome Study Short Form (SF-36)16 at baseline, 8 and 24 weeks. The PDQ-8 is a brief disease specific instrument that measures QoL and physical disability as a result of PD. The SF-36 is a generic QoL and disability instrument commonly used in medical research. Both of these self-report measures have been widely used and validated in PD.17,18 Other outcomes examined including depression severity (HAM-D and CGI) and tolerability were done at baseline, 2, 4, 8, 16, and 24 weeks. All CGI-C scores were compared to the baseline score. Nortriptyline levels, EKGs, and the Unified Parkinson’s Disease Rating Scale (UPDRS),19 which includes motor assessments as well as mood and disability measures as rated by a physician, were done at baseline, 8 and 24 weeks.
Data Analyses
For QoL and disability assessments, patients who improved during the acute phase were compared to those who had not improved. Improvement was defined as achieving a 1, 2, or 3 (at least minimally improved) on the CGI-C. An intent-to-treat approach was used in all analyses. Each outcome of interest was evaluated at baseline and 8 weeks using a mixed-model repeated measures analysis of variance as implemented in the MIXED procedure of SAS Version 9.1 with restricted maximum likelihood estimation.20 The fixed effect of interest was time 3 group (improvement; nonimprovement) interaction. Random subject intercepts were included in the model. In modeling covariance structure for all analyses, we chose a continuous AR (1) structure as a function of the square root of days from baseline that a given assessment occurred for a given patient. This model yielded the lowest AIC among all covariate structures examined. Multiple testing of subscales was adjusted for with the Bonferroni method.
A second set of mixed models was conducted for the same QoL and disability variables as above for the 20 patients who entered the extension phase. The fixed effect of interest for these analyses was time (0, 8, 24 weeks).
The relapse analysis included the 20 patients who had entered the extension phase. All patients were required by protocol to have at least a 3 on the CGI-C (minimally improved from baseline) to enter the extension phase. All subsequent CGI-C scores were compared to the study entry baseline. Although there are a variety of operational definitions of relapse in the literature, we chose to use a CGI-C deterioration of 2 points or more during the extension phase as evidence of relapse because the HAM-D scores of those entering the extension phase was very restricted (mean of 8.85) and there is no well-accepted numerical change in the HAMD that defines relapse. We believe that the CGI-C score, which is relevant to the overall estimate of the patients condition and has been widely used as an estimate of relapse, is the best available approach for a clinical study such as this and is consistent with the definition proposed by Frank et al.21 Study group (nortriptyline, paroxetine, and placebo) was then cross tabulated with relapse/nonrelapse.
Results
Sixty-seven patients signed informed consent and 15 were screened out for a variety of reasons, including cognitive impairment, having an excluded diagnosis or not meeting depression criteria. Thus, 52 patients, 27 men and 25 women, entered the acute trial. Fifty of the patients had major depression, two had dysthymia in addition to major depression and two had only dysthymia. The mean age was 62.8 and the mean duration of PD was 6.6 years. The average dose of medication over the entire 24-week trial was 28.4 mg for paroxetine, 48.5 mg for nortriptyline, and 2.7 pills for placebo. Further clinical information characterizing the sample can be found in the publication on the acute phase results.9
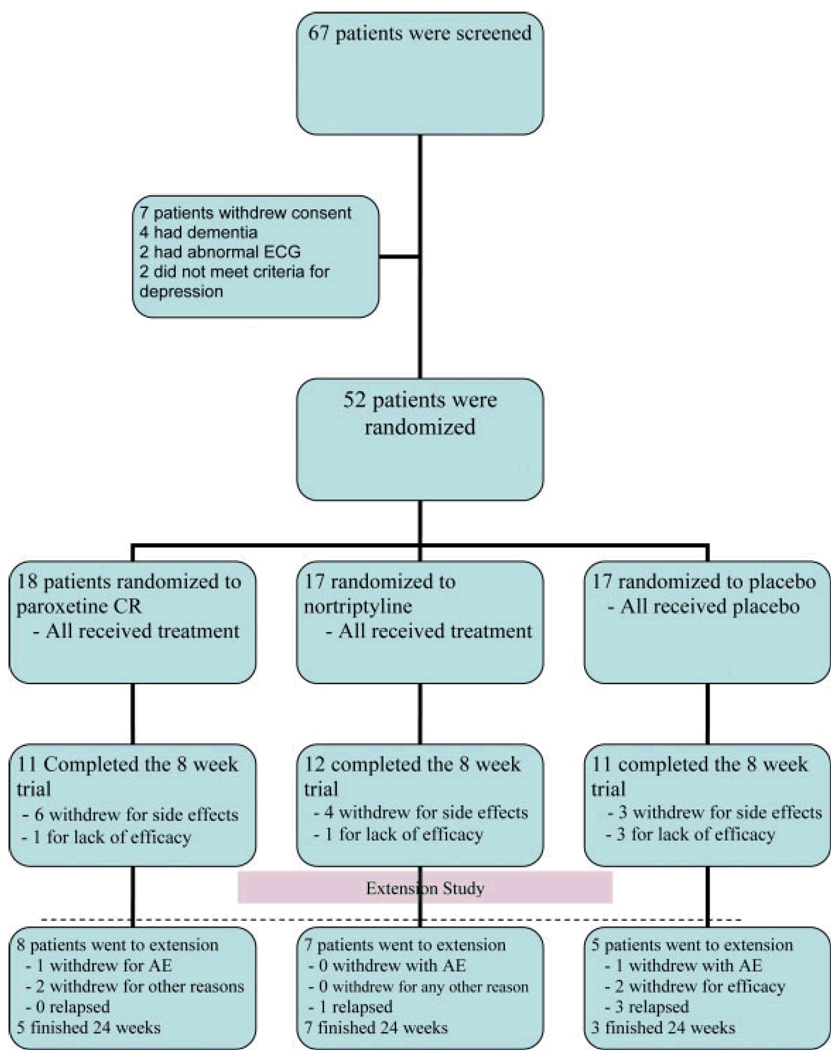
At the end of the acute phase, 30 patients met criteria for improvement and 20 did not—two patients did not contribute any data beyond baseline and were not included in any analyses reported here. Twenty patients (nortriptyline = 7, paroxetine = 8, placebo = 5) met both criteria for entry into the extension phase: a CGI-C of 1, 2, or 3 and choosing to continue blinded treatment. Fifteen patients finished the entire 24 weeks of the study; during the extension phase one patient dropped out for lack of efficacy, two for adverse events, and two for other reasons such as moving away and inability to come to study assessments. See Chart 1 for a flow chart of patients.
Chart 1.
Patient flow diagram
Quality of Life and Functioning in the Acute Eight Week Study
PDQ-8
Patients who demonstrated improvement in depression(n = 30) in the acute phase showed significantly more improvement in QoL and physical functioning on the PDQ-8 than did those who did not improve (n = 20) [mixed models test of time 3 improvement group interaction; F(1, 44) = 20.09, P = 0.0001]. See Table 1 for least squares means and standard deviations for PDQ-8 scores organized by study group and week.
Table 1.
Quality of life and disability: depression improved versus not improved in the acute phase
| Depression Not Improved |
Depression Improved |
P-value | Adjusted P | |
|---|---|---|---|---|
| Measure | Mean (SD) | Mean (SD) | ||
| PDQ-8 | ||||
| Baseline | 18.72 (5.01) | 18.09 (5.04) | 0.0001 | |
| Endpoint (8 weeks) | 20.89 (5.01) | 15.22 (5.09) | ||
| SF-36 Total | ||||
| Baseline | 48.35 (16.33) | 48.65 (16.56) | 0.0001 | |
| Endpoint (8 weeks) | 37.98 (16.92) | 57.84 (16.78) | ||
| Well Being | ||||
| Baseline | 52.37 (17.19) | 52.97 (17.43) | 0.0001 | 0.0008 |
| Endpoint (8 weeks) | 37.73 (17.78) | 64.86 (17.61) | ||
| Emotion Role Fn. | ||||
| Baseline | 49.85 (40.45) | 29.08 (41.08) | 0.0055 | 0.044 |
| Endpoint (8 weeks) | 24.07 (42.15) | 62.51 (40.04) | ||
| Social Function | ||||
| Baseline | 60.62 (23.17) | 65.52 (23.54) | 0.0244 | NS |
| Endpoint (8 weeks) | 51.81 (24.12) | 72.48 (23.81) | ||
| Energy | ||||
| Baseline | 31.07 (20.25) | 33.81 (20.62) | 0.0036 | 0.0288 |
| Endpoint (8 weeks) | 25.64 (20.52) | 49.69 (19.8) | ||
| Physical Function | ||||
| Baseline | 53.03 (25.15) | 58.39 (25.57) | 0.7118 | NS |
| Endpoint (8 weeks) | 51.34 (25.87) | 58.89 (25.79) | ||
| Physical Role Fn. | ||||
| Baseline | 30.01 (34.47) | 31.93 (34.98) | 0.0055 | 0.044 |
| Endpoint (8 weeks) | 12.87 (35.58) | 51.56 (34.24) | ||
| General Health | ||||
| Baseline | 44.25 (11.34) | 48.06 (11.55) | 0.24 | NS |
| Endpoint (8 weeks) | 41.22 (11.61) | 47.87 (11.66) | ||
| Pain | ||||
| Baseline | 64.75 (24.937) | 70.78 (25.31) | 0.17 | NS |
| Endpoint (8 weeks) | 59.29 (25.97) | 75.39 (25.63) |
SF-36
Patients who demonstrated improvement in depression in the acute phase also showed significantly more gains over time on the SF-36 than those who did not improve. The mixed-models test of the time 3 improvement group interaction for the total score on the SF-36 was statistically significant [F(1, 44) = 20.46, P = 0.0001]. As shown by the least squares means (from the mixed model) listed in Table 1, the total gain was 9 points in the improved group while the nonimproved group showed a worsening of nearly 10 points.
As would be expected given the gains in the total score of the SF-36, Table 1 also shows that those whose depression improved showed gains on several of the individual subscales of the SF-36 compared with those who did not improve. After the nominal P-levels (from mixed-models tests of time 3 improvement group interactions) for each subscale in Table 1 were corrected for the fact that there were a total of eight subscales, well-being, energy, emotional role functioning and physical role functioning all improved significantly. Social functioning, physical functioning, general health, and pain subscales did not improve significantly after adjustment for multiple testing.
Quality of life and disability in the extension phase of the study
PDQ-8 and SF-36
Table 2 shows least squares means for each of the measures of QoL and disability for the 20 patients who entered the extension phase. Both the PDQ-8 (P = 0.0046) and the SF-36 (P = 0.012) showed continued improvement over baseline for the 24 weeks of the study. P values are generated from mixed model analyses (time effect). For the SF-36, the total score improved by 10 points over the three time points in the study while the PDQ-8 score improved by 2 points.
Table 2.
Quality of life and disability: extension phase
| Measure | Baseline | 8 weeks | 24 weeks | p-value |
|---|---|---|---|---|
| PDQ Total score | 18.31(4.82) | 14.85 (4.82) | 16.18 (5.26) | 0.0046 |
| SF-36 total score | 51.45 (18.72) | 61.49 (18.72) | 61.05 (20.16) | 0.0121 |
| SF-36 emotional role functioning | 33.33 (41.8) | 61.66 (41.8) | 65.83 (47.88) | 0.0238 |
| SF-36 physical role functioning | 40 (39.87) | 51.25 (39.87) | 53.84 (43.43) | 0.2328 |
| SF-36 emotional well-being | 54.2 (18.23) | 67.2 (18.23) | 65.86 (19.81) | 0.0019 |
| SF-36 energy | 33.25 (22.05) | 46.51 (22.05) | 45.73 (24.08) | 0.0100 |
| SF-36 general health | 49.75 (12.64) | 49.75 (12.64) | 50.12 (13.36) | 0.9816 |
| SF-36 physical functioning | 60.25 (25.65) | 58.25 (25.65) | 57.24 (26.86) | 0.7401 |
| SF-36 social functioning | 55.99 (24.08) | 66.62 (24.08) | 64.38 (26.51) | 0.1056 |
| SF-36 pain | 74 (21.91) | 79.87 (21.91) | 75.62 (24.3) | 0.4841 |
Although the emotional role functioning, emotional wellbeing and energy subscales of the SF-36 were nominally significant over the course of the 6-month study, when adjusted for multiple testing, only the emotional wellbeing subscale of the SF-36 remained significant.
Relapse in the extension phase of the study
Relapse rates, defined as a CGI-C deterioration of 2 points or more, in the extension phase for patients who initially improved during the acute phase of the trial were 60% (3 of 5 patients for patients on placebo), 14% (1 of 7 patients) for those on nortriptyline, and 0% (0 of 8 patients) for those on paroxetine. There was a significant relationship (Goodman-Kruskal Tau = 0.357; Monte-Carlo estimate of P = 0.04) between relapse and study group (nortriptyline, paroxetine, placebo).
The HAM-D scores did not significantly change for the group as a whole from week 8 (8.85, SD 3.94) to week 24 (10.06, SD 4.95), P = 0.244 in mixed model analysis.
Disease measure in the extension phase of the study
There was no change in the motor and therapeutic complication subscales of the UPDRS throughout the trial. The UPDRS subscales of mood and mentation, ADLs, and Schwab Disability Scale (from the UPDRS) improved significantly, even after adjustment for multiple tests. Table 3 lists adjusted means and nominal P values (from the mixed model analyses).
Table 3.
United Parkinson’s disease rating scale: extension phase
| Measure | Baseline | 8 weeks | 24 weeks | p-value |
|---|---|---|---|---|
| UPDRS motor | 12.4 (7.32) | 12.8 (7.32) | 14.05 (7.84) | 0.5914 |
| UPDRS activities of daily living | 10.25 (4.63) | 7.95 (4.63) | 9.23 (4.82) | 0.0099 |
| UPDRS mood & mentation | 3.81 (1.36) | 3.35 (1.36) | 3.39 (1.52) | 0.0001 |
| UPDRS therapeutic complication | 4.5 (2.84) | 4 (2.84) | 3.96 (2.91) | 0.3229 |
| Schwab (functioning) | 88.25 (5.03) | 90.75 (5.03) | 91.99 (5.39) | 0.006 |
Tolerability in the extension phase
Side effects of the treatments were generally mild or moderate throughout the trial. Two patients withdrew during the extension phase because of adverse events; one on placebo developed increased rigidity and one on paroxetine broke an arm and was unable to come in for visits. The most common side effects in the extension phase were similar to those in the acute phase and are listed in Table 4. Chart 1 details all patient flow during the entire study.
Table 4.
Side effects: extension phase
| Side Effect | Placebo (%) | Nortriptyline (%) | Paroxetine (%) |
|---|---|---|---|
| Constipation | 6 | 35 | 6 |
| Dry Mouth | 0 | 41 | 6 |
| Insomnia | 24 | 12 | 0 |
| Fatigue | 12 | 0 | 17 |
| Orthostatic hypotension | 12 | 12 | 11 |
| Dizziness | 12 | 12 | 0 |
Safety in the extension phase
For the extension phase of the study, safety measures in the trial included measurement of the QTc interval in patients on nortriptyline, nortriptyline levels, vital signs and serious adverse events. The QTc of patients on nortriptyline decreased nonsignificantly from baseline (423 milliseconds) to endpoint (409 milliseconds). There was one patient with a QTc of greater than 450 milliseconds at baseline and two at endpoint. No patient had a QTc above 500 milliseconds. Nortriptyline levels ranged between 45 and 172 ng/mL with an average of 109 ng/mL at endpoint. There were no significant baseline to endpoint changes in vital signs in any of the groups. Six patients developed orthostatic hypotension at at least one visit in the trial (including the acute phase) – two on placebo, two on paroxetine and two on nortriptyline. None of these patients had falls and none stopped the trial because of this side effect. There were three serious adverse events in the acute phase and one in the extension phase: a patient in the extension phase on placebo had a severe worsening of rigidity due to an inadvertent Parkinson’s medication change.
Discussion
Depression has been found repeatedly to be a primary determinant of QoL in patients with PD6 and their caregivers.22 In fact, several studies have suggested that up to 40% of the observed variance in QoL in PD is due to depression and that the impact of depression on QoL exceeds that of the movement disorder aspects of the illness.7,23,24 Thus, the study of depression and QoL is of great importance in this population.
Despite this clear association of depression and poor QoL in PD, there is a paucity of research that examines the effect of treating depression on this important outcome. The only previous work of which we are aware was a single blind study of sertraline and amitriptyline. In this study, sertraline treatment of depression was associated with significant improvements in the PDQ-39 scale.
In the present study, we found that patients who had improvement in depression, compared to those without improvement in depression, evidenced significant gains on both the PDQ-8 and the SF-36 measures of QoL and disability during the eight-week study. Furthermore, for the subset of patients who entered the extension phase, these gains were maintained throughout the 6-month study. It is important to note that we reported in our original publication of the efficacy results of the 8-week acute study9 that there were no differences in QoL measurements between treatments, that is no treatment (nortriptyline, paroxetine, or placebo) was significantly superior to any other on QoL. In this report, we examine the difference (regardless of treatment arm) between those whose depression had improved compared to those whose depression had not improved. This addresses the important question of whether or not successfully treating depression helps to improve QoL.
We found continued improvement using the PDQ-8, a PD-specific QoL measure, and the SF-36 a more generic QoL and disability instrument. These results are comparable with results from nonPD populations, in which treatment of depression leads to an improvement in QoL of similar magnitude.26
Although we believe that these data are important and are the first of their kind, the trial has a number of limitations and the conclusions must be seen as preliminary. The sample size was small and relatively few patients finished the entire 24 weeks of the study. Thus, there is a danger of generalizing from this small, possibly nonrepresentative, sample. Only 20% (4 of 20 patients) relapsed in the extension phase, so the comparisons between placebo and active drug treatment should be viewed with caution. In general, the treatments were well tolerated, but there were 13 of 52 patients (25%) who dropped out of the acute study for adverse events and 2 of 20 patients (10%) who dropped out of the extension study for tolerability reasons. Lastly, it is important to note that cardiac conduction needs to be monitored in patients on nortriptyline.
Of note, the improvements in QoL and disability were mostly limited to emotional and psychosocial aspects of QoL, whereas parallel improvements were not seen in the physical aspects of QoL. There were also no changes in measures of PD disease severity, suggesting that the improvement seen in QoL is not the result of any impact of the medications on the disease itself, but rather is an effect on the emotional distress.
In addition, we also found that individuals who were on active drug were less likely to relapse than those on placebo, suggesting that antidepressants have an ongoing positive impact on depressive symptoms in PD. The prevention of relapse is an important goal in the treatment of depression because of the potentially chronic and disabling nature of the disorder. There is no literature on PD depression relapse rates with which to compare these results. However, in a systematic review of the literature on depression relapse in non-PD patients, the average rate of relapse was 41% in people receiving placebo compared with 18% for those continuing antidepressant treatment.27 Thus, our results are comparable to those observed in the nonPD population and suggest that antidepressants not only can treat depression in patients with PD but that the effect is sustained through at least 6 months.
Furthermore, it appears that patients tolerated up to 6 months of paroxetine and nortriptyline are relatively well. There were no apparent cardiotoxic effects of nortriptyline, though one needs to be cautious of this in a geriatric population.
Given the observation that depression in patients with PD is under-recognized and under-treated in clinical practice,28,29 we believe, despite the limitations of the trial, that the findings are important for patients with PD and their families.
Conclusions
Although relatively small, this study suggests that successful treatment of depression in patients with PD has a significant and long lasting impact on QoL and disability for patients with PD. In addition, we have demonstrated that active treatment with antidepressants is relatively well tolerated over 6 months and superior to placebo in preventing relapse in patients who have had improvements in their depressive symptoms.
Acknowledgments
This study was supported by a grant from the National Institute of Neurologic Disorders and Stroke (NINDS) RO1NS043144. GlaxoSmithKline provided free paroxetine CR and matching placebo.
Footnotes
Clinical Trials Registration: Clintrials.gov Identifier: NCT 00062738. Dr. Buyske, Department of Statistics at Rutgers University carried out the statistical analysis.
Disclosure:
Matthew Menza, MD - Research Support: National Institutes of Health (NINDS), Astra-Zeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Forest Laboratories, GlaxoSmithKline, Lilly, Pfizer, Sanofi-Aventis, Sepracor, Takeda Wyeth . Consultant: National Institutes of Health (NIMH, NINDS), GlaxoSmithKline, Kyowa, Lilly Research Laboratories, Pfizer, Sepracor, Takeda. Stocks: None. Other Financial: None
Roseanne DeFronzo Dobkin, PhD – Research Support National Institutes of Health (NINDS).
Humberto Marin, MD - Research Support: National Institutes of Health (NINDS), GlaxoSmithKline, Lilly, Sanofi-Aventis, Sepracor, Takeda. Consultant: Lilly Research Laboratories, Other - none
Margery Mark, MD: Research Support: Kyowa, Cephalon. Speaker: Allergan, Boehringer Ingelheim, GlaxoSmithKline, Valeant, Other - none
Michael Gara, PhD - None
Steven Buyske, PhD – Research Support: National Institutes of Health (NIAAA)
Karina Bienfait, PhD - None
Allison Dicke – None
References
- 1.Marras C, Tanner CM. Epidemiology of Parkinson's disease. In: Watts RL, Koller WC, editors. Movement disorders: Neurologic principles & practice. 2nd ed. New York: McGraw- Hill; 2004. pp. 177–195. [Google Scholar]
- 2.Reijnders JS, Ehrt U, Weber WE, Aarsland D, Leentjens AF. A systematic review of prevalence studies of depression in Parkinson’s disease. Mov Dis. 2008 Jan 30;23(2):183–189. doi: 10.1002/mds.21803. [DOI] [PubMed] [Google Scholar]
- 3.Weintraub D. Depression in Parkinson’s disease. In: Menza M, Marsh L, editors. Psychiatric Issues in Parkinson's Disease: A Practical Guide. London: Taylor & Francis; 2006. [Google Scholar]
- 4.Ravina B, Camicioli R, Como PG, Marsh L, Jankovic J, Weintraub D, Elm J. The impact of depressive symptoms in early Parkinson disease. Neurology. 2007 Jul 24;69(4):342–347. doi: 10.1212/01.wnl.0000268695.63392.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whetten-Goldstein K, Sloan F, Kulas E, Cutson T, Schenkman M. The burden of Parkinson’s disease on society, family, and the individual. J Am Geriatr Soc. 1997;45(7):844–849. doi: 10.1111/j.1532-5415.1997.tb01512.x. [DOI] [PubMed] [Google Scholar]
- 6.Schrag A. Quality of life and depression in Parkinson's disease. J Neurol Sci. 2006 Oct 25;248(1–2):151–157. doi: 10.1016/j.jns.2006.05.030. Epub 2006 Jun 21. [DOI] [PubMed] [Google Scholar]
- 7.Global Parkinson’s Disease Steering Committee: Factors impacting on quality of life in Parkinson’s disease: Results from an international survey. Movement Disorders. 2002;17:60–67. doi: 10.1002/mds.10010. [DOI] [PubMed] [Google Scholar]
- 8.Weintraub D, Morales KH, Moberg PJ, Bilker WB, Balderston C, Duda JE, Katz IR, Stern MB. Antidepressant studies in Parkinson's disease: a review and metaanalysis. Mov Disord. 2005 Sep;20(9):1161–1169. doi: 10.1002/mds.20555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menza M, Dobkin R, Marin H, Mark M, Gara M, Buyske S, Bienfait K, Dicke AA. Controlled Trial of Antidepressants in Patients with Parkinson’s Disease and Depression. Neurology. 2008 Dec 17th; doi: 10.1212/01.wnl.0000336340.89821.b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guy W. ECDEU assessment manual for psychopharmacology. Washington, D.C: U.S. Department of Health, Education, and Welfare; 1976. [Google Scholar]
- 11.Ward CD, Gibb WR. Research diagnostic criteria for Parkinson's disease. Adv Neurol. 1990;53:245–249. [PubMed] [Google Scholar]
- 12.First MB, Spritzer RL, Gibbon M. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 13.American Psychiatric Association. DSM-IV: Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: The Association; 1994. [Google Scholar]
- 14.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state ": a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 15.Jenkinson CR, Fitzpatrick V, Peto R, Hyman n. The PDQ-8: development and validation of a short-form Parkinson's disease questionnaire. Psychol Health. 1997;12:805–814. [Google Scholar]
- 16.Jenkinson C, Coulter A, Wright L. Short Form 36 (SF 36) Health Survey Questionnaire; normative data for adults of working age. BMJ. 1993;306:1437–1440. doi: 10.1136/bmj.306.6890.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenkinson C, Fitzpatrick R. Cross-cultural evaluation of the short form 8-item Parkinson’s disease questionnaire (PDQ-8): Results from America, Canada, Japan, Italy and Spain. Parkinsonism and Related Disorders. 2006;13:22–28. doi: 10.1016/j.parkreldis.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Muslimovic D, Post B, Speelman JD, Schmand B, de Haan RJ. Determinants of disability and quality of life in mild to moderate Parkinson disease. Neurol. 2008;70:2241–2247. doi: 10.1212/01.wnl.0000313835.33830.80. [DOI] [PubMed] [Google Scholar]
- 19.Fahn S, Elton RL. Members of the UPDRS Development Committee. Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent developments in Parkinson’s disease. Vol. 2. Florham Park (NJ): Macmillan Health Care Information; 1987. pp. 153–164. [Google Scholar]
- 20.Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York: Springer; 2000. [Google Scholar]
- 21.Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Arch Gen Psych. 1991;48:851–855. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- 22.Schrag A, Hovris A, Morley D, Quinn N, Jahanshahi M. Caregiver-burden in Parkinson's disease is closely associated with psychiatric symptoms, falls, and disability. Parkinsonism Relat Disord. 2006 Jan;12(1):35–41. doi: 10.1016/j.parkreldis.2005.06.011. Epub 2005 Nov 3. [DOI] [PubMed] [Google Scholar]
- 23.Schrag A, Jahanshahi M, Quin N. What contributes to quality of life in patients with Parkinson’s disease. J Neurol, Neurosurg Psych. 2000;69:308–312. doi: 10.1136/jnnp.69.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKinlay A, Grace RC, Dalrymple-Alford JC, Anderson T, Fink J, Roger D. A profile of neuropsychiatric problems and their relationship to quality of life for Parkinson's disease patients without dementia. Parkinsonism Relat Disord. 2008;14(1):37–42. doi: 10.1016/j.parkreldis.2007.05.009. Epub 2007 Jul 12. [DOI] [PubMed] [Google Scholar]
- 25.Antonini A, Tesei S, Zecchinelli A, Barone P, De Gaspari D, et al. Randomized study of sertraline and low-dose amitriptyline in patients with Parkinson’s disease and depression: Effect on quality of life. Mov Dis. 2006;21:1119–1122. doi: 10.1002/mds.20895. [DOI] [PubMed] [Google Scholar]
- 26.Papakostas GI, Petersen T, Mahal Y, Mischoulon D, Nierenberg AA, Fava M. Quality of life assessments in major depressive disorder: a review of the literature. General Hospital Psychiatry. 2004;26:13–17. doi: 10.1016/j.genhosppsych.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Geddes JR, Carney SM, Davies C, et al. Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet. 2003;361:653–661. doi: 10.1016/S0140-6736(03)12599-8. [DOI] [PubMed] [Google Scholar]
- 28.Shulman LM, Taback RL, Rabinstein AA, et al. Non-recognition of depression and other non-motor symptoms in Parkinson's disease. Parkinsonism and Related Disorders. 2002;8:193–197. doi: 10.1016/s1353-8020(01)00015-3. [DOI] [PubMed] [Google Scholar]
- 29.Weintraub D, Moberg PJ, Duda JE. Recognition and treatment of depression in Parkinson's disease. Journal of Geriatric Psychiatry and Neurology. 2003;16:178–183. doi: 10.1177/0891988703256053. [DOI] [PubMed] [Google Scholar]