Abstract
Despite over 25 years of concerted worldwide research, the development of a safe and effective HIV-1 vaccine remains elusive. Prototype antibody-based and T cell-based HIV-1 vaccines have failed to show efficacy in clinical trials to date. Next generation HIV-1 vaccine candidates are in various stages of preclinical and clinical development, but key scientific obstacles pose major challenges for the field. Critical hurdles include the enormous global diversity of the virus and the challenges associated with generating broadly reactive neutralizing antibody and cellular immune responses. Here we review the current state of the HIV-1 vaccine field and outline strategies that are being explored to overcome these roadblocks.
Keywords: HIV-1, Vaccine, STEP
Recent advances in our understanding of HIV-1 pathogenesis and immunology have greatly contributed to current HIV-1 vaccine development strategies. Following mucosal exposure to HIV-1, a limited number of virions cross the mucosal barrier to establish primary infection (1, 2). This may be facilitated by activated CD4+ T lymphocytes at mucosal surfaces that likely serve as initial targets of infection (3, 4). Acute infection is then characterized by early establishment of viral reservoirs (5, 6) and explosive viral replication that results in rapid and massive destruction of CD4+ T lymphocytes in the gastrointestinal mucosa (7-9). Damage to the gastrointestinal mucosa leads to bacterial translocation and chronic immune activation (10), which sets the stage for progressive immunodeficiency.
Cellular and Humoral Immune Responses
Host immune responses afford partial control of viral replication but are incapable of eradicating the virus. Virus-specific T lymphocyte responses are detectable concurrent with control of primary viremia (11, 12), suggesting an important role of cellular immune responses in immune control of viral replication. In chronic infection, the breadth of Gag-specific cellular immune responses is inversely correlated with HIV-1 RNA levels (13). Moreover, HIV-1 rapidly mutates to evade virus-specific CD8+ T lymphocyte responses, demonstrating selective pressure exerted by these immune responses (12, 14-17). In addition, genetic studies have demonstrated clear associations between specific HLA alleles and HIV-1 RNA levels (18, 19).
Additional evidence regarding the importance of virus-specific cellular immune responses has been obtained from the preclinical model of simian immunodeficiency virus (SIV) infection of rhesus monkeys. Depletion of CD8+ lymphocytes abrogates control of SIV replication during both acute and chronic infection (20), and vaccine strategies that elicit potent cellular immune responses can reduce setpoint viral loads in certain SIV challenge models (21, 22). An important limitation of T cell-based vaccines, however, is that they are unlikely to prevent infection, although efficient control at local mucosal surfaces may be achievable (23).
The importance of humoral immune responses in the control of HIV-1 replication is less clear. Antibody responses are readily detected in HIV-1-infected individuals, but neutralizing antibodies (NAbs) only arise in later stages of infection (24, 25). Recent studies have begun to reveal numerous strategies utilized by the virus to evade host antibody responses. A substantial fraction of antibodies against HIV-1 are directed against viral debris, such as monomeric gp120, rather than against the intact HIV-1 Env trimer on the virion surface (26). HIV-1 Env is also heavily glycosylated, thus shielding many potential neutralization epitopes (26, 27). The conserved CD4 binding site (CD4bs) is a target for broadly reactive NAbs (25, 28-30) but is buried in a recessed pocket (31). Moreover, the conserved chemokine receptor binding site as well as key epitopes in the membrane proximal external region (MPER) of gp41 appear to be formed only transiently during the membrane fusion process (32, 33). In addition, the virus can rapidly escape host NAbs by mutating glycans on the Env surface (34, 35).
Nevertheless, several monoclonal antibodies (mAbs) have been shown to have the capacity to neutralize a broad array of HIV-1 isolates (36). These mAbs target the CD4bs, glycans on the surface of gp120, and the MPER of gp41. However, such antibodies have proven extraordinarily difficult to elicit by vaccination, perhaps as a result of inaccessibility or transient exposure of key epitopes (31-33) and possible tolerance constraints (37). A subset of chronic HIV-1-infected individuals, however, do exhibit broadly reactive NAbs targeting either the CD4bs or a variety of epitopes on the surface of Env (25, 28-30, 38).
The development of immunogens that induce broadly reactive NAbs remains a critical unsolved problem in the HIV-1 vaccine field. Studies in rhesus monkeys have shown that administration of high doses of broadly reactive mAbs can block transmission of simian-human immunodeficiency virus (SHIV) (39, 40), suggesting the potential utility of vaccine-elicited HIV-1-specific NAbs if they could be effectively induced. It is widely believed that immunogens that induce biologically relevant antibody responses will be required to block acquisition of HIV-1 infection.
HIV-1 Vaccine Efficacy Studies
Two HIV-1 vaccine concepts have completed clinical efficacy studies to date (Table 1). The first concept involved purified monomeric Env gp120 immunogens that aimed to generate virus-specific antibody responses. Early phase clinical trials, however, revealed that antibodies elicited by this vaccine were unable to neutralize primary virus isolates and did not exert immunologic selective pressure on infecting viruses (41). Two phase 3 efficacy studies known as the AIDSVAX studies were then conducted by the biotechnology company VaxGen to evaluate the protective efficacy of gp120 vaccine candidates in the United States and Thailand. These studies were completed in 2003 and showed no efficacy against HIV-1 infection in humans (42, 43), confirming the need to develop more sophisticated Env immunogens.
Table 1. HIV-1 vaccine efficacy studies.
| Vaccine Concept | Developer | Study | Status |
|---|---|---|---|
| 1. monomeric gp120 (B/B, B/E) | VaxGen | VAX 003, VAX 004 (phase 3) | completed in 2003 (42, 43) |
| 2. rAd5-Gag/Pol/Nef | Merck | HVTN 502 “STEP” (phase 2b) | stopped in 2007 (49) |
| 3. ALVAC (vCP1521) prime, gp120 (B/E) boost | Sanofi, VaxGen | RV 144 (phase 3) | will be completed in 2009 |
| 4. DNA prime, rAd5 boost Gag/Pol/EnvA/EnvB/EnvC | NIH VRC | HVTN 505 (phase 2) | scheduled to begin in 2009 |
The challenges associated with antibody-based vaccine candidates led to intense interest in the development of novel vaccine technologies to generate cellular immune responses, including plasmid DNA vaccines and recombinant vectors. Recombinant adenovirus serotype 5 (rAd5) vectors were selected for development by Merck Research Laboratories based on a series of vector comparison studies in nonhuman primates (44). Phase 1 clinical trials revealed the induction of HIV-1-specific cellular immune responses in the majority of vaccine recipients, although subjects with pre-existing Ad5-specific NAbs exhibited blunted responses to rAd5 vectors (45, 46) as was predicted by preclinical studies (47, 48). Ad5 seroprevalence in the United States is only 30-40% but in certain areas of sub-Saharan Africa is >80%, and thus the high prevalence of pre-existing Ad5 immunity was predicted to be a major challenge for rAd5 vector-based HIV-1 vaccines.
Merck's HIV-1 vaccine candidate was formulated as a trivalent mixture of rAd5 vectors expressing HIV-1 clade B Gag, Pol, and Env antigens delivered as a homologous prime-boost regimen at months 0, 1, and 6. This vaccine was evaluated in a phase 2b proof-of-concept study known as the STEP study by Merck and the NIH-sponsored HIV Vaccine Trials Network (HVTN 502) in North America, South America, the Carribean, and Australia. A parallel study known Phambili (HVTN 503) also began in South Africa. The STEP study was unexpectedly terminated in September 2007 at the first interim review of the data safety monitoring board as a result of futility in achieving its primary endpoints (49). Moreover, a trend of increased HIV-1 infections was observed in individuals who had baseline Ad5-specific NAbs (49, 50).
The failure of the Merck rAd5 HIV-1 vaccine and the potential for increased HIV-1 acquisition in certain subsets of vaccinees led to the cancellation or modification of multiple other HIV-1 vaccine studies in the field. The Phambili study was terminated, since it utilized the same vaccine vector in a population with high levels of pre-existing Ad5 immunity. In addition, a phase 2b efficacy study known as PAVE 100 to evaluate the protective efficacy of a DNA prime, rAd5 boost vaccine expressing Gag, Pol, and three Env antigens developed by the NIH Vaccine Research Center was delayed and redesigned. A smaller version of this study known as HVTN 505 is scheduled to begin in summer 2009 in the defined subgroup of Ad5 seronegative, circumcised men. DNA priming prior to rAd5 boosting has been shown to increase vaccine-elicited T lymphocyte responses in rhesus monkeys (51, 52) but has to date failed to improve protective efficacy in terms of setpoint viral loads or survival following SIV challenge (53, 54).
The third HIV-1 vaccine concept that will complete efficacy testing in humans involves a canarypox (ALVAC) vector prime, gp120 protein boost vaccine regimen. The goal of this study is to evaluate the protective efficacy of a vaccine that aims to induce both cellular and humoral immune responses. Results from a large phase 3 efficacy trial in Thailand are expected in fall 2009.
Research Priorities After STEP
The STEP study provided a rapid and clear evaluation of the Merck rAd5 HIV-1 vaccine candidate. The efficacy failure and the potential enhancement of HIV-1 acquisition, however, led to widespread debate in the field regarding research priorities. In particular, it was unclear whether the failure of this vaccine was simply a “product failure” or whether it represented a “concept failure” of T cell-based vaccines in general. Although both remain formal possibilities, it would seem premature to conclude that the failure of one vaccine product should be generalized to all T cell-based vaccines. Consistent with this perspective, we recently demonstrated that a heterologous rAd26/rAd5 prime-boost regimen expressing SIV Gag afforded substantially greater protective efficacy than did a homologous rAd5/rAd5 regimen against a pathogenic SIV challenge in rhesus monkeys (21). It is therefore possible that vaccines that elicit improved magnitude, breadth, and quality of virus-specific T lymphocyte responses as compared with the homologous rAd5 regimen may perform better in clinical trials. The precise parameters of cellular immunity required to control viral replication, however, remain unclear and will require intensive investigation. Studies of HIV-1-infected individuals who spontaneously control viral replication and of individuals with acute HIV-1 infection hopefully will lead to an improved understanding of the immune correlates of protection.
The STEP study emphasized the need for an increased focus on discovery research, including basic, preclinical, and clinical studies. In particular, nonhuman primate challenge models have been refined in light of the results of the STEP study. Homologous rAd5 regimens effectively protected against SHIV challenges but not against more stringent SIV challenges in rhesus monkeys. As a result, SIV has emerged as the preferred challenge virus for preclinical evaluation of T cell-based vaccine candidates. It also seems reasonable to prioritize for clinical development T cell-based vaccine candidates that afford greater protective efficacy than rAd5 vectors in stringent SIV challenge models. Thus, rAd5 vectors provide a baseline against which other vaccine candidates can be compared in SIV challenge studies. It is important to recognize, however, that the predictive capacity of all preclinical challenge models remains unclear and will require validation in the setting of successful clinical efficacy studies.
The enhanced HIV-1 acquisition observed in vaccinees was unexpected and was not predicted by prior preclinical studies, highlighting our limited understanding of the events associated with HIV-1 transmission. Importantly, the potential enhancement of HIV-1 acquisition appears to diminish over time (55). An initial hypothesis was that baseline Ad5-specific NAbs may have simply been surrogate markers for Ad5-specific CD4+ T lymphocyte responses, which could have expanded rapidly following vaccination and served as increased viral targets. Recent data from several independent laboratories, however, have not supported this hypothesis. Ad5-specific NAbs were not correlated with Ad5-specific T lymphocyte responses, and Ad5-specific CD4+ T lymphocyte responses were no higher in baseline Ad5 seropositive as compared with Ad5 seronegative subjects (56-58). An alternative hypothesis is that Ad5-specific NAbs led to immune complex formation following rAd5 vaccination and resulted in altered inflammatory responses (59). It is also possible that baseline Ad5-specific NAbs were confounded by other clinical variables such as circumcision and HSV-2 status (55). Despite these unanswered questions, the STEP study emphasized the importance of developing an improved understanding of vector-specific immunity as well as mucosal and innate immunity.
A challenging issue for the field has been how to apply the results of the STEP study to optimize the current pipeline of HIV-1 vaccine candidates. A variety of DNA vaccines and improved delivery methods such as in vivo electroporation are currently being developed. Vectors under development include rare human serotype and chimeric Ads (48, 60, 61), nonhuman primate Ads (62), modified vaccinia Ankara (63), ALVAC (64), NYVAC (65), adeno-associated virus (AAV), vesicular stomatitis virus (VSV), cytomegalovirus (CMV), Venezuelan equine encephalitis virus (VEE), and certain bacteria and mycobacteria. It is hoped that some of these vectors, either alone or in heterologous prime-boost combinations, will prove more effective than rAd5 vectors. Novel HIV-1 antigens will also be needed to improve the breadth of vaccine-elicited cellular immune responses and coverage of global virus diversity.
Perhaps the most important research priority after STEP is the generation of improved Env immunogens for the generation of antibody responses. A wide variety of strategies are being pursued (36), but none to date have elicited NAbs of substantial breadth in preclinical studies. Increased understanding of the structure and diversity of HIV-1 Env will hopefully lead to stabilized Env trimers or engineered Env immunogens that will prove superior to monomeric gp120 in terms of neutralization of a substantial breadth of primary virus isolates (66, 67). Preclinical studies and clinical trials of promising antibody-based vaccine candidates therefore need to be greatly accelerated.
Novel Vaccine Strategies to Contend with HIV-1 Diversity
One of the most challenging hurdles facing the HIV-1 vaccine field is the enormous global diversity of HIV-1. The AIDSVAX and STEP studies were both based on the premise that essentially natural HIV-1 antigens might be able to elicit adequate cross-reactive immunity to afford protection. In the STEP study, the HIV-1 antigens were selected from natural sequences that were similar to the B consensus sequence (49, 50), and the extent to which responses were able to cross-react with infecting strains is still being determined. The STEP study provided favorable conditions to enhance the prospects of natural proteins eliciting a beneficial response: the inclusion of the two most conserved HIV proteins (Gag and Pol), evaluation of a B clade vaccine in the context of a B clade epidemic, and selection of natural antigens close to the consensus. The failure of this vaccine to afford protection in this setting suggests that an HIV-1 vaccine needs to elicit substantially more potent and more cross-reactive immune responses than those achieved in the STEP study. Several strategies are currently being explored to develop antigens to contend more effectively with viral diversity.
Conserved region antigens
Several groups have fused the most conserved parts of the viral proteome to form an artificial protein immunogen (68, 69). As a cautionary note, concatenating short regions from different proteins into one antigen can have undesirable immunologic consequences. For example, polyepitope vaccines have to date failed to produce robust T cell responses in phase 1 clinical studies (66). In scenarios where somewhat longer conserved peptide regions are linked (68), rather than linking narrowly defined optimal epitopes, cleavage signals for epitope processing may be preserved (70), allowing more natural epitope processing and presentation than in a polyepitope construct. In addition, in many regions there is extensive overlap of known epitopes (Figure 1). A conserved region approach thus enables responses to any of the overlapping epitopes within the included regions, which represents an advantage over the polyepitope strategy of narrowly focusing on optimally defined epitopes. There are two theoretical virtues of “immunofocusing” host vaccine responses onto conserved regions. The first is that limiting the immune responses to conserved epitopes may enhance cross-reactivity at the population level. The second is that potent immune responses in conserved regions are more likely to force escape pathways that reduce viral fitness (68, 69, 71). A limiting factor may be that some of the most conserved regions of the virus, particularly in the Pol protein, are immunologically “quiet” with diminished recognition in natural infection (66). It is also possible that artificial junctional epitopes with no biologic utility may be induced.
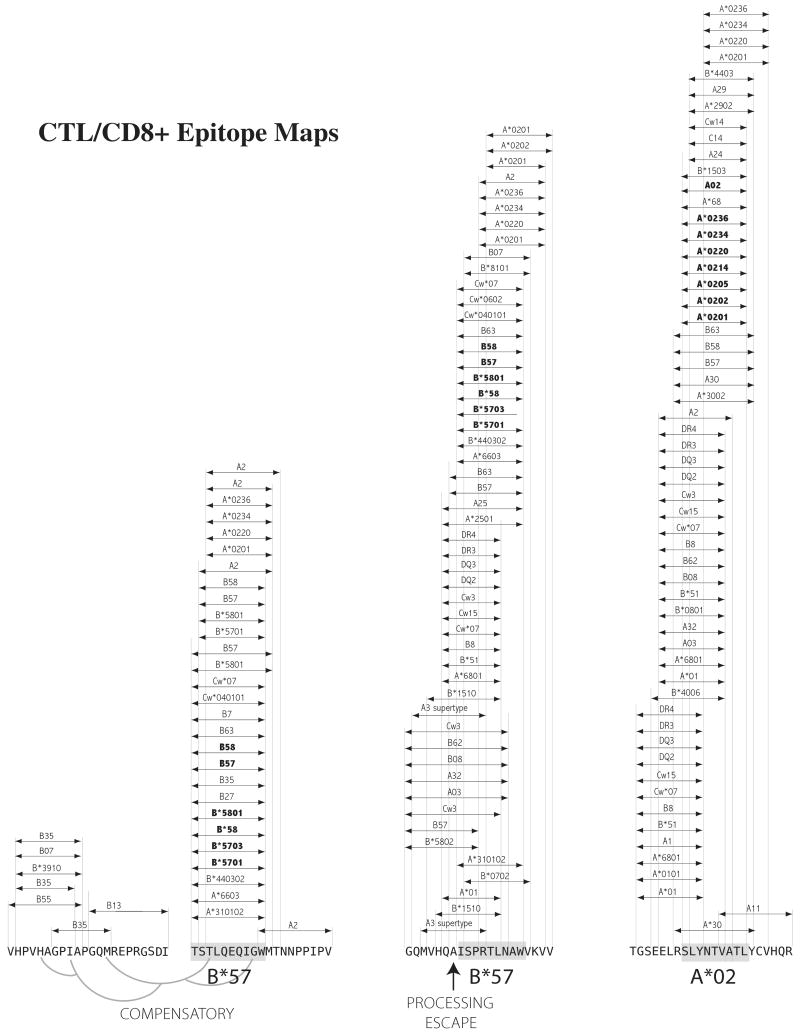
Figure 1. Overlapping epitopes.
Three well characterized HIV-1 epitopes (B*57 TW10, B*57 IW9, A*02 SL9) are shown in the context of overlapping epitopes that have been described in the literature. The compensatory mutations for the B*57 TW10 escape mutation T242N (92) are also shown embedded in overlapping epitopes to further illustrate the complexity at the population level of these immunologic targets. The epitopes that have been described in the literature are likely only the tip of the iceberg, since reagents used to screen for epitopes are seldom based on autologous sequences, which would enable detection of novel epitopes that are specific for less common variants. The common HLA molecules are in bold.
Centralized antigens
Another way to improve the cross-reactive potential of vaccine-elicited cellular immune responses is by using “central strains”, such as ancestral reconstructions, consensus sequences, or antigens designed based on their central position in a phylogenetic tree (72, 73). These three strategies proved comparable when used to design peptides for detection of natural responses (74). Central proteins can be tailored for one HIV-1 subtype (75, 76) or can be central to the entire M group, bringing distances between the vaccine candidate and natural strains from all subtypes to an intra-subtype level (72, 77-79). Despite these proteins being artificial constructs, they are similar to HIV-1 proteins and are well expressed, properly folded, and functional (66). Recently, an M Env group consensus/ancestral Env vaccine enhanced the cross-reactive potential of T cell responses relative to a typical B clade Env vaccine in rhesus monkeys (79).
Multiple antigen cocktails
Vaccinating simultaneously with multiple variants of the same protein may increase the breadth of population coverage as well as the depth of coverage of variants of a single epitope. If more T cell receptor clonotypes are expanded simultaneously, each recognizing the epitope and its variants differently, then this may effectively block common and fit viral escape routes (80). The use of multiple natural Env immunogens has been shown to elicit responses against all antigens without antigenic interference in rhesus monkeys (51). Phase 1 clinical trials have also indicated enhanced breadth of responses with the use of polyvalent cocktails of natural proteins (46, 81, 82). Should problems with immunodominance occur, utilizing different anatomic sites for vaccination may prove useful (83).
Optimizing potential epitope coverage with mosaic antigen cocktails
Computational strategies for designing vaccine antigens that optimize coverage of potential T cell epitopes have also been proposed (73, 84). Mosaic antigens are polyvalent cocktails of synthetic but intact proteins that optimize theoretical coverage of potential T cell epitopes for a given cocktail size (84). These antigens are also designed to minimize rare epitopes and to exclude junctional epitopes that are not found in natural HIV-1 sequences. In a mouse study, mosaic Env antigen cocktails elicited cellular immune responses of increased breadth as compared with equivalently sized cocktails of natural Env antigens (85). Our ongoing studies in rhesus monkeys demonstrate that 2-valent mosaic Gag/Pol/Env antigens expressed by rAd26 vectors elicited responses of substantially greater breadth (more responses to global epitopes) and greater depth (more responses to variants of a given epitope) than either consensus or optimal natural sequence antigens (86) (D.H.B. and B.K., manuscript in preparation). Thus, mosaic antigens appear to provide significant immunologic benefits with only a limited number of sequences per protein, suggesting the practical feasibility of this strategy for clinical vaccine development.
A vaccine approach that focuses on just conserved regions of HIV-1 may also benefit from a mosaic design, since conserved regions also vary. This would allow the most common alternative sequences in conserved regions to be presented simultaneously, and the mosaic design strategy itself provides a rational boundary for defining conservation. The theoretical benefit of immunofocusing vaccine responses to conserved regions with high fitness costs of escape would be maintained and potentially augmented. Simultaneous induction of responses to a typical susceptible form of an epitope and its most common escape form(s) could force the virus down unfavorable escape pathways with substantial viral fitness costs (69, 80).
HLA Haplotypes and Population Mutational Patterns
The terms “wildtype” and “escape” are often utilized as if there were two simple forms of the virus with clear phenotypes. Mutational patterns, however, are in fact highly complex and often context specific. Despite the complexity, patterns of HLA associations with mutations at the population level can be identified (87), but statistical support for such associations needs to be interpreted in a phylogenetic context (88, 89) so that associations are not simply explained by distinct lineages of the virus spreading in subpopulations with distinct HLA frequencies (88).
Two of the underlying reasons why the biology of immune escape is complicated are illustrated in Figures 1 and 2, using as examples three well studied HIV-1 epitopes: the A*0201-restricted epitope SLYTNVATL (SL9) and the two B*5701-restricted epitopes TSTLQEQIGW (TW10) and ISPRTLNAW (IW9). Figure 1 shows the many experimentally validated epitopes that are referenced in the Los Alamos HIV Database (www.hiv.lanl.gov) that overlap with these three epitopes, and thus mutations within one epitope may impact overlapping epitopes and vice versa. SL9 is an immunodominant epitope in chronic infection (16) presented by HLA-A*0201. However, HLA-A*02 associations with mutations in SL9 were not identified in two population studies, although mutations in SL9 were found to be associated with HLA-A*01, A*11, and A*29 (90, 91), each with known overlapping epitopes spanning this region. The inability of association methods to capture a mutational pattern associated with HLA-A*02 in a dominant common epitope like SL9 is likely to be a consequence of superimposed patterns of escape relative to the other T cell epitopes that can impact this region of Gag (Figure 1). An additional complication is that HLA can impact mutational patterns at a distance. For example, the most characteristic escape mutation of the B*57-restricted TW10 epitope, T242N, also requires compensatory mutations that impact interactions between the capsid protein and cyclophilin A (Figure 1) (92). Associations in mutational patterns between B*5701 escape mutations and compensatory mutations were identified at the population level (90), but both the T242N mutation within the epitope and its compensatory mutations can also be influenced by epitopes presented by other HLA molecules (Figure 1).
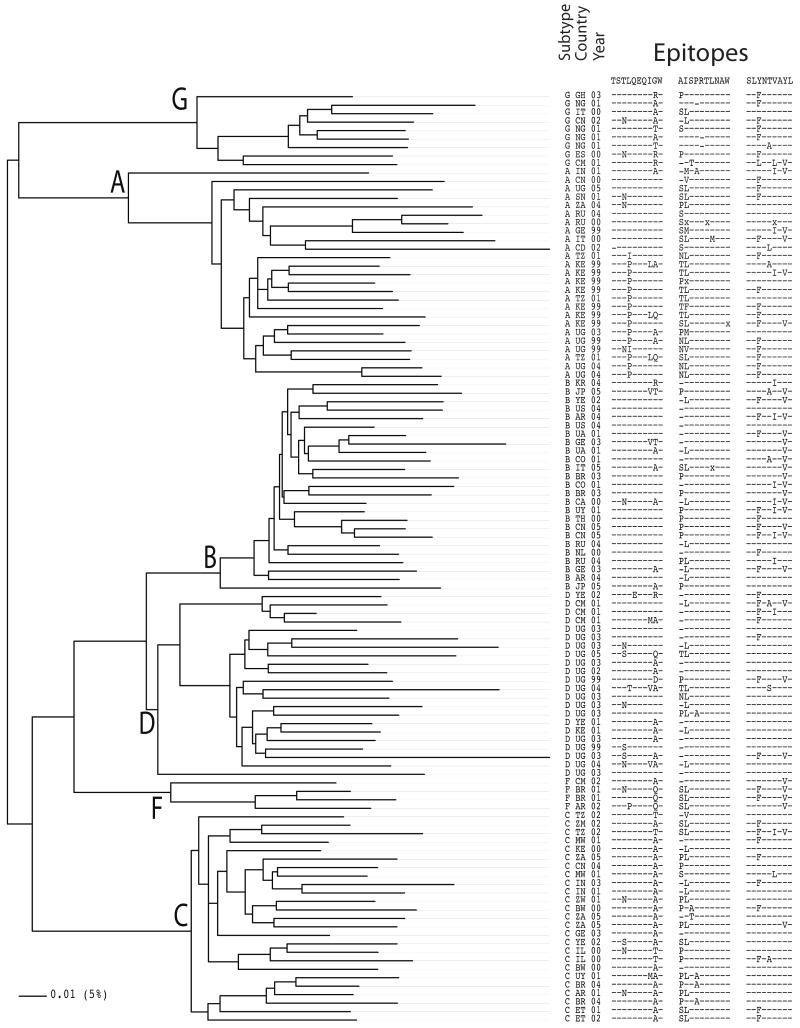
Figure 2. Global diversity.
A phylogenetic tree is shown of HIV-1 Gag from a set of isolates within the last 10 years from diverse locations throughout the world. Branch lengths reflect overall genetic distances between isolates and clades, and the epitope alignment on the right shows the impact of this diversity on three well characterized epitopes. Dashes indicated identity with the form of the epitope most often studied that is depicted at the top of each epitope alignment. The subtype, the two-letter country code indicating where the sample was obtained (www.hiv.lanl.gov/content/sequence/HelpDocs/databasecountrycode.html), and the year of isolation are indicated. The IW9 epitope also includes the preceding amino acid, as an A146P flanking substitution hinders processing and represents a common escape route (97, 98).
A further complication is that escape mutations can be susceptible forms in different individuals or at different times during infection (12, 15, 80, 88, 93, 94). The T242N escape in TW10 is an example. Whether the T242N is defined as an escape or a susceptible form depends on the individual and the transmitted form of the epitope (94). Figure 2 shows a phylogenetic tree of the major clades of HIV-1 mapping the variation in the three epitopes considered here onto a tree representing global variation. SL9 is very complex in terms of its escape pathways, such that virtually all of the mutations that are shown in Figure 2 can be either susceptible or escape forms in the context of different T cell receptor clonotypes (95, 96). In contrast to mutations within T cell epitopes, mutations that inhibit epitope processing or that prevent HLA-peptide interactions may only be escape mutations for the precise epitope in question, although they may be incorporated into a susceptible overlapping epitope. An example of a processing mutation is the A146P mutation flanking the IW9 epitope (97, 98). This mutation is positively selected in HLA-B*57 individuals and prevents proper trimming of the optimal epitope by the endoplasmic reticulum aminopeptidase I. Many variants occur in this position (Figure 2), and the proline at position 146 may sometimes become part of other epitopes (Figure 1). There are many alternate forms of each of the three epitopes considered here (Figure 2), but such levels of diversity are not atypical; in fact, these epitopes are all from the relatively conserved Gag protein. There are concentrated patterns of amino acid variants in different subclades within subtypes and in different geographic locations (Figure 2). These mutational patterns may have phenotypic consequences at the population level. When escape variants are transmitted, they revert at various rates or not at all (94, 97, 99), and the transmission of attenuating escape mutations from a donor to a recipient can thus be relevant to the extent of viral replication in a new host.
What does this imply for vaccines? Since different frequencies of variants are prevalent in different populations (Figure 2), and since multiple variants can be susceptible depending on the context, it seems prudent for a vaccine to include a variety of common sequences. Mutations that are characterized as being typical escape sequences may also be relevant for inclusion in a vaccine, since responses can often be mounted to these alternate forms (88, 94, 95). Epitope-specific responses also generally continue to vary after the initial escape forms emerge (12, 15). Therefore, blocking fit escape routes or reversion to a more fit form by vaccine-induced immune responses seems particularly desirable (84). Taken together, recent studies of acute infection, immune escape and reversion, viral fitness, population imprinting, and the ability of TCR clonotypes to recognize variant epitopes all point towards utilizing antigen cocktails that trigger improved breadth and depth of cellular immune responses. This could lead to improved immunologic coverage of global virus diversity and inhibition of common escape routes of the virus resulting in a substantial viral fitness cost.
Conclusions and Perspectives
Next generation T cell-based and antibody-based vaccine candidates will need to contend with the enormous challenge of global HIV-1 diversity. Cellular immune responses will require sufficient breadth and depth to cover extensive immunologic diversity and mutational patterns of the virus. Both humoral and cellular immune responses will need to exert antiviral activity against a substantial breadth of primary isolate viruses, and it is hoped that novel antigen strategies will begin to address these challenges. Vaccine-induced immune responses will also need to be more potent and durable in both systemic and mucosal compartments. Since an optimal HIV-1 vaccine candidate will almost certainly need to induce both humoral and cellular immunity, research should proceed in parallel in both areas with the intention that these paths may eventually converge.
It is important to recognize that HIV-1 vaccine development is part of a larger prevention portfolio that also includes behavioral modification, circumcision, pre-exposure prophylaxis, and microbicides. Recent microbicide studies have resulted in promising trends, and studies utilizing antiretroviral drugs for pre-exposure prophylaxis will be reported in the near future. Success of other prevention modalities will have major effects on the HIV-1 vaccine field, including the design of efficacy studies and the ultimate delivery of successful vaccine candidates.
The STEP study has greatly contributed to the HIV-1 vaccine field and has redirected research priorities. It is likely that the path towards a successful HIV-1 vaccine will be a long road with multiple efficacy trial failures along the way. Thus, it is critical that the field does not become paralyzed from disappointing results from any single efficacy study. There are clear reasons for optimism, such as the increased structural and functional understanding of HIV-1 Env, improved vaccine vectors that afford enhanced protective efficacy in SIV challenge studies, and novel antigens that begin to address global virus diversity. These and other promising vaccine concepts will be evaluated in clinical studies over the next several years.
Acknowledgments
D.H.B. acknowledges support from the National Institutes of Health (AI078526, AI066924, AI066305, AI058727, AI067854), the Bill & Melinda Gates Foundation (#38643, #38614), the Elizabeth Glaser Pediatric AIDS Foundation, and the Ragon Institute. B.K. acknowledges support from the National Institutes of Health (AI067854, AI061734).
Acronyms and Key Terms
- HIV-1
human immunodeficiency virus type 1
- SIV
simian immunodeficiency virus
- SHIV
simian-human immunodeficiency virus
- HLA
human leukocyte antigen
- Env
HIV-1 envelope protein
- NAb
neutralizing antibody
- MPER
membrane proximal external region
- mAb
monoclonal antibody
- rAd5
recombinant adenovirus type 5
- rAd26
recombinant adenovirus type 26
Footnotes
Disclosure Statement: The authors report no financial conflicts of interest.
Literature Cited
- 1.Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, Wei X, Jiang C, Kirchherr JL, Gao F, Anderson JA, Ping LH, Swanstrom R, Tomaras GD, Blattner WA, Goepfert PA, Kilby JM, Saag MS, Delwart EL, Busch MP, Cohen MS, Montefiori DC, Haynes BF, Gaschen B, Athreya GS, Lee HY, Wood N, Seoighe C, Perelson AS, Bhattacharya T, Korber BT, Hahn BH, Shaw GM. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7552–7. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrahams MR, Anderson JA, Giorgi EE, Seoighe C, Mlisana K, Ping LH, Athreya GS, Treurnicht FK, Keele BF, Wood N, Salazar-Gonzalez JF, Bhattacharya T, Chu H, Hoffman I, Galvin S, Mapanje C, Kazembe P, Thebus R, Fiscus S, Hide W, Cohen MS, Karim SA, Haynes BF, Shaw GM, Hahn BH, Korber BT, Swanstrom R, Williamson C. Quantitating the multiplicity of infection with human immunodeficiency virus type 1 subtype C reveals a non-poisson distribution of transmitted variants. Journal of virology. 2009;83:3556–67. doi: 10.1128/JVI.02132-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q, Skinner PJ, Ha SJ, Duan L, Mattila TL, Hage A, White C, Barber DL, O'Mara L, Southern PJ, Reilly CS, Carlis JV, Miller CJ, Ahmed R, Haase AT. Visualizing antigen-specific and infected cells in situ predicts outcomes in early viral infection. Science (New York, NY. 2009;323:1726–9. doi: 10.1126/science.1168676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, Reilly CS, Peterson ML, Schultz-Darken N, Brunner KG, Nephew KR, Pambuccian S, Lifson JD, Carlis JV, Haase AT. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–8. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho DD, Richman DD, Siliciano RF. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 6.Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, Quinn TC, Chaisson RE, Rosenberg E, Walker B, Gange S, Gallant J, Siliciano RF. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–7. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 7.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–31. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 8.Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, Reilly C, Carlis J, Miller CJ, Haase AT. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–52. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 9.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–7. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 10.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nature medicine. 2006;12:1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 11.Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowsky W, Farthing C, Ho DD. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–5. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goonetilleke N, Liu MKP, Salazar-Gonzalez JF, Ferrari G, Giorgi EE, Ganusov VV, Keele BF, Turnbull EL, Salazar MG, Weinhold K, Moore S, Letvin NL, Haynes B, Cohen MS, Hraber P, Bhattacharya T, Borrow P, Perelson AS, Hahn BH, Shaw GM, Korber B, McMichael A. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J Exp Med. 2009 doi: 10.1084/jem.20090365. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiepiela P, Ngumbela K, Thobakgale C, Ramduth D, Honeyborne I, Moodley E, Reddy S, de Pierres C, Mncube Z, Mkhwanazi N, Bishop K, van der Stok M, Nair K, Khan N, Crawford H, Payne R, Leslie A, Prado J, Prendergast A, Frater J, McCarthy N, Brander C, Learn GH, Nickle D, Rousseau C, Coovadia H, Mullins JI, Heckerman D, Walker BD, Goulder P. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med. 2007;13:46–53. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- 14.Phillips RE, Rowland-Jones S, Nixon DF, Gotch FM, Edwards JP, Ogunlesi AO, Elvin JG, Rothbard JA, Bangham CR, Rizza CR, et al. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature. 1991;354:453–9. doi: 10.1038/354453a0. [DOI] [PubMed] [Google Scholar]
- 15.Duda A, Lee-Turner L, Fox J, Robinson N, Dustan S, Kaye S, Fryer H, Carrington M, McClure M, McLean AR, Fidler S, Weber J, Phillips RE, Frater AJ. HLA-associated clinical progression correlates with epitope reversion rates in early human immunodeficiency virus infection. Journal of virology. 2009;83:1228–39. doi: 10.1128/JVI.01545-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goulder PJ, Altfeld MA, Rosenberg ES, Nguyen T, Tang Y, Eldridge RL, Addo MM, He S, Mukherjee JS, Phillips MN, Bunce M, Kalams SA, Sekaly RP, Walker BD, Brander C. Substantial differences in specificity of HIV-specific cytotoxic T cells in acute and chronic HIV infection. The Journal of experimental medicine. 2001;193:181–94. doi: 10.1084/jem.193.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawashima Y, Pfafferott K, Frater J, Matthews P, Payne R, Addo M, Gatanaga H, Fujiwara M, Hachiya A, Koizumi H, Kuse N, Oka S, Duda A, Prendergast A, Crawford H, Leslie A, Brumme Z, Brumme C, Allen T, Brander C, Kaslow R, Tang J, Hunter E, Allen S, Mulenga J, Branch S, Roach T, John M, Mallal S, Ogwu A, Shapiro R, Prado JG, Fidler S, Weber J, Pybus OG, Klenerman P, Ndung'u T, Phillips R, Heckerman D, Harrigan PR, Walker BD, Takiguchi M, Goulder P. Adaptation of HIV-1 to human leukocyte antigen class I. Nature. 2009;458:641–5. doi: 10.1038/nature07746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fellay J, Shianna KV, Ge D, Colombo S, Ledergerber B, Weale M, Zhang K, Gumbs C, Castagna A, Cossarizza A, Cozzi-Lepri A, De Luca A, Easterbrook P, Francioli P, Mallal S, Martinez-Picado J, Miro JM, Obel N, Smith JP, Wyniger J, Descombes P, Antonarakis SE, Letvin NL, McMichael AJ, Haynes BF, Telenti A, Goldstein DB. A whole-genome association study of major determinants for host control of HIV-1. Science (New York, NY. 2007;317:944–7. doi: 10.1126/science.1143767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao X, Nelson GW, Karacki P, Martin MP, Phair J, Kaslow R, Goedert JJ, Buchbinder S, Hoots K, Vlahov D, O'Brien SJ, Carrington M. Effect of a single amino acid change in MHC class I molecules on the rate of progression to AIDS. N Engl J Med. 2001;344:1668–75. doi: 10.1056/NEJM200105313442203. [DOI] [PubMed] [Google Scholar]
- 20.Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, Ghrayeb J, Forman MA, Montefiori DC, Rieber EP, Letvin NL, Reimann KA. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–60. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, O'Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM, Abbink P, Coffey RT, Grandpre LE, Seaman MS, Landucci G, Forthal DN, Montefiori DC, Carville A, Mansfield KG, Havenga MJ, Pau MG, Goudsmit J, Barouch DH. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009;457:87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson NA, Keele BF, Reed JS, Piaskowski SM, Mac Nair CE, Bett AJ, Liang X, Wang F, Thoryk E, Heidecker GJ, Citron MR, Huang L, Lin J, Vitelli S, Ahn CD, Kaizu M, Maness NJ, Reynolds MR, Friedrich TC, Loffredo JT, Rakasz EG, Erickson S, Allison DB, Piatak M, Jr, Lifson JD, Shiver JW, Casimiro DR, Shaw GM, Hahn BH, Watkins DI. Vaccine-induced Cellular Responses Control SIV Replication After Heterologous Challenge. Journal of virology. 2009 doi: 10.1128/JVI.00272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, Piatak M, Jr, Lifson JD, Nelson JA, Jarvis MA, Picker LJ. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nature medicine. 2009;15:293–9. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomaras GD, Yates NL, Liu P, Qin L, Fouda GG, Chavez LL, Decamp AC, Parks RJ, Ashley VC, Lucas JT, Cohen M, Eron J, Hicks CB, Liao HX, Self SG, Landucci G, Forthal DN, Weinhold KJ, Keele BF, Hahn BH, Greenberg ML, Morris L, Karim SS, Blattner WA, Montefiori DC, Shaw GM, Perelson AS, Haynes BF. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. Journal of virology. 2008;82:12449–63. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sather DN, Armann J, Ching LK, Mavrantoni A, Sellhorn G, Caldwell Z, Yu X, Wood B, Self S, Kalams S, Stamatatos L. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. Journal of virology. 2009;83:757–69. doi: 10.1128/JVI.02036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wyatt R, Kwong PD, Desjardins E, Sweet RW, Robinson J, Hendrickson WA, Sodroski JG. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature. 1998;393:705–11. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 27.Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–59. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scheid JF, Mouquet H, Feldhahn N, Seaman MS, Velinzon K, Pietzsch J, Ott RG, Anthony RM, Zebroski H, Hurley A, Phogat A, Chakrabarti B, Li Y, Connors M, Pereyra F, Walker BD, Wardemann H, Ho D, Wyatt RT, Mascola JR, Ravetch JV, Nussenzweig MC. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009;458:636–40. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Svehla K, Louder MK, Wycuff D, Phogat S, Tang M, Migueles SA, Wu X, Phogat A, Shaw GM, Connors M, Hoxie J, Mascola JR, Wyatt R. Analysis of neutralization specificities in polyclonal sera derived from human immunodeficiency virus type 1-infected individuals. Journal of virology. 2009;83:1045–59. doi: 10.1128/JVI.01992-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Binley JM, Lybarger EA, Crooks ET, Seaman MS, Gray E, Davis KL, Decker JM, Wycuff D, Harris L, Hawkins N, Wood B, Nathe C, Richman D, Tomaras GD, Bibollet-Ruche F, Robinson JE, Morris L, Shaw GM, Montefiori DC, Mascola JR. Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C. Journal of virology. 2008;82:11651–68. doi: 10.1128/JVI.01762-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou T, Xu L, Dey B, Hessell AJ, Van Ryk D, Xiang SH, Yang X, Zhang MY, Zwick MB, Arthos J, Burton DR, Dimitrov DS, Sodroski J, Wyatt R, Nabel GJ, Kwong PD. Structural definition of a conserved neutralization epitope on HIV-1 gp120. Nature. 2007;445:732–7. doi: 10.1038/nature05580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen B, Vogan EM, Gong H, Skehel JJ, Wiley DC, Harrison SC. Structure of an unliganded simian immunodeficiency virus gp120 core. Nature. 2005;433:834–41. doi: 10.1038/nature03327. [DOI] [PubMed] [Google Scholar]
- 33.Frey G, Peng H, Rits-Volloch S, Morelli M, Cheng Y, Chen B. A fusion-intermediate state of HIV-1 gp41 targeted by broadly neutralizing antibodies. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3739–44. doi: 10.1073/pnas.0800255105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Kwong PD, Shaw GM. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–12. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 35.Richman DD, Wrin T, Little SJ, Petropoulos CJ. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4144–9. doi: 10.1073/pnas.0630530100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montefiori D, Sattentau Q, Flores J, Esparza J, Mascola J. Antibody-based HIV-1 vaccines: recent developments and future directions. PLoS medicine. 2007;4:e348. doi: 10.1371/journal.pmed.0040348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haynes BF, Fleming J, St Clair EW, Katinger H, Stiegler G, Kunert R, Robinson J, Scearce RM, Plonk K, Staats HF, Ortel TL, Liao HX, Alam SM. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science (New York, NY. 2005;308:1906–8. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Migueles SA, Welcher B, Svehla K, Phogat A, Louder MK, Wu X, Shaw GM, Connors M, Wyatt RT, Mascola JR. Broad HIV-1 neutralization mediated by CD4-binding site antibodies. Nature medicine. 2007;13:1032–4. doi: 10.1038/nm1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, Lewis MG. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–10. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 40.Veazey RS, Shattock RJ, Pope M, Kirijan JC, Jones J, Hu Q, Ketas T, Marx PA, Klasse PJ, Burton DR, Moore JP. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nature medicine. 2003;9:343–6. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- 41.Connor RI, Korber BT, Graham BS, Hahn BH, Ho DD, Walker BD, Neumann AU, Vermund SH, Mestecky J, Jackson S, Fenamore E, Cao Y, Gao F, Kalams S, Kunstman KJ, McDonald D, McWilliams N, Trkola A, Moore JP, Wolinsky SM. Immunological and virological analyses of persons infected by human immunodeficiency virus type 1 while participating in trials of recombinant gp120 subunit vaccines. J Virol. 1998;72:1552–76. doi: 10.1128/jvi.72.2.1552-1576.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. The Journal of infectious diseases. 2005;191:654–65. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 43.Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M, van Griensven F, Hu D, Tappero JW, Choopanya K. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. The Journal of infectious diseases. 2006;194:1661–71. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- 44.Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, Zhang ZQ, Simon AJ, Trigona WL, Dubey SA, Huang L, Harris VA, Long RS, Liang X, Handt L, Schleif WA, Zhu L, Freed DC, Persaud NV, Guan L, Punt KS, Tang A, Chen M, Wilson KA, Collins KB, Heidecker GJ, Fernandez VR, Perry HC, Joyce JG, Grimm KM, Cook JC, Keller PM, Kresock DS, Mach H, Troutman RD, Isopi LA, Williams DM, Xu Z, Bohannon KE, Volkin DB, Montefiori DC, Miura A, Krivulka GR, Lifton MA, Kuroda MJ, Schmitz JE, Letvin NL, Caulfield MJ, Bett AJ, Youil R, Kaslow DC, Emini EA. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415:331–5. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- 45.Priddy FH, Brown D, Kublin J, Monahan K, Wright DP, Lalezari J, Santiago S, Marmor M, Lally M, Novak RM, Brown SJ, Kulkarni P, Dubey SA, Kierstead LS, Casimiro DR, Mogg R, DiNubile MJ, Shiver JW, Leavitt RY, Robertson MN, Mehrotra DV, Quirk E. Safety and immunogenicity of a replication-incompetent adenovirus type 5 HIV-1 clade B gag/pol/nef vaccine in healthy adults. Clinical infectious diseases. 2008;46:1769–81. doi: 10.1086/587993. [DOI] [PubMed] [Google Scholar]
- 46.Catanzaro AT, Koup RA, Roederer M, Bailer RT, Enama ME, Moodie Z, Gu L, Martin JE, Novik L, Chakrabarti BK, Butman BT, Gall JG, King CR, Andrews CA, Sheets R, Gomez PL, Mascola JR, Nabel GJ, Graham BS. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective recombinant adenovirus vector. J Infect Dis. 2006;194:1638–49. doi: 10.1086/509258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Casimiro DR, Chen L, Fu TM, Evans RK, Caulfield MJ, Davies ME, Tang A, Chen M, Huang L, Harris V, Freed DC, Wilson KA, Dubey S, Zhu DM, Nawrocki D, Mach H, Troutman R, Isopi L, Williams D, Hurni W, Xu Z, Smith JG, Wang S, Liu X, Guan L, Long R, Trigona W, Heidecker GJ, Perry HC, Persaud N, Toner TJ, Su Q, Liang X, Youil R, Chastain M, Bett AJ, Volkin DB, Emini EA, Shiver JW. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J Virol. 2003;77:6305–13. doi: 10.1128/JVI.77.11.6305-6313.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts DM, Nanda A, Havenga MJ, Abbink P, Lynch DM, Ewald BA, Liu J, Thorner AR, Swanson PE, Gorgone DA, Lifton MA, Lemckert AA, Holterman L, Chen B, Dilraj A, Carville A, Mansfield KG, Goudsmit J, Barouch DH. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature. 2006;441:239–43. doi: 10.1038/nature04721. [DOI] [PubMed] [Google Scholar]
- 49.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, McElrath MJ, Casimiro DR, Gottesdiener KM, Chodakewitz JA, Corey L, Robertson MN. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–93. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, Defawe OD, Carter DK, Hural J, Akondy R, Buchbinder SP, Robertson MN, Mehrotra DV, Self SG, Corey L, Shiver JW, Casimiro DR. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seaman MS, Xu L, Beaudry K, Martin KL, Beddall MH, Miura A, Sambor A, Chakrabarti BK, Huang Y, Bailer R, Koup RA, Mascola JR, Nabel GJ, Letvin NL. Multiclade human immunodeficiency virus type 1 envelope immunogens elicit broad cellular and humoral immunity in rhesus monkeys. J Virol. 2005;79:2956–63. doi: 10.1128/JVI.79.5.2956-2963.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santra S, Seaman MS, Xu L, Barouch DH, Lord CI, Lifton MA, Gorgone DA, Beaudry KR, Svehla K, Welcher B, Chakrabarti BK, Huang Y, Yang ZY, Mascola JR, Nabel GJ, Letvin NL. Replication-defective adenovirus serotype 5 vectors elicit durable cellular and humoral immune responses in nonhuman primates. J Virol. 2005;79:6516–22. doi: 10.1128/JVI.79.10.6516-6522.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Letvin NL, Mascola JR, Sun Y, Gorgone DA, Buzby AP, Xu L, Yang ZY, Chakrabarti B, Rao SS, Schmitz JE, Montefiori DC, Barker BR, Bookstein FL, Nabel GJ. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science. 2006;312:1530–3. doi: 10.1126/science.1124226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Casimiro DR, Wang F, Schleif WA, Liang X, Zhang ZQ, Tobery TW, Davies ME, McDermott AB, O'Connor DH, Fridman A, Bagchi A, Tussey LG, Bett AJ, Finnefrock AC, Fu TM, Tang A, Wilson KA, Chen M, Perry HC, Heidecker GJ, Freed DC, Carella A, Punt KS, Sykes KJ, Huang L, Ausensi VI, Bachinsky M, Sadasivan-Nair U, Watkins DI, Emini EA, Shiver JW. Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with dna and recombinant adenoviral vaccine vectors expressing Gag. J Virol. 2005;79:15547–55. doi: 10.1128/JVI.79.24.15547-15555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buchbinder S. Clinical endpoints in the Step study. Keystone Symposium on HIV Vaccines; Keystone, CO. 2009. [Google Scholar]
- 56.O'Brien KL, Liu J, King SL, Sun YH, Schmitz JE, Lifton MA, Hutnick NA, Betts MR, Dubey S, Goudsmit J, Shiver JW, Robertson MN, Casimiro D, Barouch DH. Adenovirus-specific immunity following immunization with an Ad5 HIV-1 vaccine candidate in humans. Nat Med. 2009 doi: 10.1038/nm.1991. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hutnick NA, Carnathan DG, Dubey S, Cox K, Ratcliffe S, Robertson MN, Casimiro DR, Ertl HC, Betts MR. The effects of adenovirus based HIV-1 vaccines on CD4+ T cells in humans. Nat Med. 2009 doi: 10.1038/nm.1989. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McElrath J. AIDS Vaccine 2008. Cape Town, South Africa: 2008. Vaccine-induced immunity in T-cell based candidate HIV vaccines. [Google Scholar]
- 59.Perreau M, Pantaleo G, Kremer EJ. Activation of a dendritic cell-T cell axis by Ad5 immune complexes creates an improved environment for replication of HIV in T cells. The Journal of experimental medicine. 2008;205:2717–25. doi: 10.1084/jem.20081786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abbink P, Lemckert AA, Ewald BA, Lynch DM, Denholtz M, Smits S, Holterman L, Damen I, Vogels R, Thorner AR, O'Brien KL, Carville A, Mansfield KG, Goudsmit J, Havenga MJ, Barouch DH. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol. 2007;81:4654–63. doi: 10.1128/JVI.02696-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barouch DH, Pau MG, Custers JH, Koudstaal W, Kostense S, Havenga MJ, Truitt DM, Sumida SM, Kishko MG, Arthur JC, Korioth-Schmitz B, Newberg MH, Gorgone DA, Lifton MA, Panicali DL, Nabel GJ, Letvin NL, Goudsmit J. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J Immunol. 2004;172:6290–7. doi: 10.4049/jimmunol.172.10.6290. [DOI] [PubMed] [Google Scholar]
- 62.Fitzgerald JC, Gao GP, Reyes-Sandoval A, Pavlakis GN, Xiang ZQ, Wlazlo AP, Giles-Davis W, Wilson JM, Ertl HC. A simian replication-defective adenoviral recombinant vaccine to HIV-1 gag. J Immunol. 2003;170:1416–22. doi: 10.4049/jimmunol.170.3.1416. [DOI] [PubMed] [Google Scholar]
- 63.Amara RR, Villinger F, Altman JD, Lydy SL, O'Neil SP, Staprans SI, Montefiori DC, Xu Y, Herndon JG, Wyatt LS, Candido MA, Kozyr NL, Earl PL, Smith JM, Ma HL, Grimm BD, Hulsey ML, Miller J, McClure HM, McNicholl JM, Moss B, Robinson HL. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science. 2001;292:69–74. doi: 10.1126/science.1058915. [DOI] [PubMed] [Google Scholar]
- 64.Ferrari G, Humphrey W, McElrath MJ, Excler JL, Duliege AM, Clements ML, Corey LC, Bolognesi DP, Weinhold KJ. Clade B-based HIV-1 vaccines elicit cross-clade cytotoxic T lymphocyte reactivities in uninfected volunteers. Proc Natl Acad Sci U S A. 1997;94:1396–401. doi: 10.1073/pnas.94.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harari A, Bart PA, Stohr W, Tapia G, Garcia M, Medjitna-Rais E, Burnet S, Cellerai C, Erlwein O, Barber T, Moog C, Liljestrom P, Wagner R, Wolf H, Kraehenbuhl JP, Esteban M, Heeney J, Frachette MJ, Tartaglia J, McCormack S, Babiker A, Weber J, Pantaleo G. An HIV-1 clade C DNA prime, NYVAC boost vaccine regimen induces reliable, polyfunctional, and long-lasting T cell responses. The Journal of experimental medicine. 2008;205:63–77. doi: 10.1084/jem.20071331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Korber BT, Letvin NL, Haynes BF. T cell Vaccine Strategies for HIV, the Virus With a Thousand Faces. Journal of virology. 2009 doi: 10.1128/JVI.00114-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barouch DH. Challenges in the development of an HIV-1 vaccine. Nature. 2008;455:613–9. doi: 10.1038/nature07352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Letourneau S, Im EJ, Mashishi T, Brereton C, Bridgeman A, Yang H, Dorrell L, Dong T, Korber B, McMichael AJ, Hanke T. Design and pre-clinical evaluation of a universal HIV-1 vaccine. PLoS ONE. 2007;2:e984. doi: 10.1371/journal.pone.0000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rolland M, Nickle DC, Mullins JI. HIV-1 group M conserved elements vaccine. PLoS pathogens. 2007;3:e157. doi: 10.1371/journal.ppat.0030157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nielsen M, Lundegaard C, Lund O, Kesmir C. The role of the proteasome in generating cytotoxic T-cell epitopes: insights obtained from improved predictions of proteasomal cleavage. Immunogenetics. 2005;57:33–41. doi: 10.1007/s00251-005-0781-7. [DOI] [PubMed] [Google Scholar]
- 71.Miura T, Brockman MA, Schneidewind A, Lobritz M, Pereyra F, Rathod A, Block BL, Brumme ZL, Brumme CJ, Baker B, Rothchild AC, Li B, Trocha A, Cutrell E, Frahm N, Brander C, Toth I, Arts EJ, Allen TM, Walker BD. HLA-B57/B*5801 human immunodeficiency virus type 1 elite controllers select for rare gag variants associated with reduced viral replication capacity and strong cytotoxic T-lymphotye recognition. Journal of virology. 2009;83:2743–55. doi: 10.1128/JVI.02265-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gaschen B, Taylor J, Yusim K, Foley B, Gao F, Lang D, Novitsky V, Haynes B, Hahn BH, Bhattacharya T, Korber B. Diversity considerations in HIV-1 vaccine selection. Science. 2002;296:2354–60. doi: 10.1126/science.1070441. [DOI] [PubMed] [Google Scholar]
- 73.Nickle DC, Jensen MA, Gottlieb GS, Shriner D, Learn GH, Rodrigo AG, Mullins JI. Consensus and ancestral state HIV vaccines. Science (New York, NY. 2003;299:1515–8. doi: 10.1126/science.299.5612.1515c. author reply -8. [DOI] [PubMed] [Google Scholar]
- 74.Frahm N, Nickle DC, Linde CH, Cohen DE, Zuniga R, Lucchetti A, Roach T, Walker BD, Allen TM, Korber BT, Mullins JI, Brander C. Increased detection of HIV-specific T cell responses by combination of central sequences with comparable immunogenicity. AIDS (London, England) 2008;22:447–56. doi: 10.1097/QAD.0b013e3282f42412. [DOI] [PubMed] [Google Scholar]
- 75.Doria-Rose NA, Learn GH, Rodrigo AG, Nickle DC, Li F, Mahalanabis M, Hensel MT, McLaughlin S, Edmonson PF, Montefiori D, Barnett SW, Haigwood NL, Mullins JI. Human immunodeficiency virus type 1 subtype B ancestral envelope protein is functional and elicits neutralizing antibodies in rabbits similar to those elicited by a circulating subtype B envelope. Journal of virology. 2005;79:11214–24. doi: 10.1128/JVI.79.17.11214-11224.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kothe DL, Decker JM, Li Y, Weng Z, Bibollet-Ruche F, Zammit KP, Salazar MG, Chen Y, Salazar-Gonzalez JF, Moldoveanu Z, Mestecky J, Gao F, Haynes BF, Shaw GM, Muldoon M, Korber BT, Hahn BH. Antigenicity and immunogenicity of HIV-1 consensus subtype B envelope glycoproteins. Virology. 2007;360:218–34. doi: 10.1016/j.virol.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weaver EA, Lu Z, Camacho ZT, Moukdar F, Liao HX, Ma BJ, Muldoon M, Theiler J, Nabel GJ, Letvin NL, Korber BT, Hahn BH, Haynes BF, Gao F. Cross-subtype T-cell immune responses induced by a human immunodeficiency virus type 1 group m consensus env immunogen. J Virol. 2006;80:6745–56. doi: 10.1128/JVI.02484-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liao HX, Sutherland LL, Xia SM, Brock ME, Scearce RM, Vanleeuwen S, Alam SM, McAdams M, Weaver EA, Camacho Z, Ma BJ, Li Y, Decker JM, Nabel GJ, Montefiori DC, Hahn BH, Korber BT, Gao F, Haynes BF. A group M consensus envelope glycoprotein induces antibodies that neutralize subsets of subtype B and C HIV-1 primary viruses. Virology. 2006;353:268–82. doi: 10.1016/j.virol.2006.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Santra S, Korber BT, Muldoon M, Barouch DH, Nabel GJ, Gao F, Hahn BH, Haynes BF, Letvin NL. A centralized gene-based HIV-1 vaccine elicits broad cross-clade cellular immune responses in rhesus monkeys. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:10489–94. doi: 10.1073/pnas.0803352105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Douek DC, Betts MR, Brenchley JM, Hill BJ, Ambrozak DR, Ngai KL, Karandikar NJ, Casazza JP, Koup RA. A novel approach to the analysis of specificity, clonality, and frequency of HIV-specific T cell responses reveals a potential mechanism for control of viral escape. Journal of immunology (Baltimore Md. 2002;168:3099–104. doi: 10.4049/jimmunol.168.6.3099. [DOI] [PubMed] [Google Scholar]
- 81.Graham BS, Koup RA, Roederer M, Bailer RT, Enama ME, Moodie Z, Martin JE, McCluskey MM, Chakrabarti BK, Lamoreaux L, Andrews CA, Gomez PL, Mascola JR, Nabel GJ. Phase 1 Safety and Immunogenicity Evaluation of a Multiclade HIV-1 DNA Candidate Vaccine. J Infect Dis. 2006;194:1650–60. doi: 10.1086/509259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang S, Kennedy JS, West K, Montefiori DC, Coley S, Lawrence J, Shen S, Green S, Rothman AL, Ennis FA, Arthos J, Pal R, Markham P, Lu S. Cross-subtype antibody and cellular immune responses induced by a polyvalent DNA prime-protein boost HIV-1 vaccine in healthy human volunteers. Vaccine. 2008;26:3947–57. doi: 10.1016/j.vaccine.2007.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu J, Ewald BA, Lynch DM, Nanda A, Sumida SM, Barouch DH. Modulation of DNA vaccine-elicited CD8+ T-lymphocyte epitope immunodominance hierarchies. J Virol. 2006;80:11991–7. doi: 10.1128/JVI.01348-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fischer W, Perkins S, Theiler J, Bhattacharya T, Yusim K, Funkhouser R, Kuiken C, Haynes B, Letvin NL, Walker BD, Hahn BH, Korber BT. Polyvalent vaccines for optimal coverage of potential T-cell epitopes in global HIV-1 variants. Nat Med. 2007;13:100–6. doi: 10.1038/nm1461. [DOI] [PubMed] [Google Scholar]
- 85.Kong WP, Wu L, Wallstrom TC, Fischer W, Yang ZY, Ko SY, Letvin NL, Haynes BF, Hahn BH, Korber B, Nabel GJ. Expanded breadth of the T-cell response to mosaic human immunodeficiency virus type 1 envelope DNA vaccination. Journal of virology. 2009;83:2201–15. doi: 10.1128/JVI.02256-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Barouch DH. Novel adenovirus vector-based vaccines for HIV-1. Keystone Symposium on HIV Vaccines; Keystone, CO. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moore CB, John M, James IR, Christiansen FT, Witt CS, Mallal SA. Evidence of HIV-1 adaptation to HLA-restricted immune responses at a population level. Science. 2002;296:1439–43. doi: 10.1126/science.1069660. [DOI] [PubMed] [Google Scholar]
- 88.Bhattacharya T, Daniels M, Heckerman D, Foley B, Frahm N, Kadie C, Carlson J, Yusim K, McMahon B, Gaschen B, Mallal S, Mullins JI, Nickle DC, Herbeck J, Rousseau C, Learn GH, Miura T, Brander C, Walker B, Korber B. Founder effects in the assessment of HIV polymorphisms and HLA allele associations. Science (New York, NY. 2007;315:1583–6. doi: 10.1126/science.1131528. [DOI] [PubMed] [Google Scholar]
- 89.Carlson J, Kadie C, Mallal S, Heckerman D. Leveraging hierarchical population structure in discrete association studies. PLoS ONE. 2007;2:e591. doi: 10.1371/journal.pone.0000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Carlson JM, Brumme ZL, Rousseau CM, Brumme CJ, Matthews P, Kadie C, Mullins JI, Walker BD, Harrigan PR, Goulder PJ, Heckerman D. Phylogenetic dependency networks: inferring patterns of CTL escape and codon covariation in HIV-1 Gag. PLoS computational biology. 2008;4:e1000225. doi: 10.1371/journal.pcbi.1000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rousseau CM, Daniels MG, Carlson JM, Kadie C, Crawford H, Prendergast A, Matthews P, Payne R, Rolland M, Raugi DN, Maust BS, Learn GH, Nickle DC, Coovadia H, Ndung'u T, Frahm N, Brander C, Walker BD, Goulder PJ, Bhattacharya T, Heckerman DE, Korber BT, Mullins JI. HLA class I-driven evolution of human immunodeficiency virus type 1 subtype c proteome: immune escape and viral load. Journal of virology. 2008;82:6434–46. doi: 10.1128/JVI.02455-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brockman MA, Schneidewind A, Lahaie M, Schmidt A, Miura T, Desouza I, Ryvkin F, Derdeyn CA, Allen S, Hunter E, Mulenga J, Goepfert PA, Walker BD, Allen TM. Escape and compensation from early HLA-B57-mediated cytotoxic T-lymphocyte pressure on human immunodeficiency virus type 1 Gag alter capsid interactions with cyclophilin A. Journal of virology. 2007;81:12608–18. doi: 10.1128/JVI.01369-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Karlsson AC, Iversen AK, Chapman JM, de Oliviera T, Spotts G, McMichael AJ, Davenport MP, Hecht FM, Nixon DF. Sequential broadening of CTL responses in early HIV-1 infection is associated with viral escape. PLoS ONE. 2007;2:e225. doi: 10.1371/journal.pone.0000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Feeney ME, Tang Y, Pfafferott K, Roosevelt KA, Draenert R, Trocha A, Yu XG, Verrill C, Allen T, Moore C, Mallal S, Burchett S, McIntosh K, Pelton SI, St John MA, Hazra R, Klenerman P, Altfeld M, Walker BD, Goulder PJ. HIV-1 viral escape in infancy followed by emergence of a variant-specific CTL response. Journal of immunology (Baltimore Md. 2005;174:7524–30. doi: 10.4049/jimmunol.174.12.7524. [DOI] [PubMed] [Google Scholar]
- 95.Iversen AK, Stewart-Jones G, Learn GH, Christie N, Sylvester-Hviid C, Armitage AE, Kaul R, Beattie T, Lee JK, Li Y, Chotiyarnwong P, Dong T, Xu X, Luscher MA, MacDonald K, Ullum H, Klarlund-Pedersen B, Skinhoj P, Fugger L, Buus S, Mullins JI, Jones EY, van der Merwe PA, McMichael AJ. Conflicting selective forces affect T cell receptor contacts in an immunodominant human immunodeficiency virus epitope. Nature immunology. 2006;7:179–89. doi: 10.1038/ni1298. [DOI] [PubMed] [Google Scholar]
- 96.Yang OO, Sarkis PT, Ali A, Harlow JD, Brander C, Kalams SA, Walker BD. Determinant of HIV-1 mutational escape from cytotoxic T lymphocytes. The Journal of experimental medicine. 2003;197:1365–75. doi: 10.1084/jem.20022138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Allen TM, Altfeld M, Yu XG, O'Sullivan KM, Lichterfeld M, Le Gall S, John M, Mothe BR, Lee PK, Kalife ET, Cohen DE, Freedberg KA, Strick DA, Johnston MN, Sette A, Rosenberg ES, Mallal SA, Goulder PJ, Brander C, Walker BD. Selection, transmission, and reversion of an antigen-processing cytotoxic T-lymphocyte escape mutation in human immunodeficiency virus type 1 infection. Journal of virology. 2004;78:7069–78. doi: 10.1128/JVI.78.13.7069-7078.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Draenert R, Le Gall S, Pfafferott KJ, Leslie AJ, Chetty P, Brander C, Holmes EC, Chang SC, Feeney ME, Addo MM, Ruiz L, Ramduth D, Jeena P, Altfeld M, Thomas S, Tang Y, Verrill CL, Dixon C, Prado JG, Kiepiela P, Martinez-Picado J, Walker BD, Goulder PJ. Immune selection for altered antigen processing leads to cytotoxic T lymphocyte escape in chronic HIV-1 infection. The Journal of experimental medicine. 2004;199:905–15. doi: 10.1084/jem.20031982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Goepfert PA, Lumm W, Farmer P, Matthews P, Prendergast A, Carlson JM, Derdeyn CA, Tang J, Kaslow RA, Bansal A, Yusim K, Heckerman D, Mulenga J, Allen S, Goulder PJ, Hunter E. Transmission of HIV-1 Gag immune escape mutations is associated with reduced viral load in linked recipients. The Journal of experimental medicine. 2008;205:1009–17. doi: 10.1084/jem.20072457. [DOI] [PMC free article] [PubMed] [Google Scholar]