Abstract
Impairment of memory functions has been frequently reported in models of sleep deprivation. Similarly, hippocampal long-term synaptic plasticity has been shown to be sensitive to sleep loss due to acute sleep restriction. However, such approaches are limited by the stressful nature of sleep deprivation, and because it is difficult to study long term sleep restriction in animals. Here we report the effects of chronic sleep loss on hippocampal long-term potentiation (LTP) in a rodent model for chronic partial sleep deprivation. We studied LTP of the Schaffer collateral-CA1 synapses in hippocampal slices prepared from rats with lesions of the ventrolateral preoptic nucleus (VLPO), who suffer reduction in total sleep time for several weeks after lesions. In slices prepared from VLPO-lesioned rats, LTP was impaired proportional to the amount of sleep loss and the decline in LTP followed a single exponential function over the amount of accumulated sleep debt. As compared to sham-lesioned controls, hippocampal slices from VLPO-lesioned rats showed a greater response to adenosine antagonists and greater paired-pulse facilitation (PPF). However, exogenous adenosine depressed evoked synaptic transmission and increased PPF in VLPO-lesioned and sham-lesioned rats by equal amounts, suggesting that the greater endogenous adenosine inhibitory tone in the VLPO-lesioned rats is associated with greater ligand accumulation rather than a change in adenosine receptor sensitivity or adenosine-mediated neurotransmitter release probability. LTP in VLPO-lesioned animals was partially restored by adenosine antagonists suggesting that adenosine accumulation in VLPO-lesioned animals can account for some of the observed synaptic plasticity deficits.
Keywords: LTP, VLPO lesions, sleep deprivation, adenosine, in vitro
Introduction
In humans, insufficient sleep produces progressive impairment in cognitive and motor performance (Dinges et al., 1997; Durmer & Dinges, 2005), and many studies have indicated an important role for sleep in acquisition and consolidation of memory (Maquet, 2001; Siegel, 2001; Stickgold et al., 2001; Hobson, 2005; Stickgold & Walker, 2005). In rodents, sleep deprivation impairs hippocampal-dependent learning and reduces hippocampal long-term potentiation (LTP) both in vivo (Romcy-Pereira & Pavlides, 2004; Kim et al., 2005; Marks & Wayner, 2005; Ishikawa et al., 2006) and in vitro (Campbell et al., 2002; Davis et al., 2003; McDermott et al., 2003; Chen et al., 2006; Tartar et al., 2006). These effects have been associated with the suppression of the expression of plasticity-related genes (Davis et al., 2006; Guzman-Marin et al., 2006), changes in NMDA receptor expression and subunit composition (Kopp et al., 2006; McDermott et al., 2006; Lopez et al., 2008) and reductions in phosphorylated-extracellular-signal-regulated kinases (p-ERK) (Guan et al., 2004; Ravassard et al., 2009). In addition, extracellular adenosine progressively accumulates during wakefulness in several brain regions including the hippocampus (Huston et al., 1996; Porkka-Heiskanen et al., 1997; Porkka-Heiskanen et al., 2000) and adenosine is known to suppress hippocampal LTP (Arai et al., 1990; de Mendonca & Ribeiro, 1990; Liang et al., 2008). Thus during sleep deprivation, the accumulation of adenosine may also contribute to the impairment of hippocampal LTP (de Mendonca & Ribeiro, 1997; Huang & Hsu, 2001).
The effects of sleep deprivation on memory consolidation have been studied using different sleep restriction methods and regimes. While effective in producing sleep loss, most of these methods require stressful manipulations, such as interaction with humans or confinement to a small platform suspended over water, or forcing them to exercise. As a result, it is difficult to carry out these paradigms for more than a few days, and the effects of chronic stress and sleep loss cannot be dissociated.
In the present study, we used rats with bilateral lesions of the ventrolateral preoptic (VLPO) nucleus, an alternative experimental model for chronic partial sleep restriction. The VLPO nucleus is a cluster of sleep-active, GABA/galanin containing neurons located just dorsal and lateral to the optic chiasm that plays a critical role in promoting sleep (Sherin et al., 1996; Szymusiak et al., 1998; Saper et al., 2001). Mechanical or electrolytic lesions of the preoptic area and adjacent basal forebrain cause prolonged insomnia in rats (Nauta, 1946; McGinty & Sterman, 1968). Previous studies by Lu and colleagues (2000) have shown that smaller, cell-body specific lesions of the VLPO nuclei produce a comparable insomnia phenotype. Because this condition persists for at least 8 weeks (the longest survival period studied to date), the VLPO-lesioned rats provide a unique model for studying the effects of increased homeostatic sleep pressure without stressful external intervention.
In the presents study, we used field recordings of synaptic transmission in the CA1 area to study LTP in hippocampal slices prepared from VLPO-lesioned rats and further determined whether accumulation of adenosine affected hippocampal LTP in the VLPO-lesioned animals.
Materials and Methods
Animals
Fifty-one adult male Sprague Dawley rats (275-300 grams, Harlan Laboratories, USA) were used in this study. All rats were individually housed and the cages were housed inside isolation chambers, which provided ventilation, computer-controlled lighting (12:12 light-dark cycle, lights on at 07:00; 200 lux), an ambient temperature of 22 ± 1°C, and visual isolation. Care of the rats in these experiments met the National Institutes of Health standards, as set forth in the Guide for the Care and Use of Laboratory Animals and all protocols were approved by the BIDMC and Harvard Medical School Institutional Animal Care and Use Committees.
Rat surgery
Under chloral hydrate anesthesia (7% solution, 350 mg/kg), a burr hole was made, and a fine glass pipette (1 mm glass stock, tapering slowly to a 10-20 um tip) containing 0.1% orexin-saporin (OX-SAP; 0.1% solution, Advanced Targeting Systems, San Diego, CA, USA, n = 31 rats) or artificial cerebrospinal fluid (ACSF, for sham-lesions; n = 20 rats) was lowered into the VLPO on each side of the brain. Coordinates for the VLPO were AP −0.6 mm, ML ±1.0, DV −8.5mm (Paxinos & Watson, 2007). 200 nl of OX-SAP was slowly injected (over five minutes) by an air-pressure delivery system (Scammell et al., 1998). Following the microinjections, the animals were implanted with four EEG screw electrodes into the frontal (two) and parietal (two) bones of each side, and two flexible EMG wire electrodes were placed into the neck muscles. The free ends of the leads were soldered into a socket that was attached to the skull with dental cement, and the incision was then closed by wound clips.
Recording of sleep-wakefulness and data analysis
EEG/EMG recordings from the VLPO-lesioned rats (VLPO-L) and sham-lesioned (Con-L) rats were conducted at 1-week, 3-weeks and 6-weeks after the surgical procedure. In each group, animals were first acclimated to the recording chamber with the EEG/EMG cable attached for 24 hours, then recorded for 48 hours, then the LTP measurements were done on the fourth day. This took place over days 7-10 post-lesion for the 1 week group, and on days 21-24 for the 3 week group, and 42-45 for the 6 week group. EEG/EMG recordings were carried out in a sound-attenuated chamber using a polygraph (Grass Instruments model 7B, West Warwick, RI, USA), and after a 24 hour acclimatization period. Continuous recordings were conducted for a 48-h period (7 A.M. to 7 A.M.) prior to euthanizing the rat for in vitro recordings (10 A.M). Amplified EEG/EMG signals were digitized and analyzed offline using either ICELUS (University of Michigan Programming by g system Dr. Mark Opp) or SLEEPSIGN (Kissei Comtek, Matsumoto, Japan) acquisition and analysis software.
The EEG/EMG recordings were divided into 12-sec epochs and manually scored into one of 3 stages of sleep-wakefulness: wake, non rapid eye movement sleep (NREM) and rapid eye movement sleep (REM) based on the criteria described in earlier reports (Lu et al., 2000, 2002). In brief, wakefulness was identified by the presence of a desynchronized-EEG and high-EMG activity. NREM sleep was identified by the presence of a high-amplitude, slow-wave EEG and low-EMG activity relative to that of wakefulness. REM sleep was identified by the presence of regular theta activity on EEG, coupled with low-EMG activity relative to that of NREM sleep. When two states (for example, NREM sleep and wake) occurred within a 12-sec epoch, the epoch was scored for the state that predominated (Lu et al., 2000), 2001). The amount of time spent in different stages of sleep-wakefulness (wake, NREM and REM) and total sleep time (NREM+ REM) per day were calculated from each 48-h recording period (i.e., 1-week, 3-weeks and 6-weeks post-injection) from VLPO-L and Con-L rats. The amount of accumulated sleep loss (total sleep, NREM and REM sleep) in each VLPO-L rat at 1, 3 and 6 weeks was therefore calculated by subtracting the total sleep, NREM sleep, and REM sleep time per day from the mean sleep times (total sleep, NREM and REM sleep time) for the CON-L rat group, and multiplying by the number of days post lesion. In other words, accumulated total sleep loss for each VLPO-L rat = (average total sleep time per day of Con-L rats – total sleep time per day of that VLPO-L rat) * number of days post-lesion (from day 7 after surgery to the day of hippocampal slice recording: 2 days for 1 week in vitro recordings, 16 days for 3 week recordings and, 37 days for 6 week recordings). In this calculation we subtracted 7 days from the number of days post-lesion as it takes about 7-10 days for neurons to die after being exposed to OX-SAP. The accumulated NREM and REM sleep losses were calculated in the same way: accumulated NREM and REM sleep losses = (average NREM and REM sleep time per day of Con-L rats – NREM and REM sleep time per day of each VLPO-L rat) * number of days post-lesion (from 7 days after surgery to the day of hippocampal slice recording). Mean total sleep time per day in Con-L rats was 12.7 ± 0.59 h.
Hippocampal slice preparation
Rats were sacrificed for preparation of hippocampal slices at 10:00 A.M. Under isoflurane anesthesia, rats were decapitated and the brain rapidly removed and placed in cold ACSF containing (in mM): 128 NaCl, 3 KCl, 0.5 NaH2PO4, 1 MgSO4, 4 CaCl2, 23.5 NaHCO3, and 10 glucose, (315-320 mOsm) pH 7.35 when equilibrated with 95% O2 and 5% CO2. Coronal forebrain slices containing the hippocampus (400 μm thick) were cut using a vibrating microtome (VT1000; Leica, Bannockburn, IL, USA) while maintained in ice cold oxygenated ACSF. Slices were hemisected and kept at 22°C in oxygenated ACSF for 1 hour prior to recording.
Extracellular field recordings and data analysis
Hippocampal slices were recorded submerged and perfused (2 ml/min) with ACSF maintained at 30°C using a temperature controller (TC-344B; Warner Instruments, Hamden, CT, USA). Field excitatory postsynaptic potentials (fEPSPs) were recorded from the CA1 stratum radiatum with glass electrodes filled with 2 M NaCl (2-4 MΩ) using a Multiclamp 700A amplifier (Axon Instruments, Foster City, CA, USA). Signals were filtered at 1-2 kHz and digitized at 40 kHz with a Digidata 1200 interface and pClamp 8.2 software (Axon Instruments). Schaffer collateral fibers were stimulated with a concentric stimulating electrode (Frederick Haer & Co, Bowdoinham, ME, USA) placed in the stratum radiatum adjacent to the CA2 field, using constant current pulses (0.2 ms duration, every 20 s). Stimulation pulses were delivered with a constant-current source (Stimulus Isolator A360; World Precision Instruments, Sarasota, FL, USA), triggered by Clampex software (Axon Instruments), and the stimulus strength was adjusted to give ~75% of maximum fEPSP amplitude (0.5-1.5 mV). LTP was induced by high frequency stimulation (HFS) using a single train of 100 pulses delivered at 100 Hz. Unless specified, all reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). The stock solution of 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) was prepared in dimethyl sulfoxide (DMSO). The final concentration of DMSO in the ACSF was < 0.1%.
Evoked field potentials were quantified as the slope of the fEPSP measured between 10% and 90% of the fEPSP peak amplitude. Percentage of fEPSP slope was normalized relative to the mean values obtained during 20 min recordings prior to HFS, and represented every 1 min. The amount of LTP was determined as the mean percentage change in fEPSP slope of 6 consecutive traces over 2-min period at the end of 60-min post HFS. Decays in LTP vs accumulation of sleep loss (total sleep, NREM sleep and REM sleep losses) were estimated by fitting the data with a single exponential function (y = A e−x/τ + C) using a non-linear least-squares fitting algorithm (Clampfit, Axon Instruments). The paired-pulse test was evoked by two electrical stimulating pulses (0.2 ms duration) in rapid sequence (40-50 msec intervals). For this test, stimulus strength was adjusted to give ~50% of maximum fEPSP amplitude. Paired-pulse ratio (PPR) was calculated by dividing the fEPSC slope of the 2nd pulse by fEPSC slope of the 1st pulse. PPR values were calculated as the average of 6 consecutive traces (PPF test repeated every 20 sec). The effects of the adenosine A1 antagonist DPCPX on PPF were represented by scaling the traces in DPCPX so that the fEPSCs (1st pulse) matches the control fEPSC (1st pulse) (Fig. 4B1). Statistical significance was established by one-way ANOVA followed by Fisher's protected least significant difference (PLSD) test for pair-wise multiple comparison. Other data were analyzed using a two-population paired or unpaired t test. All statistical analyses were done using Statview (SAS Institute Inc. Cary, NC, USA). A p value less then 0.05 was considered significant. Results are expressed as mean ± S.E.M and unless specified, n refers to the number of recordings.
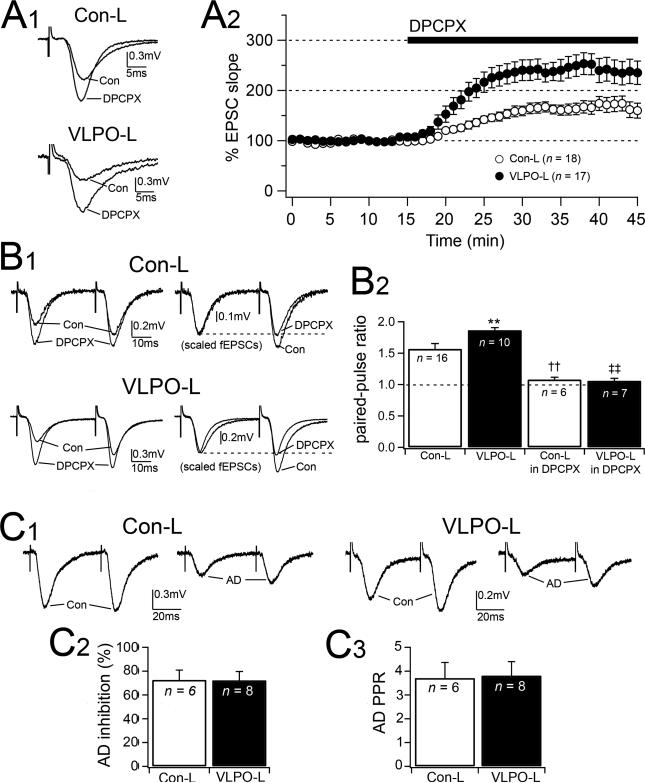
Figure 4.
Hippocampal slices from VLPO-lesioned animals are under greater inhibitory tone by endogenous adenosine. A1-2, The adenosine A1 antagonist DPCPX (400 nM) increases fEPSC slope and this effect is greater in slices prepared from VLPO-L rats (6-weeks VLPOL) compared to Con-L rats (6-weeks Con-L). A1, Representative responses to DPCPX in Con-L and VLPO-L animals (average of 6 consecutive fEPSCs recorded at the end of: 10 min in control ACSF; “Con” and 20 min in DPCPX). A2, Shows the time course of the response to DPCPX (100% is the averaged fEPSP slopes during 15 min preceding DPCPX). B1-2, Paired-pulse facilitation mediated by endogenous adenosine is greater in VLPO-lesioned animals compared to control lesioned animals. B1, Representative fEPSCs (paired-pulses test: 40 msec interstimulus interval) recorded before and during DPCPX. Scaled traces to match fEPSC (1st pulses) are shown on the right. B2, Shows the averaged results of PPR in Con-L and VLPO-L animals recorded in control ACSF and in DPCPX (one-way ANOVA test between animal groups and treatments: F = 30.6, p < 0.001; Fisher's PLSD: ** VLPO-L > Con-L, p < 0.001; †† Con-L > Con-L in DPCPX, p < 0.001; ‡‡ VLPO-L > VLPO-L in DPCPX, p < 0.001). C1-3, Effects of exogenous adenosine on fEPSC slope and PPF in slices from Con-L and VLPO-L rats. C1 shows fEPSCs (paired-pulse test: 50 msec interstimulus interval) recorded before (Con) and during adenosine 20 μM (AD). C2-3, Two graphs showing the effects of exogenous adenosine on fEPSC slope and PPF in slices from Con-L and VLPO-L rats. There is no statistically significant difference in the response to adenosine between Con-L and VLPO-L rats (AD-inhibition: p = 0.59, unpaired t test; AD-PPR: p = 0.75, unpaired t test). All data are represented as mean ± S.E.M. (n = number of recordings).
Histological processing
The verification of the lesion sites in the VLPO was determined by Nissl-staining. During in vitro slice preparation, the brain was divided in two using a coronal cut behind the optic chiasm (between AP levels −1 and −1.6) (Paxinos & Watson, 2007). The posterior portion was used for the preparation of hippocampal in vitro slices. The anterior portion containing the anterior hypothalamus was fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for three days, then cryoprotected in 30% sucrose (in PBS) and cut in 40 μm sections on a freezing microtome. Sections were mounted on gelatin-coated slides, stained with 0.25% thionin in 0.1 M acetate buffer solution (2 min), dehydrated in a series of graded ethanols, cleared in xylene and then coverslipped. The locations of the lesions in the VLPO region were confirmed based on local neuronal loss and gliosis. However, due to brain distortion caused by the razor blade cut and subsequent immersion fixation and by the damage of the area around the optic chiasm with rapid brain removal, the tissue was not sufficiently intact for accurate quantitative analysis of VLPO cell loss. We therefore assessed the effectiveness of the lesions physiologically, by the amount of sleep loss, rather than histologically, by VLPO neuron loss.
Results
Hippocampal LTP is impaired in hippocampal slices from VLPO-lesioned rats
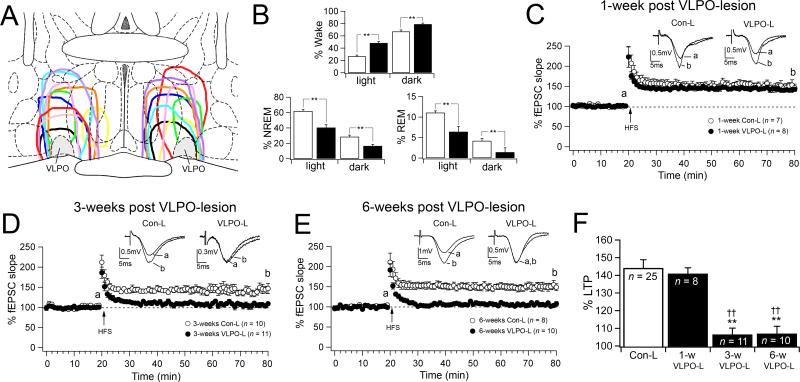
We studied the effects of sleep loss on hippocampal LTP in in vitro slices prepared from rats with bilateral lesions of the VLPO nuclei (Fig. 1A) as a model for chronic partial sleep deprivation. VLPO-lesioned (VLPO-L) rats showed significant reduction in total sleep time compared to the sham-lesioned (Con-L) rats. On average, VLPO-L rats (n = 31) lost 27.5 ± 2.9% total sleep (NREM + REM sleep) per day, with sleep loss in individual animals ranging from 4.4 to 53.2%. This variability provided an important internal control, as our animals by 3 and 6 weeks post-operatively had a wide range of number of hours of cumulative sleep loss, despite having similar lesions. Reductions were observed in the amounts of both NREM sleep and REM sleep in VLPO-lesioned rats (Fig. 1B). The times spent in NREM sleep and REM sleep were reduced during the light cycle (7:00 – 19:00) (NREM sleep: from 62.0 ± 1.85 % in CON-L rats to 43.8 ± 2.04% in VLPO-L rats; p < 0.001, unpaired t test; REM sleep: from 11.1 ± 0.41 % in CON-L rats to 7.4 ± 0.83% in VLPO-L rats; p < 0.001, unpaired t test) and during the dark cycle (19:00 – 7:00) (NREM sleep: from 28.6 ± 2.14% in CON-L rats to 20.6 ± 2.74% in VLPO-L rats; p = 0.03, unpaired t test; REM sleep: from 4.2 ± 0.57 % in CON-L rats to 1.93 ± 0.32% in VLPO-L rats; p = 0.008, unpaired t test;). In addition, in VLPO-lesioned rats, the durations of NREM and REM sleep bouts were reduced. The mean duration of the NREM sleep bouts was significantly reduced in both light and dark phases but not the number of NREM bouts (light cycle: from 193.8 ± 18.2 sec in CON-L rats to 139.2 ± 11.7 sec in VLPO-L rats; p = 0.031 unpaired t test and from 143 ± 12.9 NREM bouts in CON-L rats to 111.4 ± 10.3 bouts in VLPO-L rats; p = 0.16 unpaired t test; dark cycle: from 131.5 ± 12.7 sec in CON-L rats to 97.5 ± 9.1 sec in VLPO-L rats; p = 0.053 unpaired t test and from 95.7 ± 7.5 NREM bouts in Con-L rats to 94.5 ± 10.1 bouts in VLPO-L rats; p = 0.93 unpaired t test ). Similarly REM sleep bout duration was reduced in VLPO-lesioned rats but not the number of REM bouts (light cycle: from 94.33 ± 4.46 sec in CON-L rats to 63.65 ± 7.75 sec in VLPO-L rats; p = 0.002 unpaired t test and from 51.2 ± 3.6 REM bouts in CON-L rats and 41.4 ± 5.8 bouts in VLPO-L rats; p = 0.16 unpaired t test; dark cycle: from 75.83 ± 5.09 sec in CON-L rats to 30.78 ± 4.12 sec in VLPO-L rats; p < 0.001 and from 24.3 ± 3.8 REM bouts in CON-L rats and 17.2 ± 3.1 bouts in VLPO-L rats; p = 0.17 unpaired t test).
Figure 1.
Hippocampal LTP is impaired in VLPO-lesioned animals. A, Camera lucida drawings illustrating 10 examples of lesions in the VLPO region. B, Graphs represent the % of wake, NREM sleep and REM sleep during the light (7:00 – 19:00) and dark (19:00 7:00) cycles in the Con-L (white bars) and the VLPO-L (black bars) rats used for the LTP experiments in panels C-F. Con-L (n = 13 rats) and VLPO-L (n = 16 rats), ** p < 0.01 unpaired t test. C, D, E, Graphs compare CA1-schaffer collateral LTP in slices prepared from VLPO-L (total sleep loss > 20%) and Con-L at three time points: 1-week, 3-weeks and 6-weeks post VLPO-lesions and post sham-lesions. Data are represented as mean ± S.E.M. percent changes in fEPSP slope before and after high frequency stimulation (HFS, 100 pulses at 100 Hz) and n = number of recordings. Representative fEPSCs (average of 6 consecutive traces) were recorded over 2 minute periods before HSF (a) and 60 minutes after HSF (b). F, Summarized results of mean percent changes in fEPSP slope (60 min after HFS) in slices from control-lesioned animals (Con-L = grouped data from 1-week, 3-week and 6-week Con-L animals; one-way ANOVA between Con-L groups, F = 0.43, p = 0.654) and 1-week, 3-week and 6-week VLPO lesioned animals (one-way ANOVA test between animal groups: F = 17.5, p < 0.001; Fisher's PLSD: ** Con-L > 3-W VLPO-L, p < 0.01 and Con-L > 6-week VLPO-L, p < 0.01; †† 1-W VLPO-L > 3-W VLPO-L, p < 0.01; and 1-W VLPO-L > 6-week VLPO-L, p < 0.01).
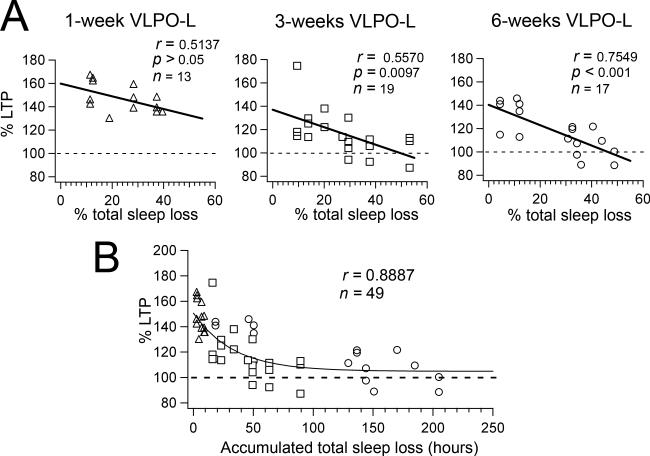
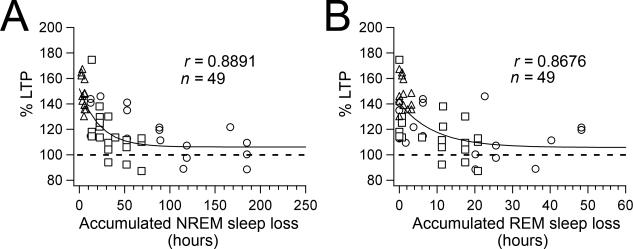
We measured hippocampal LTP of the CA1-Schaffer collateral synaptic input in hippocampal in vitro slices prepared from VLPO-L (n = 24) and Con-L (n = 13) rats at three post-lesion time points: 1-week, 3-weeks and 6-weeks (Fig. 1 C-F). In the initial study we averaged data at each time point from rats that, following VLPO bilateral lesions, showed more then 20% total sleep loss (average total sleep loss: 1-week VLPO rats, 29.2 ± 4.1% n = 4 rats; 3-week VLPO rats, 36.2 ± 4.8% n = 5 rats; 6-week VLPO rats, 38.4 ± 2.6%; n = 7 rats; one-way ANOVA between VLPO-L groups, F = 1.7, p = 0.22). As expected, we found that 1-week post VLPO lesion LTP was unaffected (as the lesions in these animals had only been present for few days at time of death). Hence, the animals studied at one week provide a control for the effects of acute VLPO lesions on LTP. In this context, it is remarkable that LTP was impaired in slices prepared 3-weeks and 6-weeks post VLPO lesions. At 60 min post-tetanus the mean fEPSPs in slices from 1-week VLPO-L rats (141.0 ± 3.3%, n = 8) did not differ from slices taken from 1-week Con-L rats (142.8 ± 10.2%, n = 7; p = 0.87 unpaired t test), however fEPSCs in slices from 3-week VLPO-L rats (106.5 ± 3.6%, n = 11) and 6-week VLPO-L rats (107.0 ± 4.1%, n = 10) were less potentiated than their respective Con-L groups (3-week Con-L rats: 140.2 ± 7.3, n = 10, p = 0.001 unpaired t test; and 6-week Con-L rats: 150.5 ± 7.6, n = 8, p < 0.001 unpaired t test ). The similar reduction in LTP at 3 and 6 weeks prompted us to study the dose-response effect of sleep loss on LTP more closely. Because some lesions were more effective than others, we reanalyzed our data by correlating the impairment of hippocampal LTP with total accumulated sleep loss for each animal (VLPO-L with total sleep loss > 20%, n = 16 rats Fig.1, and VLPO-L rats with total sleep loss ≤ 20%, n = 8 rats). We found that in addition, the effects of sleep loss of the VLPO-lesioned animals were apparent in the individual rats of the 3-week and 6-week VLPO-lesioned animal groups, but not in the rats of the 1-week VLPO-lesiond animal group (a group which has little if any accumulated sleep loss) (Fig. 2A). Regression analysis across animals of the three animal groups (1-week, 3-week, and 6-week VLPO-L rats) showed a strong negative correlation between the amount of sleep loss and percentage of hippocampal LTP in the 3-week VLPO-L rats (r = 0.5570; n = 19; p = 0.0097) and the 6-week VLPO-L rats (r = 0.7549; n = 17; p < 0.001), but there was no statistically significant correlation in the 1-week VLPO-L rats (r = 0.5137; n = 13; p = 0.072). Because OX-SAP lesions cause cell loss that does not become complete until about 7-10 days post injection (Gerashchenko et al., 2001), the animals that were examined at the 1-week time point (i.e., sleep recordings on days 8 and 9) had limited cumulative sleep loss despite a significant reduction in daily sleep time in the recording just prior to LTP assessment. Thus these animals provided an important control because they had the acute effects of loss of VLPO neurons with minimal cumulative sleep loss. Plotting fEPSC slope at 60 min post-tetanus of all VLPO-lesioned rats versus sleep loss accumulated up to the time of the day when the in vitro recordings were done (see Materials and Methods) showed a single exponential decay with a time constant of 33.4 hours (Fig. 2B). Similarly we found that LTP declined following a single exponential function over both accumulated NREM and REM sleep (Fig. 3). LTP declined over the accumulated NREM sleep loss with a time constant of 22.3 hours and over the accumulated REM sleep loss with a faster time constant of 9.2 hours. Thus, despite variability of lesion effectiveness and length of survival, the loss of LTP for all animals was best explained by the cumulative loss of NREM sleep, REM sleep and total sleep.
Figure 2.
LTP is impaired in proportion to the amount of sleep loss and LTP progressively decays with the accumulated sleep loss. A, Correlation of the percent LTP (% change of fEPSP slope 60 min after HFS) vs % daily total sleep loss (NREM + REM sleep) across animals of the three animal groups (1-week, 3-week, and 6-week VLPO-L rats). Regression analysis shows a strong negative correlation between the amount of sleep loss and percentage of hippocampal LTP in the 3-week VLPO-L rats and the 6-week VLPO-L rats, but no statistically significant correlation in the 1-week VLPO-L rats. B, The graph represents percent LTP (% change of fEPSP slope 60 min after HFS) vs accumulated total sleep loss (see Materials and Methods) from all VLPO-lesioned animals (triangles = 1-week VLPO-L; square = 3-week VLPO-L and circles = 6-week VLPO-L animals). Data was fit with a single exponential function (fitting parameters ± standard deviation: A = 45.7 ± 5.8%; τ = 33.4 ± 11.4 h; C = 104.8 ± 4.7%).
Figure 3.
LTP declines as a single exponential function over both accumulated NREM and REM sleep losses. A, Percent LTP (% change of fEPSP slope 60 min after HFS) vs accumulated NREM sleep loss (see Materials and Methods) from all VLPO-lesioned animals (triangles = 1-week VLPO-L; square = 3-week VLPO-L and circles = 6-week VLPO-L animals). Data was fit with a single exponential function (fitting parameters ± standard deviation: A = 45.7 ± 5.7%; τ = 22.3 ± 7.7 h; C = 106.1 ± 4.3%). B, Percent LTP vs accumulated REM sleep loss (triangles = 1-week VLPO-L; square = 3-week VLPO-L and circles = 6-week VLPO-L animals). Data was fit with a single exponential function (fitting parameters ± standard deviation: A = 34.9 ± 7.6%; τ = 9.2 ± 5.9 h; C = 105.8 ± 7.4%).
Inhibitory tone by endogenous adenosine is greater in slices from VLPO-lesioned rats
It has been shown that adenosine accumulates in the hippocampus during wakefulness (Huston et al., 1996) and inhibition of the Schaffer collateral input by endogenous adenosine is greater in slices prepared from rats euthanized when they are awake compared to slices taken from animals that are asleep (Liu et al., 2000). To determine whether hippocampal slices prepared from VLPO-L rats are under a greater inhibitory tone by endogenous adenosine we used three tests: 1) we tested the effects of blocking endogenous adenosine with an adenosine A1 antagonist, 2) we measured the adenosine-mediated paired-pulse facilitation (PFF) and 3) we tested the effects of exogenous adenosine. Recordings were conducted in slices from 6-week Con-L (n = 4) and 6-week VLPO-L (n = 4) rats.
Application of the A1 antagonist DPCPX increased the fEPSP slope in slices prepared from both Con-L and VLPO-L rats, but the response to DPCPX was greater in VLPO-L animals (Fig. 4A). After thirty minutes in DPCPX (400 nM) the fEPSP slope was 245.1 ± 19.8% (n = 17) in slices from VLPO-L rats compared to only 174.8 ± 19.1% (n = 18) in slices from Con-L rats (VLPO-L > Con-L rats p = 0.001, unpaired t test).
Adenosine is known to reduce hippocampal fEPSPs and to increase PPF (Dunwiddie & Haas, 1985) and increases in endogenous adenosine levels are associated with an increase in paired pulse ratio (PPR) (Tanaka et al., 2001). We examined the PPF mediated by endogenous adenosine in VLPO-L and Con-L rats (Fig. 4B). We found that PPR was greater in VLPO-L rats compared to Con-L rats (1.86 ± 0.04; n = 10 in VLPO-L rats and 1.54 ± 0.07, n = 16 in Con-L rats p < 0.001, unpaired t test) and PPF mediated by the endogenous adenosine (PPRcon – PPRDPCPX) was 2.3 times greater in slices from VLPO-L rats compared to the Con-L rats (VLPO-L > Con-L, p = 0.002, unpaired t test). In addition, we found that in the presence of DPCPX there was no significant difference in PPR between the two groups (PPR in DPCPX was 1.06 ± 0.04; n = 7 in VLPO-L rats and 1.08 ± 0.04; n = 6 in Con-L rats; p = 0.734, unpaired t test), indicating that when the effects of endogenous adenosine were antagonized there was no difference in Schaffer collateral excitatory neurotransmitter release probability between VLPO-L and Con-L rats.
To determine whether the greater increase in fEPSP slope by DPCPX in VLPO-L could be due to a difference in sensitivity to adenosine, we tested exogenous adenosine in Con-L and VLPO-L rats. We found no significant difference in the responses to adenosine (Fig. 4C). Adenosine (20 μM) reduced the fEPSC slope by 54.6 ± 4.3% (n = 6) in VLPO-L and by 59.3 ± 7.2% (n = 6) in Con-L (p = 0.59; unpaired t test) and PPR was 1.71 ± 0.18% (n = 6) in Con-L and 1.78 ± 0.08% (n = 6) in VLPO-L (p = 0.75; unpaired t test).
LTP of VLPO-lesioned rats improves with adenosine A1 receptor antagonists
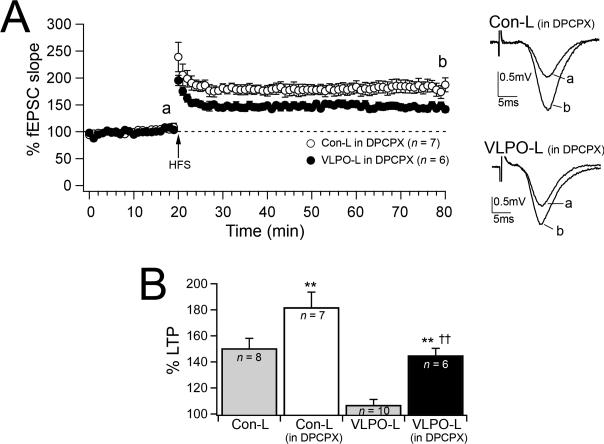
Hippocampal LTP is depressed by endogenous adenosine (Arai & Lynch, 1992; de Mendonca & Ribeiro, 1994; Forghani & Krnjevic, 1995). To determine whether the LTP impairment in VLPO-L rats was due to adenosine accumulation, we compared the effects of DPCPX on LTP in 6-week Con-L (n = 3) and 6-week VLPO-L (n = 4) rats. We found that DPCPX (400 nM) restored LTP in 6-week VLPO-L rats (Fig. 5). In the presence of DPCPX LTP was increased in both VLPO-L and Con-L rats, but DPCPX produced a greater increase in LTP in VLPO-L than in Con-L rats. At 60 min post-tetanus the mean fEPSPs in slices from 6-week Con-L rats was 182.0 ± 11.6%, n = 7 and 145.0 ± 5.4%, n = 6 in VLPO-L rats (in DPCPX, Con-L > VLPO-L rats p = 0.02 unpaired t test). The DPCPX-mediated increase in LTP in VLPO-L rats was 8 times greater than in Con-L rats, indicating that long term potentiation of the Schaffer collateral input is inhibited by endogenous adenosine by a greater extent in VLPO-L rats than in the control group and blocking endogenous adenosine can restore LTP in VLPO-L animals.
Figure 5.
Effects of blocking adenosine A1 receptors. A, LTP in the presence of DPCPX (400 nM) in slices from Con-L and VLPO-L rats (6-week Con-L and 6-week VLPO-L rats; mean ± S.E.M. percent changes in fEPSP slope before and after HFS; n = number of recordings). Representative fEPSCs (average of 6 consecutive traces) in slices from Con-L and VLPO-L rats recorded in the presence of DPCPX (400 nM) before HSF (a) and 60 minutes after HSF (b). B, Summarized results of mean percent change in fEPSP slope (60 min after HFS) in slices from Con-L and VLPO-L rats recorded in control ACSF and in the presence of DPCPX (one-way ANOVA test between animal groups and treatments: F = 19.2, p < 0.001; Fisher's PLSD: ** Con-L (in DPCPX) > Con-L, p = 0.006 and VLPO-L (in DPCPX) > VLPO-L, p =0.001; †† Con-L (in DPCPX) > VLPO-L (in DPCPX), p = 0.003).
Discussion
We found that hippocampal in vitro LTP is reduced in proportion to the amount of sleep loss in VLPO-lesioned rats. VLPO-lesioned animals also exhibited greater endogenous adenosine inhibitory tone, which may contribute to the impairments in LTP.
VLPO-lesioned rats as a model for chronic partial sleep deprivation
In this study we used VPLO-lesioned rats as an experimental model for chronic partial sleep deprivation. Cell-body specific lesions of the VLPO nuclei have been shown to produce profound insomnia (Saper et al., 2001)(for a review). Loss of neurons in the VLPO cluster correlated closely with loss of NREM sleep and delta power (Lu et al., 2000) while loss of neurons in the extended VLPO correlated with the loss of REM sleep (Lu et al., 2002). VLPO-lesioned rats that were allowed to survive for 8 weeks appeared healthy, corticosterone levels were not elevated and did not show recovery of sleep behavior (Vetrivelan, Lu and Saper; unpublished observations). On the basis of these findings, we believe that VLPO-lesioned rats represent an ideal model system to study the cumulative effect of partial sleep deprivation over long periods and without stressful external intervention. In this regard, the VLPO lesioned animals may model voluntary sleep loss in young people, or the type of sleep loss seen in old age in humans, better than methods that rely upon external manipulation.
A disadvantage of this VLPO lesion model is that it depends on stereotaxic injections that produce lesions. The cell loss in these animals is variable in extent and location with a consequently large variation in the amount of sleep loss between individual VLPO-lesioned animals. Importantly, the variability of the lesions themselves can provide a critical anatomic control. In our analysis, we made use of this variability and correlated the amount of sleep loss with the amount of LTP to determine the decline in LTP with accumulated sleep debt.
Another limitation of the model is that the effects of the lesions themselves cannot be entirely isolated from the effects produced by the sleep deprivation. However, our experimental design incorporated several controls that indicate that the sleep loss is more likely the cause of the LTP impairment: 1) in the VLPO-lesioned rats tested at 3-weeks and 6-weeks after lesions there was a strong negative correlation between the daily total amount of sleep loss and the amount of LTP. 2) The animals which were examined at the one-week time point showed no impaired LTP and no statistically significant correlation between the amount of daily sleep loss and LTP. Because lesion takes about one week to kill the VLPO cells, these animals provided an important control because they demonstrate the acute effects of loss of VLPO neurons (as indicated both histologically and by acute sleep loss), but minimal cumulative sleep loss. 3) Between the 3 and 6-week lesion groups not only was no improvement in LTP observed, but the animals were more impaired depending upon their total cumulative sleep loss, and LTP declined over the accumulated total sleep debt following a single exponential decay. Single exponential declines in LTP were also observed with accumulated NREM and REM sleep losses showing a faster decay with accumulated REM sleep loss compared to NREM sleep loss and total sleep loss. Thus, although it cannot be excluded that the cell loss in the VLPO per se might have had an effect on the memory system, overall our results indicate that the lack of sleep these animals suffered after VLPO lesions played the causal role in the deficit of hippocampal synaptic plasticity.
Greater accumulation of extracellular endogenous adenosine in VLPO-lesioned rats
Many in vivo studies using microdialysis probes have shown that extracellular adenosine levels progressively rise during wakefulness and prolonged waking in basal forebrain, cortex and hippocampus, and gradually decline during sleep (Huston et al., 1996; Porkka-Heiskanen et al., 1997; Porkka-Heiskanen et al., 2000; Basheer et al., 2004). In the present study we examined adenosine accumulation in slices by using the increase of the fEPSPs by adenosine A1 antagonists as a measurement of tonic inhibition produced by endogenous adenosine. This method has been used to study adenosine accumulation in response to acute conditions (Latini et al., 1999; Masino & Dunwiddie, 1999; Pearson et al., 2001; Arrigoni & Rosenberg, 2006) and, as in our case, to compare adenosine levels in slices taken from animals of different treatment groups (Bauman et al., 1992; Liu et al., 2000; Rebola et al., 2003). As an example, endogenous inhibitory tone was found to be higher in hippocampal slices prepared from awake rats compared to slices taken from animals that were asleep (Liu et al., 2000). Consistent with this report, we found that the responses to adenosine A1 antagonists were greater in slices prepared from VLPO-lesioned rats compared to the sham-lesions rats suggesting a greater inhibitory tone by the endogenous adenosine signal in VLPO-lesioned rats.
One caveat of this type of experiments is that adenosine concentration in slices is highly sensitive to the experimental conditions such us temperature, hypoxia, and stimulation rate (Lloyd et al., 1993; Brundege & Dunwiddie, 1997) and thus we have taken great care to maintain these conditions constant across recordings. Temperature and flow rate in the recording chamber were carefully monitored and preparation of the slice and the recordings were all conducted at the same time of day (see Materials and Methods).
Adenosine depresses synaptic transmission by decreasing transmitter release probability, resulting in a corresponding increase in PPF (Dunwiddie & Haas, 1985; Wu & Saggau, 1994). Thus, an increase in extracellular endogenous adenosine level is associated with both an increased response to A1 and increased PPF (Tanaka et al., 2001). In general agreement with these previous studies, we found that the greater response to adenosine A1 antagonists of the VLPO-L rats was accompanied by greater PPF. Also, and similar to studies using adenosine A1 receptor knockout mice (Gimenez-Llort et al., 2005), we found that adenosine A1 antagonists significantly depressed PPF, strongly suggesting that endogenous adenosine has a major role in determining the degree of PPF. Interestingly, blocking endogenous adenosine eliminated the difference in PPF between VLPO-lesioned and sham-lesioned rats indicating that in the VLPO-L rats there was no change in neurotransmitter release probability per se. We also found no differences in the responses to exogenous adenosine. For example, adenosine reduced fEPSPs by the same extent in the two animal groups and there was no difference in adenosine-mediated PPF. These results suggest that the greater increase in fEPSPs by adenosine A1 antagonists in VLPO-lesioned rats is more consistent with greater ligand levels rather than a change in adenosine receptor function. Overall, our results suggest that adenosine may accumulate more in the VLPO-lesioned rats, but adenosine receptor sensitivity and adenosine-mediated neurotransmitter release probability are unaffected.
Extracellular adenosine levels are strictly controlled by the rate of its production and the rate of its degradation or reuptake (Fredholm et al., 2000; Dunwiddie & Masino, 2001; Basheer et al., 2004). There are indications that variation of levels or activity of adenosine metabolic enzymes and adenosine membrane transporters occur across the sleep-wake cycle and in response to sleep deprivation, possibly contributing to adenosine accumulation during waking and prolonged wakefulness (Alanko et al., 2003a; Alanko et al., 2003b; Mackiewicz et al., 2003). It is conceivable that high levels of endogenous adenosine in the in vitro slices from VLPO-lesioned rats may be the result of upregulation of adenosine synthesis or downregulation of its degradation or reuptake. This hypothesis can only be tested in experiments in which levels of adenosine and the activity of enzymes that regulate it are measured. Thus, additional experiments will be required to give a definitive answer as to whether VLPO-lesioned rats and slices taken from them have elevated adenosine levels.
Adenosine A1 antagonist restores LTP in VLPO-lesioned rats
Exogenous and endogenous adenosine are known to suppress hippocampal LTP through the activation of A1 receptors (Arai et al., 1990; de Mendonca & Ribeiro, 1990; Arai & Lynch, 1992; de Mendonca & Ribeiro, 1994; Fujii et al., 1997; Liang et al., 2008) but surprisingly, adenosine A1 receptor knockout mice have normal LTP and have normal memory functions unless they are sleep deprived (Gimenez-Llort et al., 2005; Bjorness et al., 2009). Recent studies have shown that activation of A1 receptors produces depontentiation (Staubli & Chun, 1996; Fujii et al., 1997) by PKA and p38 MAPK signaling (Huang et al., 2001; Liang et al., 2008), but the mechanism by which adenosine suppresses LTP is still not fully understood.
Our results show that adenosine antagonists restored LTP in the VLPO-lesioned rats. Although adenosine antagonists increased LTP in both VLPO-lesioned and sham-lesioned rats, this effect was much greater in the VLPO-lesioned group suggesting that the greater endogenous adenosine accumulation in VLPO-lesioned animals can account for a portion of their synaptic plasticity deficit. Thus if blocking endogenous adenosine reduced the difference in LTP between VLPO-lesioned animals and the sham-lesioned group it did not close the gap completely, suggesting additional contributing mechanisms.
Recent data have shown that inhibition of hippocampal LTP by total sleep and REM sleep deprivation is associated with downregulation of pERK (Guan et al., 2004; Ravassard et al., 2009), suppression in the expression of hippocampal plasticity-related genes (Davis et al., 2006; Guzman-Marin et al., 2006) and changes in NMDA receptor levels and subunit composition (Kopp et al., 2006; McDermott et al., 2006) shifting toward less plastic circuitry (Tang et al., 1999; Barth & Malenka, 2001). We don't know whether these changes occur in the VLPO-lesioned rats and whether they are responsible for the decreased LTP level that remains after the effect of endogenous adenosine was antagonized. This issue should be addressed in future studies.
In conclusion, our findings demonstrate that LTP of the Schaffer collateral-CA1 input is impaired in slices from rats with chronic partial sleep deprivation and LTP is impaired in proportion to the amount of sleep loss. Decreased LTP was associated with greater inhibitory tone by endogenous adenosine, presumably due to greater adenosine accumulation and not changes in adenosine receptor signaling. Hence, endogenous adenosine tone is responsible for the suppression of LTP in our chronically sleep-deprived animals and likely accounts, at least in part, for the observed deficits in synaptic plasticity.
Acknowledgements
The authors wish to thank Quan Ha for performing the animal surgeries and Dr. Patrick Fuller for his helpful comments on this work. This study was supported by NIH grants: NHLBI P50-HL60292 and NINDS R01NS061863.
Abbreviations
- ACSF
artificial cerebral spinal fluid
- AD
adenosine
- Con-L
sham-lesioned animals
- DMSO
dimethyl sulfoxide
- DPCPX
8-cyclopentyl-1,3-dipropylxanthine
- fEPSP
field excitatory postsynaptic potential
- HFS
high frequency stimulation
- LTP
long term potentiation
- NREM
non rapid eye movement sleep
- OX-SAP
orexin-saporin
- PBS
phosphate-buffered saline
- PPF
paired-pulse facilitation
- PPR
paired-pulse ratio
- REM
rapid-eye-movement
- VLPO
ventrolateral preoptic nucleus
- VLPO-L
VLPO-lesioned animals
References
- Alanko L, Heiskanen S, Stenberg D, Porkka-Heiskanen T. Adenosine kinase and 5'-nucleotidase activity after prolonged wakefulness in the cortex and the basal forebrain of rat. Neurochem Int. 2003a;42:449–454. doi: 10.1016/s0197-0186(02)00155-9. [DOI] [PubMed] [Google Scholar]
- Alanko L, Stenberg D, Porkka-Heiskanen T. Nitrobenzylthioinosine (NBMPR) binding and nucleoside transporter ENT1 mRNA expression after prolonged wakefulness and recovery sleep in the cortex and basal forebrain of rat. J Sleep Res. 2003b;12:299–304. doi: 10.1046/j.0962-1105.2003.00372.x. [DOI] [PubMed] [Google Scholar]
- Arai A, Kessler M, Lynch G. The effects of adenosine on the development of long-term potentiation. Neurosci Lett. 1990;119:41–44. doi: 10.1016/0304-3940(90)90750-4. [DOI] [PubMed] [Google Scholar]
- Arai A, Lynch G. Factors regulating the magnitude of long-term potentiation induced by theta pattern stimulation. Brain Res. 1992;598:173–184. doi: 10.1016/0006-8993(92)90181-8. [DOI] [PubMed] [Google Scholar]
- Arrigoni E, Rosenberg PA. Nitric oxide-induced adenosine inhibition of hippocampal synaptic transmission depends on adenosine kinase inhibition and is cyclic GMP independent. Eur J Neurosci. 2006;24:2471–2480. doi: 10.1111/j.1460-9568.2006.05124.x. [DOI] [PubMed] [Google Scholar]
- Barth AL, Malenka RC. NMDAR EPSC kinetics do not regulate the critical period for LTP at thalamocortical synapses. Nat Neurosci. 2001;4:235–236. doi: 10.1038/85070. [DOI] [PubMed] [Google Scholar]
- Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Prog Neurobiol. 2004;73:379–396. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Bauman LA, Mahle CD, Boissard CG, Gribkoff VK. Age-dependence of effects of A1 adenosine receptor antagonism in rat hippocampal slices. J Neurophysiol. 1992;68:629–638. doi: 10.1152/jn.1992.68.2.629. [DOI] [PubMed] [Google Scholar]
- Bjorness TE, Kelly CL, Gao T, Poffenberger V, Greene RW. Control and function of the homeostatic sleep response by adenosine A1 receptors. J Neurosci. 2009;29:1267–1276. doi: 10.1523/JNEUROSCI.2942-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundege JM, Dunwiddie TV. Role of adenosine as a modulator of synaptic activity in the central nervous system. Adv Pharmacol. 1997;39:353–391. doi: 10.1016/s1054-3589(08)60076-9. [DOI] [PubMed] [Google Scholar]
- Campbell IG, Guinan MJ, Horowitz JM. Sleep deprivation impairs long-term potentiation in rat hippocampal slices. J Neurophysiol. 2002;88:1073–1076. doi: 10.1152/jn.2002.88.2.1073. [DOI] [PubMed] [Google Scholar]
- Chen C, Hardy M, Zhang J, LaHoste GJ, Bazan NG. Altered NMDA receptor trafficking contributes to sleep deprivation-induced hippocampal synaptic and cognitive impairments. Biochem Biophys Res Commun. 2006;340:435–440. doi: 10.1016/j.bbrc.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Davis CJ, Harding JW, Wright JW. REM sleep deprivation-induced deficits in the latency-to-peak induction and maintenance of long-term potentiation within the CA1 region of the hippocampus. Brain Res. 2003;973:293–297. doi: 10.1016/s0006-8993(03)02508-3. [DOI] [PubMed] [Google Scholar]
- Davis CJ, Meighan PC, Taishi P, Krueger JM, Harding JW, Wright JW. REM sleep deprivation attenuates actin-binding protein cortactin: a link between sleep and hippocampal plasticity. Neurosci Lett. 2006;400:191–196. doi: 10.1016/j.neulet.2006.02.046. [DOI] [PubMed] [Google Scholar]
- de Mendonca A, Ribeiro JA. 2-Chloroadenosine decreases long-term potentiation in the hippocampal CA1 area of the rat. Neurosci Lett. 1990;118:107–111. doi: 10.1016/0304-3940(90)90260-g. [DOI] [PubMed] [Google Scholar]
- de Mendonca A, Ribeiro JA. Endogenous adenosine modulates long-term potentiation in the hippocampus. Neuroscience. 1994;62:385–390. doi: 10.1016/0306-4522(94)90373-5. [DOI] [PubMed] [Google Scholar]
- de Mendonca A, Ribeiro JA. Adenosine and neuronal plasticity. Life Sci. 1997;60:245–251. doi: 10.1016/s0024-3205(96)00544-9. [DOI] [PubMed] [Google Scholar]
- Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, Ott GE, Aptowicz C, Pack AI. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20:267–277. [PubMed] [Google Scholar]
- Dunwiddie TV, Haas HL. Adenosine increases synaptic facilitation in the in vitro rat hippocampus: evidence for a presynaptic site of action. J Physiol. 1985;369:365–377. doi: 10.1113/jphysiol.1985.sp015907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25:117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- Forghani R, Krnjevic K. Adenosine antagonists have differential effects on induction of long-term potentiation in hippocampal slices. Hippocampus. 1995;5:71–77. doi: 10.1002/hipo.450050109. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Arslan G, Halldner L, Kull B, Schulte G, Wasserman W. Structure and function of adenosine receptors and their genes. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:364–374. doi: 10.1007/s002100000313. [DOI] [PubMed] [Google Scholar]
- Fujii S, Sekino Y, Kuroda Y, Sasaki H, Ito K, Kato H. 8-cyclopentyltheophylline, an adenosine A1 receptor antagonist, inhibits the reversal of long-term potentiation in hippocampal CA1 neurons. Eur J Pharmacol. 1997;331:9–14. doi: 10.1016/s0014-2999(97)01024-8. [DOI] [PubMed] [Google Scholar]
- Gerashchenko D, Kohls MD, Greco M, Waleh NS, Salin-Pascual R, Kilduff TS, Lappi DA, Shiromani PJ. Hypocretin-2-saporin lesions of the lateral hypothalamus produce narcoleptic-like sleep behavior in the rat. J Neurosci. 2001;21:7273–7283. doi: 10.1523/JNEUROSCI.21-18-07273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez-Llort L, Masino SA, Diao L, Fernandez-Teruel A, Tobena A, Halldner L, Fredholm BB. Mice lacking the adenosine A1 receptor have normal spatial learning and plasticity in the CA1 region of the hippocampus, but they habituate more slowly. Synapse. 2005;57:8–16. doi: 10.1002/syn.20146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Z, Peng X, Fang J. Sleep deprivation impairs spatial memory and decreases extracellular signal-regulated kinase phosphorylation in the hippocampus. Brain Res. 2004;1018:38–47. doi: 10.1016/j.brainres.2004.05.032. [DOI] [PubMed] [Google Scholar]
- Guzman-Marin R, Ying Z, Suntsova N, Methippara M, Bashir T, Szymusiak R, Gomez-Pinilla F, McGinty D. Suppression of hippocampal plasticity-related gene expression by sleep deprivation in rats. J Physiol. 2006;575:807–819. doi: 10.1113/jphysiol.2006.115287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson JA. Sleep is of the brain, by the brain and for the brain. Nature. 2005;437:1254–1256. doi: 10.1038/nature04283. [DOI] [PubMed] [Google Scholar]
- Huang CC, Hsu KS. Progress in understanding the factors regulating reversibility of long-term potentiation. Rev Neurosci. 2001;12:51–68. doi: 10.1515/revneuro.2001.12.1.51. [DOI] [PubMed] [Google Scholar]
- Huang CC, Liang YC, Hsu KS. Characterization of the mechanism underlying the reversal of long term potentiation by low frequency stimulation at hippocampal CA1 synapses. J Biol Chem. 2001;276:48108–48117. doi: 10.1074/jbc.M106388200. [DOI] [PubMed] [Google Scholar]
- Huston JP, Haas HL, Boix F, Pfister M, Decking U, Schrader J, Schwarting RK. Extracellular adenosine levels in neostriatum and hippocampus during rest and activity periods of rats. Neuroscience. 1996;73:99–107. doi: 10.1016/0306-4522(96)00021-8. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Kanayama Y, Matsumura H, Tsuchimochi H, Ishida Y, Nakamura S. Selective rapid eye movement sleep deprivation impairs the maintenance of long-term potentiation in the rat hippocampus. Eur J Neurosci. 2006;24:243–248. doi: 10.1111/j.1460-9568.2006.04874.x. [DOI] [PubMed] [Google Scholar]
- Kim EY, Mahmoud GS, Grover LM. REM sleep deprivation inhibits LTP in vivo in area CA1 of rat hippocampus. Neurosci Lett. 2005;388:163–167. doi: 10.1016/j.neulet.2005.06.057. [DOI] [PubMed] [Google Scholar]
- Kopp C, Longordo F, Nicholson JR, Luthi A. Insufficient sleep reversibly alters bidirectional synaptic plasticity and NMDA receptor function. J Neurosci. 2006;26:12456–12465. doi: 10.1523/JNEUROSCI.2702-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latini S, Bordoni F, Pedata F, Corradetti R. Extracellular adenosine concentrations during in vitro ischaemia in rat hippocampal slices. Br J Pharmacol. 1999;127:729–739. doi: 10.1038/sj.bjp.0702591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang YC, Huang CC, Hsu KS. A role of p38 mitogen-activated protein kinase in adenosine A1 receptor-mediated synaptic depotentiation in area CA1 of the rat hippocampus. Mol Brain. 2008;1:13. doi: 10.1186/1756-6606-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu DK, Horner RL, Wojtowicz JM. Time of day determines modulation of synaptic transmission by adenosine in the rat hippocampal slices. Neurosci Lett. 2000;282:200–202. doi: 10.1016/s0304-3940(00)00881-8. [DOI] [PubMed] [Google Scholar]
- Lloyd HG, Lindstrom K, Fredholm BB. Intracellular formation and release of adenosine from rat hippocampal slices evoked by electrical stimulation or energy depletion. Neurochem Int. 1993;23:173–185. doi: 10.1016/0197-0186(93)90095-m. [DOI] [PubMed] [Google Scholar]
- Lopez J, Roffwarg HP, Dreher A, Bissette G, Karolewicz B, Shaffery JP. Rapid eye movement sleep deprivation decreases long-term potentiation stability and affects some glutamatergic signaling proteins during hippocampal development. Neuroscience. 2008;153:44–53. doi: 10.1016/j.neuroscience.2008.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Bjorkum AA, Xu M, Gaus SE, Shiromani PJ, Saper CB. Selective activation of the extended ventrolateral preoptic nucleus during rapid eye movement sleep. J Neurosci. 2002;22:4568–4576. doi: 10.1523/JNEUROSCI.22-11-04568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Greco MA, Shiromani P, Saper CB. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J Neurosci. 2000;20:3830–3842. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackiewicz M, Nikonova EV, Zimmerman JE, Galante RJ, Zhang L, Cater JR, Geiger JD, Pack AI. Enzymes of adenosine metabolism in the brain: diurnal rhythm and the effect of sleep deprivation. J Neurochem. 2003;85:348–357. doi: 10.1046/j.1471-4159.2003.01687.x. [DOI] [PubMed] [Google Scholar]
- Maquet P. The role of sleep in learning and memory. Science. 2001;294:1048–1052. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- Marks CA, Wayner MJ. Effects of sleep disruption on rat dentate granule cell LTP in vivo. Brain Res Bull. 2005;66:114–119. doi: 10.1016/j.brainresbull.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Masino SA, Dunwiddie TV. Temperature-dependent modulation of excitatory transmission in hippocampal slices is mediated by extracellular adenosine. J Neurosci. 1999;19:1932–1939. doi: 10.1523/JNEUROSCI.19-06-01932.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott CM, Hardy MN, Bazan NG, Magee JC. Sleep deprivation-induced alterations in excitatory synaptic transmission in the CA1 region of the rat hippocampus. J Physiol. 2006;570:553–565. doi: 10.1113/jphysiol.2005.093781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott CM, LaHoste GJ, Chen C, Musto A, Bazan NG, Magee JC. Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. J Neurosci. 2003;23:9687–9695. doi: 10.1523/JNEUROSCI.23-29-09687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty DJ, Sterman MB. Sleep suppression after basal forebrain lesions in the cat. Science. 1968;160:1253–1255. doi: 10.1126/science.160.3833.1253. [DOI] [PubMed] [Google Scholar]
- Nauta W. Hypothalamic regulation of sleep in rats. An experimental study. J Neurophysiol. 1946;9:285–314. doi: 10.1152/jn.1946.9.4.285. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego CA: 2007. [Google Scholar]
- Pearson T, Nuritova F, Caldwell D, Dale N, Frenguelli BG. A depletable pool of adenosine in area CA1 of the rat hippocampus. J Neurosci. 2001;21:2298–2307. doi: 10.1523/JNEUROSCI.21-07-02298.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, McCarley RW. Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: an in vivo microdialysis study. Neuroscience. 2000;99:507–517. doi: 10.1016/s0306-4522(00)00220-7. [DOI] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravassard P, Pachoud B, Comte JC, Mejia-Perez C, Scote-Blachon C, Gay N, Claustrat B, Touret M, Luppi PH, Salin PA. Paradoxical (REM) sleep deprivation causes a large and rapidly reversible decrease in long-term potentiation, synaptic transmission, glutamate receptor protein levels, and ERK/MAPK activation in the dorsal hippocampus. Sleep. 2009;32:227–240. doi: 10.1093/sleep/32.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebola N, Coelho JE, Costenla AR, Lopes LV, Parada A, Oliveira CR, Soares-da-Silva P, de Mendonca A, Cunha RA. Decrease of adenosine A1 receptor density and of adenosine neuromodulation in the hippocampus of kindled rats. Eur J Neurosci. 2003;18:820–828. doi: 10.1046/j.1460-9568.2003.02815.x. [DOI] [PubMed] [Google Scholar]
- Romcy-Pereira R, Pavlides C. Distinct modulatory effects of sleep on the maintenance of hippocampal and medial prefrontal cortex LTP. Eur J Neurosci. 2004;20:3453–3462. doi: 10.1111/j.1460-9568.2004.03808.x. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- Scammell TE, Griffin JD, Elmquist JK, Saper CB. Microinjection of a cyclooxygenase inhibitor into the anteroventral preoptic region attenuates LPS fever. Am J Physiol. 1998;274:R783–789. doi: 10.1152/ajpregu.1998.274.3.R783. [DOI] [PubMed] [Google Scholar]
- Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271:216–219. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- Siegel JM. The REM sleep-memory consolidation hypothesis. Science. 2001;294:1058–1063. doi: 10.1126/science.1063049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staubli U, Chun D. Factors regulating the reversibility of long-term potentiation. J Neurosci. 1996;16:853–860. doi: 10.1523/JNEUROSCI.16-02-00853.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickgold R, Hobson JA, Fosse R, Fosse M. Sleep, learning, and dreams: off-line memory reprocessing. Science. 2001;294:1052–1057. doi: 10.1126/science.1063530. [DOI] [PubMed] [Google Scholar]
- Stickgold R, Walker MP. Memory consolidation and reconsolidation: what is the role of sleep? Trends Neurosci. 2005;28:408–415. doi: 10.1016/j.tins.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Szymusiak R, Alam N, Steininger TL, McGinty D. Sleep-waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Res. 1998;803:178–188. doi: 10.1016/s0006-8993(98)00631-3. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Yasumoto S, Hattori G, Niiyama S, Matsuyama S, Higashi H. Mechanisms underlying the depression of evoked fast EPSCs following in vitro ischemia in rat hippocampal CA1 neurons. J Neurophysiol. 2001;86:1095–1103. doi: 10.1152/jn.2001.86.3.1095. [DOI] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Tartar JL, Ward CP, McKenna JT, Thakkar M, Arrigoni E, McCarley RW, Brown RE, Strecker RE. Hippocampal synaptic plasticity and spatial learning are impaired in a rat model of sleep fragmentation. Eur J Neurosci. 2006;23:2739–2748. doi: 10.1111/j.1460-9568.2006.04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LG, Saggau P. Adenosine inhibits evoked synaptic transmission primarily by reducing presynaptic calcium influx in area CA1 of hippocampus. Neuron. 1994;12:1139–1148. doi: 10.1016/0896-6273(94)90321-2. [DOI] [PubMed] [Google Scholar]