Abstract
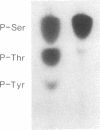
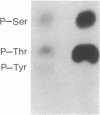
The amino acid sequence of the Alzheimer disease amyloid precursor (ADAP) has been deduced from the corresponding cDNA, and hydropathy analysis of the sequence suggests a receptor-like structure with a single transmembrane domain. The putative cytoplasmic domain of ADAP contains potential sites for serine and threonine phosphorylation. In the present study, synthetic peptides derived from this domain were used as model substrates for various purified protein kinases. Protein kinase C rapidly catalyzed the phosphorylation of a peptide corresponding to amino acid residues 645-661 of ADAP [ADAP peptide(645-661)] on Ser-655. Ca2+/calmodulin-dependent protein kinase II phosphorylated ADAP peptide (645-661) on Thr-654 and Ser-655. This peptide was virtually ineffective as a substrate for cAMP-dependent protein kinase, cGMP-dependent protein kinase, casein kinase II, or insulin receptor protein-tyrosine kinase. When a homogenate of rat cerebral cortex was used as the source of protein kinase, phosphorylation of ADAP peptide(645-661) was stimulated by calcium/phosphatidylserine/diolein to a level 4.6-fold above the basal level of phosphorylation, consistent with a prominent stimulation by protein kinase C. Using rat cerebral cortex synaptosomes prelabeled with 32Pi, a 32P-labeled phosphoprotein of approximately equal to 135 kDa was immunoprecipitated by using antisera prepared against ADAP peptide(597-624), consistent with the possibility that the holoform of ADAP in rat brain is a phosphoprotein. Based on analogy with the effect of phosphorylation by protein kinase C of juxtamembrane residues in the cytoplasmic domain of the epidermal growth factor receptor and the interleukin 2 receptor, phosphorylation of ADAP may target it for internalization.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert K. A., Nairn A. C., Greengard P. The 87-kDa protein, a major specific substrate for protein kinase C: purification from bovine brain and characterization. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7046–7050. doi: 10.1073/pnas.84.20.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beguinot L., Hanover J. A., Ito S., Richert N. D., Willingham M. C., Pastan I. Phorbol esters induce transient internalization without degradation of unoccupied epidermal growth factor receptors. Proc Natl Acad Sci U S A. 1985 May;82(9):2774–2778. doi: 10.1073/pnas.82.9.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czernik A. J., Pang D. T., Greengard P. Amino acid sequences surrounding the cAMP-dependent and calcium/calmodulin-dependent phosphorylation sites in rat and bovine synapsin I. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7518–7522. doi: 10.1073/pnas.84.21.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EIGEN M., HAMMES G. G. ELEMENTARY STEPS IN ENZYME REACTIONS (AS STUDIED BY RELAXATION SPECTROMETRY). Adv Enzymol Relat Areas Mol Biol. 1963;25:1–38. doi: 10.1002/9780470122709.ch1. [DOI] [PubMed] [Google Scholar]
- Gallis B., Lewis A., Wignall J., Alpert A., Mochizuki D. Y., Cosman D., Hopp T., Urdal D. Phosphorylation of the human interleukin-2 receptor and a synthetic peptide identical to its C-terminal, cytoplasmic domain. J Biol Chem. 1986 Apr 15;261(11):5075–5080. [PubMed] [Google Scholar]
- Glass D. B., Krebs E. G. Comparison of the substrate specificity of adenosine 3':5'-monophosphate- and guanosine 3':5'-monophosphate-dependent protein kinases. Kinetic studies using synthetic peptides corresponding to phosphorylation sites in histone H2B. J Biol Chem. 1979 Oct 10;254(19):9728–9738. [PubMed] [Google Scholar]
- Goldgaber D., Lerman M. I., McBride O. W., Saffiotti U., Gajdusek D. C. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer's disease. Science. 1987 Feb 20;235(4791):877–880. doi: 10.1126/science.3810169. [DOI] [PubMed] [Google Scholar]
- Hathaway G. M., Traugh J. A. Casein kinase II. Methods Enzymol. 1983;99:317–331. doi: 10.1016/0076-6879(83)99067-5. [DOI] [PubMed] [Google Scholar]
- Hemmings H. C., Jr, Nairn A. C., Greengard P. DARPP-32, a dopamine- and adenosine 3':5'-monophosphate-regulated neuronal phosphoprotein. II. Comparison of the kinetics of phosphorylation of DARPP-32 and phosphatase inhibitor 1. J Biol Chem. 1984 Dec 10;259(23):14491–14497. [PubMed] [Google Scholar]
- House C., Wettenhall R. E., Kemp B. E. The influence of basic residues on the substrate specificity of protein kinase C. J Biol Chem. 1987 Jan 15;262(2):772–777. [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kuenzel E. A., Mulligan J. A., Sommercorn J., Krebs E. G. Substrate specificity determinants for casein kinase II as deduced from studies with synthetic peptides. J Biol Chem. 1987 Jul 5;262(19):9136–9140. [PubMed] [Google Scholar]
- Lai Y., Nairn A. C., Greengard P. Autophosphorylation reversibly regulates the Ca2+/calmodulin-dependence of Ca2+/calmodulin-dependent protein kinase II. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4253–4257. doi: 10.1073/pnas.83.12.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. R., Chen W. S., Lazar C. S., Carpenter C. D., Gill G. N., Evans R. M., Rosenfeld M. G. Protein kinase C phosphorylation at Thr 654 of the unoccupied EGF receptor and EGF binding regulate functional receptor loss by independent mechanisms. Cell. 1986 Mar 28;44(6):839–848. doi: 10.1016/0092-8674(86)90006-1. [DOI] [PubMed] [Google Scholar]
- Nairn A. C., Greengard P. Purification and characterization of Ca2+/calmodulin-dependent protein kinase I from bovine brain. J Biol Chem. 1987 May 25;262(15):7273–7281. [PubMed] [Google Scholar]
- Pang D. T., Sharma B. R., Shafer J. A. Purification of the catalytically active phosphorylated form of insulin receptor kinase by affinity chromatography with O-phosphotyrosyl-binding antibodies. Arch Biochem Biophys. 1985 Oct;242(1):176–186. doi: 10.1016/0003-9861(85)90491-6. [DOI] [PubMed] [Google Scholar]
- Robakis N. K., Wisniewski H. M., Jenkins E. C., Devine-Gage E. A., Houck G. E., Yao X. L., Ramakrishna N., Wolfe G., Silverman W. P., Brown W. T. Chromosome 21q21 sublocalisation of gene encoding beta-amyloid peptide in cerebral vessels and neuritic (senile) plaques of people with Alzheimer disease and Down syndrome. Lancet. 1987 Feb 14;1(8529):384–385. doi: 10.1016/s0140-6736(87)91754-5. [DOI] [PubMed] [Google Scholar]
- Shackelford D. A., Trowbridge I. S. Identification of lymphocyte integral membrane proteins as substrates for protein kinase C. Phosphorylation of the interleukin-2 receptor, class I HLA antigens, and T200 glycoprotein. J Biol Chem. 1986 Jun 25;261(18):8334–8341. [PubMed] [Google Scholar]
- Tanzi R. E., Gusella J. F., Watkins P. C., Bruns G. A., St George-Hyslop P., Van Keuren M. L., Patterson D., Pagan S., Kurnit D. M., Neve R. L. Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987 Feb 20;235(4791):880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- Turner R. S., Kemp B. E., Su H. D., Kuo J. F. Substrate specificity of phospholipid/Ca2+-dependent protein kinase as probed with synthetic peptide fragments of the bovine myelin basic protein. J Biol Chem. 1985 Sep 25;260(21):11503–11507. [PubMed] [Google Scholar]
- Wang J. K., Walaas S. I., Greengard P. Protein phosphorylation in nerve terminals: comparison of calcium/calmodulin-dependent and calcium/diacylglycerol-dependent systems. J Neurosci. 1988 Jan;8(1):281–288. doi: 10.1523/JNEUROSCI.08-01-00281.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]