Abstract
We used diffusion tensor imaging to investigate fractional anisotropy (FA), a measure of fiber tract integrity, in attention-deficit hyperactivity disorder (ADHD). Using a tract-based atlasing approach on six-direction diffusion tensor imaging data, we examined FA within the cingulum, corpus callosum, corticospinal tract, fornix, optic radiations, superior longitudinal fasciculus, uncinate fasciculus, and the superior and inferior occipitofrontal fasciculi in an all-male sample of17 children and adolescents with ADHD and 16 age-matched controls. ADHD patients had significantly lower FA in the corticospinal tract (P=0.02) and the superior longitudinal fasciculus (P=0.017) compared with controls. Results support that disruptions in motor and attentional networks may contribute toward ADHD pathophysiology. Future research may clarify how ADHD subtype and psychiatric comorbidities affect diffusion measures.
Keywords: corticospinal tract, diffusion tensor imaging, fractional anisotropy, superior longitudinal fasciculus
Introduction
Attention-deficit hyperactivity disorder (ADHD) is characterized by inattentiveness, hyperactivity, impulsivity, and cognitive deficits [1]. Disruptions in neural circuits mediating attention, motor behaviors, and executive function are thought to contribute to ADHD pathophysiology. Structural imaging studies report reduced white matter volumes [2,3], midsagittal corpus callosum areas [4], and cortical thickness [5] in ADHD patients compared with controls. Fiber tract disturbances, however, are better addressed by diffusion tensor imaging (DTI). From DTI data, one can compute fractional anisotropy (FA), which reflects the directionality of water diffusion through tissue [6]. FA values range from 0 to 1 and are higher in more organized white matter fibers where myelinated tracts restrict diffusion. Higher FA is thus associated with greater fiber integrity.
White matter tracts connecting frontocortical regions and the striatum are thought to be involved in executive functioning as well as motor and oculomotor behaviors [7,8], and are thus implicated in ADHD. Still, few studies have examined FA within major white matter tracts in ADHD. In a voxel-based DTI study, below-normal white matter FA was seen in right premotor, right striatal, right cerebral peduncle, left middle cerebellar peduncle, left cerebellum, and left parieto-occipital regions in children with ADHD [7]. In adults with childhood ADHD, below-normal FA was reported in the cingulum bundle and superior longitudinal fasciculus [9].
Discrepancies in earlier findings may be attributable to sample heterogeneity or differing analysis strategies. Voxel-based analyses of DTI, for example, are sensitive to potential registration confounds. We used a tract-based atlasing method to examine changes in white matter circuitry in children and adolescents with ADHD. By averaging FA within specific regions of interest (ROIs), this method is less influenced by small errors in registration and provides a potential increase in statistical power by comparing larger ROIs. As ADHD remains relatively underexplored with DTI, we applied a broad field mapping approach to investigate FA in nine major white matter tracts in ADHD patients and controls. Based on earlier findings [9], we expected to see lower FA in the cingulum and superior longitudinal fasciculus in ADHD.
Methods
Patients
We scanned 17 patients with ADHD and 16 typically developing controls, all male and matched for age (Table 1). Most patients were included in an earlier study on callosal thickness in ADHD [5]. ADHD and controls were recruited from local clinics, schools, and health organizations. Additional controls were recruited from ongoing studies of normal development at the University of California, Los Angeles (UCLA) [10]. Written informed assent from the patient and consent from his or her guardian was obtained after explaining experimental protocols. The UCLA Institutional Review Board approved all experimental procedures.
Table 1.
Patient demographic information
| Control group | ADHD group | Significance | |
|---|---|---|---|
| Age in years (mean±SD) | 11.72±2.48 | 11.96±2.32 | P=0.778, F=0.081 |
| Handedness (right/left) | 15/1 | 16/1 | P=0.965, χ2=0.002 |
| Ethnicity (black/hispanic/white/unknown) | 2/5/5/4 | 11/6/0/0 | P=0.002, χ2=15.305 |
| WISC-III full estimated IQ (mean±SD) | 103.35±10.09 | 92.25±14.55 | P=0.008, F=7.861 |
| ADHD subtype (combined/hyperactive-impulsive/inattentive/NOS/unknown) | – | 1/9/5/1/1 | – |
| Hyperactive-impulsive symptoms score (SNAP-IV; mean±SD) | – | 6.3±3.0 | – |
| Inattentive symptoms score (SNAP-IV; mean±SD) | – | 8.7±0.6 | – |
ADHD, attention-deficit hyperactivity disorder; WISC-III, Wechsler Intelligence Scale for Children, Third Edition; SNAP-IV, Swanson, Nolan, and Pelham, version IV.
ADHD diagnosis was confirmed by parental interview using Diagnostic and Statistical Manual Of Mental Disorder-IV criteria for ADHD, the NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV) [11], and obtaining a score >1.5 SD from the mean on the Swanson, Nolan, and Pelham, version IV Rating Scale for Inattention or Hyperactive-Impulsive Scales [12]. Five patients had been taking stimulant medication at time of study, including Concerta (methylphenidate HCl, 18 mg; n=1), Ritalin (methylphenidate, 5 mg; n=1), Dexadrine (dextroamphetamine, 20 mg; n=1), Adderall (dextroamphetamine/racemic DL-amphetamine salts, 15 mg; n=1), and Straterra (atomoxetine, 20 mg; n=1). ADHD patients ceased medication for >24 h before scanning and were excluded for taking any nonstimulant psychotropic medication. Patients with co-occurring syndromes such as fragile X, tuberous sclerosis, or generalized resistance to thyroid hormone were excluded as well. Ten ADHD patients presented with comorbid oppositional defiant disorder (ODD). Four of these 10 patients also had comorbid conduct disorder, enuresis, separation anxiety, and motor tic, respectively.
Controls were required to be free from any major Axis I mental disorder as assessed by the DISC interview [11]. Controls with a history of serious illness or closed head trauma were excluded. Children with weights or heights <5th or >95th percentile were excluded. Intelligence was estimated using the Block Design and Vocabulary subtests of the Wechsler Intelligence Scale for Children, Third Edition (WISC-III, 1991; Table 1) [13].
Image acquisition and preprocessing
Spin-echo echo-planar DTI was performed with a 1.5 Tesla Siemens Sonata MRI scanner (Erlangen, Germany) using six noncollinear directions of diffusion sensitization (b=1000 s/mm2) with one nondiffusion weighted acquisition (b=0 s/mm2). The DTI pulse sequence used dual bipolar diffusion gradient pulses and a double spin echo to suppress eddy current distortions. Fifty brain images (3 × 3 × 3 mm3 spatial resolution) oriented parallel to the anterior and posterior commissure line were acquired for each direction, and the entire acquisition was repeated four times. T1-weighted structural images were acquired using a three-dimensional sagittal spoiled gradient echo pulse sequence (TR=24 ms, TE=12.6 ms, flip angle=22°, acquisition matrix 256 × 196, FOV=240 × 240 mm2, in-plane voxel size=1.3 × 0.9 mm2, slice thickness=1.2 mm, two averages). Structural images were corrected for intensity inhomogeneities [14] and manually skull-stripped for use in registration procedures with DTI data.
We computed the diffusion tensor within each voxel according to the methods of Basser et al. [6] using software developed at the UCLA Laboratory of Neuro Imaging. Six-parameter rigid body registrations between the b0 image and the images from each noncollinear direction at b=1000 s/mm2 were performed within each patient to correct for minor head movements. FA measures were derived from the diffusion tensor [6]. FA images were skull-stripped using rigid body registered masks from T1 data.
Nine white matter ROIs from the SPM Anatomy Toolbox [15] (http://www.fz-juelich.de/inb/inb-3/spm_anatomy_toolbox) were registered to each patient’s FA map using Automated Image Registration with a 12-parameter affine registration followed by a second order (30-parameter) through fourth order (105-parameter) nonlinear warp (Fig. 1) [16]. ROIs included the cingulum, corpus callosum, corticospinal tract, fornix, optic radiations, superior longitudinal fasciculus, uncinate fasciculus, and the superior and inferior occipitofrontal fasciculi. The cerebellum was not included in all scans and thus was not analyzed. FA was averaged within each ROI without separating hemispheres.
Fig. 1.
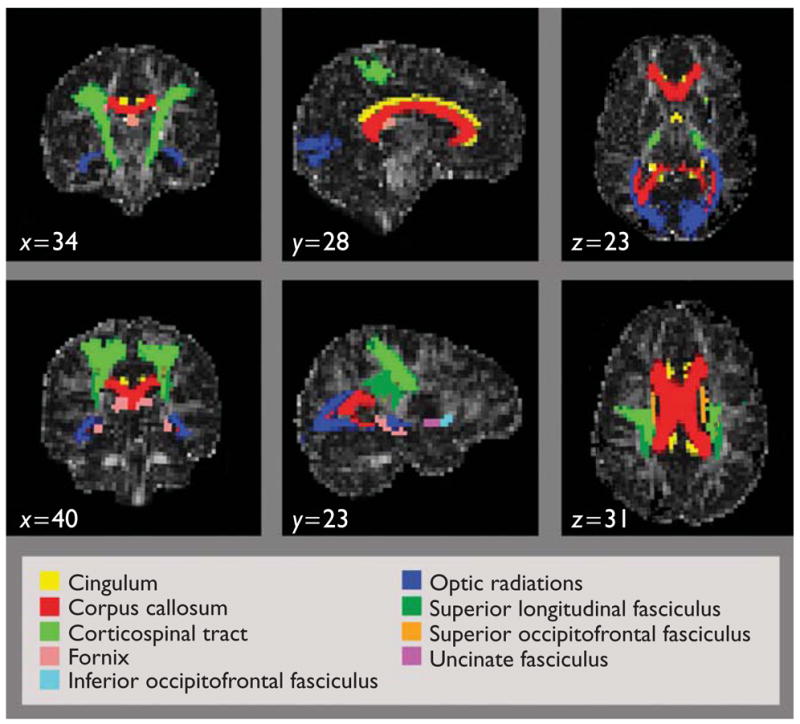
Registered white matter tract regions of interest from the SPM Anatomy Toolbox overlaid on a control’s fractional anisotropy image (coronal, sagittal, and axial views).
Statistical analyses
We tested for group differences in FA using univariate analyses of variance with diagnosis as a fixed factor, mean FA within each ROI as the dependent variable, and age as a covariate. In a post-hoc analysis, previously medicated ADHD patients were removed from the analysis. Correlation analyses between FA in each ROI and hyperactivity scores (Swanson, Nolan, and Pelham, version IV; Table 1) were also performed, controlling for age. Given the paucity of studies analyzing FA in ADHD, a two-tailed α level of P<0.05 was considered significant.
Results
FA values in each ROI did not deviate from normality (Kolmogorov–Smirnov Test; P>0.5 for all white matter ROIs). ADHD patients showed significantly lower FA compared with controls in the corticospinal tract [F(1,32)=6.058, P=0.02] and the superior longitudinal fasciculus [F(1,32)=6.377, P=0.017] (Table 2). No other ROIs showed significant FA differences across groups. Removing medicated ADHD patients did not change the presence or directionality of effects, and no significant correlations were observed between hyperactivity scores and FA.
Table 2.
Mean fractional anisotropy (FA) values (mean±SD) for white matter ROIs in ADHD versus controls, residualized for age
| Tract | Mean FA controls | Mean FA ADHD | F | Significance (Pvalue) |
|---|---|---|---|---|
| Cingulum | 0.28±0.05 | 0.28±0.07 | 0.000 | 0.999 |
| Corpus callosum | 0.41±0.05 | 0.39±0.04 | 0.732 | 0.399 |
| Corticospinal tract | 0.36±0.03 | 0.33±0.05 | 6.058 | 0.020** |
| Fornix | 0.30±0.09 | 0.31±0.09 | 0.255 | 0.617 |
| Inferior occipitofrontal Fasciculus | 0.33±0.06 | 0.34±0.08 | 0.137 | 0.714 |
| Optic radiation | 0.30±0.04 | 0.28±0.03 | 3.069 | 0.090 |
| Superior longitudinal Fasciculus | 0.39±0.06 | 0.34±0.06 | 6.377 | 0.017** |
| Superior occipitofrontal fasciculus | 0.29±0.05 | 0.31±0.06 | 0.639 | 0.430 |
| Uncinate fasciculus | 0.24±0.05 | 0.26±0.06 | 0.334 | 0.567 |
ADHD, attention-deficit hyperactivity disorder; ROIs, regions of interest.
Significant.
Discussion
We detected below-normal FA in ADHD patients in the corticospinal tract and the superior longitudinal fasciculus, white matter tracts involved in motor and attentional circuits. Below-normal FA has been observed in the superior longitudinal fasciculus in adults with childhood ADHD, and may be related to impaired attention and executive function [9]. Casey et al. [8] found that FA in frontostriatal white matter tracts (possibly including fibers from the superior longitudinal fasciculus) was positively correlated with task performance in a go/no-go task. Our finding could reflect below-normal myelination of the superior longitudinal fasciculus or higher than normal disorganization of fibers within the tract, resulting in impaired intracortical communication. Lower FA in the corticospinal tract could be related to observed abnormalities in motor inhibition in ADHD or dysregulation of dopamine in downstream pathways [17]. This may also be consistent with findings of reduced FA in ADHD patients in the cerebral peduncle, which includes part of the corticospinal tract [7]. Below-normal FA in the left corticospinal tract is shown to correlate with lower IQ [18], which is part of the clinical presentation of ADHD.
To our knowledge, this is the first study to use a validated white matter atlas to examine FA in children with ADHD. Our findings may differ from Ashtari et al. [7] because of differing analysis strategies or heterogeneous sample characteristics (e.g. their study included males and females). By averaging across voxels in an ROI, we may have had more power to detect FA changes across regions, but may have missed smaller, regionally localized effects.
Limitations
Although using more than six directions of diffusion sensitization is now advocated for precise FA determination [19], bias or imprecision in FA values resulting from the use of only six directions of diffusion sensitization would affect FA measures in controls and ADHD patients equally and thus cannot lead to false positive findings. Our multiple repeated image acquisitions enhanced the precision of the FA measures, and statistically significant FA differences between ADHD and controls were detected, despite the intrinsic precision limitations imposed by the six-direction procedure.
ADHD frequently cooccurs with other psychiatric disorders, including ODD [20]. Our sample size was not sufficient to address the effects of ODD or ADHD subtypes separately. Five of the 17 ADHD children in our sample had received stimulant medication, but removing these patients did not change the observed effects. Although ADHD is more prevalent in males, subsequent studies may assess whether differing rates of myelination during development in males and females influence results [21]. Finally, white matter ROIs were unavailable for the cerebellum, a region widely implicated in ADHD [7]. Despite these limitations, our research reveals important information to drive future tractography studies investigating attentional circuits in ADHD.
Conclusion
This study supports that motor and attentional networks are selectively disturbed in ADHD. Reduced FA in the corticospinal tract and the superior longitudinal fasciculus may contribute to symptoms of reduced attention, impulsivity, and hyperactivity in the disorder.
Acknowledgments
This work was supported by the National Center for Research Resources (P41 RR13642), the NIH Roadmap for Medical Research, Grant U54 RR021813, entitled Center for Computational Biology (CCB), grant P50 MH073466-01, and a Career Development Award (KO1 MH073990 to K.L.N.).
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 2.Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, et al. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. J Am Med Assoc. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- 3.Filipek PA, Semrud-Clikeman M, Steingard RJ, Renshaw PF, Kennedy DN, Biederman J. Volumetric MRI analysis comparing subjects having attention-deficit hyperactivity disorder with normal controls. Neurology. 1997;48:589–601. doi: 10.1212/wnl.48.3.589. [DOI] [PubMed] [Google Scholar]
- 4.Krain AL, Castellanos FX. Brain development and ADHD. Clin Psychol Rev. 2006;26:433–444. doi: 10.1016/j.cpr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Luders E, Narr KL, Hamilton LS, Phillips OR, Thompson PM, Valle JS, et al. Decreased callosal thickness in attention deficit/hyperactivity disorder (ADHD) Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.08.027. http://www.journals.elsevierhealth.com/periodicals/bps/content/0800343abs. [DOI] [PMC free article] [PubMed]
- 6.Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashtari M, Kumra S, Bhaskar SL, Clarke T, Thaden E, Cervellione KL, et al. Attention-deficit/hyperactivity disorder: a preliminary diffusion tensor imaging study. Biol Psychiatry. 2005;57:448–455. doi: 10.1016/j.biopsych.2004.11.047. [DOI] [PubMed] [Google Scholar]
- 8.Casey BJ, Epstein JN, Buhle J, Liston C, Davidson MC, Tonev ST, et al. Frontostriatal connectivity and its role in cognitive control in parent-child dyads with ADHD. Am J Psychiatry. 2007;164:1729–1736. doi: 10.1176/appi.ajp.2007.06101754. [DOI] [PubMed] [Google Scholar]
- 9.Makris N, Buka SL, Biederman J, Papadimitriou GM, Hodge SM, Valera EM, et al. Attention and executive systems abnormalities in adults with childhood ADHD: a DT-MRI study of connections. Cereb Cortex. 2008;18:1210–1220. doi: 10.1093/cercor/bhm156. [DOI] [PubMed] [Google Scholar]
- 10.Evans AC. The NIH MRI study of normal brain development. Neuroimage. 2006;30:184–202. doi: 10.1016/j.neuroimage.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 11.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Swanson J. School-based assessments and interventions for ADD students. Irvine, California: K C Press; 1992. [Google Scholar]
- 13.Wechsler D. Wechsler Intelligence Scale for Children. 3. San Antonio, TX: Psychological Corp; 1991. [Google Scholar]
- 14.Shattuck DW, Sandor-Leahy SR, Schaper KA, Rottenberg DA, Leahy RM. Magnetic resonance image tissue classification using a partial volume model. Neuroimage. 2001;13:856–876. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- 15.Burgel U, Amunts K, Hoemke L, Mohlberg H, Gilsbach JM, Zilles K. White matter fiber tracts of the human brain: three-dimensional mapping at microscopic resolution, topography and intersubject variability. Neuroimage. 2006;29:1092–1105. doi: 10.1016/j.neuroimage.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 16.Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC. Automated image registration: II. Intersubject validation of linear and nonlinear models. J Comput Assist Tomogr. 1998;22:153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- 17.Heijtz RD, Kolb B, Forssberg H. Motor inhibitory role of dopamine D1 receptors: implications for ADHD. Physiol Behav. 2007;92:155–160. doi: 10.1016/j.physbeh.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 18.Yu C, Li J, Liu Y, Qin W, Li Y, Shu N, et al. White matter tract integrity and intelligence in patients with mental retardation and healthy adults. Neuroimage. 2008;40:1533–1541. doi: 10.1016/j.neuroimage.2008.01.063. [DOI] [PubMed] [Google Scholar]
- 19.Jones DK. The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: a Monte Carlo study. Magn Reson Med. 2004;51:807–815. doi: 10.1002/mrm.20033. [DOI] [PubMed] [Google Scholar]
- 20.Pliszka SR. Comorbidity of attention-deficit/hyperactivity disorder with psychiatric disorder: an overview. J Clin Psychiatry. 1998;59 (Suppl 7):50–58. [PubMed] [Google Scholar]
- 21.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]


