Abstract
Dentate granule cell axon (mossy fiber) sprouting is a common abnormality in patients with temporal lobe epilepsy. Mossy fiber sprouting creates an aberrant positive-feedback network among granule cells that does not normally exist. Its role in epileptogenesis is unclear and controversial. If it were possible to block mossy fiber sprouting from developing after epileptogenic treatments, its potential role in the pathogenesis of epilepsy could be tested. Previous attempts to block mossy fiber sprouting have been unsuccessful. The present study targeted the mammalian target of rapamycin (mTOR) signaling pathway, which regulates cell growth and is blocked by rapamycin. Rapamycin was focally, continuously, and unilaterally infused into the dorsal hippocampus for prolonged periods beginning within hours after rats sustained pilocarpine-induced status epilepticus. Infusion for 1 month reduced aberrant Timm staining (a marker of mossy fibers) in the granule cell layer and molecular layer. Infusion for 2 months inhibited mossy fiber sprouting more. However, after rapamycin infusion ceased, aberrant Timm staining developed and approached untreated levels. When onset of infusion began after mossy fiber sprouting had developed for 2 months, rapamycin did not reverse aberrant Timm staining. These findings suggest that inhibition of the mTOR signaling pathway suppressed development of mossy fiber sprouting. However, suppression required continual treatment, and rapamycin treatment did not reverse already established axon reorganization.
Introduction
Temporal lobe epilepsy is common and difficult to treat, and its pathogenesis remains unclear (Engel et al., 1997). Aberrant sprouting of granule cell axons (mossy fibers) is evident in the dentate gyrus of many patients with temporal lobe epilepsy (de Lanerolle et al., 1989; Sutula et al., 1989; Houser et al., 1990; Babb et al., 1991) and in different laboratory animal models of epilepsy (Nadler et al., 1980; Sutula et al., 1988; Mello et al., 1993; Qiao and Noebels, 1993; Ribak et al., 1998; Nissinen et al., 2000; Kharatishvili et al., 2006; Kadam and Dudek, 2007). The role of mossy fiber sprouting in generation of spontaneous seizures is controversial. It has been proposed as a compensatory mechanism that restores excitatory synaptic input to inhibitory interneurons (Sloviter, 1992; Sloviter et al., 2006). However, sprouted mossy fibers predominantly synapse with granule cells (Buckmaster et al., 2002), resulting in a positive-feedback circuit (Lynch and Sutula, 2000; Scharfman et al., 2003) that may reduce seizure threshold (Tauck and Nadler, 1985; Wuarin and Dudek, 1996). It would be useful experimentally, and perhaps therapeutically, to selectively block mossy fiber sprouting after epileptogenic injuries. Prior attempts neutralized nerve growth factor with antibodies (Holtzman and Lowenstein, 1995), inhibited neural activity with tetrodotoxin (Buckmaster, 2004b), blocked protein synthesis with cycloheximide around the time of epileptogenic injury (Williams et al., 2002; Toyoda and Buckmaster, 2005), and targeted growth cone function by inhibiting calcineurin with cyclosporin A and FK506 or L-type calcium channels with nicardipine (Ingram et al., 2009), but none prevented mossy fiber sprouting (cf. Moriwaki et al., 1996; Longo and Mello, 1997, 1998; Ikegaya, 1999; Ikegaya et al., 2000).
Mammalian target of rapamycin (mTOR) is an evolutionarily conserved serine/threonine kinase that plays a central role in control of cell growth (Schmelzle and Hall, 2000). It is expressed in many tissues, including brain (Kim et al., 2002), where its activation is controlled by a variety of extracellular signals, including glutamate (Cammalleri et al., 2003; Hou and Klann, 2004; Gong et al., 2006) and BDNF (Takei et al., 2004). The mTOR signaling pathway has been reviewed (Harris and Lawrence, 2003; Swiech et al., 2008). Briefly, ligands bind receptors and activate phosphoinositide 3-kinase, which activates the protein kinase Akt, which modulates activity of proteins involved in tuberous sclerosis (TSC1 and TSC2) and the small guanosine triphosphatase, Rheb, which is immediately upstream of mTOR. mTOR, when coupled with other proteins, inhibits 4EBP1, which is a translation repressor. mTOR also activates S6K-1, which phosphorylates ribosomal protein S6, resulting in increased translation.
Rapamycin specifically inhibits mTOR (Sabatini et al., 1994). Rapamycin is anticonvulsant in phosphatase and tensin homolog (PTEN)-deficient mice (Kwon et al., 2003) and antiepileptogenic in a rodent model of tuberous sclerosis (Zeng et al., 2008). Traumatic brain injury activates the mTOR signaling pathway (Chen et al., 2007) and can cause mossy fiber sprouting and epilepsy (Kharatishvili et al., 2006). Thus, previous studies suggest that mossy fiber sprouting may result, in part, from activation of the mTOR signaling pathway. In some neuronal types, rapamycin inhibits regeneration (Verma et al., 2005; Park et al., 2008) and guidance of axonal growth cones (Campbell and Holt, 2001). The present study addressed the following questions. Is the mTOR pathway activated when mossy fiber sprouting occurs after status epilepticus? Does prolonged, continuous, focal infusion of rapamycin suppress development of mossy fiber sprouting? If so, does mossy fiber sprouting remain suppressed after rapamycin treatment ceases? And does rapamycin infusion reverse previously established mossy fiber sprouting?
Materials and Methods
All experiments were approved by the Stanford University Institutional Animal Care and Use Committee and performed in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals. Pilocarpine treatment was performed as described previously (Buckmaster, 2004a). Briefly, male Sprague Dawley rats (34 ± 1 d old; range 27–45) were treated with atropine methylbromide (5 mg/kg, i.p.), then 20 min later with pilocarpine hydrochloride (380 mg/kg, i.p.) to induce status epilepticus. All chemicals were from Sigma-Aldrich unless specified otherwise. After 2 h of status epilepticus, convulsions were suppressed with diazepam (10 mg/kg, i.p., repeated as needed; Hospira), and lactated Ringer's solution was administered subcutaneously. All rats in this study experienced at least 2 h of status epilepticus.
Osmotic pumps and cannulae were implanted to focally and continuously deliver rapamycin (Alexis Biochemicals or LC Laboratories) to the dorsal, left dentate gyrus. Rats were anesthetized with 2% isoflurane (Baxter). Body temperature was monitored and controlled with a heating pad with feedback control. Rats were placed in a stereotaxic apparatus, and their head and dorsal neck were prepared for aseptic surgery. A scalp incision was made, and a <1-mm-diameter hole was drilled through the skull, 4.6 mm caudal and 2.8 mm left of bregma. A 3.5-mm-long 28 gauge cannula (Alzet brain infusion kit II; Durect Corporation) was inserted and secured to the skull with cranioplastic cement and a jeweler's screw. An osmotic pump (model 2004; Durect Corporation) and tubing leading to the cannula were implanted subcutaneously over the back. The manufacturer's specifications indicate that at body temperature (37°C), model 2004 pumps deliver 0.25 ± 0.05 μl/h for at least 28 d. Pumps contained vehicle solution consisting of 50% DMSO, 15% ethanol, and 0.1% fluorescein (Invitrogen) with or without rapamycin (0.01–10 mm). In most experiments, infusions began 1–8 h after administering the first dose of diazepam, which was 2 h after onset of status epilepticus. In one experiment, surgery and rapamycin infusion was delayed until 2 months after status epilepticus. Rapamycin was infused for 1 or 2 months. For 2-month-long infusions, pumps were replaced after 1 month. In most experiments, rats were perfused immediately after 1- or 2-month-long infusions. In one experiment, perfusion was delayed until 2 months after rapamycin infusion ceased.
Rats were perfused and hippocampi sectioned and processed as described previously (Buckmaster, 2004b). Briefly, rats were killed by urethane overdose (2 g/kg, i.p.), perfused through the ascending aorta at 30 ml/min for 2 min with 0.9% sodium chloride, 5 min with 0.37% sodium sulfide, 1 min with 0.9% sodium chloride, and 30 min with 4% paraformaldehyde in 0.1 m phosphate buffer (PB, pH 7.4). Brains postfixed overnight at 4°C. Then, both hippocampi were isolated, cryoprotected in 30% sucrose in 0.1 m PB, gently straightened, frozen, and sectioned transversely with a microtome set at 30 μm. Starting at a random point near the septal pole, a 1-in-6 series of sections from each hippocampus was mounted, dried, and developed for 45 min in 120 ml of 50% gum arabic, 20 ml of 2 m citrate buffer, 60 ml of 0.5 m hydroquinone, and 1 ml of 19% silver nitrate. Infused and contralateral noninfused hippocampi from the same animal developed together. An adjacent 1-in-6 series of sections was processed for Nissl staining with 0.25% thionin. A third 1-in-6 series was mounted, dried, coverslipped with Vectashield (Vector Laboratories), and checked for fluorescein labeling to verify delivery of pump contents in infused hippocampi.
Mossy fiber sprouting was measured as described previously (Buckmaster and Dudek, 1997; Buckmaster, 2004b). Briefly, sections were analyzed using a light microscope equipped with a 10× objective, Lucivid, and Neurolucida software (MBF Bioscience). An investigator blind to the rats' treatment made contours around the granule cell layer + molecular layer and the Timm-positive parts of the granule cell layer + molecular layer. Figure 1 illustrates a typical example of contours drawn on a Timm-stained section of dentate gyrus. Density of Timm staining within Timm-positive contours was measured using identical camera and microscope settings for all samples. Brightness was measured and expressed on an optical density scale from 0 to 1, in which 0 was the minimal density value when no tissue was in the light path, and 1 was the maximal density value when the microscope light source was turned off. Extent of mossy fiber sprouting varies among individual rats treated systemically with chemoconvulsants (Buckmaster and Dudek, 1997). To avoid this potential confound, the contralateral noninfused hippocampus served as a control in each rat.
Figure 1.

Aberrant mossy fiber sprouting was measured by drawing contours around the entire granule cell layer (g) + molecular layer (m) (magenta line) and the Timm-positive part (cyan line) (A). h, Hilus; CA3, CA3 pyramidal cell layer. B, Isolated outlines with Timm-positive contour filled. Areas were recorded, and the percentage of the entire granule cell layer + molecular layer that was Timm positive was calculated.
To measure activation of the mTOR pathway, ribosomal protein S6 was evaluated, which is a downstream phosphorylation target of the mTOR signaling pathway (Chung et al., 1992). In an experiment to measure mTOR activity after status epilepticus, male Sprague Dawley rats (41 d old) were vehicle or pilocarpine treated, as described above. Tissue was collected for Western blot analysis 24 h or 7 d later. Control groups consisted of vehicle-treated rats (n = 5) and pilocarpine-treated rats that did not develop status epilepticus (n = 6). Control tissue was collected 24 h after treatment. Tissue was collected from pilocarpine-treated rats that experienced status epilepticus 24 h (n = 6) or 7 d previously (n = 6). To collect tissue, rats were killed by urethane overdose (2 g/kg, i.p.) and decapitated. Brains were quickly removed from the skull, and both hippocampi were immediately isolated and frozen in liquid nitrogen and preserved at −80°C. Tissue samples (25 mg) were homogenized at 4°C in 150 μl of lysis buffer consisting of 62.5 mm Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 50 mm DTT, and 0.01% bromophenol blue. Samples were sonicated for 15 s, and aliquots were stored at −80°C. Expression levels of total ribosomal protein S6 and phosphorylated ribosomal protein S6 were measured by Western blotting. Isolated protein samples were heated to 95–100°C for 5 min, chilled on ice, and centrifuged. Isolated proteins (3 μl, ∼35 μg) were diluted and loaded on 4–20% SDS-polyacrylamide gels and electrophoresed at 20 mA/gel for 110 min, before being transferred onto pure nitrocellulose membranes (Schleicher and Schuell Bioscience) at 200 mA for 108 min. Blotted nitrocellulose membranes were blocked with freshly prepared Tris-buffered saline with 0.1% Tween 20 (TBS/T) containing 5% nonfat milk for 1 h, then incubated in rabbit anti-phospho-ribosomal protein S6 (Ser235/236) serum (1:900; Cell Signaling Technology) in TBS/T containing 5% bovine serum albumin overnight with agitation at 4°C. After a wash, nitrocellulose membranes incubated in anti-rabbit horseradish peroxidase-linked conjugate (1:5000; GE Healthcare) in TBS/T for 50 min at room temperature with agitation. Nitrocellulose membranes then were washed with TBS/T. ECL Western blotting detection reagents and autoradiography film (GE Healthcare) were used to detect bands. After completing analysis of phospho-S6 bands, blotted nitrocellulose membranes were washed in Restore Western blot stripping buffer (Pierce Biotechnology) for 15 min at room temperature and incubated with anti-total ribosomal protein S6 serum (1:2500; 5G10, Cell Signaling Technology) using methods described above. Phosphorylated S6 and total S6 levels were quantified by densitometry using NIH Image software. Ratiometric data for each animal, consisting of duplicate sample tubes, were averaged together.
In an experiment to test the effect of rapamycin infusion on mTOR activity in the hippocampus close to the infusion site, six naive male Sprague Dawley rats (35 d old) were implanted for rapamycin infusion as described above. After 14 d of infusion, rats were killed by urethane overdose (2 g/kg, i.p.) and decapitated. Brains were quickly removed from the skull, placed in a chilled rat brain matrix (ASI Instruments), and blocked coronally to isolate a 3-mm-thick slice that contained the infusion site. On a chilled platform, infused and contralateral hippocampi were isolated from slices, immediately frozen in liquid nitrogen, and preserved at −80°C. Sample processing and Western analysis were performed as described above.
Values are reported as mean ± SEM. Statistics were performed using SigmaStat (Systat). A value of p < 0.05 was considered significant.
Results
Status epilepticus activates the mTOR pathway
To test whether status epilepticus activated the mTOR signaling pathway in hippocampus, a downstream target of the pathway was evaluated. S6K-1, which is phosphorylated and activated by mTOR, in turn, phosphorylates ribosomal protein S6 (Chung et al., 1992), which is a commonly used readout of mTOR activity (Kwon et al., 2003; Huang et al., 2007; Meikle et al., 2008; Park et al., 2008; Zeng et al., 2008). Hippocampal tissue was evaluated in rats 24 h (n = 6) and 7 d (n = 6) after pilocarpine-induced status epilepticus. Control groups consisted of vehicle-treated rats (n = 5) and pilocarpine-treated rats that did not develop status epilepticus (n = 6). Western blot analysis was used to measure ratios of phospho-S6 to total S6 (Fig. 2). Results from the two control groups were similar (0.28 ± 0.04 vs 0.30 ± 0.11 for vehicle- and pilocarpine-treated controls, respectively, p = 0.62, t test), so they were combined. At 24 h and 7 d after status epilepticus, average phospho-S6-to-total-S6 ratios were greater than twice control values (p < 0.05, Kruskal–Wallis ANOVA on ranks). These findings suggest that status epilepticus activates the mTOR signaling pathway.
Figure 2.
The mTOR signaling pathway in hippocampus was activated by status epilepticus and inhibited by prolonged infusion of rapamycin. Ribosomal protein S6 is phosphorylated by the mTOR signaling pathway. Ratios of phospho-S6 to total S6 were measured by Western blot analysis to evaluate activity of the mTOR pathway. Each lane is a different sample, and all bands were at 32 kDa. A, Ratios were similar in vehicle-treated (n = 5) and pilocarpine-treated (n = 6) controls. Ratios 24 h (n = 6) and 7 d after status epilepticus (n = 6) were significantly greater than controls (C) (*p < 0.05, Kruskal–Wallis ANOVA on ranks). B, After 14 d of continuous infusion of 10 mm rapamycin, S6 phosphorylation was reduced compared with contralateral hippocampal tissue. C, Average ratios of phospho-S6 to total S6 in infused versus noninfused, contralateral hippocampi were significantly different (**p = 0.004, paired t test, n = 6). Error bars indicate SEM.
Rapamycin infusion inhibited the mTOR signaling pathway in hippocampus
Osmotic pumps continuously delivered rapamycin to a focal region of the left, dorsal dentate gyrus, which was compared with the contralateral noninfused region. Vehicle contained fluorescein, and sections were examined to verify fluorescence in infused hippocampi. Fluorescence was clearly evident in sections near sites of infusion, but not in corresponding sections from contralateral hippocampi (Fig. 3). In three rats, fluorescence was not observed, and they were excluded from analysis. These findings suggest that in all rats included in this study, pumps delivered their contents, which was concentrated in the infused but not contralateral dentate gyrus.
Figure 3.
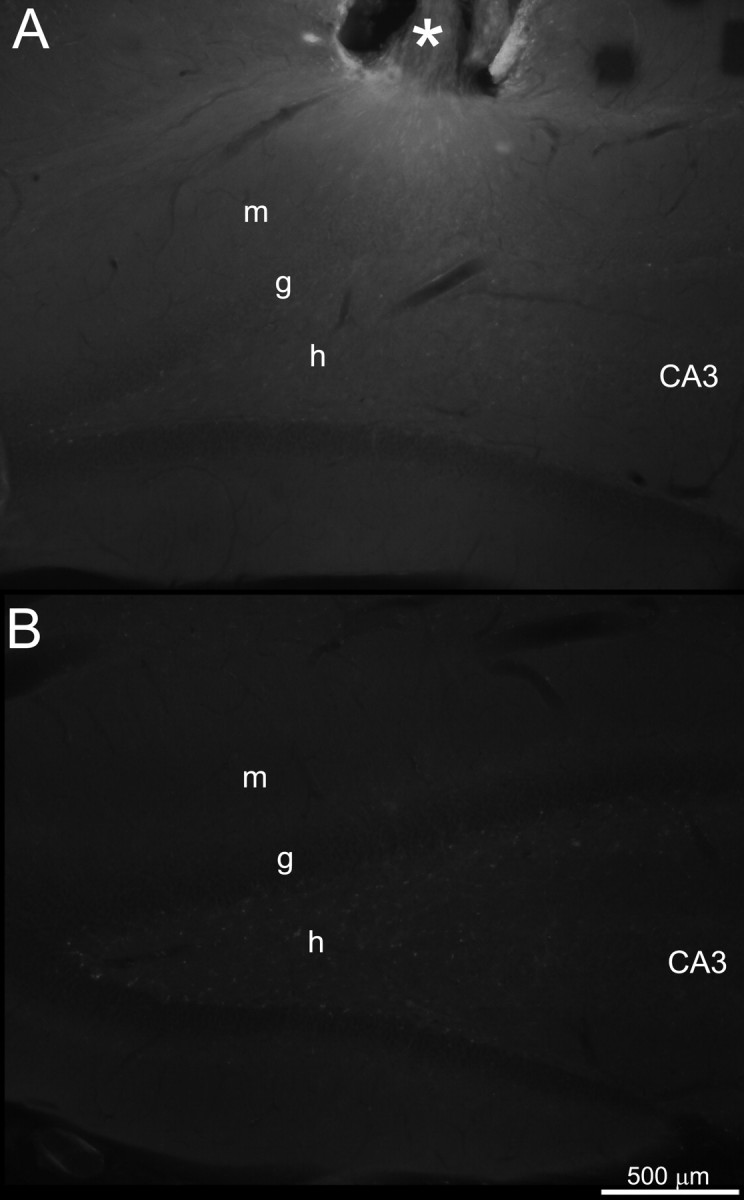
Fluorescence verified delivery of osmotic pump contents to infused but not contralateral dentate gyrus. A, Left, dorsal dentate gyrus of a rat infused for 1 month with 0.01 mm rapamycin dissolved in vehicle containing 0.1% fluorescein. The asterisk indicates the cannula site. B, Corresponding septotemporal level of right, contralateral, noninfused dentate gyrus. Images were acquired with identical microscope and camera settings. m, Molecular layer; g, granule cell layer; h, hilus; CA3, CA3 pyramidal cell layer.
Inhibition of the mTOR signaling pathway would be expected to reduce levels of phospho-S6. Using the same rapamycin-infusion methods as used for experiments in which Timm staining was measured, six rats were treated with 10 mm rapamycin for 14 d. Subjects were naive, not pilocarpine treated, but we expect both groups to respond similarly. Hippocampal tissue (3 mm thick, anterior–posterior) was isolated, which included dentate gyrus, CA3, CA1, and subiculum and contained the infusion site. The same region was isolated from contralateral hippocampi. Western blot analysis was used to measure ratios of phospho-S6 to total S6 in infused and contralateral noninfused hippocampi (Fig. 2). Ratios of phospho-S6 to total S6 were lower in infused versus contralateral hippocampi in all individual animals. Group averages were significantly different (0.58 ± 0.05 vs 0.92 ± 0.09, p = 0.004, paired t test). These findings suggest that the rapamycin treatment protocol used in this study partially suppressed the mTOR pathway in hippocampi near infusion sites.
Extent of inhibition is likely to be greatest near sites of infusion, where rapamycin concentration is likely to be highest with this delivery method (Sendelbeck and Urquhart, 1985). In the present Western blot analysis, samples included all parts of hippocampus, not just dentate gyrus, and 3-mm-thick sections were collected to provide sufficient material for analysis. This sampling method may have diluted focal rapamycin-infusion effects. Results, therefore, may underestimate inhibition of the mTOR pathway in regions closest to infusion sites.
Rapamycin infusion for 1 month suppressed mossy fiber sprouting
Mossy fiber sprouting develops gradually over a period of weeks to months after pilocarpine-induced status epilepticus (Mello et al., 1993). By 1 month after status epilepticus, mossy fiber sprouting is developed to a sufficient baseline level for quantitative analysis (Buckmaster, 2004b). In eight rats, 10 mm rapamycin was infused continuously and focally into the left, dorsal dentate gyrus for 1 month after status epilepticus. Extent of aberrant Timm staining was measured as the percentage of the area of the granule cell layer + molecular layer that was Timm positive. In sections ±200 μm along the septotemporal axis from the infusion site, percentage of the granule cell layer + molecular layer that was Timm positive was less than that of the corresponding region in contralateral noninfused hippocampi (Fig. 4). Inhibition of aberrant Timm staining appeared maximal closest to infusion sites. In each animal, extent of aberrant Timm staining was calculated by averaging values of the three sections closest to infusion sites and the corresponding three sections in the contralateral noninfused hippocampus. In all rats, hippocampi infused with 10 mm rapamycin for 1 month displayed less aberrant Timm staining than contralateral noninfused hippocampi. Group averages were significantly different (22.7 ± 2.1 vs 27.2 ± 1.9%, p = 0.001, paired t test).
Figure 4.
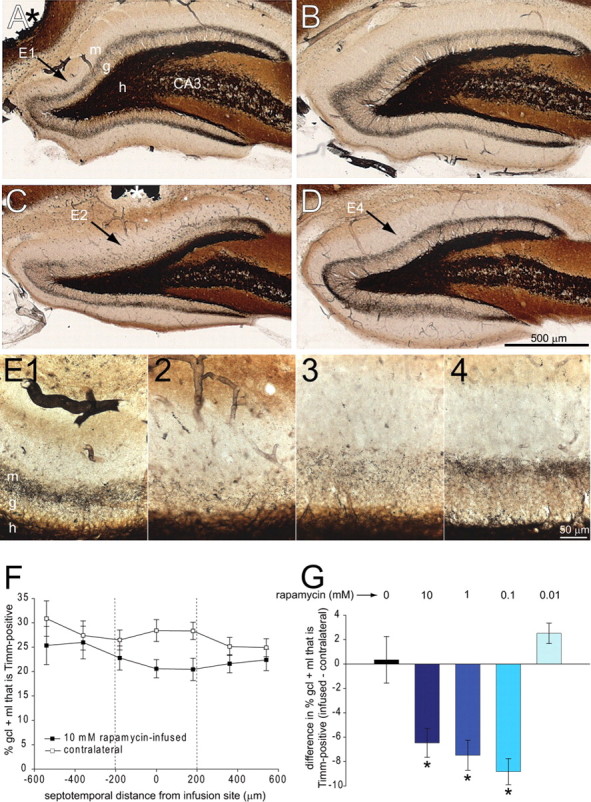
Prolonged, 1 month infusion of rapamycin reduced aberrant Timm staining. Timm stained dentate gyrus in vehicle-infused (A) and contralateral noninfused (B) hippocampus and in 10 mm rapamycin-infused (C) and contralateral noninfused (D) hippocampus. Asterisks indicate infusion sites (A, C). g, Granule cell layer; m, molecular layer; h, hilus; CA3, CA3 pyramidal cell layer. E, Higher-magnification views reveal less aberrant Timm staining in rapamycin-infused regions. E1, Vehicle-infused hippocampus (arrow in A). E2, Rapamycin-infused hippocampus at septotemporal level of cannula (arrow in C). E3, Rapamycin-infused hippocampus at 180 μm toward temporal pole of hippocampus from section shown in C and E2. E4, Contralateral noninfused hippocampus (arrow in D). F, The average percentage of area of the granule cell layer (gcl) + molecular layer (ml) that is Timm positive versus section position along the septotemporal axis relative to infusion site in rats infused with 10 mm rapamycin (n = 8). Infusion site = 0. The septal pole is to the left (negative values). The temporal pole is to the right (positive values). G, Difference in the percentage of the granule cell layer + molecular layer that is Timm positive in infused minus contralateral noninfused hippocampi. Negative values indicate reduced aberrant Timm staining in infused hippocampi. Values calculated in individual animals by averaging results of the three sections closest to the infusion site (between dashed vertical lines in F) and corresponding sections in the contralateral hippocampus. Hippocampi infused with 10 mm (n = 8), 1 mm (n = 4), and 0.1 mm (n = 3) rapamycin displayed less aberrant Timm staining than those infused with 0.01 mm rapamycin (n = 4) or vehicle alone (n = 6) (*p < 0.05, ANOVA with Bonferroni t test). Error bars indicate SEM.
To test whether suppression of aberrant Timm staining was specific to rapamycin, a range of concentrations was tested. Identical infusion methods were used to test 1 mm rapamycin (n = 4), 0.1 mm (n = 3), 0.01 mm (n = 4), and vehicle alone (n = 6). In each animal, average percentage of the granule cell layer + molecular layer that was Timm positive was calculated for infused and contralateral hippocampi, as described above. Then, the difference was calculated by subtracting the average value of the contralateral hippocampus from the average of the infused hippocampus. Accordingly, negative values indicate reduced aberrant Timm staining in infused hippocampi. Group averages for 10 mm, 1 mm, 0.1 mm, 0.01 mm, and vehicle alone were −6.5 ± 1.2, −7.5 ± 1.2, −8.8 ± 1.1, 2.5 ± 0.8, and 0.3 ± 1.9%, respectively. ANOVA revealed a significant difference between groups (p < 0.001). Pairwise multiple comparisons revealed significantly less aberrant Timm staining in hippocampi infused with 10, 1, or 0.1 mm rapamycin (no significant differences between these three groups) compared with 0.01 or 0 mm rapamycin (no significant differences between these two groups) (p < 0.05, Bonferroni t test). These findings suggest that infusion of rapamycin, but not vehicle alone, suppresses mossy fiber sprouting.
Rapamycin infusion for 2 months suppressed mossy fiber sprouting more
Development of mossy fiber sprouting is incomplete 1 month after pilocarpine-induced status epilepticus (Mello et al., 1993). To test whether longer treatment would further suppress mossy fiber sprouting, three rats were infused with 10 mm rapamycin for 2 months (Fig. 5). Infusion methods were identical to those described above, except that osmotic pumps were replaced after 1 month, which is the limit of their delivery duration. All rats displayed less aberrant Timm staining in infused than in contralateral noninfused hippocampi. The average difference in percentage of the granule cell layer + molecular layer that was Timm positive (infused − contralateral) was −11.8 ± 1.6%, which was greater than the average difference after only 1 month infusion with 10 mm rapamycin (−6.5 ± 1.2%, p = 0.04, unpaired t test). These findings suggest that longer treatment with rapamycin suppressed mossy fiber sprouting more.
Figure 5.

Longer rapamycin infusion suppressed mossy fiber sprouting more, but the effect reversed after infusion ceased. Timm stained dentate gyrus after 2 months of continuous infusion with 10 mm rapamycin (A) and contralateral noninfused hippocampus (B). The asterisk indicates the infusion site. Arrows indicate reduced Timm staining in the granule cell layer (g) and molecular layer (m) in the rapamycin-infused hippocampus. h, Hilus; CA3, CA3 pyramidal cell layer. Timm stained dentate gyrus after 2 months of continuous infusion with 10 mm rapamycin followed by 2 more months without infusion (C) and contralateral noninfused hippocampus (D). E, Experimental time line indicating duration of treatment (colored horizontal bars) and timing of perfusion (red “x”). F, Difference in the percentage of the granule cell layer (gcl) + molecular layer (ml) that is Timm positive in infused minus contralateral noninfused hippocampi. Negative values indicate reduced aberrant Timm staining in infused hippocampi. Values were calculated in individual animals by averaging results of the three sections closest to the infusion site and corresponding sections in the contralateral hippocampus. The color of the vertical bars corresponds to the color of the horizontal bars in E, which indicate experimental group. Aberrant Timm staining was reduced more in rats infused with 10 mm rapamycin for 2 months (n = 3) than in those infused only 1 month (n = 8, p = 0.04, unpaired t test) and those infused for 2 months but then allowed to survive another 2 months after infusion ceased (n = 4, p = 0.01, unpaired t test). Results from vehicle-infused rats (n = 6) are displayed for comparison. Error bars indicate SEM.
Suppression of mossy fiber sprouting diminished after rapamycin infusion stopped
To test whether rapamycin infusion permanently suppressed mossy fiber sprouting, four rats were infused with 10 mm rapamycin for 2 months and then survived 2 more months after pump removal. Infusion methods were identical to those described above, except that pumps were removed and tubing sealed after 2 months of infusion, and rats were perfused 2 months later. Intense, diffuse fluorescence was not evident as it was in rats perfused immediately after infusion. Instead, moderate levels of particulate fluorescence were observed in all rats (data not shown). These findings suggest that fluorescein had been delivered and largely but incompletely cleared over the 2 months after pump removal. The average percentage of the granule cell layer + molecular layer that was Timm positive in sections within ±200 μm of infusion sites was similar to that in contralateral noninfused hippocampi (30.5 ± 2.5 vs 32.2 ± 1.7%, respectively, p = 0.42, paired t test) (Fig. 5). The average difference in percentage of the granule cell layer + molecular layer that was Timm positive (infused − contralateral) in rats that survived 2 months beyond a 2 month period of 10 mm rapamycin infusion was significantly less than that of rats perfused immediately after 2 months infusion with 10 mm rapamycin (−1.7 ± 1.8 vs −11.8 ± 1.6%, respectively, p = 0.01, unpaired t test). These findings suggest that suppression of mossy fiber sprouting by rapamycin is not permanent and instead may require continuous treatment.
Rapamycin infusion did not reverse established mossy fiber sprouting
To test whether rapamycin could reverse mossy fiber sprouting after it had developed, infusion began 2 months after status epilepticus and lasted 1 month (n = 6). The average percentage of the granule cell layer + molecular layer that was Timm positive in sections within ±200 μm of infusion sites was similar to that in contralateral noninfused hippocampi (29.2 ± 2.5 vs 29.7 ± 2.5%, respectively, p = 0.72, paired t test) (Fig. 6). The average difference in percentage of the granule cell layer + molecular layer that was Timm positive (infused − contralateral) in rats treated for 1 month with 10 mm rapamycin after a latency of 2 months was significantly less than that of rats treated beginning immediately after status epilepticus (−0.3 ± 0.9 vs −6.5 ± 1.2%, respectively, p = 0.002, unpaired t test). These findings suggest that rapamycin infusion did not reverse mossy fiber sprouting after it had developed.
Figure 6.

Rapamycin infusion did not reverse mossy fiber sprouting after it had developed. Timm stained dentate gyrus after 1 month infusion with 10 mm rapamycin, beginning 2 months after status epilepticus (A) and contralateral noninfused hippocampus (B). The asterisk indicates the infusion site. m, Molecular layer; g, granule cell layer; h, hilus; CA3, CA3 pyramidal cell layer. C, Experimental time line indicating duration of treatment (colored horizontal bars) and timing of perfusion (red “x”). D, Difference in the percentage of the granule cell layer (gcl) + molecular layer (ml) that is Timm positive in infused minus contralateral noninfused hippocampi. Negative values indicate reduced aberrant Timm staining in infused hippocampi. Values were calculated in individual animals by averaging results of the three sections closest to the infusion site and corresponding sections in the contralateral hippocampus. The color of the vertical bars corresponds to the color of the horizontal bars in C, which indicate experimental group. Aberrant Timm staining is reduced more in rats when 10 mm rapamycin is infused beginning immediately after status epilepticus (n = 8) than in those in which onset of infusion was delayed 2 months (n = 6, p = 0.002, unpaired t test). Results from vehicle-infused rats (n = 6) are displayed for comparison. Error bars indicate SEM.
Rapamycin infusion did not prevent status epilepticus-induced hilar neuron loss
Extent of aberrant Timm staining correlates with extent of hilar neuron loss (Babb et al., 1991; Masukawa et al., 1996; Buckmaster and Dudek, 1997; Nissinen et al., 2001; Jiao and Nadler, 2007), and rapamycin has neuroprotective effects (Alirezaei et al., 2008; Pan et al., 2008). These findings raise the possibility that rapamycin might have protected hilar neurons from status epilepticus-induced excitotoxicity and thereby indirectly reduced mossy fiber sprouting. To avoid this potential confounding factor, rapamycin infusion did not begin until at least 1 h after suppression of status epilepticus with diazepam. Nevertheless, to test whether hilar neurons were spared in rapamycin-infused hippocampi, numbers of hilar neuron profiles were counted in the three sections closest to infusion sites and corresponding three sections in contralateral noninfused hippocampi. Average values from each hippocampus were calculated. Then, averages for experimental groups were calculated (Fig. 7). Average numbers of hilar neuron profiles were slightly and consistently lower in infused versus contralateral noninfused hippocampi (ANOVA, p = 0.006). The effect was apparent in all experimental groups, including those infused with only 0.01 mm rapamycin or vehicle alone. These findings suggest that hilar neuron loss was slightly more severe in infused hippocampi, regardless of whether rapamycin was present. Perhaps surgical implantation of cannulae contributed to hilar neuron loss. These findings also suggest that rapamycin infusion did not prevent status epilepticus-induced hilar neuron loss. Therefore, suppression of mossy fiber sprouting is not likely attributable to a secondary effect on hilar neuron survival.
Figure 7.
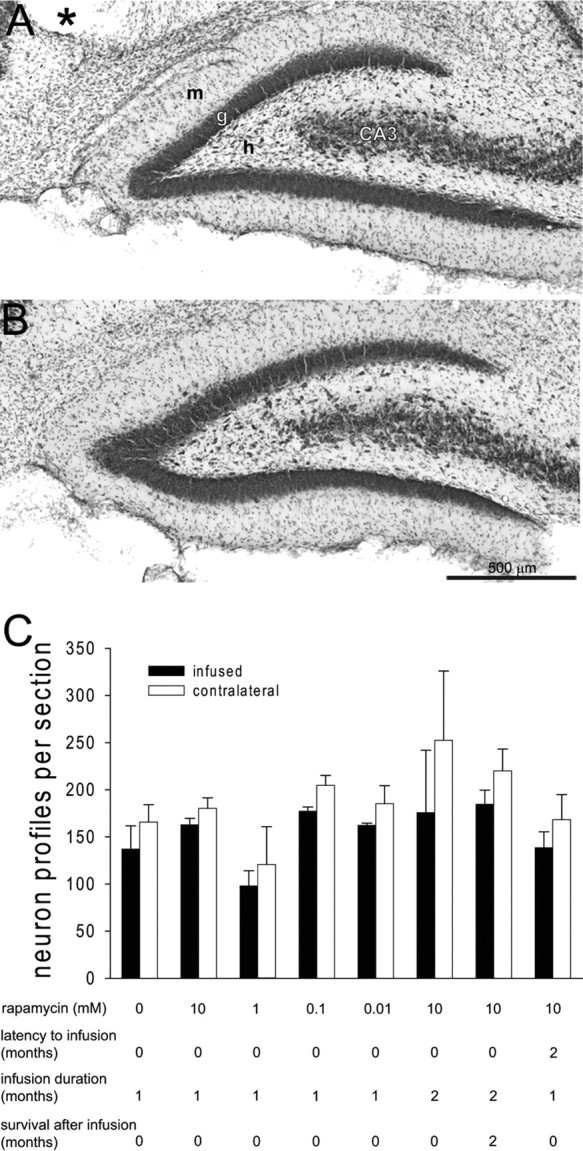
Rapamycin infusion did not protect hilar neurons from status epilepticus-induced loss. Nissl stained dentate gyrus after 1 month infusion with 10 mm rapamycin (A) and contralateral noninfused hippocampus (B). The asterisk indicates the infusion site. m, Molecular layer; g, granule cell layer; h, hilus; CA3, CA3 pyramidal cell layer. C, The average number of hilar neuron profiles per section in the three sections closest to the infusion site and corresponding sections of contralateral hippocampus for all experimental groups. The number of hilar neuron profiles was slightly and consistently lower in infused than in contralateral hippocampi (p = 0.006, ANOVA), regardless of experimental group. Error bars indicate SEM.
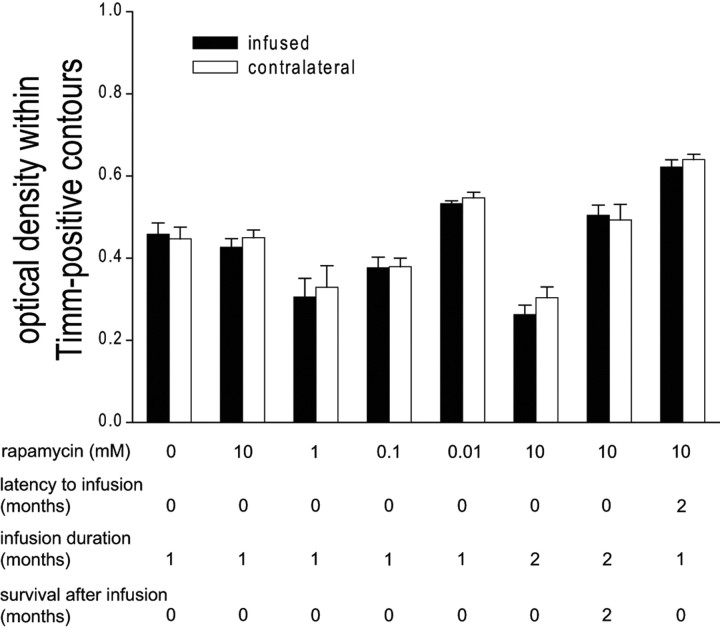
Another potential confound is density of aberrant Timm staining. To test whether intensities of Timm staining in the granule cell layer + molecular layer were different in infused versus noninfused contralateral hippocampi, optical densities within Timm-positive contours were measured (Fig. 8). Densities of aberrant Timm staining were similar within outlined areas of infused versus noninfused contralateral hippocampi (ANOVA, p = 0.4), including groups infused with maximal doses of rapamycin and maximal durations. These findings suggest that reduced areas of aberrant Timm staining in rapamycin-infused hippocampi were not compensated by more intense sprouting.
Figure 8.
Density of staining within Timm-positive contours was not more intense in rapamycin-infused hippocampi. The average optical density (0, no tissue in light path; 1, microscope light source turned off) within Timm-positive contours in three sections within ±200 μm of infusion site and corresponding sections of contralateral noninfused hippocampus for all experimental groups is shown. Within each experimental group, densities of aberrant Timm staining were similar in infused and contralateral, noninfused hippocampi (ANOVA, p = 0.4). Error bars indicate SEM.
Discussion
The principal findings of the present study are the following. Prolonged, continuous, focal infusion of rapamycin suppressed development of aberrant mossy fiber sprouting in a rat model of temporal lobe epilepsy. Longer infusions suppressed sprouting more. Sprouting that had been suppressed initially with rapamycin developed after infusion ceased, and rapamycin failed to reverse established sprouting. These findings suggest that the mTOR signaling pathway may be a useful target for reducing granule cell axon reorganization after epileptogenic injuries.
Status epilepticus activates the mTOR pathway
Pilocarpine was used to cause status epilepticus, which resulted in development of mossy fiber sprouting. Oxotremorine, another muscarinic acetylcholine receptor agonist, acutely increases phosphorylation of S6-kinase, and to a lesser extent mTOR, in hippocampus, but levels return to baseline by 16 h (Deguil et al., 2008). In the present study, there was no significant difference in levels of phosphorylated S6 in pilocarpine- versus vehicle-treated controls 24 h after treatment. On the other hand, pilocarpine-treated rats that experienced status epilepticus displayed significantly elevated levels of phosphorylated S6 24 h and 7 d after treatment. These findings suggest that the mTOR signaling pathway is activated by status epilepticus, but not by pilocarpine alone, during the period when mossy fibers sprout. Similarly, traumatic brain injury activates the mTOR pathway (Chen et al., 2007) and can cause mossy fiber sprouting (Golarai et al., 2001; Kharatishvili et al., 2006).
Rapamycin suppressed mossy fiber sprouting
In the present study, granule cell axon terminals were identified by black Timm staining. The Timm staining protocol generates opaque silver particles at sites at which heavy metals are concentrated (Haug, 1967). The Timm stain is a specific marker for granule cell axons because mossy fibers concentrate zinc in synaptic vesicles (Wenzel et al., 1997). Did rapamycin artifactually interfere with Timm staining instead of suppressing mossy fiber sprouting? This is unlikely, because Timm staining was reduced specifically in regions of sprouting, not in adjacent areas, including hilus and stratum lucidum of CA3. Furthermore, when infusion was delayed until after mossy fiber sprouting developed, Timm staining in the granule cell layer and molecular layer was intense, despite rapamycin treatment. In the present study, mossy fiber sprouting was quantified by drawing contours around Timm-positive areas within the granule cell layer + molecular layer. Timm-positive areas consist of many small, black spots, and defining borders of those areas can be subjective. To avoid bias, investigators were blind to experimental groups. Similar optical densities within Timm-positive regions of infused versus contralateral hippocampi in each experimental group support the validity of comparing relative areas of Timm-positive regions within the granule cell layer and molecular layer.
Suppression of mossy fiber sprouting appeared to be specific to rapamycin treatment. Sprouting occurred at untreated levels when vehicle or low-dose rapamycin was infused and was suppressed by higher doses. Rapamycin is a specific inhibitor of mTOR complex 1 (Loewith et al., 2002), which is part of a signaling pathway that phosphorylates and activates ribosomal protein S6 (Chung et al., 1992). Reduced phospho-S6 levels after infusion of 10 mm rapamycin are consistent with inhibition of mTOR complex 1. Prolonged rapamycin treatment can sequester mTOR and indirectly inhibit mTOR complex 2 (Sarbassov et al., 2006), which is part of another signaling pathway that controls cell proliferation and survival through the actin cytoskeleton and Akt/PKB (Jacinto et al., 2004). The present study cannot distinguish between rapamycin's effects on mTOR complex 1 and its effects on mTOR complex 2. Despite mTOR's role in cell survival, rapamycin infusion did not spare hilar neurons from excitotoxicity in the present study, probably because infusion began after rats had experienced status epilepticus.
Hilar neuron loss was slightly more severe in infused than in noninfused hippocampi. However, the effect was not specific to rapamycin. All groups displayed the difference, even those infused with little or no rapamycin. Slightly more severe hilar neuron loss in infused hippocampi might be attributable to surgical placement of cannulae. Nevertheless, rapamycin's suppression of mossy fiber sprouting was not attributable to hilar neuron survival.
Rapamycin binds FK506 binding protein 12 (Heitman et al., 1991), which is part of yet another signaling pathway that affects activity of calcineurin, a calcium-activated phosphatase that has been proposed to play a role in mossy fiber sprouting (Moriwaki et al., 1996). However, this mechanism is unlikely to account for rapamycin's effect, because infusion of FK506, an inhibitor of calcineurin, fails to suppress mossy fiber sprouting (Ingram et al., 2009). Rapamycin impairs potentiation of excitatory synapses (Casadio et al., 1999; Tang et al., 2002) and can reduce network excitability (Rüegg et al., 2007), suggesting that it might have suppressed mossy fiber sprouting indirectly by affecting excitability or synapse strength. This possibility seems unlikely, however, because prolonged infusion of the sodium channel blocker tetrodotoxin, which reduces neuronal activity substantially (Galvan et al., 2000), fails to suppress mossy fiber sprouting (Buckmaster, 2004b).
Findings of the present study suggest that rapamycin suppressed development of mossy fiber sprouting in a rodent model of temporal lobe epilepsy. These findings also suggest that targeting the mTOR signaling pathway may be useful for testing the role of mossy fiber sprouting on epileptogenesis. However, rapamycin also impairs long-term potentiation (Casadio et al., 1999; Tang et al., 2002) and learning (Dash et al., 2006; Parsons et al., 2006), alters neuronal expression of transporters (Li et al., 2006), voltage-gated channels (Raab-Graham et al., 2006), and ligand-gated channels (Sabatini et al., 1999; Wang et al., 2006), inhibits dendritic growth (Jaworski et al., 2005; Kumar et al., 2005; Huang et al., 2007), and changes dendritic spine morphology (Tavazoie et al., 2005). Therefore, the mTOR signaling pathway's effects on epileptogenesis and seizure threshold may be complex.
Mossy fiber sprouting may be permanent once established
The hypothesis that rapamycin might reverse already established mossy fiber sprouting was motivated by a report that mTOR inhibition reverses dentate granule cell hypertrophy in adult PTEN-deficient mice (Kwon et al., 2003). However, in the present study, 1 month treatment with rapamycin was insufficient to significantly reduce already established aberrant Timm staining. Although these findings do not exclude the possibility that longer treatments may have been more effective, they suggest that mossy fiber sprouting may be irreversible. Similarly, in rodent models of tuberous sclerosis, rapamycin administered after completion of neuronal differentiation failed to reverse abnormally oriented neocortical dendrites (Meikle et al., 2008), and beneficial effects of rapamycin, including reduced seizure frequency and increased survival, require continued long-term treatment and reverse after treatment stops (Zeng et al., 2008). In PTEN-deficient mice, dentate granule cell hypertrophy resumes after mTOR inhibition stops (Kwon et al., 2003). These results are not surprising, because genetic disease models involve mutations that persist after rapamycin treatment ends. In the present study, continuous rapamycin treatment was required, which suggests that signals that promote mossy fiber sprouting may be persistent and, unfortunately, not transiently expressed only during a critical window during which time targeted, temporary treatment could permanently block axon reorganization.
Mossy fiber sprouting is resilient
Mossy fiber sprouting is a common abnormality in patients, occurs in many laboratory animal models of epilepsy, and has resisted previous attempts to suppress it (see Introduction). In the present study, mossy fiber sprouting was reduced but not blocked entirely by rapamycin, even at high concentrations. These findings suggest redundancy in signaling pathways, such that inhibition of a single important node may partially suppress but not abrogate axon sprouting (Bromberg et al., 2008). More complete blockade of mossy fiber sprouting may require targeting additional steps in the mTOR cascade and in other signaling pathways.
Footnotes
This work was supported by National Institutes of Health (National Institute of Neurological Disorders and Stroke and National Center for Research Resources).
References
- Alirezaei M, Kiosses WB, Flynn CT, Brady NR, Fox HS. Disruption of neuronal autophagy by infected microglia results in neurodegeneration. PLoS ONE. 2008;3:e2906. doi: 10.1371/journal.pone.0002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb TL, Kupfer WR, Pretorius JK, Crandall PH, Levesque MF. Synaptic reorganization by mossy fibers in human epileptic fascia dentata. Neuroscience. 1991;42:351–363. doi: 10.1016/0306-4522(91)90380-7. [DOI] [PubMed] [Google Scholar]
- Bromberg KD, Ma'ayan A, Neves SR, Iyengar R. Design logic of a cannabinoid receptor signaling network that triggers neurite outgrowth. Science. 2008;320:903–909. doi: 10.1126/science.1152662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS. Laboratory animal models of temporal lobe epilepsy. Comp Med. 2004a;54:473–485. [PubMed] [Google Scholar]
- Buckmaster PS. Prolonged infusion of tetrodotoxin does not block mossy fiber sprouting in pilocarpine-treated rats. Epilepsia. 2004b;45:452–458. doi: 10.1111/j.0013-9580.2004.67103.x. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Dudek FE. Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats. J Comp Neurol. 1997;385:385–404. [PubMed] [Google Scholar]
- Buckmaster PS, Zhang GF, Yamawaki R. Axon sprouting in a model of temporal lobe epilepsy creates a predominantly excitatory feedback circuit. J Neurosci. 2002;22:6650–6658. doi: 10.1523/JNEUROSCI.22-15-06650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammalleri M, Lütjens R, Berton F, King AR, Simpson C, Francesconi W, Sanna PP. Time-restricted role for dendritic activation of the mTOR-p70S6K pathway in the induction of late-phase long-term potentiation in the CA1. Proc Natl Acad Sci U S A. 2003;100:14368–14373. doi: 10.1073/pnas.2336098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DS, Holt CE. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32:1013–1026. doi: 10.1016/s0896-6273(01)00551-7. [DOI] [PubMed] [Google Scholar]
- Casadio A, Martin KC, Giustetto M, Zhu H, Chen M, Bartsch D, Bailey CH, Kandel ER. A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell. 1999;99:221–237. doi: 10.1016/s0092-8674(00)81653-0. [DOI] [PubMed] [Google Scholar]
- Chen S, Atkins CM, Liu CL, Alonso OF, Dietrich WD, Hu BR. Alterations in mammalian target of rapamycin signaling pathways after traumatic brain injury. J Cereb Blood Flow Metab. 2007;27:939–949. doi: 10.1038/sj.jcbfm.9600393. [DOI] [PubMed] [Google Scholar]
- Chung J, Kuo CJ, Crabtree GR, Blenis J. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell. 1992;69:1227–1236. doi: 10.1016/0092-8674(92)90643-q. [DOI] [PubMed] [Google Scholar]
- Dash PK, Orsi SA, Moore AN. Spatial memory formation and memory-enhancing effect of glucose involves activation of the tuberous sclerosis complex-mammalian target of rapamycin pathway. J Neurosci. 2006;26:8048–8056. doi: 10.1523/JNEUROSCI.0671-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguil J, Perault-Pochat MC, Chavant F, Lafay-Chebassier C, Faucconneau B, Pain S. Activation of the protein p70S6K via ERK phosphorylation by cholinergic muscarinic receptors stimulation in human neuroblastoma cells and in mice brain. Toxicol Lett. 2008;182:91–96. doi: 10.1016/j.toxlet.2008.08.012. [DOI] [PubMed] [Google Scholar]
- de Lanerolle NC, Kim JH, Robbins RJ, Spencer DD. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res. 1989;495:387–395. doi: 10.1016/0006-8993(89)90234-5. [DOI] [PubMed] [Google Scholar]
- Engel J, Jr, Williamson PD, Wieser HG. Mesial temporal lobe epilepsy. In: Engel J Jr, Pedley TA, editors. Epilepsy: a comprehensive textbook. Philadelphia: Lippincott-Raven; 1997. pp. 2417–2426. [Google Scholar]
- Galvan CD, Hrachovy RA, Smith KL, Swann JW. Blockade of neuronal activity during hippocampal development produces a chronic focal epilepsy in the rat. J Neurosci. 2000;20:2904–2916. doi: 10.1523/JNEUROSCI.20-08-02904.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golarai G, Greenwood AC, Feeney DM, Connor JA. Physiological and structural evidence for hippocampal involvement in persistent seizure susceptibility after traumatic brain injury. J Neurosci. 2001;21:8523–8537. doi: 10.1523/JNEUROSCI.21-21-08523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong R, Park CS, Abbassi NR, Tang SJ. Roles of glutamate receptors and the mammalian target of rapamycin (mTOR) signaling pathway in activity-dependent dendritic protein synthesis in hippocampal neurons. J Biol Chem. 2006;281:18802–18815. doi: 10.1074/jbc.M512524200. [DOI] [PubMed] [Google Scholar]
- Harris TE, Lawrence JC., Jr TOR signaling. Sci STKE. 2003;2003:re15. doi: 10.1126/stke.2122003re15. [DOI] [PubMed] [Google Scholar]
- Haug FM. Electron microscopical localization of the zinc in hippocampal mossy fibre synapses by a modified sulfide silver procedure. Histochemie. 1967;8:355–368. doi: 10.1007/BF00401978. [DOI] [PubMed] [Google Scholar]
- Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Lowenstein DH. Selective inhibition of axon outgrowth by antibodies to NGF in a model of temporal lobe epilepsy. J Neurosci. 1995;15:7062–7070. doi: 10.1523/JNEUROSCI.15-11-07062.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Klann E. Activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2004;24:6352–6361. doi: 10.1523/JNEUROSCI.0995-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser CR, Miyashiro JE, Swartz BE, Walsh GO, Rich JR, Delgado-Escueta AV. Altered patterns of dynorphin immunoreactivity suggest mossy fiber reorganization in human hippocampal epilepsy. J Neurosci. 1990;10:267–282. doi: 10.1523/JNEUROSCI.10-01-00267.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Kang BN, Tian J, Liu Y, Luo HR, Hester L, Snyder SH. The cationic amino acid transporters CAT1 and CAT3 mediate NMDA receptor activation-dependent changes in elaboration of neuronal processes via the mammalian target of rapamycin mTOR pathway. J Neurosci. 2007;27:449–458. doi: 10.1523/JNEUROSCI.4489-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegaya Y. Abnormal targeting of developing hippocampal mossy fibers after epileptiform activities via L-type Ca2+ channel activation in vitro. J Neurosci. 1999;19:802–812. doi: 10.1523/JNEUROSCI.19-02-00802.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegaya Y, Nishiyama N, Matsuki N. L-type Ca2+ channel blocker inhibits mossy fiber sprouting and cognitive deficits following pilocarpine seizures in immature mice. Neuroscience. 2000;98:647–659. doi: 10.1016/s0306-4522(00)00188-3. [DOI] [PubMed] [Google Scholar]
- Ingram EA, Toyoda I, Wen X, Buckmaster PS. Prolonged infusion of inhibitors of calcineurin or L-type calcium channels does not block mossy fiber sprouting in a model of temporal lobe epilepsy. Epilepsia. 2009;50:56–64. doi: 10.1111/j.1528-1167.2008.01704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacinto E, Loewith R, Schmidt A, Lin S, Rüegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- Jaworski J, Spangler S, Seeburg DP, Hoogenraad CC, Sheng M. Control of dendritic arborization by the phosphoinositide-3′-kinase-Akt-mammalian target of rapamycin pathway. J Neurosci. 2005;25:11300–11312. doi: 10.1523/JNEUROSCI.2270-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Nadler JV. Stereological analysis of GluR2-immunoreactive hilar neurons in the pilocarpine model of temporal lobe epilepsy: correlation of cell loss with mossy fiber sprouting. Exp Neurol. 2007;205:569–582. doi: 10.1016/j.expneurol.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam SD, Dudek FE. Neuropathological features of a rat model of perinatal hypoxic-ischemic encephalopathy with associated epilepsy. J Comp Neurol. 2007;505:716–737. doi: 10.1002/cne.21533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharatishvili I, Nissinen JP, McIntosh TK, Pitkänen A. A model of posttraumatic epilepsy induced by lateral fluid-percussion brain injury in rats. Neuroscience. 2006;140:685–697. doi: 10.1016/j.neuroscience.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- Kumar V, Zhang MX, Swank MW, Kunz J, Wu GY. Regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK signaling pathways. J Neurosci. 2005;25:11288–11299. doi: 10.1523/JNEUROSCI.2284-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon CH, Zhu X, Zhang J, Baker SJ. mTor is required for hypertrophy of Pten-deficient neuronal soma in vivo. Proc Natl Acad Sci U S A. 2003;100:12923–12928. doi: 10.1073/pnas.2132711100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LB, Toan SV, Zelenaia O, Watson DJ, Wolfe JH, Rothstein JD, Robinson MB. Regulation of astrocytic glutamate transporter expression by Akt: evidence for a selective transcriptional effect on the GLT-1/EAAT2 subtype. J Neurochem. 2006;97:759–771. doi: 10.1111/j.1471-4159.2006.03743.x. [DOI] [PubMed] [Google Scholar]
- Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10:457–468. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- Longo BM, Mello LEAM. Blockade of pilocarpine- or kainate-induced mossy fiber sprouting by cycloheximide does not prevent subsequent epileptogenesis in rats. Neurosci Lett. 1997;226:163–166. doi: 10.1016/s0304-3940(97)00267-x. [DOI] [PubMed] [Google Scholar]
- Longo BM, Mello LEAM. Supragranular mossy fiber sprouting is not necessary for spontaneous epileptogenesis in rats. Epilepsy Res. 1998;32:172–182. doi: 10.1016/s0920-1211(98)00049-7. [DOI] [PubMed] [Google Scholar]
- Lynch M, Sutula T. Recurrent excitatory connectivity in the dentate gyrus of kindled and kainic acid-treated rats. J Neurophysiol. 2000;83:693–704. doi: 10.1152/jn.2000.83.2.693. [DOI] [PubMed] [Google Scholar]
- Masukawa LM, Wang H, O'Connor MJ, Uruno K. Prolonged field potentials evoked by 1 Hz stimulation in the dentate gyrus of temporal lobe epileptic human brain slices. Brain Res. 1996;721:132–139. doi: 10.1016/0006-8993(96)00153-9. [DOI] [PubMed] [Google Scholar]
- Meikle L, Pollizzi K, Egnor A, Kramvis I, Lane H, Sahin M, Kwiatkowski DJ. Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J Neurosci. 2008;28:5422–5432. doi: 10.1523/JNEUROSCI.0955-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello LEAM, Cavalheiro EA, Tan AM, Kupfer WR, Pretorius JK, Babb TL, Finch DM. Circuit mechanisms of seizures in the pilocarpine model of chronic epilepsy: cell loss and mossy fiber sprouting. Epilepsia. 1993;34:985–995. doi: 10.1111/j.1528-1157.1993.tb02123.x. [DOI] [PubMed] [Google Scholar]
- Moriwaki A, Lu YF, Hayashi Y, Tomizawa K, Tokuda M, Itano T, Hatase O, Matsui H. Immunosuppressant FK506 prevents mossy fiber sprouting induced by kindling stimulation. Neurosci Res. 1996;25:191–194. doi: 10.1016/0168-0102(96)01036-x. [DOI] [PubMed] [Google Scholar]
- Nadler JV, Perry BW, Cotman CW. Selective reinnervation of hippocampal area CA1 and the fascia dentata after destruction of CA3-CA4 afferents with kainic acid. Brain Res. 1980;182:1–9. doi: 10.1016/0006-8993(80)90825-2. [DOI] [PubMed] [Google Scholar]
- Nissinen J, Halonen T, Koivisto E, Pitkänen A. A new model of chronic temporal lobe epilepsy induced by electrical stimulation of the amygdala in rats. Epilepsy Res. 2000;38:177–205. doi: 10.1016/s0920-1211(99)00088-1. [DOI] [PubMed] [Google Scholar]
- Nissinen J, Lukasiuk K, Pitkänen A. Is mossy fiber sprouting present at the time of the first spontaneous seizures in rat experimental temporal lobe epilepsy? Hippocampus. 2001;11:299–310. doi: 10.1002/hipo.1044. [DOI] [PubMed] [Google Scholar]
- Pan T, Kondo S, Zhu W, Xie W, Jankovic J, Le W. Neuroprotection of rapamycin in lactacystin-induced neurodegeneration via autophagy enhancement. Neurobiol Dis. 2008;32:16–25. doi: 10.1016/j.nbd.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons RG, Gafford GM, Helmstetter FJ. Translational control via the mammalian target of rapamycin pathway is critical for the formation and stability of long-term fear memory in amygdala neurons. J Neurosci. 2006;26:12977–12983. doi: 10.1523/JNEUROSCI.4209-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao X, Noebels JL. Developmental analysis of hippocampal mossy fiber outgrowth in mutant mouse with inherited spike-wave seizures. J Neurosci. 1993;13:4622–4635. doi: 10.1523/JNEUROSCI.13-11-04622.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab-Graham KF, Haddick PCG, Jan YN, Jan LY. Activity- and mTOR-dependent suppression of Kv1.1 channel mRNA translation in dendrites. Science. 2006;314:144–148. doi: 10.1126/science.1131693. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Seress L, Weber P, Epstein CM, Henry TR, Bakay RAE. Alumina gel injections into the temporal lobe of rhesus monkeys cause complex partial seizures and morphological changes found in human temporal lobe epilepsy. J Comp Neurol. 1998;401:266–290. [PubMed] [Google Scholar]
- Rüegg S, Baybis M, Juul H, Dichter M, Crino PB. Effects of rapamycin on gene expression, morphology, and electrophysiological properties of rat hippocampal neurons. Epilepsy Res. 2007;77:85–92. doi: 10.1016/j.eplepsyres.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994;78:35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- Sabatini DM, Barrow RK, Blackshaw S, Burnett PE, Lai MM, Field ME, Bahr BA, Kirsch J, Betz H, Snyder SH. Interaction of RAFT1 with gephyrin required for rapamycin-sensitive signaling. Science. 1999;284:1161–1164. doi: 10.1126/science.284.5417.1161. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Sollas AL, Berger RE, Goodman JH. Electrophysiological evidence of monosynaptic excitatory transmission between granule cells after seizure-induced mossy fiber sprouting. J Neurophysiol. 2003;90:2536–2547. doi: 10.1152/jn.00251.2003. [DOI] [PubMed] [Google Scholar]
- Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell. 2000;103:253–262. doi: 10.1016/s0092-8674(00)00117-3. [DOI] [PubMed] [Google Scholar]
- Sendelbeck SL, Urquhart J. Spatial distribution of dopamine, methotrexate and antipyrine during continuous intracerebral microperfusion. Brain Res. 1985;328:251–258. doi: 10.1016/0006-8993(85)91036-4. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Possible functional consequences of synaptic reorganization in the dentate gyrus of kainate-treated rats. Neurosci Lett. 1992;137:91–96. doi: 10.1016/0304-3940(92)90306-r. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Zappone CA, Harvey BD, Frotscher M. Kainic acid-induced recurrent mossy fiber innervation of dentate gyrus inhibitory interneurons: possible anatomical substrate of granule cell hyperinhibition in chronically epileptic rats. J Comp Neurol. 2006;494:944–960. doi: 10.1002/cne.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutula T, He XX, Cavazos J, Scott G. Synaptic reorganization in the hippocampus induced by abnormal functional activity. Science. 1988;239:1147–1150. doi: 10.1126/science.2449733. [DOI] [PubMed] [Google Scholar]
- Sutula T, Cascino G, Cavazos J, Parada I, Ramirez L. Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann Neurol. 1989;26:321–330. doi: 10.1002/ana.410260303. [DOI] [PubMed] [Google Scholar]
- Swiech L, Perycz M, Malik A, Jaworski J. Role of mTOR in physiology and pathology of the nervous system. Biochem Biophys Acta. 2008;1784:116–132. doi: 10.1016/j.bbapap.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Takei N, Inamura N, Kawamura M, Namba H, Hara K, Yonezawa K, Nawa H. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J Neurosci. 2004;24:9760–9769. doi: 10.1523/JNEUROSCI.1427-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci U S A. 2002;99:467–472. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauck DL, Nadler JV. Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid-treated rats. J Neurosci. 1985;5:1016–1022. doi: 10.1523/JNEUROSCI.05-04-01016.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie SF, Alvarez VA, Ridenour DA, Kwiatkowski DJ, Sabatini BL. Regulation of neuronal morphology and function by the tumor suppressors Tsc1 and Tsc2. Nat Neurosci. 2005;8:1727–1734. doi: 10.1038/nn1566. [DOI] [PubMed] [Google Scholar]
- Toyoda I, Buckmaster PS. Prolonged infusion of cycloheximide does not block mossy fiber sprouting in a model of temporal lobe epilepsy. Epilepsia. 2005;46:1017–1020. doi: 10.1111/j.1528-1167.2005.04605.x. [DOI] [PubMed] [Google Scholar]
- Verma P, Chierzi S, Codd AM, Campbell DS, Meyer RL, Holt CE, Fawcett JW. Axonal protein synthesis and degradation are necessary for efficient growth cone regeneration. J Neurosci. 2005;25:331–342. doi: 10.1523/JNEUROSCI.3073-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Barbaro MF, Baraban SC. A role for the mTOR pathway in surface expression of AMPA receptors. Neurosci Lett. 2006;401:35–39. doi: 10.1016/j.neulet.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Wenzel HJ, Cole TB, Born DE, Schwartzkroin PA, Palmiter RD. Ultrastructural localization of zinc transporter-3 (ZnT-3) to synaptic vesicle membranes within mossy fiber boutons in the hippocampus of mouse and monkey. Proc Natl Acad Sci U S A. 1997;94:12676–12681. doi: 10.1073/pnas.94.23.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PA, Wuarin JP, Dou P, Ferraro DJ, Dudek FE. Reassessment of the effects of cycloheximide on mossy fiber sprouting and epileptogenesis in the pilocarpine model of temporal lobe epilepsy. J Neurophysiol. 2002;88:2075–2087. doi: 10.1152/jn.2002.88.4.2075. [DOI] [PubMed] [Google Scholar]
- Wuarin JP, Dudek FE. Electrographic seizures and new recurrent excitatory circuits in the dentate gyrus of hippocampal slices from kainate-treated epileptic rats. J Neurosci. 1996;16:4438–4448. doi: 10.1523/JNEUROSCI.16-14-04438.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LH, Xu L, Gutmann DH, Wong M. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol. 2008;63:444–453. doi: 10.1002/ana.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]