Abstract
Background
The majority of patients randomized to percutaneous coronary intervention (PCI) in the Occluded Artery Trial (OAT) and its angiographic substudy, the Total Occlusion Study of Canada 2 (TOSCA-2) were treated with bare metal stents (BMS). We aimed to determine if stenting of the target occlusion in OAT with drug-eluting stents (DES) was associated with more favorable angiographic results and clinical outcome when compared with treatment with BMS.
Methods
TOSCA-2 DES was a prospective nonrandomized substudy that provided 1-year angiographic comparison of late loss and reocclusion in 25 patients treated with DES and in 128 treated with BMS. In addition, all PCI-assigned patients enrolled from the time when DES were first utilized were similarly categorized (DES n = 77, and BMS n = 386) and compared using the 3-year cumulative OAT primary combined endpoint of death, myocardial infarction, or Class-IV heart failure, as well as angina.
Results
In-segment late loss was 0.14 ± 0.45 mm for DES and 0.75 ± 0.86 mm for BMS (P < 0.001). Corresponding binary restenosis rates were 13.0% and 44.3% (P = 0.005). Occlusion at 1 year was observed in 4.0 and 12.1%, respectively (P = 0.23). The 3-year cumulative primary event rate was 13.8% with DES and 12.5% with BMS (hazard ratio 1.08, 99% confidence intervals 0.44, 2.64; P = 0.83). Angina over time occurred less frequently in the DES group (P = 0.01).
Conclusions
Although the reduction of late loss and trend to reduction in reocclusion with the use of DES for PCI of persistently occluded IRA 3–28 days post myocardial infarction did not translate into a signal for reduction in death, reinfarction, or Class IV heart failure, DES use was associated with less angina over time. Further follow-up is warranted.
Keywords: open artery hypothesis, percutaneous coronary intervention, drug-eluting stent, bare metal stent, angina
INTRODUCTION
The Occluded Artery Trial (OAT) was a randomized trial of percutaneous coronary intervention (PCI) in stable patients with persistent occlusion of the infarct-related artery 3–28 days after myocardial infarction [1]. Our group previously reported 1-year angiographic outcomes in the ancillary angiographic study of OAT, the Total Occlusion Study of Canada (TOSCA)-2 [2]. Most PCI-assigned patients enrolled in TOSCA-2 were treated with bare metal stents (BMS). The observed incidence of restenosis and reocclusion (45.7% and 11%, respectively) was concordant with previous angiographic studies employing BMS during PCI for nonacute coronary occlusions [2–4]. We now report 1-year angiographic findings in TOSCA-2 DES (a prospectively defined substudy of TOSCA-2), the aim of which was to determine if deployment of drug-eluting stents (DES) in PCI-assigned patients was associated with reduced restenosis and reocclusion. Secondly, we report the clinical outcomes through 3 years amongst PCI-assigned patients treated with DES versus BMS in the overall trial.
MATERIALS AND METHODS
Study Design
The design of OAT and TOSCA-2 have been previously described [2,5]. Briefly, 2,201(2166 between Feb 2000 and Dec 2005 in the main OAT trial and 35 in the extension phase of the OAT-NUC substudy in 2006) stable myocardial infarction survivors with persistent infarct-related artery occlusion were randomly assigned to PCI with stenting plus optimal medical therapy versus optimal medical therapy alone. The predefined primary clinical outcome combined death, rein-farction, or development of New York Heart Association Class IV heart failure. TOSCA-2, an ancillary study of OAT, provided quantitative and qualitative coronary angiographic follow-up in 381 participants of whom 195 were assigned to PCI.
Patient Sample
The inclusion criteria for OAT and TOSCA-2 have been previously published [5]. Briefly, patients were eligible for OAT and TOSCA-2 if they were found to have an occluded infarct-related artery defined as a coronary artery with a 100% stenosis with Thrombolysis in Myocardial Infarction grade 0 or 1 antegrade flow suitable for PCI and stenting on coronary angiography performed 3–28 calendar days after a documented myocardial infarction. Because the date of symptom onset was labeled day 1, patients could be randomized after the 24th hour postmyocardial infarction. Patients were additionally required to either have an occlusion that was proximal and subtended at least 25% of the left ventricle or left ventricular ejection fraction <50%. These are features known to confer increased risk for future events in prior studies [5].
Angiographic Cohort
TOSCA-2 DES was a nonrandomized substudy conducted in 12 TOSCA-2 sites in the latter period of study enrollment, beginning in February 2003. The purpose was to compare 1-year quantitative and qualitative angiographic outcomes of the subjects treated with the CYPHER stent to those treated with bare-metal stents. The TOSCA-2 DES substudy received separate local institutional IRB approval and participating patients (who would then receive a CYPHER stent during the index procedure of the culprit vessel) provided informed consent. CYPHER stents were provided free of charge by Cordis, Johnson&Johnson (Miami Lakes, FL). The intent was deploy CYPHER stents in the culprit vessels of consecutive patients who consented to participate in the substudy until the end of study enrollment in December 2005. Because of logistical challenges with stent supply, only 20 patients were ultimately enrolled. Ten additional PCI-assigned TOSCA-2 subjects were treated exclusively with paclitaxel-eluting Taxus stents (Boston Scientific Corporation, Boston, MA) in the qualifying culprit occlusion. Deployment of DES in these patients was by operator preference. These patients were grouped with those receiving sirolimus-eluting CYPHER stents within TOSCA-2 DES for the purpose of examining angiographic outcomes. All other prespecified aspects of TOSCA-2, including its angiographic analysis, were retained. All angiographic analyses reported in this manuscript refer to this group of patients.
Clinical Outcomes Cohort
To determine if primary use of DES in the target occlusion influenced clinical outcome, we categorized all successfully stented PCI-assigned OAT subjects enrolled after the first DES patient was enrolled in February 2003. Patients were designated DES if at least one CYPHER or TAXUS DES device was employed within the target occlusion. The primary OAT outcome (mortality and morbidity classification committee adjudicated death, myocardial infarction, or new class-IV heart failure) and its components were then analyzed though 3 years of follow-up. All clinical outcomes reported in the manuscript are based on this cohort, of which the angiographic cohort is a subset.
Study Procedures
Patients assigned PCI were to undergo the procedure within 24 h of randomization, deploying whenever possible, one or more locally approved coronary stents and utilizing a glycoprotein IIb/IIIa inhibitor. Drug-eluting stents were deployed in 77 OAT patients. Thienopyridine therapy, in accordance with contemporaneous practice, was initially recommended for 2–4 weeks following BMS and 3–6 months following DES deployment, Following publication of data supporting longer-term therapy following acute coronary syndrome, clopidogrel was recommended for 1 year in both groups [6]. In the TOSCA-2 DES subset, angio-graphic follow-up was to be obtained 12 ± 3 months following randomization.
Angiographic Analysis
OAT/TOSCA-2 sites and operators were certified after core laboratory review of angiograms of PCI of total coronary occlusions and procedural statistics [5]. Study operators were instructed to extend cine runs for antegrade and collateral flow assessment, to use intra-coronary nitroglycerin, and to reproduce baseline views at follow-up.
Angiographic images were analyzed in a blinded fashion at the Cardiovascular Imaging Research Core Laboratory, Vancouver Hospital, University of British Columbia, Vancouver, Canada, using the Image-Comm system (Quinton). Coronary luminal diameter, including 5 mm long peri-lesional segments, was measured to obtain in-stent and in-segment minimal luminal diameter (MLD). Restenosis was defined as >50% diameter stenosis at the point of in-lesion MLD utilizing proximal-only reference segments. Patency at follow-up was defined as antegrade flow grades 2 or 3 irrespective of lumen dimensions [7]. Collateral flow was graded utilizing standard criteria [8]. The area-length method was utilized to measure left ventricular ejection fraction and left ventricular volumes when calibration images were provided. Regional wall motion was measured using the centerline method [9].
The primary endpoint in TOSCA-2 DES was mean late loss (DES versus BMS groups). Late loss was calculated as the minimum in-segment diameter immediately post PCI minus the minimum in-segment diameter at follow-up. The secondary angiographic endpoint was mean percent stenosis at 1 year. Other prespecified 1-year angiographic endpoints included infarct-related artery reocclusion, left ventricular ejection fraction, infarct segment regional wall motion, and left ventricular volumes.
Clinical outcomes included the primary OAT outcome and its components, repeat revascularization, and angina.
Statistical Considerations
The inclusion of 22 patients with baseline and follow-up angiographic studies for the primary late loss outcome who received a DES (CYPHER n = 16 and TAXUS n = 6) stent during the index procedure and 128 patients who received a bare metal stent during the index procedure provided 90% power to detect a reduction in the late loss of 0.66 mm and 80% power to detect a difference of 0.57 mm. For the difference in percent diameter stenosis at follow-up, the sample provided 90% power to detect an absolute difference of 14.6% and 80% power to detect a difference of 17.0%.
Analysis of binary end points (such as binary restenosis to >50% diameter stenosis and patency) was accomplished using contingency table analysis. Significance of results was assessed with the Chi-square test uncorrected for continuity. Estimates of the cumulative event rate were calculated by the Kaplan-Meier product-limit method [10] and groups were compared with the use of log-rank tests of the 3-year curves [11]. Hazard ratio and 99% confidence intervals were calculated by Cox proportional-hazards regression [12]. For continuous variables, comparisons of groups were accomplished using Student's t-test or the Wilcoxon rank sum test depending on the distributional properties of the data.
To examine the relationship between stent type and presence or absence of angina over time, longitudinal logistic regression models were fitted using the generalized estimating equation (GEE) method [13]. Least square estimates of model coefficients were obtained and residuals from the fitted model were used to adjust the standard errors of model coefficients to take into account within-individual correlations in repeated measurements of the dependent variable. Two independent indicator variables and one interaction term were included in each model: visit (with visit 4 months as the reference group) and stent type (with BMS as the reference group). Differences by stent type in the trends with visit in presence of angina were tested using visit-by-stent type interaction terms. Testing for main effects of visit, stent type, and visit-by-stent type interactions was performed using global tests before testing for significance of any individual predictors.
RESULTS
From February 2003 when the first OAT participant randomized to PCI underwent deployment of a DES until December 2005 when enrollment in the main OAT was completed, 386 patients were treated with at least one BMS and 77 patients received DES (Cypher n = 46, Taxus n = 31). Of the 195 TOSCA-2 patients randomized to PCI, 178 underwent a successful PCI of the IRA with stent deployment. Of these, 148 underwent deployment of a BMS, whereas 30 were treated with DES (Cypher n = 20, Taxus n = 10).
Assignment to DES was by protocol in the TOSCA-2 DES substudy (n = 20) in that consecutive patients in participating centers were approached to participate during the period of TOSCA-2 DES enrollment. The remaining patients received DES or BMS according to operator preference. In sites not participating in TOSCA-2 DES, the DES utilization rate was 41.1% (23/56) in the USA, 7.4% (2/27) in Canada, and 5.8% (15/258) in all other countries (P < 0.001). The DES and BMS groups in both the TOSCA-2 substudy and OAT cohorts were similar in most baseline characteristics (Table I). The DES patients in TOSCA-2 tended to have better renal function but worse baseline left ventricular function.
TABLE I.
Baseline Clinical Characteristics of Patients With Deployment of a DES and Those Receiving BMS in OAT, and in the TOSCA-2 DES Substudy
| OAT |
TOSCA-2 DES |
|||
|---|---|---|---|---|
| DES |
BMS |
DES |
BMS |
|
| Variable | N = 77 | N = 386 | N = 30 | N = 148 |
| Age | 57.1 ± 11.4 | 57.2 ± 10.4 | 54.4 ± 11.5 | 57.6 ± 10.3 |
| Female gender (%) | 26.0 | 22.0 | 16.7 | 17.6 |
| Race | ||||
| White (%) | 77.9 | 79.5 | 70.0 | 81.1 |
| Black (%) | 3.9 | 2.3 | 6.7 | 1.4 |
| Hispanic (%) | 9.1 | 15.8 | 6.7 | 8.1 |
| Other (%) | 9.1 | 2.3 | 16.7 | 9.5 |
| Diabetes (%) | 16.9 | 18.1 | 13.3 | 18.2 |
| Hypertension (%) | 49.4 | 48.7 | 50.0 | 47.3 |
| Hypelipidemia (%) | 53.2 | 53.6 | 50.0 | 56.1 |
| Current smoker (%) | 48.1 | 39.4 | 43.3 | 31.8 |
| History of | ||||
| Angina (%) | 11.7 | 20.2 | 10.0 | 23.0 |
| Myocardial infarction (%) | 5.2 | 9.3 | 6.7 | 13.5 |
| Peripheral vascular disease (%) | 2.6 | 2.6 | 0 | 2.7 |
| Conestive heart failure (%) | 0 | 2.6 | 0 | 2.0 |
| Prior percutaneous coronary intervention (%) | 3.9 | 2.3 | 6.7 | 2.7 |
| Prior coronary artery bypass grafting (%) | 0 | 0.5 | 0 | 0 |
| New York Heart Association Class | ||||
| I (%) | 85.7 | 85.2 | 90.0 | 93.9 |
| II (%) | 14.3 | 14.8 | 10.0 | 6.1 |
| ST-elevation, Q-wave or loss of R-wave (%) | 85.7 | 89.1 | 93.3 | 81.1 |
| Estimated glomerular filtration rate ml/min/1.73 m2 | 89.2 ± 24.1 | 81.3 ± 19.3 | 92.4 ± 28.9 | 80.8 ± 16.8 |
| Baseline physical exam | ||||
| Heart rate | 73.1 ± 12.0 | 71.2 ± 11.3 | 72.7 ± 13.2 | 68.2 ± 11.5b |
| Systolic blood pressure | 118.4 ± 17.9 | 119.3 ± 15.1 | 114.8 ± 16.7 | 116.8 ± 16.0 |
| Diastolic blood pressure | 71.2 ± 12.2 | 72.0 ± 10.2 | 71.9 ± 12.1 | 70.3 ± 11.0 |
| Multivessel disease (%) | 22.1 | 16.7 | 23.3 | 17.6 |
| Infarct-related artery | ||||
| Left anterior descending (%) | 33.8 | 35.5 | 26.7 | 33.8 |
| Circumflex (%) | 14.3 | 15.3 | 3.3 | 15.5 |
| Right coronary artery (%) | 51.9 | 49.2 | 70.0 | 50.7b |
| TIMI flow grade | ||||
| 0 (%) | 76.6 | 76.2 | 86.7 | 76.9 |
| I (%) | 23.4 | 23.0 | 13.3 | 21.8 |
| II | 0 | 0.8 | 0 | 1.4 |
| Any collaterals present (%) | 89.6 | 88.5 | 90.0 | 88.4 |
| Left ventricular ejection fraction | 48.1 ± 11.1 | 47.7 ± 9.9 | 45.9 ± 12.3 | 48.7 ± 10.1 |
| Left ventricular ejection fraction <40% (%) | 24.7 | 17.1 | 33.3 | 15.5a |
P < 0.05.
P < 0.10.
Postrandomization Therapy
Glycoprotein IIb/IIIa inhibitors were utilized in 81%. TIMI grade 3 flow was achieved in 86.7% DES and 93.9% BMS patients (Table II). The utilization of proven medical therapies was high and similar in all groups (Table III). The rates of thienopyridine utilization at 1 year were low in both groups and only 50% in the DES group.
TABLE II.
Infarct-Related Artery PCI Procedural and One-Year Core Angiographic Laboratory Results
| DES |
BMS |
||||||
|---|---|---|---|---|---|---|---|
| Baseline |
Post PCI |
1 Year |
Baseline |
Post PCI |
1 Year |
||
| Variable | N = 30 | N = 30 | N = 25 | N = 148 | N = 148 | N = 132 | P value |
| TIMI grade flowa | |||||||
| Grade 0 | 26 (86.7%) | 0 | 0 | 113 (76.9%) | 0 | 12 (9.1%) | |
| Grade 1 | 4 (13.0%) | 1 (3.3%) | 1(4.0%) | 32 (21.8%) | 3 (2.0%) | 4 (3.0%) | |
| Grade 2 | 0 | 3 (10.0%) | 3 (12.0%) | 2 (1.4%) | 6 (4.1%) | 9 (6.8%) | |
| Grade 3 | 0 | 26 (86.7) | 21 (84.0%) | 0 | 139 (93.9%) | 107 (81.1%) | 0.38 |
| TIMI Grade 0 or 1a | 30 (100%) | 1 (3.3%) | 1 (4.0%) | 145 (98.5%) | 3 (2.0%) | 16 (12.1%) | 0.23 |
| Reference diameter (mm) (n = 22, 117)b | 3.31 ± 0.65 | 3.23 ± 0.62 | 0.58 | ||||
| In-stent | |||||||
| Minimal lumen diameter (mm) (n = 22, 128)b | 0 | 2.63 ± 0.47 | 2.39 ± 0.62 | 0 | 2.79 ± 0.49 | 1.67 ± 0.91 | <0.001 |
| Percent diameter stenosis (n = 24, 132)b | 100 | 20.4 ± 15.5 | 28.5 ± 17.3 | 100 | 12.8 ± 12.2 | 47.5 ± 25.9 | <0.001 |
| Late loss, mm (n = 22, 128)b | –0.24 ± 0.56 | –1.12 ± 0.93 | <0.001 | ||||
| In-segment | |||||||
| Minimal lumen diameter mm (n = 22, 128)b | 0 | 2.36 ± 0.50 | 2.22 ± 0.61 | 0.002 ± 0.02 | 2.30 ± 0.53 | 1.54 ± 0.82 | <0.001 |
| Percent diameter stenosis (n = 24, 132)b | 100 | 29.5 ± 12.0 | 34.6 ± 14.9 | 99.7 ± 3.0 | 27.9 ± 15.1 | 51.3 ± 23.9 | <0.001 |
| Late loss, mm (n = 22, 128)b | –0.14 ± 0.45 | –0.75 ± 0.86 | <0.001 | ||||
| Restenosis | 3/23 (13.0%) | 54/122 (44.3%) | 0.005 | ||||
P values are for comparison of 1-year values
The N's for comparison of changes in minimal lumen diameter and percent diameter stenosis differ from stated 1-year N's (25 and 132) due to inability to measure the lumen diameter at ostial occlusions or reocclusions.
TABLE III Medical Therapy at 1 Year in the OAT Group and in the TOSCA-2 Group Stratified by DES Use
| OAT |
TOSCA-2 DES |
|||
|---|---|---|---|---|
| DES |
BMS |
DES |
BMS |
|
| Variable | N = 63 | N = 341 | N = 28 | N = 140 |
| Aspirin (%) | 92.1 | 93.8 | 92.9 | 95.0 |
| Thienopyridine (%) | 49.2 | 23.2a | 50.0 | 20.0a |
| Aspirin and thienopyridine (%) | 44.4 | 20.8a | 46.4 | 18.6a |
| Aspirin or thienopyridine (%) | 96.8 | 96.2 | 96.4 | 96.4 |
| Beta blocker (%) | 87.3 | 83.0 | 85.7 | 85.0 |
| Angiotensin-converting enzyme inhibitor or angiotensin receptor blocker (%) | 81.0 | 82.4 | 89.3 | 86.4 |
| Lipid-lowering drug (%) | 88.9 | 89.1 | 96.4 | 86.4 |
| Spironolactone (%) | 4.8 | 6.5 | 10.7 | 7.9 |
| Calcium blocker (%) | 4.8 | 8.5 | 3.6 | 7.9 |
P < 0.001.
Coronary Angiographic Endpoints
In-segment late loss (Table II) was −0.14 ± 0.45 mm in the DES and −0.75 ± 0.86 mm in the BMS patients (P < 0.001). Binary restenosis was observed in three (13.0%, 99% confidence intervals 0, 31.1%) patients in the DES group and 54 (44.3%, 99% confidence intervals 32.7, 55.9%) of those in the BMS group (P = 0.005), whereas Failure of patency (TIMI 0 or 1) was observed in one (4.0%, 99% confidence intervals 0, 14.1%) and 16 (12.1%, 99% confidence intervals 4.8, 19.4%) patients in these groups, respectively (P = 0.23). Adjusting for differences in estimated glomerular filtration rate and left ventricular ejection fraction between the two groups did not influence these results. Similar angiographic outcomes were observed in the DES group when only patients treated with sirolimus-eluting stents were included.
Left Ventricular Function and Volumes
Paired left ventricular ejection fraction and regional wall motion measurements were available in 22 of 30 DES and 113 of 148 BMS patients, whereas paired volume measurements were available in 15 of the DES and 66 of the BMS group. Left ventricular ejection fraction improved in a similar fashion in both groups, with no difference in the change between the groups (Table IV). There were no significant differences between the groups in the change in infarct zone regional wall motion score, left ventricular end-systolic volume, or left ventricular end-diastolic volume.
TABLE IV.
Comparison of Changes in Left Ventricular Systolic Function and Volumes Between TOSCA-2 DES Patients Treated With DES and Those Treated With BMS
| DES |
BMS |
||||||
|---|---|---|---|---|---|---|---|
| Baseline |
1 Year |
Baseline |
1 Year |
Between groups change P value | |||
| Variable | N = 30 | N = 25 | P value | N = 148 | N = 132 | P value | |
| Left ventricular ejection fraction (n = 22, 113) | 44.8 ± 9.8 | 49.0 ± 9.6 | 0.06 | 49.1 ± 10.0 | 53.3 ± 10.2 | <0.001 | 0.96 |
| Change in left ventricular ejection fraction | 4.2 ± 9.9 | 4.3 ± 8.9 | |||||
| Left ventricular systolic volume index (n = 15, 66) | 47.7 ± 33.2 | 45.7 ± 45.8 | 0.71 | 33.1 ± 17.0 | 30.7 ± 17.5 | 0.17 | 0.94 |
| Change in left ventricular systolic volume index | –2.1 ± 21.1 | –2.4 ± 14.1 | |||||
| Left ventricular diastolic volume index (n = 15, 66) | 83.6 ± 55.1 | 84.6 ± 58.1 | 0.79 | 66.7 ± 28.6 | 66.2 ± 26.7 | 0.88 | |
| Change in left ventricular end-diastolic volume index | 1.0 ± 13.5 | –0.46 ± 24.6 | 0.83 | ||||
| Regional wall motion score (n = 22, 112) | –3.35 ± 1.00 | –2.82 ± 0.87 | 0.01 | –2.93 ± 0.86 | –2.20 ± 1.10 | <0.001 | |
| Change in regional wall motion score | 0.53 ± 0.90 | 0.74 ± 1.04 | 0.38 | ||||
Clinical Primary Composite Endpoint
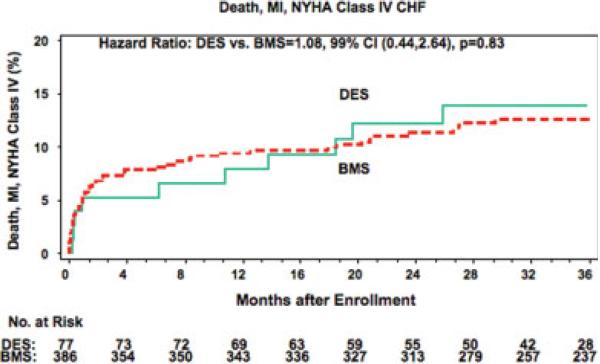
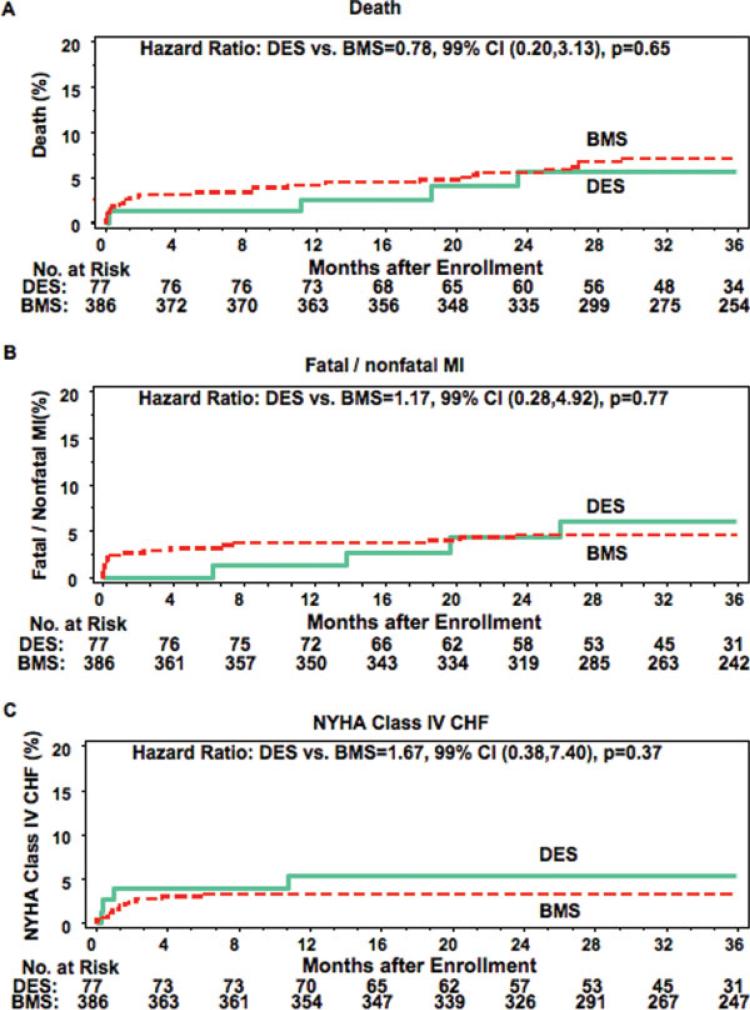
The 36-month cumulative event rate for the primary OAT endpoint of death, myocardial infarction, or New York Heart Association Class IV heart failure (Fig. 1) was 13.8% in the DES and 12.5% of BMS group (hazard ratio 1.08, 99% confidence intervals 0.44, 2.64; P = 0.83). A sensitivity analysis that compared the DES group with all patients who underwent BMS deployment for the entire OAT enrollment period from February 2000 through June 2006 (n = 79 DES, 865 BMS) revealed a similar result (DES 14.7% versus BMS 13.6, HR 5 1.09, 99% confidence intervals (0.48, 2.46, P = 0.79,) The adjudicated myocardial infarction or death rate was 9.9% and 11.0% in these two groups (hazard ratio 0.85, 99% confidence intervals 0.29, 2.45; P = 0.70). Comparison of individual clinical event rates is found in Fig. 2. This study was not powered to detect differences in these event rates.
Fig. 1.
Kaplan-Meier curves for the OAT combined primary endpoint comparing the DES- and BMS-treated patients in the PCI arm of OAT. red = BMS, green = DES. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Fig. 2.
Kaplan-Meier curves for the individual secondary clinical endpoints death, reinfarction, and NYHA Class IV heart failure comparing the DES- and BMS-treated patients in the PCI arm of OAT. red = BMS, blue = DES. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Repeat Revascularization and Angina Status
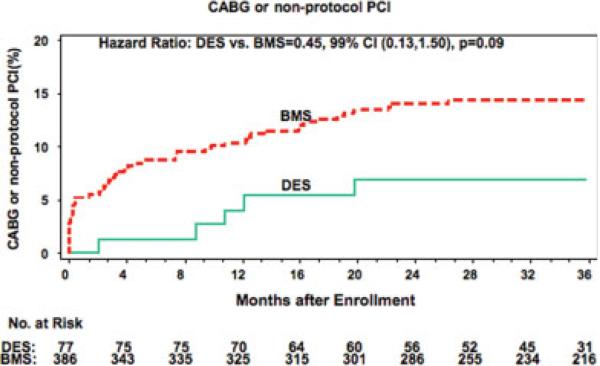
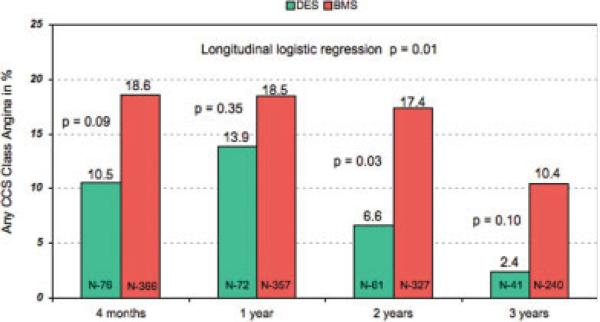
Repeat revascularization by either PCI or coronary artery bypass surgery, shown in Fig. 3, was performed in 6.9% of the DES-treated patients and 14.3% of the BMS group (hazard ratio 0.45, 99% confidence intervals 0.13, 1.50; P = 0.09). This was primarily a function of a decrease in the need for repeat PCI (6.9% vs. 13.5%; hazard ratio 0.48, 99% confidence intervals 0.14, 1.59; P = 0.11). Utilizing longitudinal analysis, angina of any severity occurred less frequently in the DES (P = 0.01) than in the BMS group (Fig. 4).
Fig. 3.
Kaplan-Meier curves for repeat revascularization by PCI or CABG comparing the DES- and BMS-treated patients in the PCI arm of OAT. red = BMS, green = DES. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Fig. 4.
Rates of any angina in the OAT DES- and BMS-treated patients at 4 months and 1, 2, and 3 years of follow-up. red = BMS, green = DES. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
Our data are the first to examine angiographic and clinical outcome after DES vs. BMS deployment in patients with recently occluded infarct-related arteries up to 28 days after myocardial infarction. The main findings include a much lower rate of restenosis and a possible reduction in reocclusion at 1 year with the use of DES. We observed no difference in the primary composite outcome of death, myocardial infarction, or Class-IV heart failure between patients treated with DES and those treated with BMS over the time period when DES were available. A favorable trend toward a lower rate of repeat revascularization procedures in the DES group is consistent with prior comparisons of DES and BMS in other settings, including the setting of more chronic occlusions. The occurrence of less angina over time in the DES-treated patients is a novel observation.
The 1-year angiographic results extend our knowledge to a population with recent occlusions that are complicated by thrombus of various degrees of severity in all cases. Such patients were specifically excluded from at least some trials of DES deployment in patients with totally occluded coronary arteries [14], whereas the presence of thrombus in other trials was unlikely due to the exclusion of patients with occlusion less than 2 weeks in duration [15]. The reocclusion rate observed at 1 year in the BMS group is similar to that reported in recent studies of BMS deployment in similar patient populations. In The Open Artery Trial (TOAT), the reocclusion rate in the PCI group was 11.1% [16], whereas in the DEsobstruction COronaire en Post-Infarctus (DECOPI) study, the reocclusion rate was 12.6% [17]. Although restenosis was not studied in these trials, the restenosis rate observed in DES-treated patients in TOSCA-2 DES is similar to that reported by the Primary Stenting of Totally Occluded Native Coronary Arteries II (PRISON II) study, a randomized trial of sirolimus-eluting stenting versus BMS deployment in 200 patients with nonacute coronary occlusions, with an in-segment binary restenosis rate of 12% in the DES group and 46% in the BMS-treated patients [15].
The superior angiographic results attained with DES in these patients with persistent infarct-related artery occlusion after a recent myocardial infarction were not associated with a reduction of clinical events as defined by the OAT combined primary endpoint of death, reinfarction, or development New York Heart Association Class IV heart failure or any of its individual components. Although this analysis was not powered to detect differences in these clinical outcomes, there is no signal to suggest that there might be a benefit from DES deployment with respect to these endpoints. Neither did we definitively demonstrate harm from deployment of DES in this patient population at this stage of follow-up. It is notable in this regard that only approximately half of patients with DES and one quarter of those with BMS were taking a thienopyri-dine at 1 year. These observations will require the corroboration of longer-term follow-up of the entire cohort, in light of recent conflicting observations. Thus, even though there are concerns regarding a possible increased risk of late stent thrombosis after DES implantation [18,19], more recent data indicate that this incremental risk of late stent thrombosis does not translate into a higher mortality risk [20,21].
There was a strong signal indicating that deployment of DES in patients with recent coronary occlusions reduces the need for repeat revascularization. The study was underpowered to detect differences but the longer-term follow-up of the entire cohort, currently under way will provide an opportunity to observe if this trend persists. Although based on current data do not suggest that universal use of DES in OAT would have favorably influenced the outcome of death, reinfarction or development of Class IV heart failure in the PCI arm, the need for repeat revascularization would likely have decreased, and it is possible that there may have been a larger and more sustained effect of PCI relative to optimal medical therapy alone on angina.
This study has several limitations. First, selection of patients to receive DES was not randomized. This may have led to important differences in the characteristics of patients in whom DES were deployed compared with those treated with BMS. Although we did not document such differences, it is possible that the two groups may have differed in other unrecorded clinical or angiographic characteristics that may have influenced outcome. Furthermore, the number of patients treated with DES was relatively low and thus the analyses are relatively underpowered. The analysis of the DES group includes patients treated with two different stents, eluting different drugs. By virtue of exclusion of patients with 3-vessel and left main disease suitable for coronary artery bypass surgery, the proportion of patients with multivessel disease is relatively low compared with other trials in patients with acute myocardial infarction. Finally, in spite of recommendations to extend dual antiplatelet therapy to 1 year early in the study, and well before the introduction of DES into practice, fewer than half of DES- and one fifth of BMS-treated patients were on this combination at 1 year. The study was conducted prior to the more recent recommendations for 1-year duration of dual antiplatelet therapy after DES implantation.
In summary, the improved angiographic outcome at 1 year associated with DES versus BMS use in persistently occluded infarct-related arteries treated days to weeks after myocardial infarction was not associated with a concomitant reduction in death, reinfarction, or development of New York Heart Association Class IV heart failure up to 3 years. These data suggest that the main OAT results were unlikely to have been influenced by the rate of DES use. The improvement in angina status and a trend toward a reduction in repeat revascularization with DES warrants further investigation.
ACKNOWLEDGMENTS
Medtronic Canada Inc provided free bare-metal stents in Canada. Cordis, Johnson&Johnson (Miami Lakes, FL) provided free bare-metals stents in Australia, Canada and Poland, and specifically provided free sirolimus-eluting stents and support for the TOSCA-2 DES substudy. Glycoprotein IIb/IIIa inhibitors were provided by Schering-Plough and Millenium Pharmaceuticals.
Grant sponsor: NHLBI, National Institutes of Health; Grant numbers: U01 HL062509, Dr. Dz̆avík is supported in part by the Brompton Funds Professorship in Interventional Cardiology, U01 HL062511, U01 HL062257, HL67683-01A1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute or the National Institutes of Health; Brompton Funds Professorship in Interventional Cardiology
Footnotes
Conflict of interest: Dr. Dzavik reports research, honorarium and Advisory Board member funds from Cordis, Johnson&Johnson, and honoraria from Boston Scientific. Dr. Rankin reports an educational grant from Cordis, Johnson&Johnson. Dr. Buszman owns stock in American Heart of Poland Ltd. and NAFIS SA (Poland). Dr Hochman reports grant support to her institution from Eli Lilly and Bristol Myers Squibb Medical Imaging and product donation from Millennium Pharmaceuticals, Schering-Plough, Guidant, and Merck for OAT and received consultation fees from Bristol Myers Squibb, honoraria for Steering Committee service from CV Therapeutics, Eli Lilly and Glaxo Smith Kline and honoraria for serving on the Data Safety Monitoring Board of a trial supported by Schering-Plough.
REFERENCES
- 1.Hochman JS, Lamas GA, Buller CE, et al. Coronary intervention for persistent occlusion after myocardial infarction. N Engl J Med. 2006;355:2395–2407. doi: 10.1056/NEJMoa066139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dzavik V, Buller CE, Lamas GA, et al. Randomized trial of percutaneous coronary intervention for subacute infarct-related coronary artery occlusion to achieve long-term patency and improve ventricular function: The total occlusion study of Canada (TOSCA)-2 trial. Circulation. 2006;114:2449–2457. doi: 10.1161/CIRCULATIONAHA.106.669432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubartelli P, Niccoli L, Verna E, Giachero C, Zimarino M, Fontanelli A, Vassanelli C, Campolo L, Martuscelli E, Tommasini G. Stent implantation versus balloon angioplasty in chronic coronary occlusions: Results from the GISSOC trial. Gruppo Italiano di Studio sullo Stent nelle Occlusioni Coronariche. J Am Coll Cardiol. 1998;32:90–96. doi: 10.1016/s0735-1097(98)00193-4. [DOI] [PubMed] [Google Scholar]
- 4.Sirnes PA, Golf S, Myreng Y, Molstad P, Emanuelsson H, Albertsson P, Brekke M, Mangschau A, Endresen K, Kjekshus J. Stenting in chronic coronary occlusion (SICCO): A randomized, controlled trial of adding stent implantation after successful angioplasty. J Am Coll Cardiol. 1996;28:1444–1451. doi: 10.1016/s0735-1097(96)00349-x. [DOI] [PubMed] [Google Scholar]
- 5.Hochman JS, Lamas GA, Knatterud GL, Buller CE, Dzavik V, Mark DB, Reynolds HR, White HD. Design and methodology of the occluded artery trial (OAT). Am Heart J. 2005;150:627–642. doi: 10.1016/j.ahj.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Mehta SR, Yusuf S, Peters RJ, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: The PCI-CURE study. Lancet. 2001;358:527–533. doi: 10.1016/s0140-6736(01)05701-4. [DOI] [PubMed] [Google Scholar]
- 7.Chesebro JH, Knatterud G, Roberts R, et al. Thrombolysis in myocardial infarction (TIMI) trial, phase I: A comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation. 1987;76:142–154. doi: 10.1161/01.cir.76.1.142. [DOI] [PubMed] [Google Scholar]
- 8.Rentrop KP, Cohen M, Blanke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol. 1985;5:587–592. doi: 10.1016/s0735-1097(85)80380-6. [DOI] [PubMed] [Google Scholar]
- 9.Sheehan FH, Bolson EL, Dodge HT, Mathey DG, Schofer J, Woo HW. Advantages and applications of the centerline method for characterizing regional ventricular function. Circulation. 1986;74:293–305. doi: 10.1161/01.cir.74.2.293. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 11.Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, Mantel N, McPherson K, Peto J, Smith PG. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. Analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox D. Regression models and life tables. J R Stat Soc Ser B. 1972;34:187–220. [Google Scholar]
- 13.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 14.Kelbaek H, Thuesen L, Helqvist S, et al. The stenting coronary arteries in non-stress/benestent disease (SCANDSTENT) trial. J Am Coll Cardiol. 2006;47:449–455. doi: 10.1016/j.jacc.2005.10.045. [DOI] [PubMed] [Google Scholar]
- 15.Suttorp MJ, Laarman GJ, Rahel BM, et al. Primary stenting of totally occluded native coronary arteries II (PRISON II): A randomized comparison of bare metal stent implantation with sirolimus-eluting stent implantation for the treatment of total coronary occlusions. Circulation. 2006;114:921–928. doi: 10.1161/CIRCULATIONAHA.106.613588. [DOI] [PubMed] [Google Scholar]
- 16.Yousef ZR, Redwood SR, Bucknall CA, Sulke AN, Marber MS. Late intervention after anterior myocardial infarction: Effects on left ventricular size, function, quality of life, and exercise tolerance: Results of the open artery trial (TOAT Study). J Am Coll Cardiol. 2002;40:869–876. doi: 10.1016/s0735-1097(02)02058-2. [DOI] [PubMed] [Google Scholar]
- 17.Steg PG, Thuaire C, Himbert D, et al. DECOPI (DEsobstruction COronaire en Post-Infarctus): A randomized multi-centre trial of occluded artery angioplasty after acute myocardial infarction. Eur Heart J. 2004;25:2187–2194. doi: 10.1016/j.ehj.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 18.Laskey WK, Yancy CW, Maisel WH. Thrombosis in coronary drug-eluting stents: Report from the meeting of the circulatory system medical devices advisory panel of the food and drug administration center for devices and radiologic health. December 7–8, 2006. Circulation. 2007;115:2352–2357. doi: 10.1161/CIRCULATIONAHA.107.688416. [DOI] [PubMed] [Google Scholar]
- 19.Jaffe R, Strauss BH. Late and very late thrombosis of drug-eluting stents: Evolving concepts and perspectives. J Am Coll Cardiol. 2007;50:119–127. doi: 10.1016/j.jacc.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 20.Stettler C, Wandel S, Allemann S, et al. Outcomes associated with drug-eluting and bare-metal stents: A collaborative network meta-analysis. Lancet. 2007;370:937–948. doi: 10.1016/S0140-6736(07)61444-5. [DOI] [PubMed] [Google Scholar]
- 21.Tu JV, Bowen J, Chiu M, et al. Effectiveness and safety of drug-eluting stents in Ontario. N Engl J Med. 2007;357:1393–1402. doi: 10.1056/NEJMoa071076. [DOI] [PubMed] [Google Scholar]