Abstract
Loss of Nkx3.1 function in mice results in defects in prostate development and epithelial hyperplasia, indicating that this gene plays important roles in both the initiation and maintenance of prostate differentiation. In humans, decreased NKX3.1 expression is associated with the progression of prostate cancer. Despite these roles in prostate development and disease, the transcriptional regulation of Nkx3.1 has not been systematically addressed. A reporter gene approach in transgenic mice was used to identify regulatory regions that dictate the expression pattern of Nkx3.1. A 32-kb DNA fragment from the Nkx3.1 locus that specifies the expected expression pattern during embryogenesis and postnatal life has been identified. Deletion analyses demonstrated that cis-regulatory elements that mediate expression in distinct sites are separable. A 5-kb fragment downstream of the Nkx3.1 coding region contains elements that support expression in the prostate and bulbourethral glands, whereas an upstream fragment contains elements that direct expression in somites and testes. Reporter gene expression analyses also revealed several previously unknown sites of Nkx3.1 expression in males, including urethral glands, glandular cells in the urethral diverticulum and basal epithelial cells in the prostate. In addition, these analyses revealed Nkx3.1 expression in female urethral glands. The identification of Nkx3.1 cis-regulatory elements provides a unique starting point to dissect signaling pathways involved in prostate organogenesis and pathogenesis and provides a system to perturb gene expression throughout prostate development.
Keywords: Nkx3.1, prostate development, transgenic mice, reporter gene, urethral diverticulum, cis-regulatory elements
INTRODUCTION
The NK subfamily of homeobox genes was first identified in Drosophila (Kim and Nirenberg, 1989), and subsequent analyses have identified multiple vertebrate homologs. In both insects and vertebrates, NK genes have been implicated in cell fate specification and organogenesis. The Drosophila NK-3 gene (bagpipe) is required for visceral mesoderm development (Azpiazu and Frasch, 1993), and the NK-4 gene (tinman) is required for visceral mesoderm development and heart formation from the dorsal mesoderm (Azpiazu and Frasch, 1993). Essential roles during development have also been demonstrated for several vertebrate NK genes. Nkx2.5 is critical for heart and blood vessel development and severe defects in vascular formation and hematopoiesis are also observed in Nkx2.5 mutant yolk sacs (Lyons et al., 1995; Tanaka et al., 1999a). Nkx2.1 is required for the formation of the thyroid and pituitary glands, and Nkx2.1 mutant mice also display defects in lung and brain (Kimura et al., 1996). Nkx2.2 mutant mice undergo a ventral-to-dorsal transformation in ventral neuronal patterning (Briscoe et al., 1999) and have defects in cell specification in pancreatic development (Sussel et al., 1998), whereas Nkx6.1 and Nkx6.2 regulate oligodendrocyte generation in ventral spinal cord and hindbrain (Vallstedt et al., 2005). Loss-of-function mutations in Nkx2.3 result in severe morphological abnormalities of the small intestine and spleen (Pabst et al., 1999), and disruption of Nkx3.2 results in malformation or absence of specific vertebral and cranial bones of mesodermal origin. In addition, Nkx3.2 is also required for spleen development (Lettice et al., 1999; Tribioli and Lufkin, 1999).
Nkx3.1 is a mouse homolog of Drosophila NK-3. Loss of Nkx3.1 function in mice results in reduced branching morphogenesis in the prostate, altered secretory protein production, and epithelial hyperplasia and dysplasia (Bhatia-Gaur et al., 1999; Schneider et al., 2000; Tanaka et al., 2000). The epithelial hyperplasia and dysplasia in knockout mice histologically resemble prostatic intraepithelial neoplasia, a likely precursor of prostate cancer (Abdulkadir et al., 2002; Kim et al., 2002). Moreover, human NKX3.1 maps to human chromosome 8p21 (He et al., 1997), a region frequently deleted in prostate cancer (Abate-Shen and Shen, 2000). These data suggest that Nkx3.1 plays important regulatory roles in prostate organogenesis and in prostate cancer (Shen and Abate-Shen, 2003).
The Nkx3.1 gene is expressed in a dynamic pattern during development. During embryogenesis, Nkx3.1 expression is first observed in somites and is later found in the dorsal aorta and in epithelia at multiple sites of epithelial–mesenchymal interaction. Nkx3.1 is also expressed in the emerging palatine, bulbourethral, and prostate glands (Tanaka et al., 1999b). Postnatally, Nkx3.1 is expressed in tongue epithelium, small salivary glands, prostate, bulbourethral gland, and testis (Bieberich et al., 1996; Sciavolino et al., 1997; Tanaka et al., 1999b). To date, the transcriptional regulatory elements that mediate expression in these sites have not been defined.
Transcriptional regulatory regions of several other prostate-restricted genes, including the human prostate-specific antigen (PSA) gene (Riegman et al., 1991; Cleutjens et al., 1996, 1997b; Schuur et al., 1996), the rat probasin gene (Greenberg et al., 1994; Yan et al., 1997), the rat C3(1) gene (Allison et al., 1989), the human prostatic acid phosphatase gene (Zelivianski et al., 1998, 2000), the prostatic membrane antigen gene (Watt et al., 2001), and the human kallikrein 2 gene (Murtha et al., 1993) have been identified. However, only the PSA, probasin, and C3(1) regulatory regions have been functionally analyzed in transgenic mice. These three promoters are androgen-responsive, and the initiation of their transcriptional activity has been reported to occur in the second week of postnatal life or in adult mice. The earliest of these promoters is C3(1), which is active 10 days postnatally. In contrast, the initial expression of Nkx3.1 is likely to be androgen-independent, since androgen receptor expression has not been detected in the urogenital sinus epithelium. As an early marker of prostate differentiation, the initiation of Nkx3.1 expression may provide a unique model to elucidate the signaling pathways that underlie prostate development.
In this study, we identify a region of the Nkx3.1 locus that mediates expression of a reporter gene in a manner that parallels the endogenous gene in most sites. Deletion analyses demonstrate that the cis-regulatory elements that mediate distinct expression domains are separable. The data reported here also show that Nkx3.1 is expressed in a population of basal prostate epithelial cells. Several previously unknown sites of Nkx3.1 expression in both the male and female urogenital system are also reported. These data provide the first insights into the transcriptional regulation of Nkx3.1 in vivo and identify a novel set of genetic elements that can be used to manipulate gene expression in vivo.
RESULTS
A 10-kb Region Surrounding Nkx3.1 Is Devoid of Regulatory Elements
To characterize the cis-regulatory elements of Nkx3.1, a cosmid containing 32 kb of the Nkx3.1 locus was isolated from a 129/SJ genomic library (C.J.B., unpublished data). Initially, a 10-kb fragment containing approximately 5 kb upstream and 5 kb downstream of the Nkx3.1 start codon was subcloned and the Escherichia coli reporter gene lacZ with its own polyadenylation signal was inserted directly upstream of the start codon of Nkx3.1. The resulting construct was designated NK10/lacZ (Fig. 1A). Twelve independent transgenic mice, including six transgenic embryos and six adults, were analyzed for transgene expression by X-gal staining (Table 1). No expression of the reporter gene was observed in any of the sites expected for Nkx3.1 in any of the 12 independent lines. Several lines exhibited weak expression in ectopic sites, indicating that the lacZ gene was functional in the NK10/lacZ construct (data not shown).
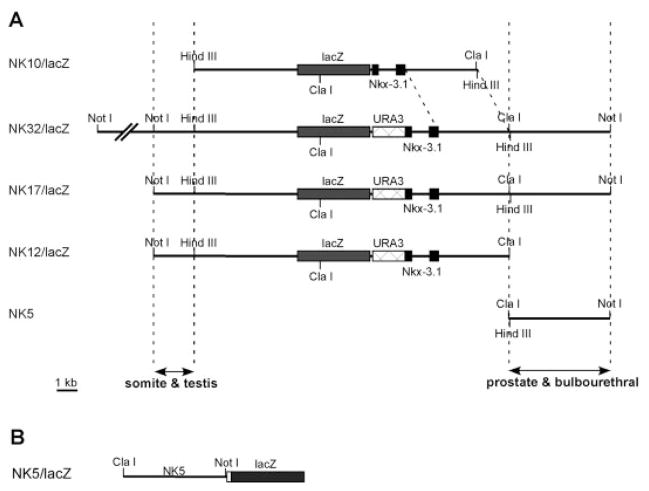
Fig. 1.
Transgene constructs. A: NK10/lacZ, reporter gene cassette inserted upstream of the start codon; NK32/lacZ, NK17/lacZ and NK12/lacZ, reporter gene cassette inserted in-frame downstream of the start codon by homologous recombination in yeast. B: NK5/lacZ, reporter gene was inserted upstream of a β-globin basal promoter (open box).
TABLE 1.
Penetrance of Reporter Gene Expression in Somites and Prostate
| Transgene | Sites/lines surveyed | LacZ-expressing lines | Description of expression |
|---|---|---|---|
| NK10/lacZ | Somites 6 | 0 | None detected |
| Prostate 6 | 0 | None detected | |
| NK32/lacZ | Somites 4 | 3 | Strong |
| Prostate 6 | 4 | Strong (3/4), patched (1/4) | |
| NK17/lacZ | Somites 1 | 1 | Strong |
| Prostate 4 | 4 | Strong | |
| NK12/lacZ | Somites 5 | 4 | Weak |
| Prostate 10 | 0 | None detected | |
| NK5/lacZ | Somites 3 | 0 | None detected |
| Prostate 7 | 5 | Moderate in all lobes (1/5); patchy or distal tip only (4/5) |
Generation of NK32/lacZ and NK17/lacZ Reporter Strains
To survey a larger region of the genome for Nkx3.1 cis-regulatory elements, a homologous recombination approach in yeast was used. The entire 32-kb insert of the Nkx3.1 cosmid was subcloned to the bacterium–yeast shuttle vector pClasper (Bradshaw et al., 1995). This 32-kb fragment contains approximately 20 kb upstream and 12 kb downstream of the start codon of Nkx3.1 (Fig. 1A). A reporter gene cassette flanked with homologous regions surrounding the start codon region of Nkx3.1 (pLZURA-Nkx) was cotransformed into yeast with pClasper/NK32, and recombinants were sequenced to confirm correct insertion of the lacZ gene. A deletion derivative (termed NK17/lacZ) lacking 15 kb of the upstream region of NK32/lacZ was also constructed. The inserts of NK32/lacZ and NK17/lacZ were released from the vector and injected into fertilized FVB/N eggs to generate transgenic mice. Southern blot analysis identified eight independent transgenic founders carrying the NK32/lacZ transgene and four carrying NK17/lacZ (Table 1). Founders were mated to FVB/N mice to derive transgenic F1 offspring.
Analysis of Reporter Gene Activity in Embryos
Whole-mount microscopic analysis of transgenic embryos carrying NK32/lacZ or NK17/lacZ revealed strong reporter gene expression in somites, a predicted site of Nkx3.1 promoter activity. One line representing each transgene was chosen for detailed temporal and spatial analyses of reporter gene expression in 7.5–15.5 days post coitum (dpc) embryos (Fig. 2). With the exception of a neuroepithelial site of expression (see below), the expression domains observed in NK32/lacZ and NK17/lacZ embryos were essentially identical. No reporter gene activity was observed in 7.5 dpc transgenic embryos. Expression was first observed in somites in 8.0 dpc embryos (Fig. 2A), indicating that the initiation of reporter gene activity occurred between 7.5 and 8.0 dpc. No reporter gene expression was observed in the unsegmented presomitic mesoderm at any stage (Fig. 2B,C). Although immediately after condensation the cell density within each somite is fairly uniform, a denser caudal compartment can soon be distinguished from a less dense rostral compartment in 8.5 dpc embryos. The initiation of β-galactosidase (β-gal) activity appeared to be coincident with this compartmentalization, with reporter gene activity becoming apparent in the caudal compartment first, then spreading to the rostral compartment. Typically, the youngest recognizable somite did not show reporter gene expression. Expression was initially restricted to the medial portion of the somite; however, the domain of expression within the somite expanded as maturation progressed.
Fig. 2.
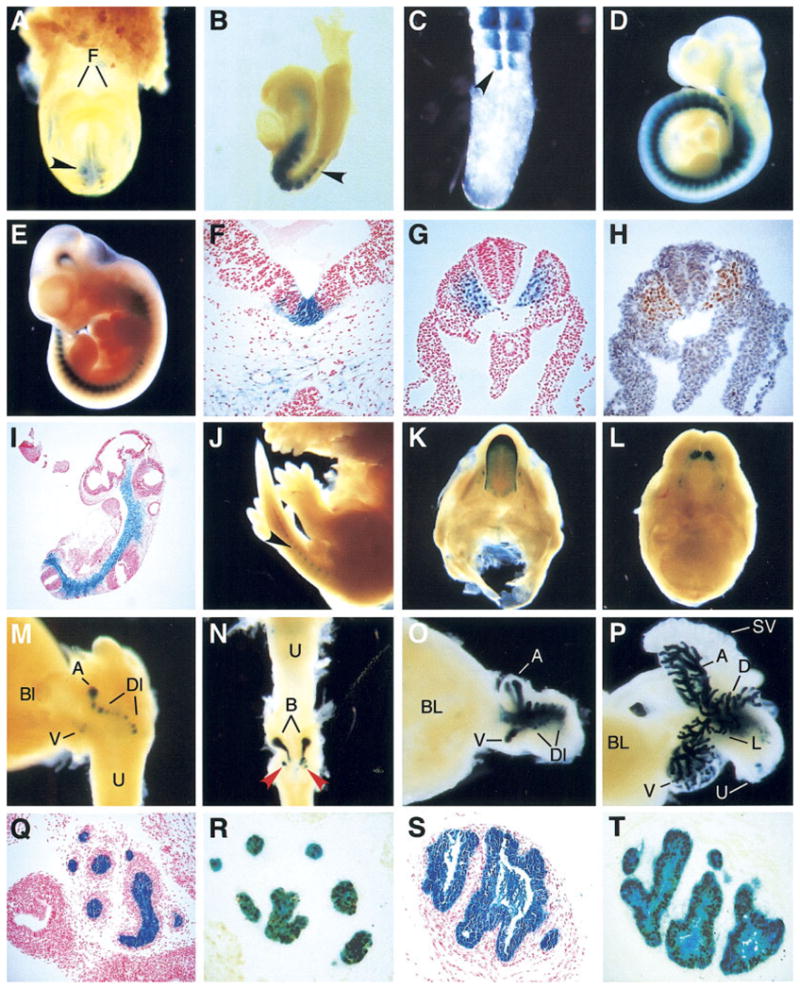
Expression of NK32/lacZ and NK17/lacZ transgenes in embryos and neonates. A: NK32/lacZ, 8.5 days post coitum (dpc), frontal view. B: NK32/lacZ, 8.5 dpc, sagittal view. C: NK32/lacZ, 11.5 dpc tail bud, dorsal view. D: NK17/lacZ, 10.5 dpc. E: NK32/lacZ, 11.5 dpc. F–I: Sections from lacZ stained NK32/lacZ embryos. F: NK32/lacZ, transverse section near the midbrain/hind brain junction, 11.5 dpc. G: NK32/lacZ, transverse section posterior to the hindlimb, 9.5 dpc embryo. H: Adjacent section, immunohistochemical (IHC) detection of Nkx3.1 (brown). I: NK32/lacZ, section, 10.5 dpc. J: NK32/lacZ, 15.5 dpc. K: NK32/lacZ, 15.5 dpc lower palate and tongue. L: NK32/lacZ, 15.5 dpc upper palate. M: NK32/lacZ, 17.5 dpc, bladder and prostatic urethra with emerging prostate buds, sagittal view. N: NK32/lacZ, postnatal day (PND) 2, urethra, bulbourethral gland, and urethral diverticulum anlage, dorsal view. O: NK32/lacZ, PND2 bladder and prostate, sagittal view. P: NK32/lacZ, PND6, bladder, prostate, and seminal vesicles, sagittal view. Q: NK32/lacZ, PND6 anterior prostate. R: Section nearby to Q, IHC detection of Nkx3.1 after X-gal staining. S: NK32/lacZ, PND6, bulbourethral gland. T: Section nearby to S, IHC detection of Nkx3.1 after X-gal staining. F,G,I,Q,S: Counterstaining with nuclear fast red. H, counterstaining with hematoxylin. H,R,T: Diaminobenzidine (DAB) used as Chromagen. A, anterior prostate lobe; B, bulbourethral gland; Bl, bladder; D, dorsal prostate lobe; Dl, dorsolateral prostate lobe; F, forebrain; L, lateral prostate lobe; SV, seminal vesicle; U, urethra. Black arrowheads indicate somites. Red arrowheads indicate the position of glandular buds in the urethral diverticulum anlage.
By 9.5 dpc, expression had clearly spread to the anterior part of the somite, but expression appeared to remain stronger in the posterior compartment (Fig. 2C). The posterior somitic expression domain also extended further dorsally than the anterior. Expression remained strong among all but the youngest somites along the body axis and was also observed in the cephalic mesenchyme extending to the junction between the forebrain and the midbrain in 8.5–11.5 dpc embryos (Fig. 2B,D,E).
Histological analyses of X-gal–stained embryos further clarified the distribution of β-gal activity in 9.5 dpc embryos. Transverse sections revealed that β-gal activity was strongest in somites in the ventromedial portion of the sclerotome, and diminished somewhat more laterally toward the dermatome (Fig. 2G). Expression was not observed in either the dermatome or the myotome. This distribution agreed well with the distribution of Nkx3.1 revealed by immunohistochemical (IHC) staining with anti-Nkx3.1 antibodies (Fig. 2H). Strong expression was also observed in nearly all of the axial mesenchyme underlying the ventral floor of the neural tube and surrounding the no-tochord and extending ventrally to the dorsal aorta (Fig. 2I). The staining in the axial mesenchyme seemed to flow uninterrupted from the sclerotome. In sections, the staining in the axial mesenchyme appeared to merge with somitic staining in the posterior and with the cephalic mesenchyme in the anterior (Fig. 2I). The net result was a continuous line of axially located mesenchymal staining from the fore-brain–midbrain junction to the tail bud. Scattered cells along the wall of the dorsal aorta showed reporter gene expression.
In 10.5 and 11.5 dpc embryos, expression continued to be strong in the cephalic mesenchyme and in all but the youngest somites (Fig. 2D,E). Expression was also observed in an area in the roof of the fourth ventricle and in a few cells at the junction between the first and second and the second and third branchial arches. Histological analyses of 11.5 dpc embryos showed a distribution similar to that seen at 9.5 dpc. Cells associated with dorsal and ventral but not lateral aspects of the dorsal aorta wall showed reporter gene expression (data not shown).
By 15.5 dpc, several new sites of β-gal activity were observed by whole-mount microscopic analysis in NK32/lacZ and NK17/lacZ embryos. Strong β-gal activity was observed in the tongue (Fig. 2K), where expression was strongest at the distal tip, decreased in a graded manner more proximally, and ended at the base of the tongue. β-Gal activity was also observed in developing molars and incisors (Fig. 2L). Reporter gene expression could also be seen in somites in the developing tail, albeit much weaker than was observed earlier (Fig. 2J). Dissection of the developing urogenital system of 15.5 dpc embryos revealed expression only in the emerging bulbourethral glands (data not shown). Expression continued in essentially the same pattern in 16.5 dpc embryos.
By 17.5 dpc, expression was observed in epithelial buds of the developing prostate (Fig. 2M). Staining was not observed in prostatic mesenchyme. Emerging ventral, anterior, and dorsolateral buds all exhibited reporter gene activity. Expression also continued in the bulbourethral glands (Fig. 2N). Several sites of expression were observed budding from the urethra at a position just distal to the developing bulbourethral glands (Fig. 2N). Multiple areas of staining were observed in this region moving into the mesenchyme surrounding the urethra. These structures most likely represent glands emerging within the urethral diverticulum anlage.
Reporter Gene Activity in Early Postnatal Life
On the day of birth, designated postnatal day 1 (PND1), β-gal activity was observed in NK32/lacZ and NK/17lacZ males in the developing prostate, bulbourethral gland, and tongue. In PND2 mice, a similar pattern was observed in the prostate (Fig. 2O), bulbourethral gland, and tongue; however, expression was also apparent in restricted areas of the urethra (data not shown). By PND 6, the prostate had undergone extensive branching (Fig. 2P) and strong β-gal expression continued along the length of the emerging ducts. Histological analyses revealed reporter gene activity confined to the epithelial component of the emerging prostate ducts (Fig. 2Q). Within the bulbourethral gland, expression was also confined to the epithelial component (Fig. 2S). To determine whether this distribution of reporter gene activity reflected the endogenous Nkx3.1 expression pattern in the developing urogenital system, immunohistochemistry was performed on histological sections of PND6 prostate and bulbourethral glands that had been analyzed as whole-mounts for β-gal activity. Near-perfect concordance was observed between cytoplasmic X-gal staining and the nuclear signal indicating Nkx3.1 immunoreactivity (Fig. 2R,T). In PND1–6 females, reporter gene activity was confined to the tongue epithelium (data not shown).
Reporter Gene Activity in the Male Urogenital System at 2–24 Weeks
To determine whether the expression of the reporter gene was maintained in later postnatal life, NK32/lacZ and NK17/lacZ transgenic animals were analyzed for reporter gene activity at various stages from 15 days through 6 months of age. Within the male urogenital system, strong activity continued to be observed in the prostate gland, urethra, the bulbourethral gland and its associated excretory ducts, and in the urethral diverticulum at the junction of the pelvic and penile urethra (Fig. 3A,B). Weak expression was observed in testis, and was confined to cells at various stages of spermatogenesis (Fig. 3C,D).
Fig. 3.
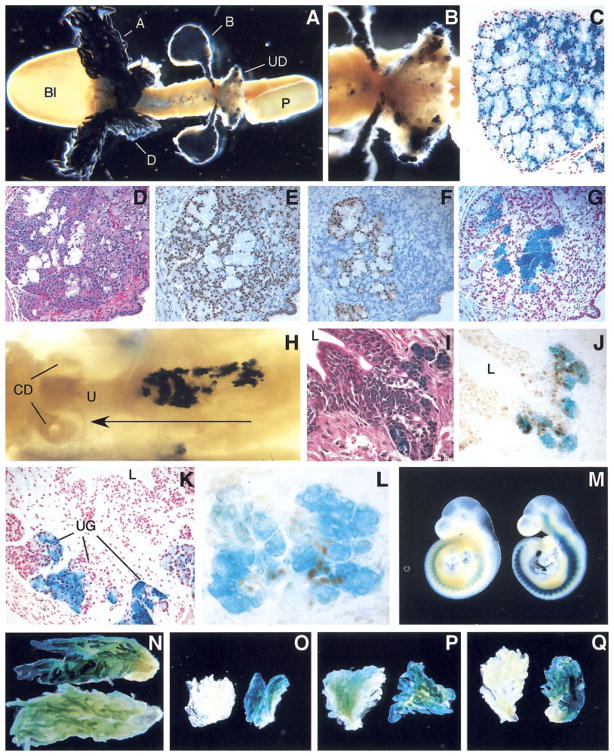
Nkx3.1 reporter gene expression in prepubertal and adult urogenital organs. A–C,E–H: Whole-mount staining of lacZ in NK32/lacZ mice. A: Postnatal day (PND) 15, urinary axis, sagittal view. B: Five-week-old bladder and prostate. C: Testis, FVB mouse (top) shown as negative control. D: NK32/lacZ testes, section counterstained with nuclear fast red. E–H: NK32/lacZ prostate lobes (right) with age-matched FVB lobes (left). E: Anterior prostate lobe. F: Dorsal prostate lobe. G: Lateral prostate lobe. H: Ventral prostate lobe. I–O: NK32/lacZ, paraffin sections of X-gal–stained adult prostate tissue. I–M: Counterstaining with nuclear fast red. I: Anterior prostate. J: Dorsal prostate. K: Lateral prostate. L: Ventral prostate. M: High magnification of adult ventral prostate section showing X-gal staining in basal cells. N: Immunohistochemical detection of Nkx3.1 in ventral prostate. O: High magnification view of section shown in N. P–R: Coimmunofluorescence detection of Nkx3.1 and p63 in adult FVB lateral prostate. P: Detection of Nkx3.1. Q: Detection p63 expression in the same section shown in P. R: Overlay of (P) and (Q).
Dissection of the prostate into anterior, dorsal, lateral, and ventral lobes showed expression in all lobes (Fig. 3E–H). Along the length of the prostatic ducts, reporter gene expression was strongest in distal tips and weakest in the most proximal regions near the junction with the urethra. Histological analyses of X-gal–stained adult prostate and bulbourethral glands revealed reporter gene activity in most luminal epithelial cells in all four prostate lobes (Fig. 3I–L) and in most epithelial cells of the bulbourethral gland (Fig. 4C). Nonexpressing cells lining the prostate ducts were also consistently observed, often in clusters.
Fig. 4.
Nkx3.1 reporter gene expression in urogenital glands and activity of the NK5/lacZ transgene. A: Adult urinary axis, dorsal view. B: High-magnification view of urethral diverticulum region shown in A. C: NK32/lacZ adult bulbourethral gland, section. D–F: Serial sections, glandular region of FVB adult urethral diverticulum wall. D: Hematoxylin and eosin (H&E) staining. E: Immunohistochemical (IHC) detection of androgen receptors (AR). F: IHC detection of Nkx3.1. G: Alcian blue staining with nuclear fast red counterstain. H: NK32/lacZ adult female distal urethra, ventral view. Arrow indicates the proximal distal axis of the urethra; the arrowhead is distal. Clitoral glands were removed for clarity, clitoral gland ducts (CD) remain. I: H&E section of an NK17/lacZ adult female distal urethra after X-gal staining. J: Adjacent section, IHC detection of Nkx3.1. K: NK32/lacZ adult male urethra, transverse section after X-gal staining showing urethral glands. L: IHC detection of endogenous Nkx3.1 in an adjacent section, high magnification view. M: Comparison of reporter gene activity in an NK12/lacZ 9.5 dpc embryo (left) and an NK17/lacZ 9.5 embryo (right). N–Q: Comparison of reporter gene activity in NK5/lacZ adult prostate lobes. Wild-type prostate lobes (bottom in N, left in O–Q) were included for comparison. N: Anterior prostate lobe. O: Dorsal prostate lobe. P: Lateral prostate lobe. Q: Ventral prostate lobe. A, anterior prostate lobe; B, bulbourethral gland; Bl, bladder; D, dorsal prostate lobe; L, lumen of urethra; U, urethra.
Expression of Nkx3.1 in Prostate Basal Epithelial Cells
Surprisingly, a significant population of cells lying beneath the luminal epithelial cells showed reporter gene expression (Fig. 3M). The location and morphology of these cells was consistent with that of basal epithelial cells. To date, the expression of Nkx3.1 has been reported to be confined to the luminal epithelial cell compartment (Bhatia-Gaur et al., 1999). To investigate the possibility that the endogenous Nkx3.1 gene was expressed in a population other than luminal epithelial cells, an IHC analysis of mouse prostate tissue was performed using affinity purified rabbit anti-mouse Nkx3.1 polyclonal antibodies. Cells with elongated nuclei arranged perpendicular to the axis of the ducts and underlying the luminal cell layer showed clear nuclear staining (Fig. 3N,O), strongly suggesting that the basally located X-gal staining observed in the NK32/lacZ prostates reflects an expression domain of the endogenous Nkx3.1 gene. The intensity of Nkx3.1 immunostaining in these nuclei was consistently lower than that observed in luminal cell nuclei.
To further explore the possibility that the Nkx3.1-expressing nonluminal cells were basal cells, lateral prostate tissue was costained with anti-mouse Nkx3.1 and anti-p63 antibodies. p63-positive cells often showed low but consistent staining for Nkx3.1 (Fig. 3P–R), demonstrating that Nkx3.1 is present in a sub-population of prostate basal epithelial cells. Similar overlap between Nkx3.1 and p63 expression were also observed in the anterior prostate (data not shown).
Expression of lacZ and Endogenous Nkx3.1 in the Urethral Diverticulum
In male rodents, the urethral diverticulum is a urethral bulb that occurs at the ischiadic arch and is surrounded by the bulbocavernosus muscle (Fig. 4A,B). Histologic examination of the diverticulum revealed proteinaceous material within the central lumen, and numerous small glands within its wall (Fig. 4D). The epithelium showed nuclear immunoreactivity for androgen receptor (AR), and stronger nuclear AR staining was observed in the embedded glands (Fig. 4E). In whole-mount preparations, a subset of these glands showed intense β-gal activity in NK32/lacZ and NK17/lacZ mice (Fig. 4A,B). To determine whether the endogenous Nkx3.1 gene was also expressed in glands within the urethral diverticulum, IHC staining for Nkx3.1 was performed on sections from FVB/N mice. Nuclear staining was apparent in cells with a mucinous appearance that was similar to the morphology of cells within the bulbourethral gland (Fig. 4F). To determine whether the Nkx3.1-expressing cells within the urethral diverticulum glands were mucin-producing, an adjacent section was stained with Alcian blue. The pattern of Alcian blue staining correlated well with the pattern of Nkx3.1 immunoreactivity (Fig. 4G). The urethral diverticulum was present in all (n ≥ 10) of the NK32/lacZ mice dissected as well as in all (n = 4) wild-type FVB/N males.
Expression of the Reporter Genes and Endogenous Nkx3.1 in Females
In adult female NK32/lacZ and NK/17 lacZ mice, strong β-gal activity was observed in the tongue epithelium (data not shown). Within the urogenital system, β-gal activity was observed in a distal portion of the urethra, approximately 2–3 mm from the urethral opening (Fig. 4H). Histologic examination of this region revealed the presence of urethral glands that were β-gal positive (Fig. 4I). To determine whether the endogenous Nkx3.1 gene was expressed in these glands, paraffin sections of X-gal–stained female urethras were analyzed by IHC staining for Nkx3.1. Nuclear immunostaining coincident with the X-gal signal was observed, indicating that the female urethral glands represent an authentic site of Nkx3.1 expression (Fig. 4J). β-Gal activity was also observed in bilaterally symmetric patches of epithelial cells lining the vaginal opening (data not shown); however, endogenous Nkx3.1 expression in these cells could not be confirmed.
Deletion Analysis to Localize Nkx3.1 cis-Regulatory Elements
The NK32/lacZ and NK17/lacZ transgenes were capable of recapitulating key features of the Nkx3.1 expression pattern. To begin to localize specific regulatory elements, further deletions were performed. The most downstream 5 kb was deleted from NK17/lacZ (Fig. 1A), and the resulting construct, termed NK12/lacZ, was used to generate 10 independent transgenic lines (Table 1). Analyses of reporter gene expression in postnatal NK12/lacZ transgenic mice revealed weak expression only in the testis. Reporter gene activity was not observed in prostate, bulbourethral glands, or other urogenital glands in any of the 10 transgenic lines. These data demonstrate that the regulatory elements that mediate testis expression are separable from those that mediate expression in urogenital glands. Embryos from five of the lines were also examined for reporter gene expression. Somitic expression was preserved in embryos from four NK12/lacZ lines, albeit at a much lower level than in either NK32/lacZ or NK17/lacZ embryos (Table 1; Fig. 4L,M). These results demonstrate that the Nkx3.1 regulatory elements required for correct spatial patterning of expression during embryogenesis are distinct from those that support expression in urogenital glands. In light of our earlier observation that the NK10/lacZ construct failed to be expressed in developing somites, the somitic expression of the NK12/lacZ construct strongly suggests that key elements required for somite expression are located within the upstream-most 2 kb of the NK12 fragment (Fig. 1A). Furthermore, the absence of reporter gene activity in urogenital glands of NK12/lacZ transgenic mice suggested that elements important for expression in prostate and bulbourethral glands were contained within the distal 5 kb that was deleted from NK17/lacZ to create NK12/lacZ (Fig. 1A).
The 5-kb Downstream Element Acts as a Urogenital Enhancer
To determine whether the 5-kb downstream region was capable of acting as an enhancer to mediate gene expression in urogenital glands, this region was cloned directly upstream of a heterologous minimal β-globin promoter to generate construct NK5/lacZ (Fig. 1B). Seven independent transgenic mice were generated carrying this construct (Table 1). Analysis of reporter gene expression in prostate glands of postnatal mice revealed uniform expression at a moderate level in the prostate of one line (Fig. 4N–O). Four of the remaining lines showed sporadic expression in a few cells within the prostate that were generally located more toward the ductal tips. For all of the lines, expression in the bulbourethral gland was similar to that observed in the prostate. These data demonstrate that the 5-kb fragment contains elements that are capable of directing expression of a heterologous promoter in the prostate and bulbourethral glands of transgenic mice.
DISCUSSION
Using a reporter gene approach in transgenic mice, we have identified regions of the Nkx3.1 locus that mediate essential features of the Nkx3.1 expression pattern. A 32-kb region of the Nkx3.1 locus was sufficient to recapitulate the pattern of expression of the endogenous Nkx3.1 gene in most of the expected sites, and detailed analyses of reporter gene expression also revealed several previously unknown sites. Deletion studies demonstrated that the transcriptional control of Nkx3.1 expression is complex and is mediated by multiple cis-acting elements that are clearly separable.
Embryonic Expression Elements
Expression was observed in developing somites and axial mesenchyme in embryos beginning between 7.5 and 8.0 dpc, in good agreement with previous reports that showed a similar time of onset (Tanaka et al., 1999b). Expression was maintained in differentiating somites throughout embryogenesis but waned as the somites developed into axial skeletal components, also in agreement with the expected pattern (Schneider et al., 2000). The expression of the endogenous Nkx3.1 gene in somitic mesoderm and axial mesenchyme has been demonstrated to be induced by the secreted signaling molecule Sonic Hedgehog (Shh; Kos et al., 1998), although it is not clear whether the regulation is direct or indirect. The 32-kb region identified by our analyses is likely to contain elements that mediate the positive response of Nkx3.1 to Shh signaling during embryogenesis. Furthermore, comparison of the expression patterns observed in NK10/lacZ and NK12/lacZ transgenic embryos demonstrated that a 2-kb sequence located 5 kb upstream of the Nkx3.1 coding region is important for mediating expression in paraxial and axial mesoderm. More detailed deletion and biochemical analyses will be required to further localize the Shh-responsive elements contained within this region. In addition, given the diminution of embryonic expression in NK12/lacZ strains compared with NK17/lacZ strains, it is clear that elements that support robust expression in somites are present in the 5-kb region that distinguishes these two constructs (Fig. 1).
Although the NK32/lacZ transgene expression pattern closely mimicked that of the endogenous Nkx3.1 gene during embryogenesis, there were several features that differed significantly. Most notably, reporter gene expression was not observed in Rathke’s pouch (Tanaka et al., 1999b). However, expression of the reporter gene directed by the 32-kb fragment was observed in a restricted area of the neuroepithelium that directly overlies Rathke’s pouch (Fig. 2I). These observations suggest that the 32-kb region of the Nkx3.1 locus assayed is missing both positively and negatively acting elements required for precise localization of Nkx3.1 expression in developing epithelial structures in the head. In addition, expression of the reporter genes along the dorsal aorta was not as robust as would be predicted by in situ hybridization analyses of Nkx3.1 expression in 9.5–11.5 dpc embryos.
Urogenital Gland Expression
Our observation that the NK32/lacZ transgene is expressed at very early stages of prostate development is of particularly high value given that the molecular basis of prostate development is currently not precisely defined. Using the Nkx3.1 regulatory region described here, it is now possible to direct gene expression into developing prostate glands in vivo to develop gain of function mutants. This approach will likely be able to shed significant new insights into the role of signaling pathways that are important for prostate differentiation by overexpressing potential ligands, or disrupting the function of receptors and signaling intermediates using dominant-negative approaches. In addition, it will be useful to generate transgenic strains expressing the bacteriophage recombinase Cre under the control on Nkx3.1 regulatory elements to enable the development of conditional mutants to study the roles of individual genes in early prostate growth and differentiation.
Although Nkx3.1 transcripts have been detected in the caudal (prostatic) region of the urogenital sinus in male 15.5 dpc embryos, expression of the Nkx3.1 reporter genes described here was not detected until 17.5 dpc. This discrepancy may reflect that the initiation of Nkx3.1 transcription at 15.5 dpc may require elements not included in the 32-kb region assayed by the reporter genes. Alternatively, the reporter gene may be weakly transcribed precluding detection of β-gal activity.
The prostate-restricted cis-regulatory elements that have been described to date in transgenic mice, PSA, probasin and C3(1), rely on high levels of circulating androgens to achieve their maximal transcriptional activity. The activity of the 6-kb PSA promoter is not detected until 8 weeks of age (Cleutjens et al., 1997a), and the 11.5-kb probasin promoter increases dramatically in activity from week 1 to 7 week of postnatal life (Yan et al., 1997). C3(1) transcriptional activity does not reach its peak level until 3 weeks of age (Zhang et al., 1988). In contrast, the Nkx3.1 regulatory region described here directs transcriptional activity in emerging prostate buds during gestation and throughout the prepubertal period of branching morphogenesis.
Analyses of the Nkx3.1 reporter mice also revealed several novel sites of Nkx3.1 expression in males, the urethral glands, and the urethral diverticulum, and endogenous Nkx3.1 expression was confirmed in both sites. The urethral glands located in the cavernous layer are believed to secrete mucus to lubricate the urethra. Our observation that Nkx3.1/lacZ activity is restricted to a subset of these urethral glands suggests that there may be functional heterogeneity among them. Alternatively, the restriction of Nkx3.1 expression to a subset may reflect different physiological states that vary in a temporal manner among the glands. The urethral diverticulum has received a dearth of attention in the urologic development literature, perhaps owing to the difficulty in dissecting this structure. Buried deep within the bulbocavernosus muscle, it is covered by a thick fibrous tunica. To our knowledge, the function of this structure has not been well defined. By virtue of the fact that it contains Nkx3.1-expressing glands, it seems likely that it bears functional similarity to the urethral and bulbourethral glands.
The expression of Nkx3.1 in female urethral glands was unexpected, and it will be of interest to determine whether these glands become hyper-plastic in Nkx3.1 knockout mice. Further experiments will be required to determine whether the epithelial cell staining observed in the vaginal opening reflects an authentic site of endogenous Nkx3.1 expression.
Deletion of the 5′-most 15 kb of the Nkx3.1 sequences contained in NK32/lacZ did not substantially alter the overall spatial distribution of reporter gene expression. However, an element that mediates the neuroepithelial expression of the reporter gene was shown to lie within this region, since NK17/lacZ embryos did not display the neuroepithelial expression domain. Perhaps most interestingly, deletion of the 15-kb region led to apparently randomized asymmetric reporter gene expression in the paired lobes of the prostate gland and in the bulbourethral glands. This observation suggests that elements that insulate the Nkx3.1 gene from left–right asymmetry may lie within the 15-kb region. Although gross left–right differences in reporter gene expression were not observed among prostate lobes of NK32/lacZ mice, it is possible that slight differences in reporter gene activity may exist. It is interesting to note that the mouse Nkx3.2 gene, with which Nkx3.1 is likely to share a common ancestor, is known to be asymmetrically expressed in lateral mesoderm during mouse embryogenesis (Schneider et al., 1999). One possible scenario is that, during the gene duplication event that gave rise to Nkx3.1, elements responsive to asymmetric signals were also duplicated. As the Nkx3.1 expression pattern evolved, these elements may have been functionally inactivated by sequences now contained within the 15-kb region.
Identification of a Urogenital Gland Enhancer
The deletion analyses suggested that a 5-kb region located downstream of the Nkx3.1 coding sequence contained an enhancer activity that could support gene expression in the prostate and bulbourethral glands. Analyses of the NK5/lacZ transgenic mice clearly showed that this region indeed harbors such elements and that they are capable of directing expression of a heterologous promoter in a tissue-restricted manner. Although the penetrance of prostate and bulbourethral expression in NK5/lacZ transgenic mice was comparable to that of strains carrying larger transgenes (5/7), the expressivity was much lower, with only one line showing moderate reporter gene activity (Table 1). These data indicate that the transcriptional activity of this construct is strongly affected by the site of transgene integration. These data further suggest that the Nkx3.1 downstream prostate and bulbourethral enhancer may exhibit a strong preference for its own promoter or that precise spacing of the enhancer and promoter is required to achieve efficient transcriptional activity. Promoter preference has been observed in transgenic analyses of cis-regulatory elements from other tissue-restricted genes, including immunoglobulin and elastase (Garcia et al., 1986; Pinkert et al., 1987). Although to our knowledge, the molecular mechanisms that underlie promoter preference of cis-regulatory regions have not been well defined, it is likely that this phenomenon reflects the complex dynamics of chromatin conformation and transcription factor access that enable precise spatial and temporal control of transcription. Although our observations provide crucial insights into the location of important regulatory elements of Nkx3.1, further deletion analyses, in combination with biochemical approaches, for example, chromatin immunoprecipitation or genomic footprinting, will be required to identify transcription factors that act through the 5-kb region to establish gene expression in the prostate and bulbourethral glands.
Whereas the transgene constructs described here are capable of directing a tissue-specific expression pattern, the expression of the transgenes was integration site-dependent and the transgene copy number did not correlate well with the expression level of the reporter gene even in NK32/lacZ, the largest transgene construct. These observations suggest that this region does not contain dominant cis-regulatory sequences similar to the locus control regions flanking the β-globin gene, which specify integration site independent expression of transgenes (Grosveld et al., 1987). It is highly likely that the transgene constructs described here are missing such regulatory sequences.
The Nkx3.1 regulatory elements described here can direct dynamic expression of the reporter gene β-galactosidase in transgenic mice that follows endogenous Nkx3.1 expression spatially and temporally. Elements responsible for different tissues are separable, suggesting that the regulatory elements are modular. This phenomenon is analogous to that observed for another NK family gene, Nkx2.5, which is the earliest marker of the cardiac lineage and plays important roles in cardiogenesis. The regulatory region of Nkx2.5 contains six different activating regions and three repressor regions, covering a 23-kb region that surrounds the Nkx2.5 gene (Schwartz and Olson, 1999). Modular regulation has also been observed in the Drosophila NK gene, tinman (Yin et al., 1997). This finding suggests that this mode of regulation may be shared among NK family members and conserved during evolution.
In this study, we also demonstrated that the Nkx3.1 gene is expressed not only in luminal epithelial cells, but also in a subpopulation of basal epithelial cells, albeit at a much lower level. Our ability to detect weak basal cell expression may be due to the fact that we used novel affinity purified antibodies raised against the N-terminus of Nkx3.1, which did not include the homeodomain. In addition, short fixation time and specific antigen retrieval conditions may have also increased the sensitivity of Nkx3.1 detection in situ. Although androgen ablation studies in rats (English et al., 1987; Evans and Chandler, 1987) have demonstrated the existence of proliferative capacity in both basal and luminal prostate epithelial cell populations, the role of each compartment in the natural history of human prostate cancer is not clear. Our observations raise the possibility that Nkx3.1 may play a role in suppressing cell proliferation in both compartments. Expression of NKX3.1 has been observed recently in a subpopulation of human basal prostate epithelial cells by immunohistochemical staining (A. DeMarzo and C. Bethel, manuscript in preparation). Further expression analyses of cytokeratins and other markers in conjunction with NKX3.1 immunostaining will be required to determine more precisely the phenotype of these cells and their relationship to prostate pathogenesis.
EXPERIMENTAL PROCEDURES
Construction of pLZURA-Nkx
Homologous recombination construct pLZURA-Nkx was constructed from pLZURA (Bradshaw et al., 1996) by inserting sequences homologous to the Nkx3.1 locus flanking the lacZ/URA3 cassette. The upstream flanking sequence was a 486-bp fragment homologous to the upstream and beginning part of the Nkx3.1 coding sequence (from −465 to +21). The downstream arm was a 236-bp fragment homologous to part of the Nkx3.1 coding sequence (from +30 to +265).
Generation of constructs NK10/lacZ, NK32/lacZ, NK17/lacZ, NK12/lacZ, and NK5/lacZ
A cosmid containing 32 kb of DNA surrounding the Nkx3.1 locus was isolated from a 129/SJ genomic library (C.J.B., unpublished data). A 10-kb HindIII fragment containing the Nkx3.1 coding region was subcloned to a modified version of pACYC184 (NEB, Beverly, MA). A unique SalI site was inserted between −12 and −13 bp (relative to the start codon) by polymerase chain reaction and a 3.7-kb SalI fragment containing a lacZ gene and SV40 polyadenylation signal from pLZRVA (Bieberich et al., 1990) was inserted into the SalI site. The transgene fragment NK10/lacZ was released from the vector by restriction digestion with HindIII.
NK32/lacZ was generated as follows: the cosmid was partially digested with NotI, and a fragment representing the entire cosmid insert was inserted into the NotI site in pClasper (Bradshaw et al., 1995) to generate pClasper/NK32. LZURA-Nkx was released from pLZURA-Nkx by digesting with XhoI and XmnI. The circular construct pClasper/NK32 and the linear LZURA-Nkx fragment were co-transformed into yeast strain YPH857 (MATa, ura3-52, lys2-801, ade2-101, his3Δ200, trp1Δ63, leu2Δ1, cyh2r; Spencer et al., 1993) for homologous recombination. Recombinants were selected on synthetic dropout medium lacking leucine and uracil. Total DNA was extracted from the Ura+Leu+ potential recombinants and transformed into E. coli. The bacterial transformants were selected on chloramphen-icol-containing LB plates. Plasmids were prepared from the bacterial transformants and digested with restriction enzymes to confirm recombination. The new construct, named pClasper/NK32/lacZ, was digested with I-SceI (New England Biolab, Beverly, MA) to release the NK32/lacZ transgene.
To generate NK17/lacZ, NK32/lacZ was digested with NotI, which generated a 15-kb fragment upstream and NK17/lacZ. The mixture was inserted into NotI-digested pClasper. The resulting plasmids were digested with restriction enzymes to identify the pClaper/NK17/lacZ construct. The pClasper/NK17/lacZ construct was digested with NotI to release the NK17/lacZ transgene.
To generate NK12/lacZ, NK17/lacZ was partially digested with ClaI. A modified version of pClasper, in which the ClaI site in the plasmid backbone had been destroyed and a new ClaI site inserted into the BamHI site, was used to clone the partially digested NK17/lacZ products. The modified pClasper was digested with NotI and ClaI was ligated with the partially digested NK17/lacZ. The resulting pClasper/NK12/lacZ was digested with NotI to release the NK12/lacZ transgene.
To generate NK5/lacZ, the pClasper/NK17lacZ fragment was digested with NotI and ClaI. A 5-kb NotI–ClaI fragment was inserted upstream of a β-globin basal promoter followed by the lacZ gene (Yee and Rigby, 1993). NK5/lacZ was released from the construct by NotI digestion.
Transgenic Mouse Generation and Genotyping
Transgene fragments were separated from vector sequences by sucrose gradient and dialyzed as described (Bieberich et al., 1990). The fragments were injected into FVB single-cell embryos, and transgenic mice were generated as described (Gordon and Ruddle, 1983). Founders or transgenic offspring were identified by Southern blot analyses. Genomic DNA was extracted from embryo yolk sacs or mouse tails and digested with BamHI. For NK32/lacZ, NK17/lacZ, NK12/lacZ, a 570-bp PstI–EcoRI fragment in the Nkx3.1 intron was used as the probe for Southern blot analysis. For NK5, a 280-bp ClaI–HindIII fragment at the 5′ end of NK5 was used as the probe for Southern blot analysis.
Detection of β-Gal Activity
Embryos or tissues were fixed before staining for β-gal activity. Embryos younger than 9.0 dpc were fixed with 0.25% glutaraldehyde in PBS for 20 min. Embryos older than 9.0 dpc and other tissues were fixed with 4% para-formaldehyde for 30 min. The staining was performed essentially as described (Bieberich et al., 1990) with 5 mM potassium ferricyanide and 4.5 mM potassium ferrocyanide in the staining cocktail.
Generation of Rabbit Anti-Nkx3.1 Antibodies
The N-terminus of the Nkx3.1 protein (amino acids 1–127) was fused in frame to a 6XHis tag. The recombinant protein was expressed in E. coli and purified by Ni+ column chromatography. The recombinant protein was used to immunize rabbits (Covance, Inc., Princeton, NJ). Antisera were purified first using a protein A column followed by an Nkx3.1 affinity column (X.L. and C.J.B.).
Immunohistochemistry
Transgenic organs were fixed with 4% paraformaldehyde (PFA) for 30 min, stained for β-gal activity, embedded, and sectioned as described above. Sections were deparaffinized with xylene and rehydrated. Sections were then microwaved three times for 5 min each in 1 mM ethylenediaminetetraacetic acid (EDTA) pH 8.0 for antigen retrieval. The sections were stained for endogenous Nkx3.1 expression using the Dako Envision Plus System (anti-rabbit, peroxidase), following the manufacturer’s recommendations (DakoCytomation, Carpinteria, CA). Affinity-purified rabbit anti-mouse Nkx3.1 antibody was used as the primary antibody. The antibody was diluted 1:1,000 in 1% bovine serum albumin, 50 mM Tris HCl and incubated at room temperature for 2 hr. After diaminobenzidine staining, the slides were dehydrated and mounted with Permount or the slides were counter-stained with nuclear fast red (Molecular Probe, Eugene, OR) and mounted. For Nkx3.1 and p63 double staining, after antigen retrieval, slides were first incubated with anti-p63 mouse monoclonal antibody clone 4A4+Y4A3 (Lab Vision, Fremont, CA) using a Mouse on Mouse (M.O.M.) Fluorescein Kit (Vector Laboratories, Burlingame, CA). Slides were then sequentially incubated with anti-Nkx3.1 antibodies as described above and with a biotinylated anti-rabbit secondary (1:1,000; Vector Labs). Nkx3.1 imunoreactivity was detected using Texas Red Avidin DCS (1:1,000; Vector Labs). Nontransgenic tissues were fixed in 4% PFA and directly processed for paraffin embedding.
Acknowledgments
The authors thank Sandy Mason for excellent animal care and Carl Pink-ert for helpful discussions.
Grant sponsor: National Institutes of Health, NIDDK; Grant number: DK059152.
References
- Abate-Shen C, Shen MM. Molecular genetics of prostate cancer. Genes Dev. 2000;14:2410–2434. doi: 10.1101/gad.819500. [DOI] [PubMed] [Google Scholar]
- Abdulkadir SA, Magee JA, Peters TJ, Kaleem Z, Naughton CK, Humphrey PA, Milbrandt J. Conditional loss of Nkx3.1 in adult mice induces prostatic intraepithelial neoplasia. Mol Cell Biol. 2002;22:1495–1503. doi: 10.1128/mcb.22.5.1495-1503.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison J, Zhang YL, Parker MG. Tissue-specific and hormonal regulation of the gene for rat prostatic steroid-binding protein in transgenic mice. Mol Cell Biol. 1989;9:2254–2257. doi: 10.1128/mcb.9.5.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azpiazu N, Frasch M. tinman and bagpipe: two homeo box genes that determine cell fates in the dorsal mesoderm of Drosophila. Genes Dev. 1993;7:1325–1340. doi: 10.1101/gad.7.7b.1325. [DOI] [PubMed] [Google Scholar]
- Bhatia-Gaur R, Donjacour AA, Sciavolino PJ, Kim M, Desai N, Young P, Norton CR, Gridley T, Cardiff RD, Cunha GR, Abate-Shen C, Shen MM. Roles for Nkx3.1 in prostate development and cancer. Genes Dev. 1999;13:966–977. doi: 10.1101/gad.13.8.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieberich CJ, Utset MF, Awgulewitsch A, Ruddle FH. Evidence for positive and negative regulation of the Hox-3.1 gene. Proc Natl Acad Sci U S A. 1990;87:8462–8466. doi: 10.1073/pnas.87.21.8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieberich CJ, Fujita K, He WW, Jay G. Prostate-specific and androgen-dependent expression of a novel homeobox gene. J Biol Chem. 1996;271:31779–31782. doi: 10.1074/jbc.271.50.31779. [DOI] [PubMed] [Google Scholar]
- Bradshaw MS, Bollekens JA, Ruddle FH. A new vector for recombination-based cloning of large DNA fragments from yeast artificial chromosomes. Nucleic Acids Res. 1995;23:4850–4856. doi: 10.1093/nar/23.23.4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw MS, Shashikant CS, Belting HG, Bollekens JA, Ruddle FH. A long-range regulatory element of Hoxc8 identified by using the pClasper vector. Proc Natl Acad Sci U S A. 1996;93:2426–2430. doi: 10.1073/pnas.93.6.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe J, Sussel L, Serup P, Hartigan-O’Connor D, Jessell TM, Rubenstein JL, Ericson J. Homeobox gene Nkx2.2 and specification of neuronal identity by graded Sonic hedgehog signalling. Nature. 1999;398:622–627. doi: 10.1038/19315. [DOI] [PubMed] [Google Scholar]
- Cleutjens KB, van Eekelen CC, van der Korput HA, Brinkmann AO, Trapman J. Two androgen response regions cooperate in steroid hormone regulated activity of the prostate-specific antigen promoter. J Biol Chem. 1996;271:6379–6388. doi: 10.1074/jbc.271.11.6379. [DOI] [PubMed] [Google Scholar]
- Cleutjens KB, van der Korput HA, Ehren-van Eekelen CC, Sikes RA, Fasciana C, Chung LW, Trapman J. A 6-kb promoter fragment mimics in transgenic mice the prostate-specific and androgen-regulated expression of the endogenous prostate-specific antigen gene in humans. Mol Endocrinol. 1997a;11:1256–1265. doi: 10.1210/mend.11.9.9974. [DOI] [PubMed] [Google Scholar]
- Cleutjens KB, van der Korput HA, van Eekelen CC, van Rooij HC, Faber PW, Trapman J. An androgen response element in a far upstream enhancer region is essential for high, androgen-regulated activity of the prostate-specific antigen promoter. Mol Endocrinol. 1997b;11:148–161. doi: 10.1210/mend.11.2.9883. [DOI] [PubMed] [Google Scholar]
- English HF, Santen RJ, Isaacs JT. Response of glandular versus basal rat ventral prostatic epithelial cells to androgen withdrawal and replacement. Prostate. 1987;11:229–242. doi: 10.1002/pros.2990110304. [DOI] [PubMed] [Google Scholar]
- Evans GS, Chandler JA. Cell proliferation studies in rat prostate. I. The proliferative role of basal and secretory epithelial cells during normal growth. Prostate. 1987;10:163–178. doi: 10.1002/pros.2990100208. [DOI] [PubMed] [Google Scholar]
- Garcia JV, Bich-Thuy LT, Stafford J, Queen C. Synergism between immunoglobulin enhancers and promoters. Nature. 1986;322:383–385. doi: 10.1038/322383a0. [DOI] [PubMed] [Google Scholar]
- Gordon JW, Ruddle FH. Gene transfer into mouse embryos: production of transgenic mice by pronuclear injection. Methods Enzymol. 1983;101:411–433. doi: 10.1016/0076-6879(83)01031-9. [DOI] [PubMed] [Google Scholar]
- Greenberg NM, DeMayo FJ, Sheppard PC, Barrios R, Lebovitz R, Finegold M, Angelopoulou R, Dodd JG, Duckworth ML, Rosen JM, et al. The rat probasin gene promoter directs hormonally and developmentally regulated expression of a heterologous gene specifically to the prostate in transgenic mice. Mol Endocrinol. 1994;8:230–239. doi: 10.1210/mend.8.2.8170479. [DOI] [PubMed] [Google Scholar]
- Grosveld F, van Assendelft GB, Greaves DR, Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987;51:975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- He WW, Sciavolino PJ, Wing J, Augustus M, Hudson P, Meissner PS, Curtis RT, Shell BK, Bostwick DG, Tindall DJ, Gelmann EP, Abate-Shen C, Carter KC. A novel human prostate-specific, androgen-regulated homeobox gene (NKX3.1) that maps to 8p21, a region frequently deleted in prostate cancer. Genomics. 1997;43:69–77. doi: 10.1006/geno.1997.4715. [DOI] [PubMed] [Google Scholar]
- Kim Y, Nirenberg M. Drosophila NK-homeobox genes. Proc Natl Acad Sci U S A. 1989;86:7716–7720. doi: 10.1073/pnas.86.20.7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Bhatia-Gaur R, Banach-Petrosky WA, Desai N, Wang Y, Hayward SW, Cunha GR, Cardiff RD, Shen MM, Abate-Shen C. Nkx3.1 mutant mice recapitulate early stages of prostate carcinogenesis. Cancer Res. 2002;62:2999–3004. [PubMed] [Google Scholar]
- Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox CH, Ward JM, Gonzalez FJ. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;10:60–69. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- Kos L, Chiang C, Mahon KA. Medio-lateral patterning of somites: multiple axial signals, including Sonic hedgehog, regulate Nkx-3.1 expression. Mech Dev. 1998;70:25–34. doi: 10.1016/s0925-4773(97)00168-8. [DOI] [PubMed] [Google Scholar]
- Lettice LA, Purdie LA, Carlson GJ, Kilanowski F, Dorin J, Hill RE. The mouse bagpipe gene controls development of axial skeleton, skull, and spleen. Proc Natl Acad Sci U S A. 1999;96:9695–9700. doi: 10.1073/pnas.96.17.9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons I, Parsons LM, Hartley L, Li R, Andrews JE, Robb L, Harvey RP. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2–5. Genes Dev. 1995;9:1654–1666. doi: 10.1101/gad.9.13.1654. [DOI] [PubMed] [Google Scholar]
- Murtha P, Tindall DJ, Young CY. Androgen induction of a human prostate-specific kallikrein, hKLK2: characterization of an androgen response element in the 5′ promoter region of the gene. Biochemistry. 1993;32:6459–6464. doi: 10.1021/bi00076a020. [DOI] [PubMed] [Google Scholar]
- Pabst O, Zweigerdt R, Arnold HH. Targeted disruption of the homeobox transcription factor Nkx2–3 in mice results in postnatal lethality and abnormal development of small intestine and spleen. Development. 1999;126:2215–2225. doi: 10.1242/dev.126.10.2215. [DOI] [PubMed] [Google Scholar]
- Pinkert CA, Ornitz DM, Brinster RL, Palmiter RD. An albumin enhancer located 10 kb upstream functions along with its promoter to direct efficient, liver-specific expression in transgenic mice. Genes Dev. 1987;1:268–276. doi: 10.1101/gad.1.3.268. [DOI] [PubMed] [Google Scholar]
- Riegman PH, Vlietstra RJ, van der Korput JA, Brinkmann AO, Trapman J. The promoter of the prostate-specific antigen gene contains a functional androgen responsive element. Mol Endocrinol. 1991;5:1921–1930. doi: 10.1210/mend-5-12-1921. [DOI] [PubMed] [Google Scholar]
- Schneider A, Mijalski T, Schlange T, Dai W, Overbeek P, Arnold HH, Brand T. The homeobox gene NKX3.2 is a target of left-right signalling and is expressed on opposite sides in chick and mouse embryos. Curr Biol. 1999;9:911–914. doi: 10.1016/s0960-9822(99)80397-2. [DOI] [PubMed] [Google Scholar]
- Schneider A, Brand T, Zweigerdt R, Arnold H. Targeted disruption of the Nkx3.1 gene in mice results in morphogenetic defects of minor salivary glands: parallels to glandular duct morphogenesis in prostate. Mech Dev. 2000;95:163–174. doi: 10.1016/s0925-4773(00)00355-5. [DOI] [PubMed] [Google Scholar]
- Schuur ER, Henderson GA, Kmetec LA, Miller JD, Lamparski HG, Henderson DR. Prostate-specific antigen expression is regulated by an upstream enhancer. J Biol Chem. 1996;271:7043–7051. doi: 10.1074/jbc.271.12.7043. [DOI] [PubMed] [Google Scholar]
- Schwartz RJ, Olson EN. Building the heart piece by piece: modularity of cis-elements regulating Nkx2–5 transcription. Development. 1999;126:4187–4192. doi: 10.1242/dev.126.19.4187. [DOI] [PubMed] [Google Scholar]
- Sciavolino PJ, Abrams EW, Yang L, Austenberg LP, Shen MM, Abate-Shen C. Tissue-specific expression of murine Nkx3.1 in the male urogenital system. Dev Dyn. 1997;209:127–138. doi: 10.1002/(SICI)1097-0177(199705)209:1<127::AID-AJA12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Shen MM, Abate-Shen C. Roles of the Nkx3.1 homeobox gene in prostate organogenesis and carcinogenesis. Dev Dyn. 2003;228:767–778. doi: 10.1002/dvdy.10397. [DOI] [PubMed] [Google Scholar]
- Spencer F, Ketner G, Connelly C, Hieter P. Targeted recombination-based cloning and manipulation of large DNA segments in yeast. Methods: A Comp Methods Enzymol. 1993;5:161–175. [Google Scholar]
- Sussel L, Kalamaras J, Hartigan-O’Connor DJ, Meneses JJ, Pedersen RA, Rubenstein JL, German MS. Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development. 1998;125:2213–2221. doi: 10.1242/dev.125.12.2213. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Chen Z, Bartunkova S, Yamasaki N, Izumo S. The cardiac homeobox gene Csx/Nkx2.5 lies genetically upstream of multiple genes essential for heart development. Development. 1999a;126:1269–1280. doi: 10.1242/dev.126.6.1269. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Lyons GE, Izumo S. Expression of the Nkx3.1 homeobox gene during pre and postnatal development. Mech Dev. 1999b;85:179–182. doi: 10.1016/s0925-4773(99)00084-2. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Komuro I, Inagaki H, Jenkins NA, Copeland NG, Izumo S. Nkx3.1, a murine homolog of Drosophila bagpipe, regulates epithelial ductal branching and proliferation of the prostate and palatine glands. Dev Dyn. 2000;219:248–260. doi: 10.1002/1097-0177(2000)9999:9999<::aid-dvdy1054>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- Tribioli C, Lufkin T. The murine Bapx1 homeobox gene plays a critical role in embryonic development of the axial skeleton and spleen. Development. 1999;126:5699–5711. doi: 10.1242/dev.126.24.5699. [DOI] [PubMed] [Google Scholar]
- Vallstedt A, Klos JM, Ericson J. Multiple dorsoventral origins of oligodendrocyte generation in the spinal cord and hindbrain. Neuron. 2005;45:55–67. doi: 10.1016/j.neuron.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Watt F, Martorana A, Brookes DE, Ho T, Kingsley E, O’Keefe DS, Russell PJ, Heston WD, Molloy PL. A tissue-specific enhancer of the prostate-specific membrane antigen gene, folh1. Genomics. 2001;73:243–254. doi: 10.1006/geno.2000.6446. [DOI] [PubMed] [Google Scholar]
- Yan Y, Sheppard PC, Kasper S, Lin L, Hoare S, Kapoor A, Dodd JG, Duckworth ML, Matusik RJ. Large fragment of the probasin promoter targets high levels of transgene expression to the prostate of transgenic mice. Prostate. 1997;32:129–139. doi: 10.1002/(sici)1097-0045(19970701)32:2<129::aid-pros8>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Yee SP, Rigby PW. The regulation of myogenin gene expression during the embryonic development of the mouse. Genes Dev. 1993;7:1277–1289. doi: 10.1101/gad.7.7a.1277. [DOI] [PubMed] [Google Scholar]
- Yin Z, Xu XL, Frasch M. Regulation of the twist target gene tinman by modular cis-regulatory elements during early mesoderm development. Development. 1997;124:4971–4982. doi: 10.1242/dev.124.24.4971. [DOI] [PubMed] [Google Scholar]
- Zelivianski S, Comeau D, Lin MF. Cloning and analysis of the promoter activity of the human prostatic acid phosphatase gene. Biochem Biophys Res Commun. 1998;245:108–112. doi: 10.1006/bbrc.1998.8386. [DOI] [PubMed] [Google Scholar]
- Zelivianski S, Larson C, Seberger J, Taylor R, Lin M. Expression of human prostatic acid phosphatase gene is regulated by upstream negative and positive elements. Biochim Biophys Acta. 2000;1491:123–132. doi: 10.1016/s0167-4781(00)00037-3. [DOI] [PubMed] [Google Scholar]
- Zhang YL, Zhou ZX, Zhang YD, Parker MG. Expression of androgen receptors and prostatic steroid-binding protein during development of the rat ventral prostate. J Endocrinol. 1988;117:361–366. doi: 10.1677/joe.0.1170361. [DOI] [PubMed] [Google Scholar]