Summary
We hypothesized that sirolimus, an mTOR inhibitor, may be effective in patients with autoimmune lymphoproliferative syndrome (ALPS) and treated patients who were intolerant to or failed other therapies. Four patients were treated for autoimmune cytopenias; all had a rapid complete or near complete response. Two patients were treated for autoimmune arthritis and colitis, demonstrating marked improvement. Three patients had complete resolution of lymphadenopathy and splenomegaly and all patients had a reduction in double negative T cells, a population hallmark of the disease. Based on these significant responses, we recommend that sirolimus be considered as second-line therapy for patients with steroid-refractory disease.
Keywords: mTOR, autoimmunity, signal transduction, rapamycin
Autoimmune lymphoproliferative syndrome (ALPS) is a disorder of disrupted lymphocyte homeostasis caused by defective Fas-mediated apoptosis. As part of the down-regulation of the immune response, activated B lymphocytes normally up-regulate Fas expression and activated B and T lymphocytes up-regulate expression of Fas-ligand (Nagata & Golstein, 1995). The binding of Fas to Fas-ligand triggers the caspase cascade, leading to cellular apoptosis. Patients with ALPS have a defect in this extrinsic apoptotic pathway, leading to chronic lymphoproliferation, autoimmune manifestations and a propensity to develop secondary malignancies (Bleesing et al, 2000).
Currently, a patient must meet three diagnostic criteria for ALPS: (i) clinically identifiable chronic non-malignant lymphoproliferation; (ii) an increased number of double-negative T cells (DNTs; cell phenotype CD4−/CD8−, CD3+, TCRαβ+) and (iii) in vitro evidence of defective Fas-mediated apoptosis (Bleesing et al, 2000). Supporting evidence for the diagnosis includes genetic mutations in the Fas pathway (FAS, FAS LG, CASP8, or CASP10), systemic autoimmunity, elevated serum levels of interleukin-10 (IL-10), elevated serum vitamin B12 and hypergammaglobulinaemia; however, these latter findings are not diagnostic (Rieux-Laucat et al, 2003).
Most patients with ALPS develop autoimmunity, usually manifested as autoimmune cytopenias. Other autoimmune manifestations are seen less frequently, including autoimmune nephritis, hepatitis, arthritis, colitis, uveitis and urticaria (Sneller et al, 1997). Some patients with ALPS require no treatment; however, many other patients require medications directed toward autoimmune manifestations, particularly autoimmune cytopenias. Patients usually respond to short bursts of immunosuppressive medications, including corticosteroids (Bleesing et al, 2000). Occasionally patients with severe cytopenias need more aggressive immunosuppression. Anecdotal reports and small series have described responses to a number of medications, including cyclosporine, mycophenolate mofetil (MMF), vincristine and rituximab (Ferrer et al, 2007). Nevertheless, these agents are often ineffective, have significant side effects and are non-specific for ALPS.
Sirolimus (rapamycin) is an immunosuppressive agent that targets the mammalian target of rapamycin (mTOR) and has been shown to induce apoptosis in both normal and abnormal lymphocytes. We hypothesized that targeting the mTOR pathway might be effective in patients with ALPS by inducing apoptosis in the abnormal lymphocytes underlying this condition. We previously tested this hypothesis using two murine models of ALPS (CBA-lprcg and MRL-lpr) and found sirolimus was more effective than conventional therapies, including MMF (Teachey et al, 2006). Based on these results, we began treating children with refractory ALPS with sirolimus, targeting a serum trough level of 4–15 ng/ml. We have treated six patients and found marked improvement in autoimmune disease markers in all of these patients.
Results
Patients with ALPS were followed under an Institutional Review Board-approved classification protocol that allowed data collection, including medication response. We treated six male ALPS patients with sirolimus. Patients were started on sirolimus at 2–3 mg/m2/d, rounding to the nearest 0·5 mg with maximum initial dose of 4 mg. Doses were adjusted to achieve a steady-state serum trough of 4–15 ng/ml. Three of these patients were type Ia because they have a documented mutation in FAS. The other three were classified as type III because no genetic mutation was found. Four corticosteroid-refractory or intolerant patients were treated for severe chronic autoimmune cytopenias. Three of the four patients had complete resolution of autoimmune cytopenias (Table I and Fig 1). The fourth patient had near complete resolution with a residual mild thrombocytopenia (platelet count >100 × 109/l). All four patients were able to completely taper off steroids within 4 weeks of initiating sirolimus. These patients have been treated for 5, 7, 17 and 27 months. Many of these patients had failed other immunosuppressive medications, including MMF (Table I). Two of the six patients were treated with sirolimus for autoimmune arthritis and colitis for 6 and 36 months respectively. Both of these patients had marked improvement in their symptoms on sirolimus after failing multiple other immunosuppressant medications. One of these patients remains on low dose every other day steroids (Patient 5) without toxicity. Prior to sirolimus he required high dose steroids (1–2 mg/kg/d) with significant side effects. The other patient (Patient 6) required re-initiation of steroids after one year and other more aggressive immunosuppressive regimens were tried, including systemic chemotherapy (mercaptopurine, cyclophosphamide and methotrexate) and biological agents (anakindra, etanercept, rituximab and MMF) with and without sirolimus. No regimen controlled his disease as well as a combination of sirolimus and steroids and whenever his sirolimus was held for alternative regimens his disease would flare significantly. Thus, he has been continued on chronic sirolimus.
Table I.
Patient characteristics and sirolimus response.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
|---|---|---|---|---|---|---|
| Age at diagnosis |
4 years | 20 months | 6 years | 4 years | 8 years | 8 years |
| LPD | Cervical (massive), auxiliary LAD; splenomegaly |
Anterior cervical, submandibular, inguinal LAD |
Cervical, axillary LAD; splenomegaly |
Cervical LAD; splenomegaly |
Splenomegaly | Mesenteric LAD, splenomegaly |
| AID | Trilineage Cytopenias (primarily ITP) |
Trilineage Cytopenias |
Trilineage Cytopenias |
Cytopenias (AIN, ITP) |
Arthritis; colitis; urticaria;mild cytopenias |
Arthritis; colitis; pancreatitis; cerebellar syndrome; BOOP; mild cytopenias |
| Prior failed or intolerant therapy |
IVIG, corticosteroids, MMF, rituximab |
Corticosteroids | Corticosteroids, hydroxychloroquine, methotrexate, MMF |
Corticosteroids | Corticosteroids, hydroxychloroquine, mercaptopurine, MMF |
Corticosteroids, MMF, methotrexate, tacrolimus, CSA, rituximab |
| Indication for sirolimus |
Refractory cytopenias |
Steroid-dependent ITP |
Refractory cytopenias |
Steroid intolerance |
Refractory arthritis and colitis; steroid intolerance |
Refractory arthritis and colitis; steroid intolerance |
| Age at treatment |
15 years | 5 years | 11 years | 18 years | 14 years | 22 years |
| Duration of sirolimus |
17+ months | 5+ months | 7+ months | 27+ months | 6+ months | 36+ months |
| Outcome | ||||||
| LPD | Near-CR* | Not evaluable | CR | LAD – no change; mild improvement in splenomegaly |
CR | CR |
| AID | CR | CR | CR† | Near-CR‡ | Near-CR‡ | Initial CR with relapse one year later |
| Toxicity | Grade I mucositis – intermittent |
Grade I hypertension |
None | Grade II mucositis – resolved |
None | Thrush; pulmonary embolism§ |
LPD, lymphoproliferative disease; AID, autoimmune disease; LAD, lymphadenopathy; AIN, autoimmune neutropenia; ITP, immune thrombocytopenic purpura; BOOP, bronchiolitis obliterans with organizing pneumonia; IVIG, intravenous immune globulin; MMF, mycophenolate mofetil; CSA, cyclosporine A; CR, complete response.
Patient 1 had single residual lymph node measuring 0·5 cm.
Patient 3 also had recurrent oral Herpes simplex virus flares and Epstein–Barr virus reactivation that resolved with initiation of sirolimus.
Sirolimus can cause mild thrombocytopenia from bone marrow suppression. Patients 4 and 5 had mild residual thrombocytopenias (platelet count >100 × 109/l) that could be immune-mediated or a drug side effect.
Patient 6 developed a pulmonary embolism while on steroids and sirolimus, most likely caused by steroids and inflammatory nature of his disease. Sirolimus is not reported to increase the risk of venous thromboembolism without concurrent microangiopathy. He had a negative anti-phospholipid antibody screen and no evidence of microangiopathy.
Fig. 1.
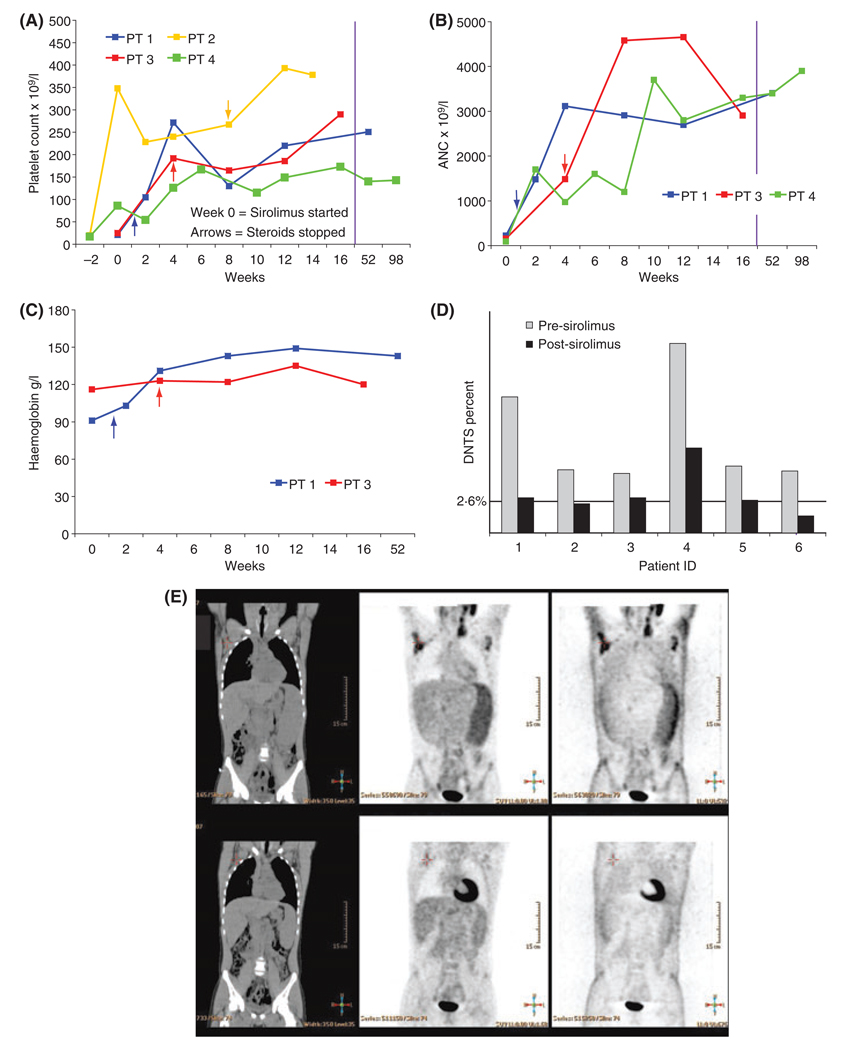
Sirolimus improves lymphoproliferation, autoimmune cytopenias and double negative T cells in patients with ALPS. Four patients were treated with sirolimus for autoimmune cytopenias. Panels depict changes in platelet count (A), ANC (B) and haemoglobin (C). Patients 1 and 3 had autoimmune pancytopenia. Patient 2 only had autoimmune thrombocytopenia. Patient 4 had autoimmune neutropenia and thrombocytopenia. The x-axis depicts time in weeks with data for every 2-week time points if available. NOTE change in scale after 16 weeks demarcated by vertical purple line. Week 0 (zero) is the time sirolimus was initiated. Arrows depict the time points when steroids were stopped. Panel D depicts peripheral blood DNTS (Double negative T cells; T cell phenotype: CD3+, CD4−, CD8−, TCRa/b+). DNTs were collected prior to initiating sirolimus and on therapy. All patients demonstrated significant reduction in DNTs. Post-treatment data point represents time 4–8 weeks after steroids stopped except for Patient 5 who had his level obtained on low dose steroids (5 mg every other day). Panel E depicts Fluorodeoxyglucose (FDG) PET–CT imaging, demonstrating a marked improvement in lymphadenopathy and splenomegaly in Patient 1 with ALPS after 3 months of treatment with sirolimus. Improvement was demonstrated in size of lymph nodes and spleen and in FDG-uptake. Upper panels represent pre-treatment (marked by arrows) and lower panels represent post-treatment.
Five patients had significant lymphoproliferation (lymphadenopathy and/or splenomegaly) at the initiation of sirolimus therapy. One patient’s lymphoproliferation was controlled with steroids and thus he had no measurable lymphadenopathy or splenomegaly when sirolimus was initiated; he has not developed lymphoproliferation on sirolimus after stopping steroids. Of the five patients with lymphoproliferation at the time of sirolimus initiation, one patient had minimal response (no change in lymphadenopathy and mild improvement in splenomegaly), three patients had a complete response and one patient had a near-complete response with one 0·5-cm residual cervical lymph node. One patient had positive disease on a positron emission tomography (PET)/computed tomography (CT) scan immediately prior to initiating sirolimus; a follow-up scan after 3 months of treatment demonstrated resolution of PET-avid disease (Fig 1). We know of no other reported therapy producing such a dramatic response in refractory ALPS patients. As a biological marker of disease response, we compared peripheral blood DNTs prior to and after starting therapy with sirolimus. The percentage of DNTs was reduced by >50% in five of six patients (Fig 1) with an average decrease of 60% (P = 0·02) (range 41–74%). Of note, despite the reduction in DNTs, no patient had a reduction in total absolute lymphocyte count (ALC). Three patients were mildly lymphopenic at the initiation of sirolimus and two of these (Patients 1 and 3) had improvement of ALC to the normal range. No grade 3 or 4 toxicities were observed in any patient.
Discussion
Sirolimus (rapamycin) is a Federal Drug Administration-approved immunosuppressant that targets mTOR. Sirolimus has been in clinical use for over 20 years and its toxicities are well-described (Abdel-Karim & Giles, 2008). We hypothesized that mTOR inhibitors would be effective in patients with ALPS for three compelling reasons: (i) mTOR inhibitors induce cell death and apoptosis in abnormal lymphocytes; (ii) mTOR inhibitors, unlike most other immunosuppressive medications, increase peripheral blood regulatory T cells (Tregs) and (iii) mTOR inhibitors are safe and well-tolerated.
Common toxicities found in patients taking sirolimus include hypercholesterolemia, hypertension and mucositis (Abdel-Karim & Giles, 2008). An increased risk of infections is very rare when used as a single agent; however, when combined with other immunosuppressive agents the risk increases. Sirolimus requires therapeutic drug monitoring to maximize effect and avoid toxicity.
Treatment for patients with refractory ALPS is challenging. Many patients do not require chronic treatment, but for those patients who do there are only a few effective and tolerable agents. Currently a number of more targeted therapies are undergoing preclinical testing and clinical trials. Pyrimethamine and sulphadoxine were shown to reduce lymphoproliferation and autoimmune cytopenias significantly in a small series of patients with ALPS; however, this combination failed to show any response in a different larger clinical trial (Ferrer et al, 2007). We have also recently shown that targeting the Notch signaling pathway may be beneficial in preclinical models of ALPS (Teachey et al, 2008), while other groups have shown that arsenic and histone deacetylase-inhibitors may be effective in preclinical models of ALPS (Ferrer et al, 2007). As with many autoimmune diseases, rituximab has been shown to be effective in a subset of ALPS patients; however, 5–10% of ALPS patients eventually develop common variable immunodeficiency (CVID) and our group (unpublished observations) and others have seen an association between rituximab use and the development of CVID in ALPS patients (Dale et al, 2007). Moreover, the effects of rituximab are generally relatively transient; patients that do respond will probably relapse and with the potential risk of CVID, we recommend avoiding use of rituximab in ALPS patients. MMF was found to be effective in another series (Koneti Rao et al, 2005). While we have had less success overall with this drug, we have also seen good responses in a few children with ALPS treated with MMF (unpublished data).
MMF inhibits lymphocyte proliferation but does not cause lymphocyte death and does not appear to increase Tregs (Noris et al, 2007). Tregs are a subset of T lymphocytes that suppress the activation of the immune system and increasing Treg numbers may improve autoimmune diseases (Brusko et al, 2008). Interestingly, some evidence suggests that peripheral blood DNTs may be dysregulated Tregs, while other evidence suggests they are cytotoxic T lymphocytes that have lost CD8 expression (Bleesing et al, 2001; Fischer et al, 2005). We have found mTOR inhibition decreases these abnormal DNTs in ALPS patients. Sirolimus has been shown to increase Tregs in healthy volunteers and patients with other autoimmune diseases (Battaglia et al, 2006). These observations, taken together, suggest the possibility that the improved responses with sirolimus seen here compared to other immunosuppressive agents, both in preclinical models and in patients, may be attributable to both induction of apoptosis in the abnormal lymphocytes and increases in normal Tregs. In subsequent patients, we plan to measure Tregs before and after initiating treatment with sirolimus. Unfortunately, as DNTs do not survive in culture, we could not directly test the in-vitro sensitivity of ALPS-patient’s DNTs to sirolimus.
As sirolimus can cause lymphocyte apoptosis and increase Tregs, it may also have activity in non-ALPS patients with autoimmune cytopenias, including patients with immune thrombocytopenia purpura (ITP), autoimmune hemolytic anemia (AIHA) and autoimmune neutropenia, either as isolated idiopathic conditions or when associated with other autoimmune syndromes, including lupus or Evans syndrome. Our findings suggest that sirolimus may be superior to other agents currently used for or being investigated in these syndromes, including mercaptopurine, MMF, cyclosporine and tacrolimus. None of these agents are both lymphotoxic to both B and T cells while simultaneously increasing Tregs. Accordingly, we plan to open a clinical trial investigating the efficacy of sirolimus in patients with chronic ITP and chronic AIHA.
We found sirolimus was very effective in ameliorating autoimmune disease and lymphoproliferation in patients with ALPS, corroborating our previous preclinical work. Based on these results, we plan to continue to treat patients with ALPS and either steroid-refractory disease or who are steroid-intolerant with sirolimus and we have initiated a phase II efficacy trial of sirolimus in ALPS. Based on this trial, we anticipate that sirolimus may move to the frontline of therapy for patients with ALPS.
Acknowledgements
This work was supported by grants from the United States Immunodeficiency Network (USIDNET, N01-A1-30070) (DTT), a Foerderer-Murray Award (DTT), the Larry and Helen Hoag Foundation Clinical Translational Research Career Development Award (DTT), the Goldman Philanthropic Partnerships and the Rockefeller Brothers Fund (DTT) and the Sanford Chair and Weinberg Fund (SAG).
Footnotes
Conflict of interest
No author has competing financial interests to declare.
References
- Abdel-Karim IA, Giles FJ. Mammalian target of rapamycin as a target in hematological malignancies. Current Problems in Cancer. 2008;32:161–177. doi: 10.1016/j.currproblcancer.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+ CD25+ FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. Journal of Immunology. 2006;177:8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- Bleesing JJ, Straus SE, Fleisher TA. Autoimmune lymphoproliferative syndrome. A human disorder of abnormal lymphocyte survival. Pediatric Clinics of North America. 2000;47:1291–1310. doi: 10.1016/s0031-3955(05)70272-8. [DOI] [PubMed] [Google Scholar]
- Bleesing JJ, Brown MR, Straus SE, Dale JK, Siegel RM, Johnson M, Lenardo MJ, Puck JM, Fleisher TA. Immunophenotypic profiles in families with autoimmune lymphoproliferative syndrome. Blood. 2001;98:2466–2473. doi: 10.1182/blood.v98.8.2466. [DOI] [PubMed] [Google Scholar]
- Brusko TM, Putnam AL, Bluestone JA. Human regulatory T cells: role in autoimmune disease and therapeutic opportunities. Immunological Reviews. 2008;223:371–390. doi: 10.1111/j.1600-065X.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- Dale J, Price S, J T, Aldridge P, Montague S, David J, Gill F, Hartmann K, Stork L, Gnarra D, Kirshnamurti L, Puck JM, Fleisher T, Rao VK. Use of rituximab for refractory autoimmune cytopenias associated with autoimmune lymphoproliferative syndrome (ALPS) Blood (ASH Annual Meeting Abstracts) 2007;110:1319. [Google Scholar]
- Ferrer E, Moral MA, Bozzo J. Spotlight on autoimmune lymphoproliferative syndrome. Drug News Perspect. 2007;20:260–262. [PubMed] [Google Scholar]
- Fischer K, Voelkl S, Heymann J, Przybylski GK, Mondal K, Laumer M, Kunz-Schughart L, Schmidt CA, Andreesen R, Mackensen A. Isolation and characterization of human antigen-specific TCR alpha beta+ CD4(−)CD8− double-negative regulatory T cells. Blood. 2005;105:2828–2835. doi: 10.1182/blood-2004-07-2583. [DOI] [PubMed] [Google Scholar]
- Koneti Rao V, Dugan F, Dale JK, Davis J, Tretler J, Hurley JK, Fleisher T, Puck J, Straus SE. Use of mycophenolate mofetil for chronic, refractory immune cytopenias in children with autoimmune lymphoproliferative syndrome. British Journal of Haematology. 2005;129:534–538. doi: 10.1111/j.1365-2141.2005.05496.x. [DOI] [PubMed] [Google Scholar]
- Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- Noris M, Casiraghi F, Todeschini M, Cravedi P, Cugini D, Monteferrante G, Aiello S, Cassis L, Gotti E, Gaspari F, Cattaneo D, Perico N, Remuzzi G. Regulatory T cells and T cell depletion: role of immunosuppressive drugs. Journal of the American Society of Nephrology. 2007;18:1007–1018. doi: 10.1681/ASN.2006101143. [DOI] [PubMed] [Google Scholar]
- Rieux-Laucat F, Le Deist F, Fischer A. Autoimmune lymphoproliferative syndromes: genetic defects of apoptosis pathways. Cell Death and Differentiation. 2003;10:124–133. doi: 10.1038/sj.cdd.4401190. [DOI] [PubMed] [Google Scholar]
- Sneller MC, Wang J, Dale JK, Strober W, Middelton LA, Choi Y, Fleisher TA, Lim MS, Jaffe ES, Puck JM, Lenardo MJ, Straus SE. Clincial, immunologic, and genetic features of an autoimmune lymphoproliferative syndrome associated with abnormal lymphocyte apoptosis. Blood. 1997;89:1341–1348. [PubMed] [Google Scholar]
- Teachey DT, Obzut DA, Axsom K, Choi JK, Goldsmith KC, Hall J, Hulitt J, Manno CS, Maris JM, Rhodin N, Sullivan KE, Brown VI, Grupp SA. Rapamycin improves lymphoproliferative disease in murine autoimmune lymphoproliferative syndrome (ALPS) Blood. 2006;108:1965–1971. doi: 10.1182/blood-2006-01-010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teachey DT, Seif AE, Brown VI, Bruno M, Bunte RM, Chang YJ, Choi JK, Fish JD, Hall J, Reid GS, Ryan T, Sheen C, Zweidler-McKay P, Grupp SA. Targeting Notch signaling in autoimmune and lymphoproliferative disease. Blood. 2008;111:705–714. doi: 10.1182/blood-2007-05-087353. [DOI] [PMC free article] [PubMed] [Google Scholar]