Summary
Increasing tensile properties and collagen content is a recognized need in articular cartilage tissue engineering. This study tested the hypothesis that multiple applications of chondroitinase ABC (C-ABC), a glycosaminoglycan (GAG) degrading enzyme, could increase construct tensile properties in a scaffold-less approach for articular cartilage tissue engineering. Developing constructs were treated with C-ABC at 2 wks, 4 wks, or both 2 and 4 wks. At 4 and 6 wks, construct sulfated GAG composition, collagen composition, and compressive and tensile biomechanical properties were assessed, along with immunohistochemistry (IHC) for collagens type I, II, and VI, and the proteoglycan decorin. At 6 wks, the tensile modulus and ultimate tensile strength of the group treated at both 2 and 4 wks were significantly increased over controls by 78% and 64%, reaching values of 3.4 and 1.4 MPa, respectively. Collagen concentration also increased 43%. Further, groups treated at either 2 wks or 4 wks alone also had increased tensile stiffness compared to controls. Surprisingly, though GAG was depleted in the treated groups, by 6 wks there were no significant differences in compressive stiffness. IHC showed abundant collagen type II and VI in all groups, with no collagen type I. Further, decorin staining was reduced following C-ABC treatment, but returned during subsequent culture. The results support the use of C-ABC in cartilage tissue engineering for increasing tensile properties.
Keywords: glycosaminoglycans, collagen, biomechanics, tensile properties, mechanobiology
Introduction
Degeneration of articular cartilage poses a significant clinical problem. The low regenerative capacity of cartilage limits healing, making the need for replacement tissue important. The primary goal of cartilage tissue engineering is to produce neotissue with sufficient mechanical properties to, when implanted, function in the native environment.
Self-assembly of chondrocytes has shown promise for cartilage tissue engineering.1, 2 In this process, chondrocytes are cultured in non-adherent agarose wells, and inter-cellular adhesion directs self-assembly through an N-cadherin binding process.3 Other scaffold-less systems, such as pellet and aggregate culture, have also been used in cartilage tissue engineering.4–6 Collectively, these approaches provide potential benefits over traditional scaffold-based strategies, including increased biocompatibility and a lack of exogenous degradation products. Self-assembly has been studied with growth factors7, 8 and mechanical stimulation,7, 9 but efforts to-date have not produced constructs with tensile properties in the range of native tissue. Engineered constructs generally have significantly less collagen than native tissue,10–13 and an insufficient collagen network reduces construct functionality by impairing their tensile stiffness and resistance to proteases.14 One way to improve upon this limitation is application of exogenous agents that modulate the matrix of the developing tissue.
One potential exogenous agent is the glycosaminoglycan (GAG) degrading agent chondroitinase ABC (C-ABC). In a recent study, C-ABC treatment of cartilage explants followed by 2 wks of culture resulted in reconstitution of GAG content and increased tensile properties.15 Moreover, C-ABC treatment of agarose encapsulated chondrocytes increased collagen concentration.16 Cartilage grown in vitro tends to lack maturational growth and overproduces GAGs, resulting in an imbalance between GAG and collagen content that reduces tissue tensile properties.17, 18 Thus, using C-ABC, which selectively degrades chondroitin and dermatan sulfates,19 could benefit the matrix by restoring the balance between GAG and collagen content.
The purpose of the present study was to elucidate the temporal effects of multiple C-ABC treatments on self-assembled articular cartilage constructs. To investigate this, constructs were treated with C-ABC at 2 wks, 4 wks, or both 2 and 4 wks, with construct assessment at 4 and 6 wks. We hypothesized that a single C-ABC treatment would increase tensile mechanical properties, and that multiple treatments would further enhance tensile properties. Additionally, by allowing additional culture time post-treatment, it was expected that constructs treated at 2 wks would regain GAG content and compressive stiffness similar to untreated controls.
Methods
Chondrocyte isolation and culture
Bovine chondrocytes were isolated and self-assembled as previously described.2 Cartilage from the distal femur and patellofemoral groove of male calves (Research 87, Boston, MA) was digested in collagenase type II for 24 hrs. Cells were counted using a hemocytometer and then frozen at −80°C in DMEM containing 20% FBS and 10% DMSO. Within days, cells were thawed and counted. Chondrocyte viability was >90% upon thawing. Cells were then seeded at ~5 million cells in 100 µL of media into 5 mm diameter cylindrical agarose wells. An additional 400 µL of media was added 4 hrs later. Chondrogenic medium composed of DMEM with 4.5 mg/mL glucose and L-glutamine (Biowhittaker/Cambrex, Walkersville, MD), 100 nM dexamethasone (Sigma, St. Louis, MO), 1% fungizone, 1% penicillin/streptomycin, 1% ITS+ (BD Scientific, Franklin Lakes, NJ), 50 µg/mL ascorbate-2-phosphate, 40 µg/mL L-proline, and 100 µg/mL sodium pyruvate (Fisher Scientific, Pittsburgh, PA) was used for seeding and subsequent media changes. This chondrogenic medium formulation contains no FBS and no stimulatory factors other than those listed above. The 500 µL of media were changed daily throughout the experiment. At 10 days, all constructs were transferred to tissue culture plates in which only the bottom of the wells was coated with a thin layer of agarose20 and randomly assigned to one of the four treatment groups. Of note, separate harvests were used for the 4 and 6 wk experiments. In the 4 wk experiment, cells from 3 distinct calves were used. Based on the promising results at 4 wks, a 6 wk study was then conducted. Cells from 4 distinct calves were used in the 6 wk study. Separate batches of agarose wells were also fabricated for each experiment.
C-ABC treatment and construct processing
In the 4 wk experiment, constructs were treated with C-ABC at 2 wks (early), 4 wks (late), or 2 and 4 wks (combined). For treatment, constructs were exposed to C-ABC (Sigma or Associates of Cape Cod, Falmouth, MA) at an activity of 2 U/mL in chondrogenic media for 4 hrs at 37°C, followed by five washes in 400 µL chondrogenic media. At 4 wks, samples were prepared for histology, quantitative biochemistry, and mechanical testing. Hence, the late and combined groups did not have any recovery time post-treatment in the 4 wk experiment. In the 6 wk experiment, constructs were treated early, late, or combined, but culture duration was lengthened to 6 wks, at which time samples were prepared for histology, quantitative biochemistry, and mechanical testing. Separate groups served as untreated controls for both experiments. Just prior to mechanical testing, constructs were removed from culture and processed. A 3 mm diameter core was removed from the construct’s center with a biopsy punch for creep indentation testing. The remaining outer ring was portioned ~60% for biochemical analysis and ~40% for tensile testing. This method of tissue processing tests tensile properties and assays the biochemical content of the outer annulus, which is presumably representative of the entire construct. However, any inhomogeneities that are specific to the annulus of tissue engineered constructs may bias the results. Indeed, inhomogeneities in tissue engineered articular cartilage have been observed at similar time points to those investigated in this study.21 However, as all specimens are prepared similarly, any bias is not likely to affect inter-group comparisons.
Gross morphology, histology, and immunohistochemistry (IHC)
Construct diameter was measured from digital photographs using ImageJ (National Institutes of Health, Bethesda, MD). For histology, constructs were cryoembedded and sectioned at 14 µm. Some sections were fixed in 10% phosphate buffered formalin and stained with Safranin O/fast green for GAG content. For IHC, slides were first fixed with acetone at 4°C for 20 min. For collagens type I, II, and VI, slides were rinsed with IHC buffer, quenched of peroxidase activity, and blocked with horse serum for collagen type I and goat serum for collagens type II and VI (Vectastain ABC kit, Vector Labs, Burlingame, CA). Sections were then incubated for 1 hr with either mouse anti-collagen type I diluted 1:1000 (Accurate Chemicals, Westbury, NY), rabbit anti-collagen type II diluted 1:300 (Cedarlane Labs, Burlington, NC), or rabbit anti-collagen type VI diluted 1:300 (US Biological, Swampscott, MA). The secondary antibody, made by adding one drop of stock solution is added to 10 mL of buffer in the mixing bottle (anti-mouse or anti-rabbit IgG, Vectastain ABC kit), was then applied for 30 min, and color was developed using the Vectastain ABC reagent and 5 min exposure to DAB. For decorin, whole rabbit serum (LF-94) was a generous gift from the laboratory of Dr. Larry W. Fisher.22 Following fixation in cold acetone and peroxidase quenching, sections were treated with 0.2 U/mL protease free C-ABC (Associates of Cape Cod) in a solution of 0.01 M Tris, 0.01 M NaCl, and 0.012 M NaAc containing 0.1% bovine serum albumin (BSA) for 1 hr at 37°C to expose the core protein. Sections were then blocked with goat serum and incubated overnight at 4°C in the presence of LF-94 diluted 1:500 in tris-buffered saline (TBS) containing 1% BSA. Finally, the secondary antibody, made as described above (anti-rabbit IgG, Vectastain ABC kit), was applied for 1 hr, and color was developed using the Vectastain ABC reagent and 5 min exposure to DAB. In addition to IHC staining of experimental groups, bovine articular cartilage was used as a positive control for collagen type II, collagen type VI, and decorin and as a negative control for type I collagen. Bovine tendon and bone were used as positive controls for collagen type I. As additional negative controls, tissues were stained for each protein as described above, but without application of the appropriate primary antibody.
Biochemical analysis
The portion of the sample assigned to biochemical analyses was weighed wet, lyophilized for 48 hrs, weighed dry, and subjected to a sequential pepsin-elastase digestion previously described.8 Briefly, samples were re-suspended in 0.8 mL of 0.05 M acetic acid containing 0.5 M sodium chloride. To this suspension, 0.1 mL of a 10 mg/mL pepsin (Sigma) solution in 0.05 M acetic acid was added, and the suspension was mixed at 4°C for 96 hrs. Next, 0.1 mL of 10x TBS buffer was added along with 0.1 mL pancreatic elastase (1 mg/mL dissolved in 1x TBS buffer). This suspension was mixed at 4°C overnight. Following this protocol, no residual neo-tissue remained. DNA content was assessed with the Quant-iT™ PicoGreen® dsDNA Assay Kit (Invitrogen, Carlsbad, CA). Following hydrolysis with 4N NaOH for 20 min at 110°C, collagen content of the samples was quantified with a modified chloramine-T hydroxyproline assay.23 Finally, sulfated GAG content was quantified using the Blyscan Glycosaminoglycan Assay kit (Accurate Chemical and Scientific Corp., Westbury, NY).
Creep indentation testing
Compressive mechanical properties were determined by creep indentation testing of the central 3 mm core biopsy assuming a linear biphasic model.24 Thickness was measured using a micrometer, and specimens were attached to a flat stainless steel surface with a thin layer of cyanoacrylate glue. The attached specimen was then placed into the creep indentation apparatus, which was used to automatically load and unload the specimens while monitoring specimen creep and recovery. In the 4 wk experiment, the late and combined treatment groups were tested using a 0.05 g tare followed by a 0.27 g test load. These lighter loads allowed control over excessive deformation that resulted immediately following GAG removal. All other specimens were tested with a tare load of 0.2 g followed by a test load of 0.7 g. The loads were applied to the specimens through a 0.8 mm diameter, flat-ended, porous tip. To determine construct mechanical properties, a semi-analytical, semi-numerical, linear biphasic model was employed,25 followed by a non-linear finite element optimization (FEO) to refine the solution and obtain values for the aggregate modulus, permeability, and Poisson’s ratio.26 To assess the appropriateness of the biphasic model for assessment of self-assembled tissue engineered constructs, a goodness-of-fit test was performed. The coefficient of determination from the FEO output and experimental displacement data was calculated using the following equation:
In this equation, yiexp are the experimental displacement data, yiFEO are the theoretical values obtained from the FEO, and yavgexp is the average of the experimental displacement data. This method has been used before to assess the appropriateness of several biomechanical models for articular cartilage.27
Tensile testing
The portion of each sample assigned to tensile testing was cut into a dog-bone shape and glued to paper tabs.28 This procedure prepares specimens with slightly curved lateral edges from the outer annulus, which may introduce artifact into the testing. However, as all specimens were prepared in the same manner, this potential artifact would be evenly distributed. ImageJ was used to determine sample gauge length and width from photographs. Gauge length was defined as the distance between the paper tabs. Thickness was measured with a micrometer. Tensile tests were performed at a strain rate of 1% gauge length per second on a mechanical testing system (Instron Model 5565, Canton, MA). There was no pre-conditioning of the specimens. The Instron monitors and records displacement, which when combined with gauge length, allows strain to be calculated. Young’s modulus was determined by linear regression of the linear portion of the stress-strain curve based on initial cross-sectional area. The ultimate tensile strength (UTS) was taken to be the maximal stress prior to failure.
Statistical analyses
Each group consisted of n = 8 samples. Two samples were randomly assigned for histology and IHC. The remaining six were used in the biochemical, compression, and tension tests. At each time point, a 2-way ANOVA based on main factors of treatment at 2 wks or treatment at 4 wks was performed to investigate additive or synergistic effects.29 Where warranted, the ANOVA was followed by a Student-Newman-Keuls post-hoc test on a cross of the main factors. Significance was set at p < 0.05. All data are presented as mean ± standard deviation (S.D.).
Results
4 wk experiment
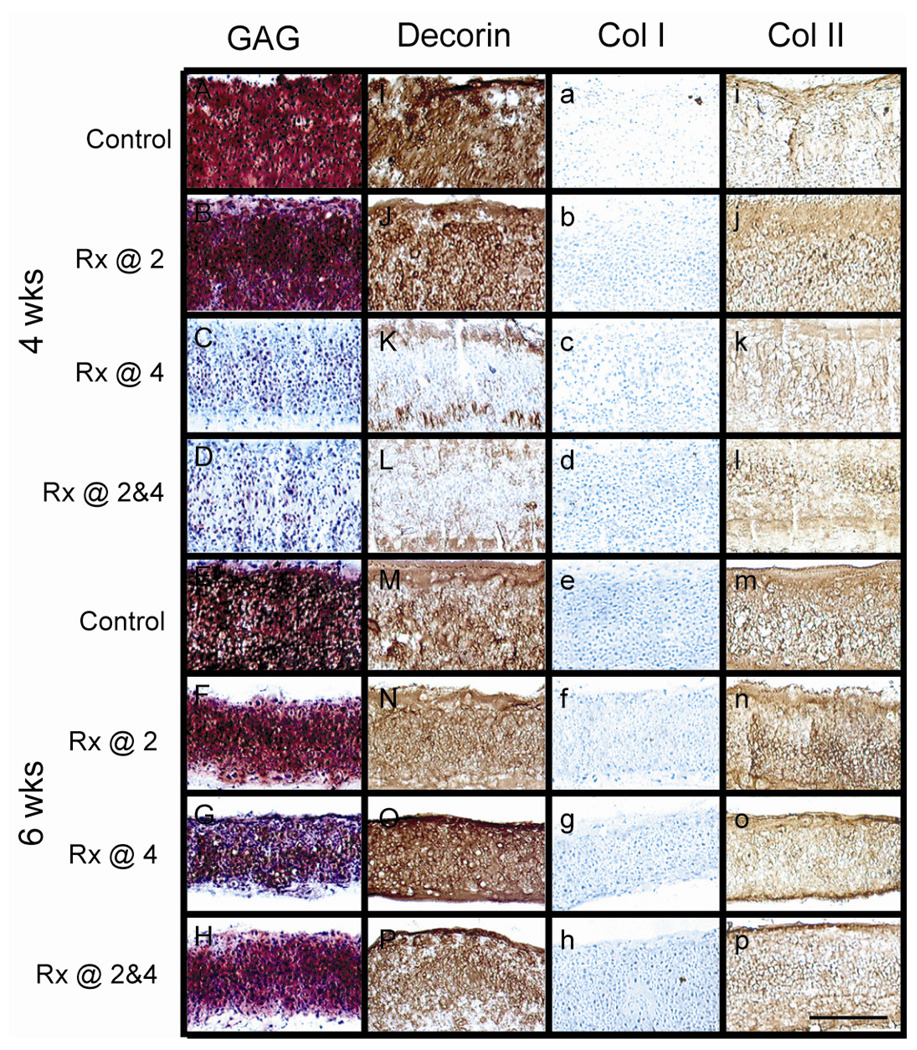
Figure 1 shows results from histology and IHC. At 4 wks, Safranin-O/fast green staining for GAGs showed GAG presence in the control and early treatment groups, and verified GAG removal in the late and combined treatment groups. Staining for collagens type II and VI (data not shown for type VI) was evident in all constructs. With respect to collagen type VI, pericellular staining was present, but it was not exclusive. There was also diffuse staining throughout the construct. Constructs did not stain for collagen type I. Decorin stained intensely throughout constructs in the control and early treatment groups, but stained only on the perimeter of constructs in the late and combined treatment groups.
Figure 1. Histology and IHC.
A-H) Safranin-O/fast green stain for GAGs, I-P) IHC for decorin, a-h) IHC for collagen type I, and i-p) IHC for collagen type II. At 4 wks, note the absence of GAG and substantially decreased decorin staining immediately following C-ABC treatment in the group treated at 4 wks and group treated at both 2 and 4 wks (C, D, K, and L, respectively). GAG and decorin staining returned in these groups by 6 wks (G, H, O, and P, respectively). Scale bar in P is 200µm.
One-time treatment at 2 wks or 4 wks was a significant factor for diameter, wet weight (WW), and thickness. Further, with respect to decreasing WW, the interaction term was significant (p = 0.03). For diameter, each group was significantly different from the others, except for early and combined treatments being similar. Diameters measured 6.02 ± 0.17, 5.45 ± 0.07, 5.71 ± 0.18, and 5.31 ± 0.07 mm for the control, early, late, and combined groups, respectively. For thickness, each group was significantly different from the others, except for late treatment being similar to early and combined. Thicknesses measured 0.45 ± 0.04, 0.36 ± 0.03, 0.34 ± 0.05, and 0.30 ± 0.03 mm for the control, early, late, and combined groups, respectively. For WW, each group was significantly different from the others, except for early and late treatments being similar. WWs measured 14 ± 1.7, 9.2 ± 0.8, 8.3 ± 1.2, and 5.5 ± 0.6 mg for the control, early, late, and combined groups, respectively. In terms of DNA content, treatment at 4 wks was a significant factor. Post-hoc testing showed the combined treatment group had significantly less DNA than control (30 ± 5.6 µg per construct compared to 21 ± 1.8 µg). The early group had 28 ± 5.2 µg and the late group had 26 ± 6.5 µg.
Turning to extracellular matrix (ECM) content, for both GAG/WW and collagen/WW treatment at 2 wks or treatment at 4 wks was a significant factor. Table 1 shows GAG/WW and collagen/WW. Post-hoc testing of GAG/WW showed each group was significantly different from the others, except for late treatment being similar to combined. Post-hoc testing of collagen/WW showed the combined treatment group was significantly different from all others. GAG or collagen per construct can be obtained by multiplying the WW% by the total wet weight. Post-hoc testing of GAG/construct was identical to GAG/WW. Post-hoc testing of collagen/construct showed the combined treatment group was significantly different from control and the late treatment groups.
Table 1.
Construct GAG and collagen content normalized to wet weight.
| 4 wks | 6 wks | |||
|---|---|---|---|---|
| Group | GAG / WW (%) | Collagen / WW (%) | GAG / WW (%) | GAG / WW (%) |
| Control | 6.9 ± 1.8a | 8.9 ± 2.1a | 6.3 ± 0.6A | 16 ± 2.1A |
| C-ABC @ 2 wks | 4.4 ± 0.8b | 19 ± 3.1a | 5.5 ± 1.1A | 20 ± 4.3A,B |
| C-ABC @ 4 wks | 3.1 ± 1.7c | 17 ± 3.4a | 2.7 ± 0.5B | 24 ± 3.9B |
| C-ABC @ 2 & 4 wks | 1.6 ± 0.8c | 38 ± 15b | 3.3 ± 0.4B | 23 ± 2.0B |
Values in table are mean ± S.D. (n = 6). WW = wet weight. Separate statistical analyses were run on each column. Within a column, groups not sharing a similar letter are significantly different from one another (p < 0.05 by Student-Newman-Keuls post-hoc test). At 6 wks, the groups that had been treated at 4 wks contained significantly less sulfated GAG/WW, but significantly more collagen/WW, than control.
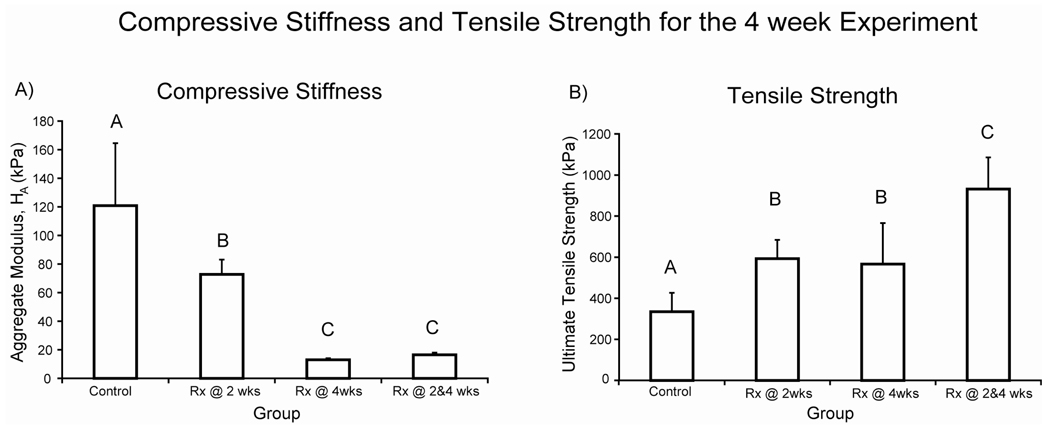
Compressive and tensile mechanical properties were also measured. Treatment at 2 wks or treatment at 4 wks was a significant factor for compressive stiffness. The interaction term was also significant (p = 0.012). Further, treatment at 4 wks was as significant factor for permeability. All treatment groups were significantly less stiff in compression compared to control (Fig. 2A). Post-hoc testing of permeability showed an increasing trend with treatment. Permeabilities measured 3.11 ± 1.73, 9.35 ± 5.92, 75.5 ± 64.6, and 86.8 ± 85.9 × 10−15 m4/N.s for the control, early, late, and combined groups, respectively. Post-hoc testing of Poisson’s ratio showed combined treatment was significantly greater than early or late treatment. Poisson’s ratio measured 0.093 ± 0.095, 0.035 ± 0.028, 0.054 ± 0.039, and 0.172 ± 0.097 for the control, early, late, and combined groups, respectively. Goodness-of-fit was assessed by calculating the coefficient of determination (r2). There were no significant differences in r2 among the groups. The r2 values were calculated to be 0.890 ± 0.046, 0.908 ± 0.041, 0.917 ± 0.033, and 0.910 ± 0.065 for the control, early, late, and combined groups, respectively.
Figure 2. 4 week biomechanical properties.
A) Compressive stiffness and B) ultimate tensile strength. Bars represent mean ± S.D. Groups not connected by the same letter are significantly different. The aggregate modulus of all treated groups was significantly less than control. The ultimate tensile strength of all treated groups was significantly greater than control, reaching 931 ± 155 kPa in the combined group. The group treated at both 2 and 4 wks was significantly greater than the groups treated at only 2 or 4 wks.
In terms of tensile properties, treatment at 2 wks or at 4 wks was a significant factor for UTS, and treatment at 2 wks was a significant factor for Young’s modulus The interaction term was also significant for Young’s modulus (p = 0.0008). All treatment groups had significantly greater UTS than control (Fig. 2B). Post-hoc testing of Young’s modulus showed all groups were significantly different from each other except control and early treatment. Young’s modulus measured 1299 ± 101, 1308 ± 257, 950 ± 284, and 1689 ± 159 kPa for the control, early, late, and combined groups, respectively.
6 wk experiment
In the 6 wk experiment, histology and IHC results were similar to the 4 wk experiment, except GAG and decorin were present in the late and combined treatment groups (Fig. 1). Treatment at 4 wks was a significant factor for diameter, WW, and thickness. Further, with respect to decreasing diameter, the interaction term was significant (p = 0.046). For diameter, each group was significantly different from the others, except for late and combined treatment being similar. Diameters measured 5.27 ± 0.10, 5.14 ± 0.07, 4.99 ± 0.09, and 5.00 ± 0.07 mm for the control, early, late, and combined groups, respectively. For thickness, late and combined treatments were significantly different than both control and early treatment, though the differences were small. Thicknesses measured 0.29 ± 0.03, 0.31 ± 0.03, 0.25 ± 0.04, and 0.24 ± 0.02 mm for the control, early, late, and combined groups, respectively. For WW, late and combined treatments were significantly different than both control and early treatment. WWs measured 5.9 ± 0.4, 5.7 ± 0.5, 4.9 ± 0.3, and 4.7 ± 0.3 mg for the control, early, late, and combined groups, respectively. DNA content at 6 wks showed a similar trend to 4 wks. Post-hoc testing showed the late and combined treatments had significantly less DNA than both control and early treatment.
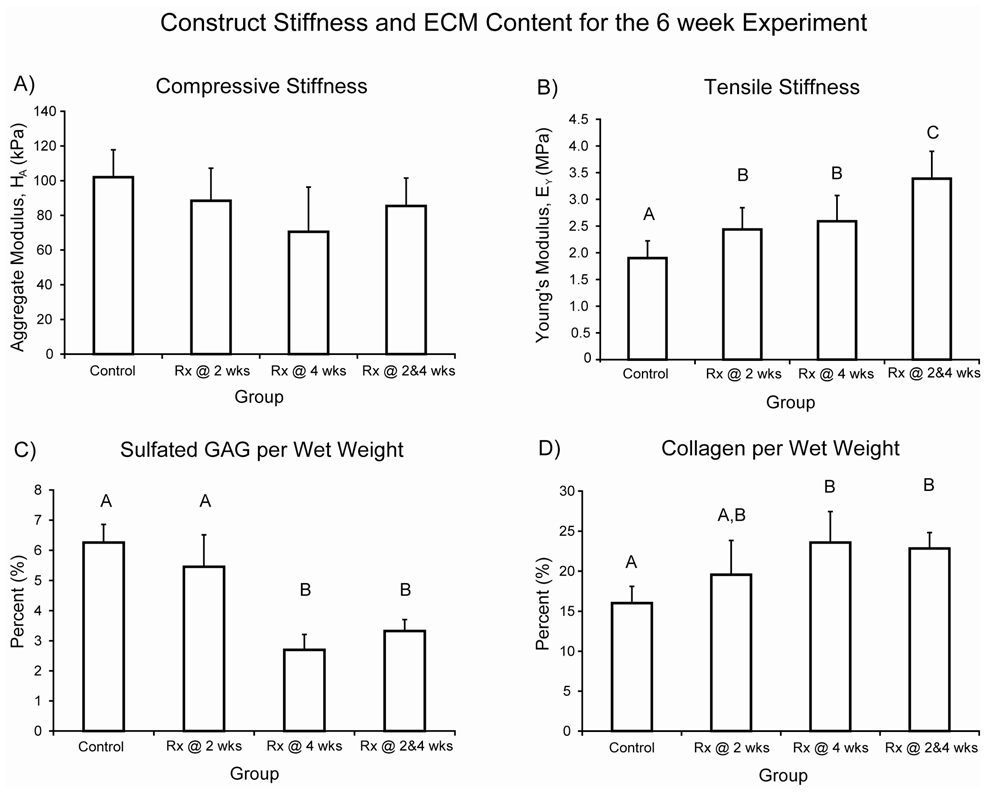
In terms of ECM content and mechanical properties, treatment at 4 wks was a significant factor for both GAG/WW and collagen/WW. Further, with respect to decreasing GAG/WW, the interaction term was significant (p = 0.02). Late or combined treatment resulted in significantly decreased GAG/WW compared to control or early treatment, while late or combined treatment resulted in significantly increased collagen/WW compared to control (Table 1). Post-hoc testing of GAG/construct showed each group was significantly different from the others, except for late treatment being similar to combined. There were no significant differences in collagen/construct.
In terms of compressive properties, treatment at 4 wks was a significant factor (p = 0.048) for compressive stiffness (Fig. 3A). There were no significant differences in aggregate modulus among the four groups. Post-hoc testing of permeability and Poisson’s ratio also showed no significant differences among the groups. Permeabilities measured 19.7 ± 19.8, 17.6 ± 16.9, 15.7 ± 8.5, and 20.7 ± 12.7×10−15 m4/N.s for the control, early, late, and combined groups, respectively. Poisson’s ratio measured 0.138 ± 0.110, 0.085 ± 0.119, 0.072 ± 0.033, and 0.133 ± 0.112 for the control, early, late, and combined groups, respectively. As seen at 4 wks, there were no significant differences among the groups for r2 values, which were 0.936 ± 0.031, 0.930 ± 0.028, 0.911 ± 0.054, and 0.907 ± 0.094 for the control, early, late, and combined groups, respectively.
Figure 3. 6 week biomechanical properties.
A) Compressive stiffness and B) tensile stiffness. Bars represent mean ± S.D. Groups not connected by the same letter are significantly different. There were no significant differences in aggregate modulus. Young’s modulus of all treated groups was significantly greater than control, reaching 3.4 ± 0.5 MPa in the combined group. Further, the group treated at both 2 and 4 wks was significantly greater than the groups treated at only 2 or 4 wks, which measured 2.4 ± 0.4 and 2.6 ± 0.5 MPa, respectively.
For tensile properties, treatment at 2 wks or at 4 wks was a significant factor for Young’s modulus, and treatment at 4 wks was a significant factor for UTS. All treated groups had significantly greater Young’s modulus than control (Fig. 3B). Additionally, the combined treatment group was greater than early or late treatment. Post-hoc testing of UTS showed the control and early treatment group were not significantly different, and the late and combined treatment groups were not significantly different. All other comparisons were significantly different. UTS measured 881 ± 181, 984 ± 143, 1343 ± 169, and 1441 ± 184 kPa for the control, early, late, and combined groups, respectively.
Discussion
In this study, we examined the effects of multiple C-ABC applications on self-assembled tissue engineered articular cartilage constructs. Results showed that the group treated at both 2 and 4 wks (combined) had significantly greater tensile properties (ultimate tensile strength and Young’s modulus) than control in both the 4 and 6 wk experiments, reaching a stiffness of 3.4 MPa at 6 wks. Further, treating at 2 wks (early) or at 4 wks (late) alone resulted in significantly greater ultimate tensile strength at 4 wks, and significantly greater Young’s modulus at 6 wks, compared to controls at these time points. Though compressive stiffness of all treatment groups was less than control in the 4 wk experiment, there were no significant differences at 6 wks. These results support our hypotheses that multiple C-ABC treatments would further enhance tensile properties compared to a single treatment, and, given longer culture time post-treatment, the early treatment group would not have a significantly different compressive stiffness compared to untreated controls.
An interesting result of this study is that the compressive properties of all C-ABC treated groups were not significantly different from controls at 6 wks. Furthermore sulfated GAG returned following depletion in the early treatment group over 4 wks of culture. Additionally, though given only 2 wks of culture post-treatment, compressive stiffness at 6 wks of the late and combined treatment groups was not significantly different from controls, despite significantly decreased sulfated GAG content. This is potentially explained by the significantly increased collagen content in these groups compared to control, reaching 23% by WW. Indeed, collagen has been shown to have a role in the compressive behavior of articular cartilage. One study performed confined compression with the expectation that compressions greater than 5% would lead to the full load being borne by the proteoglycan osmotic pressure, evidenced by equal stresses in the axial and radial directions. However, it was found that the axial and radial stresses were not equal, highlighting a role for the collagen network in compression.30 Williamson et al.31 examined bovine cartilage compressive properties as a function of age, showing that the compressive modulus increased 180% from fetus to adult. This increase correlated with increased collagen during development, as GAG content changed negligibly. These findings underscore the concept that construct material properties reflect the balance of ECM components, and both collagen and GAG contribute to compressive stiffness.
Analysis of compressive testing data also revealed that the liner biphasic theory well predicts the compressive behavior of self-assembled articular cartilage constructs. This claim is supported by the relatively high coefficients of determination that were calculated by comparing the experimental displacement data to the theoretical output of the FEO. The lowest r2 calculated was 0.89. This is surprisingly good considering the relative simplicity of the linear biphasic theory. More complicated theories for the biomechanical behavior of articular cartilage, such as the biphasic-CLE-QLV model,27 have achieved r2 values of 0.95 and 0.97 for confined and unconfined compression testing, respectively. It is likely that these models would also better predict the behavior of self-assembled tissue engineered articular cartilage; however, it is not yet clear if self-assembled cartilage exhibits non-linear behavior for the same reasons or in the same proportions that native tissue articular cartilage does. As the permeability in linear biphasic theory captures these behaviors, the permeability values should be interpreted in this light, and not necessarily as the intrinsic permeability of the engineered constructs, which can only be properly measured through direct permeation experiments. As self-assembled constructs are further studied and become more robust, more detailed assessments of their biomechanical behavior can be made.
Moreover, the results of this study show C-ABC treatment increases construct tensile properties. At 4 wks, the increase in tensile properties may be attributed to substantially decreased construct size (e.g., thickness) resulting from loss of GAG and its associated water or to increased ability of collagen to rearrange in the absence of a pre-stress generating swelling pressure.32, 33 However, as GAG returns, differences in construct size at 6 wks were markedly reduced. This suggests increased collagen concentration could be responsible for the increased tensile properties. Kempson et al.34 have shown that the tensile properties of articular cartilage are correlated to collagen content. However, another interesting possibility raised by results from this study is a role for the small proteoglycan decorin. Decorin is known to interact with type II collagen and has key roles in collagen fibrillogenesis, such as limiting fiber diameter.35, 36 For example, fibroblasts seeded onto collagen scaffolds from decorin knockout mice have been shown to increase scaffold tensile properties compared to wild-type cells.37 The decrease in decorin evident by IHC staining shown immediately post-treatment in the present study may allow larger diameter collagen fibers to be formed or previously blocked interactions between neighboring collagens to take place, both of which could lead to a more functional collagen network and increased tensile properties. This proposition is similar to a recently described mechanism whereby selective knockdown of the small proteoglycan lumican led to increased collagen deposition and fibril diameter in a polyglycolic acid scaffold based approach to cartilage engineering employing bovine nasal chondrocytes.11 In addition to decorin and lumican, aggrecan and biglycan have a role in the observed changes. Selective modulation of small, regulatory matrix molecules could be an important consideration for future articular cartilage tissue engineering experiments.
In addition to self-assembly, other scaffold-less culture systems for engineering cartilage replacement tissue have been studied.38–43 One study showed that human articular chondrocytes grown in serum free conditions exhibit an age-dependent ability to produce neo-tissue.38 However, mechanical properties were not assessed. Fedewa et al.39 plated rabbit articular chondrocytes at a concentration of 2 million cells per 25 cm2 and cultured them for up to 10 wks. Chondrocytes grew into cell sheets up to 130 µm thick and produced collagens type II, IX, and XI, indicating that cell phenotype was not affected. At 10 wks, the engineered tissue’s tensile stiffness was 1.3 MPa. Stiffness was further increased to 3.4 MPa by treatment with interleukin-1β, but the cell sheet thickness decreased to only 38 µm. Using the same culture system, it was shown that increased construct tensile stiffness correlated with greater collagen volume fraction43 and that bonds formed among collagen fibrils.42 In the present study, greater construct collagen concentration was also related to increased tensile stiffness. Further, it is possible that interfibrillar collagen bonds formed upon GAG depletion with C-ABC. This possibility should be assessed in future studies.
Another study used the plated chondrocyte culture system to assess the effects of proteoglycans on construct tear toughness.41 C-ABC was used to remove GAG from the cell sheets at 8 wks, followed by tensile tear testing. C-ABC treatment decreased the thickness of grown tissue, and material tear toughness was increased upon GAG removal, but not when adjusted for collagen content. The authors concluded that proteoglycans were not a determining factor of the grown tissue’s fracture toughness. Finally, Gemmiti and Guldberg40 demonstrated that exposure of scaffold-less grown engineered cartilage to fluid flow-induced shear stress causes increased total and type II collagen. These increases in collagen were mirrored by increased tensile properties, reaching a stiffness of 2.28 MPa at 17 days. Despite these efforts, the tensile stiffness of engineered articular cartilage remains low compared to native tissue. Further research into methods for increasing tensile properties of scaffold-less grown tissue engineered cartilage remains necessary.
In summary, C-ABC treatment is a promising approach for increasing tensile properties of self-assembled tissue engineered cartilage, perhaps by inducing maturational instead of expansive growth,15 whereby construct size changes minimally while ECM continues to be deposited. A potential limitation to scaffold-free approaches is less control over construct thickness, which is further affected by C-ABC treatment. However, construct size could be increased before treatment by using more cells44 or allowing additional culture time before C-ABC application. Using the self-assembly process, our laboratory has grown constructs greater than 1 mm thick,7 though there is considerable biological variability. In conclusion, as hypothesized, treatment with C-ABC at both 2 and 4 wks resulted in further enhancement of tensile properties compared to a single treatment at 2 or 4 wks. Future experiments should probe more deeply into the mechanism as to how temporary proteoglycan depletion affects the collagen network of tissue engineered constructs by examining aspects such as fiber size and degree of cross-linking.
Acknowledgements
The authors gratefully acknowledge funding support from NIAMS R01AR053286, Elizabeth Stephens for her assistance with decorin IHC, and Ke Xun Chen for his help with the culture. K. A. Athanasiou, has contributed to OsteoBiologics (now part of Smith & Nephew), Diabetica Solutions, and VidaCare. He is now working with Rice University to develop technologies related to cartilage repair. Other authors have no competing interests to declare.
References
- 1.Hoben GM, Hu JC, James RA, Athanasiou KA. Self-assembly of fibrochondrocytes and chondrocytes for tissue engineering of the knee meniscus. Tissue Eng. 2007;13:939–946. doi: 10.1089/ten.2006.0116. [DOI] [PubMed] [Google Scholar]
- 2.Hu JC, Athanasiou KA. A self-assembling process in articular cartilage tissue engineering. Tissue Eng. 2006;12:969–979. doi: 10.1089/ten.2006.12.969. [DOI] [PubMed] [Google Scholar]
- 3.Ofek G, Revell CM, Hu JC, Allison DD, Grande-Allen KJ, Athanasiou KA. Matrix development in self-assembly of articular cartilage. PLoS ONE. 2008;3:e2795. doi: 10.1371/journal.pone.0002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furukawa KS, Suenaga H, Toita K, Numata A, Tanaka J, Ushida T, et al. Rapid and large-scale formation of chondrocyte aggregates by rotational culture. Cell Transplant. 2003;12:475–479. doi: 10.3727/000000003108747037. [DOI] [PubMed] [Google Scholar]
- 5.Stewart MC, Saunders KM, Burton-Wurster N, Macleod JN. Phenotypic stability of articular chondrocytes in vitro: the effects of culture models, bone morphogenetic protein 2, and serum supplementation. J Bone Miner Res. 2000;15:166–174. doi: 10.1359/jbmr.2000.15.1.166. [DOI] [PubMed] [Google Scholar]
- 6.Kandel RA, Chen H, Clark J, Renlund R. Transplantation of cartilagenous tissue generated in vitro into articular joint defects. Artif Cells Blood Substit Immobil Biotechnol. 1995;23:565–577. doi: 10.3109/10731199509117971. [DOI] [PubMed] [Google Scholar]
- 7.Elder BD, Athanasiou KA. Synergistic and additive effects of hydrostatic pressure and growth factors on tissue formation. PLoS ONE. 2008;3:e2341. doi: 10.1371/journal.pone.0002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elder BD, Athanasiou KA. Systematic assessment of growth factor treatment on biochemical and biomechanical properties of engineered articular cartilage constructs. Osteoarthritis Cartilage. 2008 doi: 10.1016/j.joca.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu JC, Athanasiou KA. The effects of intermittent hydrostatic pressure on self-assembled articular cartilage constructs. Tissue Eng. 2006;12:1337–1344. doi: 10.1089/ten.2006.12.1337. [DOI] [PubMed] [Google Scholar]
- 10.Freed LE, Hollander AP, Martin I, Barry JR, Langer R, Vunjak-Novakovic G. Chondrogenesis in a cell-polymer-bioreactor system. Exp Cell Res. 1998;240:58–65. doi: 10.1006/excr.1998.4010. [DOI] [PubMed] [Google Scholar]
- 11.Kafienah W, Cheung FL, Sims T, Martin I, Miot S, Ruhland CV, et al. Lumican inhibits collagen deposition in tissue engineered cartilage. Matrix Biol. 2008 doi: 10.1016/j.matbio.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Ng KW, Saliman JD, Lin EY, Statman LY, Kugler LE, Lo SB, et al. Culture duration modulates collagen hydrolysate-induced tissue remodeling in chondrocyte-seeded agarose hydrogels. Ann Biomed Eng. 2007;35:1914–1923. doi: 10.1007/s10439-007-9373-z. [DOI] [PubMed] [Google Scholar]
- 13.Wong M, Siegrist M, Gaschen V, Park Y, Graber W, Studer D. Collagen fibrillogenesis by chondrocytes in alginate. Tissue Eng. 2002;8:979–987. doi: 10.1089/107632702320934074. [DOI] [PubMed] [Google Scholar]
- 14.Bastiaansen-Jenniskens YM, Koevoet W, de Bart AC, van der Linden JC, Zuurmond AM, Weinans H, et al. Contribution of collagen network features to functional properties of engineered cartilage. Osteoarthritis Cartilage. 2008;16:359–366. doi: 10.1016/j.joca.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Asanbaeva A, Masuda K, Thonar EJ, Klisch SM, Sah RL. Mechanisms of cartilage growth: modulation of balance between proteoglycan and collagen in vitro using chondroitinase ABC. Arthritis Rheum. 2007;56:188–198. doi: 10.1002/art.22298. [DOI] [PubMed] [Google Scholar]
- 16.Bian L, Williams DY, Mao DQ, Xu D, Ateshian GA, Hung CT. Trans Orthopaedic Res. Vol. 2008. San Francisco; 2008. Influence of Temporary Chondroitinase ABC-induced GAG Suppression on Maturation of Tissue Engineered Cartilage; p. 601. [Google Scholar]
- 17.Williamson AK, Chen AC, Masuda K, Thonar EJ, Sah RL. Tensile mechanical properties of bovine articular cartilage: variations with growth and relationships to collagen network components. J Orthop Res. 2003;21:872–880. doi: 10.1016/S0736-0266(03)00030-5. [DOI] [PubMed] [Google Scholar]
- 18.Williamson AK, Masuda K, Thonar EJ, Sah RL. Growth of immature articular cartilage in vitro: correlated variation in tensile biomechanical and collagen network properties. Tissue Eng. 2003;9:625–634. doi: 10.1089/107632703768247322. [DOI] [PubMed] [Google Scholar]
- 19.Prabhakar V, Capila I, Raman R, Srinivasan A, Bosques CJ, Pojasek K, et al. The catalytic machinery of chondroitinase ABC I utilizes a calcium coordination strategy to optimally process dermatan sulfate. Biochemistry. 2006;45:11130–11139. doi: 10.1021/bi0605484. [DOI] [PubMed] [Google Scholar]
- 20.Elder BD, Athanasiou KA. Effects of confinement on the mechanical properties of self-assembled articular cartilage constructs in the direction orthogonal to the confinement surface. J Orthop Res. 2008;26:238–246. doi: 10.1002/jor.20480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kisiday JD, Kurz B, DiMicco MA, Grodzinsky AJ. Evaluation of medium supplemented with insulin-transferrin-selenium for culture of primary bovine calf chondrocytes in three-dimensional hydrogel scaffolds. Tissue Eng. 2005;11:141–151. doi: 10.1089/ten.2005.11.141. [DOI] [PubMed] [Google Scholar]
- 22.Fisher LW, Stubbs JT, 3rd, Young MF. Antisera and cDNA probes to human and certain animal model bone matrix noncollagenous proteins. Acta Orthop Scand Suppl. 1995;266:61–65. [PubMed] [Google Scholar]
- 23.Almarza AJ, Athanasiou KA. Seeding techniques and scaffolding choice for tissue engineering of the temporomandibular joint disk. Tissue Eng. 2004;10:1787–1795. doi: 10.1089/ten.2004.10.1787. [DOI] [PubMed] [Google Scholar]
- 24.Mow VC, Kuei SC, Lai WM, Armstrong CG. Biphasic creep and stress relaxation of articular cartilage in compression? Theory and experiments. J Biomech Eng. 1980;102:73–84. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- 25.Mow VC, Gibbs MC, Lai WM, Zhu WB, Athanasiou KA. Biphasic indentation of articular cartilage--II. A numerical algorithm and an experimental study. J Biomech. 1989;22:853–861. doi: 10.1016/0021-9290(89)90069-9. [DOI] [PubMed] [Google Scholar]
- 26.Athanasiou KA, Agarwal A, Muffoletto A, Dzida FJ, Constantinides G, Clem M. Biomechanical properties of hip cartilage in experimental animal models. Clin Orthop. 1995;316:254–266. [PubMed] [Google Scholar]
- 27.Huang CY, Soltz MA, Kopacz M, Mow VC, Ateshian GA. Experimental verification of the roles of intrinsic matrix viscoelasticity and tension-compression nonlinearity in the biphasic response of cartilage. J Biomech Eng. 2003;125:84–93. doi: 10.1115/1.1531656. [DOI] [PubMed] [Google Scholar]
- 28.Aufderheide AC, Athanasiou KA. Assessment of a bovine co-culture, scaffold-free method for growing meniscus-shaped constructs. Tissue Eng. 2007;13:2195–2205. doi: 10.1089/ten.2006.0291. [DOI] [PubMed] [Google Scholar]
- 29.Slinker BK. The statistics of synergism. J Mol Cell Cardiol. 1998;30:723–731. doi: 10.1006/jmcc.1998.0655. [DOI] [PubMed] [Google Scholar]
- 30.Khalsa PS, Eisenberg SR. Compressive behavior of articular cartilage is not completely explained by proteoglycan osmotic pressure. J Biomech. 1997;30:589–594. doi: 10.1016/s0021-9290(97)84508-3. [DOI] [PubMed] [Google Scholar]
- 31.Williamson AK, Chen AC, Sah RL. Compressive properties and function-composition relationships of developing bovine articular cartilage. J Orthop Res. 2001;19:1113–1121. doi: 10.1016/S0736-0266(01)00052-3. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt MB, Mow VC, Chun LE, Eyre DR. Effects of proteoglycan extraction on the tensile behavior of articular cartilage. J Orthop Res. 1990;8:353–363. doi: 10.1002/jor.1100080307. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz MH, Leo PH, Lewis JL. A microstructural model for the elastic response of articular cartilage. J Biomech. 1994;27:865–873. doi: 10.1016/0021-9290(94)90259-3. [DOI] [PubMed] [Google Scholar]
- 34.Kempson GE, Muir H, Pollard C, Tuke M. The tensile properties of the cartilage of human femoral condyles related to the content of collagen and glycosaminoglycans. Biochim Biophys Acta. 1973;297:456–472. doi: 10.1016/0304-4165(73)90093-7. [DOI] [PubMed] [Google Scholar]
- 35.Roughley PJ, Lee ER. Cartilage proteoglycans: structure and potential functions. Microsc Res Tech. 1994;28:385–397. doi: 10.1002/jemt.1070280505. [DOI] [PubMed] [Google Scholar]
- 36.Douglas T, Heinemann S, Bierbaum S, Scharnweber D, Worch H. Fibrillogenesis of collagen types I, II, and III with small leucine-rich proteoglycans decorin and biglycan. Biomacromolecules. 2006;7:2388–2393. doi: 10.1021/bm0603746. [DOI] [PubMed] [Google Scholar]
- 37.Ferdous Z, Wei VM, Iozzo R, Hook M, Grande-Allen KJ. Decorin-transforming Growth Factor- Interaction Regulates Matrix Organization and Mechanical Characteristics of Three-dimensional Collagen Matrices. J Biol Chem. 2007;282:35887–35898. doi: 10.1074/jbc.M705180200. [DOI] [PubMed] [Google Scholar]
- 38.Adkisson HD, Gillis MP, Davis EC, Maloney W, Hruska KA. In vitro generation of scaffold independent neocartilage. Clin Orthop Relat Res. 2001:S280–S294. doi: 10.1097/00003086-200110001-00026. [DOI] [PubMed] [Google Scholar]
- 39.Fedewa MM, Oegema TR, Jr, Schwartz MH, MacLeod A, Lewis JL. Chondrocytes in culture produce a mechanically functional tissue. J Orthop Res. 1998;16:227–236. doi: 10.1002/jor.1100160210. [DOI] [PubMed] [Google Scholar]
- 40.Gemmiti CV, Guldberg RE. Fluid flow increases type II collagen deposition and tensile mechanical properties in bioreactor-grown tissue-engineered cartilage. Tissue Eng. 2006;12:469–479. doi: 10.1089/ten.2006.12.469. [DOI] [PubMed] [Google Scholar]
- 41.Koop BE, Lewis JL, Fedewa MM, Oegema TR., Jr The effects of displacement rate and proteoglycan digestion on the fracture resistance of tissue grown from chondrocyte culture. J Mater Sci Mater Med. 2002;13:823–828. doi: 10.1023/a:1016588026499. [DOI] [PubMed] [Google Scholar]
- 42.Lewis JL, Johnson SL, Oegema TR., Jr Interfibrillar collagen bonding exists in matrix produced by chondrocytes in culture: evidence by electron microscopy. Tissue Eng. 2002;8:989–995. doi: 10.1089/107632702320934083. [DOI] [PubMed] [Google Scholar]
- 43.Simha NK, Fedewa M, Leo PH, Lewis JL, Oegema T. A composites theory predicts the dependence of stiffness of cartilage culture tissues on collagen volume fraction. J Biomech. 1999;32:503–509. doi: 10.1016/s0021-9290(98)00185-7. [DOI] [PubMed] [Google Scholar]
- 44.Revell CM, Reynolds CE, Athanasiou KA. Effects of initial cell seeding in self assembly of articular cartilage. Ann Biomed Eng. 2008;36:1441–1448. doi: 10.1007/s10439-008-9524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]