Abstract
2C4 (Pertuzumab, Omnitarg) is a monoclonal antibody targeting p185her2/neu, which is overexpressed in 30% of invasive breast cancer. 2C4 is currently in phase II clinical trials for several types of cancers. This antibody has been reported to disrupt the association between p185her2/neu and ErbB3. In our studies of epidermal growth factor receptor (EGFR)–p185her2/neu heterodimerization, we noted that 2C4 formed associations with the EGFR–p185her2/neu receptor complex. Our data argue against 2C4 as a universal heterodimerization blocker for p185her2/neu, but indicate that cocktails of monoclonal antibodies binding distinct interaction surfaces of p185her2/neu will emerge as the most potent targeted therapy.
Keywords: her2, Pertuzumab, heterodimer, 4D5
p185her2/neu (Neu, c-ErbB2), a protein product related to the oncogene neu and the second member of the ErbB family of receptor tyrosine kinases, is overexpressed in many breast and ovarian cancers (van de Vijver et al., 1987; Slamon et al., 1989), early breast tumors (Lodato et al., 1990), gastrointestinal tumors (Cohen et al., 1989), lung tumors (Kern et al., 1990) as well as tumors of the pancreas (Williams et al., 1991). Extensive studies have shown that p185her2/neu, acting as the preferred co-receptor for other family members, plays a dominant role in mediating the malignant phenotype (Cohen et al., 1989; Kokai et al., 1989; Lodato et al., 1990).
p185her2/neu-targeted therapy was initiated more than 20 years ago when monoclonal antibodies were used to reverse the malignant phenotype (Drebin et al., 1984, 1985). Since then, significant efforts have been spent to improve antibodies disabling p185her2/neu. The humanized antibody ‘trastuzumab’ (rhumAb 4D5 or Herceptin) (Carter et al., 1992) is already used to treat advanced breast cancer and, more recently, as an adjuvant to prevent tumor emergence (Katsumata et al., 1995; Romond et al., 2005).
2C4 (Pertuzumab, Omnitarg) is a distinct recombinant humanized monoclonal antibody targeting a different epitope on the extracellular domain of the p185her2/neu receptor. Agus et al. (2002) suggested that 2C4 disrupts the association of p185her2/neu with all other ErbB family receptors. This hypothesis was based on the 2C4 influence on p185her2/neu–ErbB3 association.
We first identified homodimers of p185her2/neu (Weiner et al., 1989) and heterodimers of epidermal growth factor receptor (EGFR) with p185her2/neu some years ago (Wada et al., 1990b). Moreover, we have defined interface binding molecules that do interfere with all heteromeric associations of the ErbB family (Berezov et al., 2002). In this study, we sought to investigate the effect of 2C4 preincubation on heteromeric EGFR–p185her2/neu interactions. Here, ‘preincubation’ refers to contacting cells on tissue culture plate with the antibody. Cells were washed twice with phosphate-buffered saline (PBS) before they were lysed to release membrane and cytoplasmic proteins.
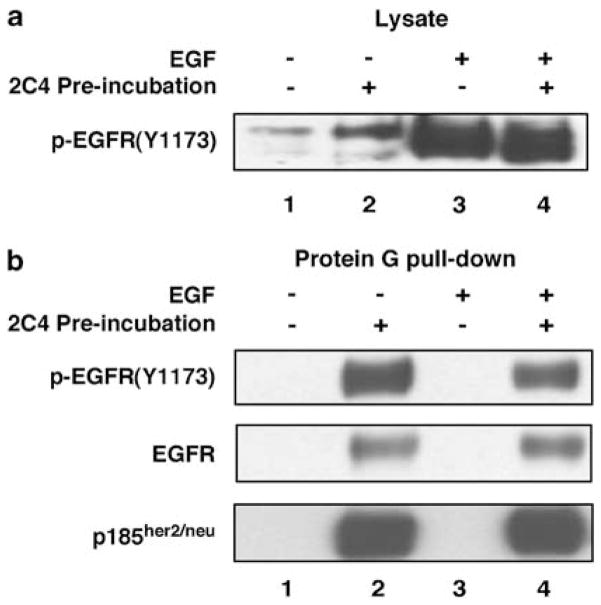
Experiments were performed with SKBR3, a breast cancer cell line expressing EGFR, p185her2/neu and ErbB3. EGF stimulation led to strong phosphorylation of EGFR, as revealed by the pEGFR(Y1173) antibody (Figure 1a). Preincubation of 2C4 did not significantly prevent phosphorylation of EGFR on residue Tyr1173. 2C4, however, formed discernible complexes with p185her2/neu–EGFR heteromers during the preincubation, as p185her2/neu and EGFR were detected in the protein G-agarose pulled down from the lysates of cells preincubated with 2C4 (Figure 1b). As 2C4 is specific to p185her2/neu, EGFR was co-precipitated with p185her2/neu. EGFR in the complex with p185her2/neu appeared to be already phosphorylated on Tyr1173, even before EGF stimulation.
Figure 1.
2C4 stably bound to a heteroreceptor complex containing p185her2/neu and epidermal growth factor receptor (EGFR). SKBR3 was routinely cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (Hyclone, Logan, UT, USA), 2mM glutamine, 1 × penicillin/streptomycin. SKBR3 cells were serum-starved overnight and preincubated with anti-p185her2/neu antibody 2C4 (20 μg ml−1, lanes 2 and 4). Cells were challenged with EGF (100ng ml−1, lanes 3 and 4) for 10min at 37 °C and washed twice with phosphate-buffered saline containing 5 mM NaF and 4 mM Na3VO4. Cell lysates were prepared in RPMI lysis buffer (Agus et al., 2002). Insoluble material was removed by centrifugation at 15 500 × g at 4°C for 15min. (a) Western blot with cell lysates. (b) Western blot with protein complexes pulled down by protein G agarose beads. Rabbit polyclonal antiphospho-EGFR-Tyr1174 and anti-EGFR clone 1005 were from Santa Cruz Biotech (Santa Cruz, CA, USA). Anti-p185her2/neu antibody cocktail (Ab-17) was purchased from Lab Vision (Fremont, CA, USA).
The observation that the p185her2/neu and associated proteins formed a stable complex with the preincubated antibodies and subsequently were pulled down by protein G-agarose is not limited to 2C4. 4D5, when preincubated with intact SKBR3 cells, also formed stable complexes with receptors. Consequently, both EGFR and ErbB3 were co-precipitated after protein G pull-down (Figure 2). Interestingly, the receptor complexes captured by 2C4 and 4D5 showed distinct contents of heteroreceptors. Using SKBR3 cells preincubated with 2C4 or 4D5 and subsequently stimulated with heregulin (HRG)-β1, we demonstrated that the 2C4 complex contained more EGFR but much less ErbB3 than the 4D5 complex (Figure 2). The less favored binding of 4D5 to the EGFR–p185her2/neu was also evident when 4D5 only co-immunoprecipitated a modest level of EGFR from lysates of SKBR3 cells stimulated by EGF (Supplementary Figure S1).
Figure 2.

2C4 and 4D5 captured receptor complexes contained different profiles of ErbB heteroreceptors. Serum-starved SKBR3 cells were first preincubated with medium control, 2C4 or 4D5 (20 μg ml−1 for both antibodies) as indicated for 1 h at 37 °C and then exposed to heregulin-β1 (R&D systems, Minneapolis, MN, USA, 250 ng ml−1) for a 10-min stimulation at 37 °C. Cells were washed twice with phosphate-buffered saline (PBS) containing 5mM NaF and 4mM Na3VO4 and lysed in RPMI lysis buffer (Agus et al., 2002). Cell lysates were cleared by centrifugation and subjected to protein G pull-down or 4D5 immunoprecipitation (IP). For protein G pull-down (lanes 1–3), cell lysates were contacted directly with protein G agarose beads. Antibodies (2C4 and 4D5) that were preincubated with cells sustained the PBS washing and remained bound to the receptors. Thus, receptor complexes captured by the preincubating antibodies were pulled down by protein G beads. For 4D5 IP, cell lysates from cells preincubated with or without antibodies were contacted first with additional 4D5 (5 μg) for 1 h at 4 °C and then with protein G agarose beads. Beads were collected by centrifugation and washed three times by lysis buffer. SDS sample buffer was added to the beads, and samples were boiled for 5 min before SDS–polyacrylamide gel electrophoresis. Separated proteins were transferred to nitrocellulose membrane for western blot. Membranes were blocked in 5% nonfat milk in PBS overnight. The following antibodies were used for blot: EGFR, clone 1005; ErbB3, C-17 (HRP) (Santa Cruz Biotech). EGFR, epidermal growth factor receptor.
The effect of 2C4 on p185her2/neu–ErbB3 dimerization is also not as simple as expected before. Although 2C4 preincubation reduced the amount of ErbB3 associated with p185her2/neu (Figure 2, lane 5 vs lane 4), it did not prevent HRGβ1 from inducing the heterodimerization of p185her2/neu with ErbB3. Protein G beads only pulled down a weak ErbB3 band from 2C4-preincubated cells (Figure 2, lane 2), but addition of 4D5 to the cell lysates from the same treatment co-precipitated a significant amount of ErbB3 (Figure 2, lane 5). The weak ErbB3 band in lane 2 was not a consequence of reduced heterodimerization by 2C4. It is simply caused by the weak 2C4 association with the p185her2/neu–ErbB3 heterodimer as we discussed later. These data indicate that significant ErbB3–p185her2/neu complexes exist in these cells, even in the presence of 2C4.
We have tested several cell lines with different levels of EGFR and p185her2/neu. Both SKBR3 and BT474 express very high level of p185her2/neu, but EGFR levels in BT474 are very low. A431 has high levels of EGFR and medium levels of p185her2/neu. Co-precipitated EGFR was detected in A431 cells in addition to SKBR3 cells, although the signal from A431 cells was weaker than that from SKBR3 cells (Supplementary Figure S1). In BT474 cells, although large amounts of p185her2/neu captured by 2C4 were pulled down by protein G beads, EGFR was rarely detected, possibly due to much lower expression levels of EGFR in this cell line (Supplementary Figure S1). MCF7, a cell line expressing low levels of EGFR and p185her2/neu, did not reveal any EGFR co-precipitation by 2C4 under similar conditions (data not shown).
In summary, our data indicated that 2C4 functions by a mechanism other than a general ErbB dimerization blocker. First, 2C4 stabilizes, instead of blocking, p185her2/neu–EGFR dimerization. Second, 2C4 also has a weak affinity for p185her2/neu–ErbB3 complexes, and the presence of 2C4 might reduce but not abolish p185her2/neu–ErbB3 association.
The effect of 2C4 on the p185her2/neu–ErbB3 heterodimers is significantly different from that reported by Agus et al. (2002), in which 2C4 dramatically and almost completely inhibited p185her2/neu–ErbB3 associations. In that report, p185her2/neu–ErbB3 interaction was studied by co-immunoprecipitation using an antibody specific to the cytoplasmic domain of p185her2/neu. However, Agus et al. did not clearly evaluate if 2C4 forms complexes with heteroreceptors.
Other laboratories also studied the effect of 2C4 on the dimerization of ErbB receptors, but the data are neither uniform nor convincing. Mendoza et al. (2002) reported that 2C4 inhibited p185her2/neu and ErbB3 association, which was induced by low-dose HRG (1 nM). Takai et al. (2005) studied the dimerization of EGFR and p185her2/neu. Although immunoprecipitation experiments were used to demonstrate EGFR and p185her2/neu dimerization in OVCA433 cells, 2C4 inhibition of dimerization was only shown in a contrived eTag assay. 2C4 was not studied by conventional biochemical immunoprecipitations. It should be noted that co-precipitation experiments disclose proteins associated in a complex, whereas the eTag assay reveals proteins in an extended proximity of 200 nm. Jackson et al. (2004) also studied EGF- and HRG-induced receptor activation. However, 2C4 was only examined for its effect on receptor phosphorylation, but not on heteromeric associations.
Our data on heteroreceptor preferences by 2C4 and 4D5, for the first time to our knowledge, also indicate that p185her2/neu may use different sets of surface residues for heterodimerizing with different ErbB family receptors. The 2C4 epitope in p185her2/neu appears to be involved in the heterodimerization with ErbB3, but less so with EGFR. In contrast, the 4D5 epitope plays a greater role in the heterodimerization with EGFR.
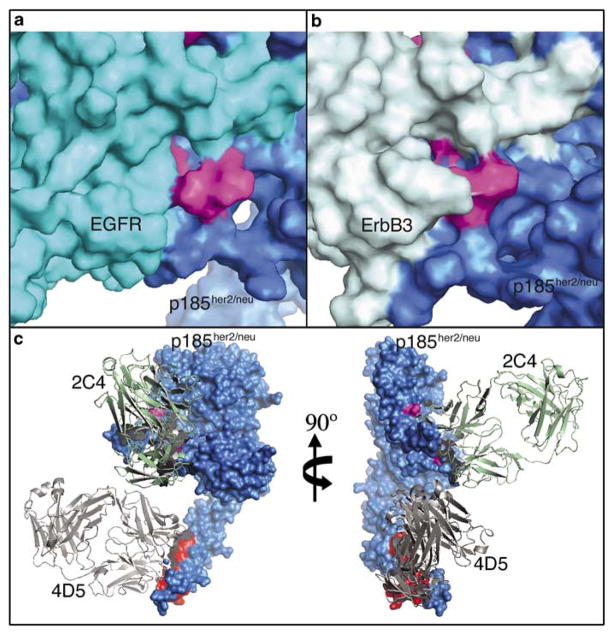
To understand the role of the 2C4 epitope, we developed a structural model of the EGFR–p185her2/neu heterodimer based on ErbB structures available from the RCSB protein data bank (PDB). The EGFR homodimer structure (PDB ID: 1IVO) (Ogiso et al., 2002) was selected as the most appropriate structural template, although information on subdomain IV was missing. By superimposing domain III and IV from the tethered form of EGFR (PDB ID: 1NQL) (Ferguson et al., 2003) onto domain III of the dimeric form, we obtained a homodimeric structure of the full-length EGFR ectodomain. Finally, we replaced one of the two EGFR molecules in the model with p185her2/neu (PDB ID: 1N8Z) (Cho et al., 2003) by superimposing residues 232–292 of p185her2/neu onto EGFR residues 226–286. The domain II region of the dimer is shown in Figure 3a.
Figure 3.
Structural model of ErbB dimers and binding of 4D5 and 2C4. (a and b) Surface representations of the epidermal growth factor receptor (EGFR)/p185her2/neu heterodimer (a) and ErbB3/p185her2/neu heterodimer (b) model, showing domain II of each receptors only. The six residues critical for 2C4 binding were colored in magenta. EGFR and p185her2/neu were colored in cyan and blue, whereas ErbB3 was colored in light cyan. (c) Orthogonal views of binding of antibodies 2C4 and 4D5 to p185her2/neu. p185her2/neu was colored in blue, with the six residues for 2C4 binding colored in magenta. 4D5-binding sites derived from the 4D5–p185her2/neu complex structure were colored in red. Ribbon diagram of 2C4 and 4D5 were colored in green and gray, respectively. We followed the approach described by Franklin et al. (2004) for the modeling of the p185her2/neu heterodimer. The superposition was performed with Swiss-Pdb Viewer (Guex and Peitsch, 1997). Energy minimization was carried out using Discover within Insight II 98.0, a molecular modeling package (Molecular Simulation Inc., San Diego, CA, USA).
The only available ErbB3 structure is in the tethered (inactive) form (PDB ID: 1M6B) (Cho and Leahy, 2002). Modeling is very unlikely to produce an accurate extended form for the whole extracellular domains. However, EGFR and ErbB3 share significant similarities in each subdomain. Therefore, we have superimposed domain II of ErbB3 onto the domain II of EGFR in the EGFR–p185her2/neu heterodimer model to generate the ErbB3-domainII/p185her2/neu heterodimer model (Figure 3b).
The crystal structure of 2C4–p185her2/neu complex (Franklin et al., 2004) indicates that 2C4 interacts predominantly with the domain II of p185her2/neu. Several residues in p185her2/neu have been identified by mutagenesis to be critical for 2C4 binding: His245, Val286, Ser288, Leu295, His296 and Lys311. Among these residues, Leu295 and His296 are critically involved in the association of p185her2/neu with ErbB3. Double mutation of these two residues (L295A and H296A) completely disabled the capability of p185her2/neu to heterodimerize with ErbB3 (Franklin et al., 2004). Consistent with that observation, our ErbB3-domainII/p185her2/neu model (Figure 3b) revealed that the side chains of Leu295 and His296 of p185her2/neu were relatively buried in a pocket and were in close proximity of the loop (Lys295–Leu298) of ErbB3.
However, this region was structurally different in the EGFR–p185her2/neu heterodimer model. The side chains of Leu295 and His296 along with those of Val286, Ser288 and Lys311 form a compact 2C4-contacting interface on the surface of the heterodimer. In particular, side chains of Leu295 and His296 of p185her2/neu were almost completely exposed on the heterodimer surface and can be fully accessible to 2C4. The corresponding loop in EGFR is relatively far away from Leu295 and His296 of p185her2/neu (Figure 3a).
The models are consistent with our data, revealing that the 2C4 epitope in p185her2/neu, although important for the heterodimerization with ErbB3, is not as critical for the heterodimerization with EGFR. In addition, using a docking program Autodock 4.0.1 (Sanner, 1999), we were able to demonstrate that 2C4 can be docked back to p185her2/neu as well as the EGFR–p185her2/neu heterodimers, but not to the p185her2/neu–ErbB3 heterodimer (Supplementary Figure S2). According to the docking program, 2C4 binds to p185her2/neu monoreceptor with lower energy than it does with the EGFR–p185her2/neu heterodimers (−11.93 vs −6.8 kcal mol−1).
The dimers of ErbB receptors adopt the shape of an elliptic disk with a hollow center. The two halves of the disk contact each other mainly through domain II and IV. Whereas 2C4 binds to domain II of p185her2/neu, 4D5 binds to a different epitope in domain IV. In addition, 2C4 and 4D5 orient differently with respect to the dimer disk after binding to p185her2/neu. 2C4 binds to domain II perpendicularly, whereas 4D5 binds to domain IV in a parallel position to the disk (Figure 3c).
The 4D5 epitope appears to be involved in ErbB receptor dimerization. Using constrained peptides mimicking cystine knot in domain IV, Berezov et al. (2002) have observed in Biacore experiments that B2-S22-APE, which contains the fragment 590PIWKFP-DEE598 of p185her2/neu, has higher affinity to EGFR than ErbB3. Another peptide B2-S23-BPT, which contains a sequence C terminal to B2-S22-APE (amino acid 605–632), prefers ErbB3 to EGFR. Interestingly, the 4D5 epitope covers B2-S22-APE but not B2-S23-BPT (Cho et al., 2003). By binding to a sequence critical to EGFR–p185her2/neu complexes, 4D5 in the parallel orientation can pose as a steric hindrance for the proper alignment of EGFR. This explains why in the presence of 4D5 there were less EGFR–p185her2/neu complexes. The subtle effect of 4D5, if any, on the p185her2/neu–ErbB3 complexes is also due to the steric disturbance to the dimerization region. In contrast, because of the way 2C4 latches onto p185her2/neu, 2C4 will mediate strong inhibition only when its epitope is critically involved in dimerization.
Our studies have indicated that 2C4 and 4D5 complement each other in their ability to capture different sets of heteroreceptors. We are optimistic about using 2C4 together with other antibodies targeting different epitopes (for example, 4D5) or different ErbB receptors (for example, C225) in an antibody-cocktail therapy, a strategy we proposed first many years ago for this family of receptors (Drebin et al., 1988; Wada et al., 1990a). Interactions among ErbB receptors have been implicated in the acquired resistance to tyrosine kinase inhibitors to EGFR (Kwak et al., 2005; Sergina et al., 2007). An antibody cocktail might efficiently disable p185her2/neu homomeric and heteromeric associations and limit the emergence of resistance caused by ErbB3 hetero-associations.
Supplementary Material
Acknowledgments
This work was partially funded by a grant from NCI (5P01 CA 89480).
Footnotes
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
References
- Agus DB, Akita RW, Fox WD, Lewis GD, Higgins B, Pisacane PI, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127–137. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- Berezov A, Chen J, Liu Q, Zhang HT, Greene MI, Murali R. Disabling receptor ensembles with rationally designed interface peptidomimetics. J Biol Chem. 2002;277:28330–28339. doi: 10.1074/jbc.M202880200. [DOI] [PubMed] [Google Scholar]
- Carter P, Presta L, Gorman CM, Ridgway JB, Henner D, Wong WL, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci USA. 1992;89:4285–4289. doi: 10.1073/pnas.89.10.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HS, Leahy DJ. Structure of the extracellular region of HER3 reveals an interdomain tether. Science. 2002;297:1330–1333. doi: 10.1126/science.1074611. [DOI] [PubMed] [Google Scholar]
- Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW, Jr, et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421:756–760. doi: 10.1038/nature01392. [DOI] [PubMed] [Google Scholar]
- Cohen JA, Weiner DB, More KF, Kokai Y, Williams WV, Maguire HC, Jr, et al. Expression pattern of the neu (NGL) gene-encoded growth factor receptor protein (p185neu) in normal and transformed epithelial tissues of the digestive tract. Oncogene. 1989;4:81–88. [PubMed] [Google Scholar]
- Drebin JA, Link VC, Greene MI. Monoclonal antibodies reactive with distinct domains of the neu oncogene-encoded p185 molecule exert synergistic anti-tumor effects in vivo. Oncogene. 1988;2:273–277. [PubMed] [Google Scholar]
- Drebin JA, Link VC, Stern DF, Weinberg RA, Greene MI. Down-modulation of an oncogene protein product and reversion of the transformed phenotype by monoclonal antibodies. Cell. 1985;41:697–706. doi: 10.1016/s0092-8674(85)80050-7. [DOI] [PubMed] [Google Scholar]
- Drebin JA, Stern DF, Link VC, Weinberg RA, Greene MI. Monoclonal antibodies identify a cell-surface antigen associated with an activated cellular oncogene. Nature. 1984;312:545–548. doi: 10.1038/312545a0. [DOI] [PubMed] [Google Scholar]
- Ferguson KM, Berger MB, Mendrola JM, Cho HS, Leahy DJ, Lemmon MA. EGF activates its receptor by removing interactions that autoinhibit ectodomain dimerization. Mol Cell. 2003;11:507–517. doi: 10.1016/s1097-2765(03)00047-9. [DOI] [PubMed] [Google Scholar]
- Franklin MC, Carey KD, Vajdos FF, Leahy DJ, De Vos AM, Sliwkowski MX. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex.[see comment] Cancer Cell. 2004;5:317–328. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- Jackson JG, St Clair P, Sliwkowski MX, Brattain MG. Blockade of epidermal growth factor- or heregulin-dependent ErbB2 activation with the anti-ErbB2 monoclonal antibody 2C4 has divergent downstream signaling and growth effects. Cancer Res. 2004;64:2601–2609. doi: 10.1158/0008-5472.can-03-3106. [DOI] [PubMed] [Google Scholar]
- Katsumata M, Okudaira T, Samanta A, Clark DP, Drebin JA, Jolicoeur P, et al. Prevention of breast tumour development in vivo by downregulation of the p185neu receptor. Nat Med. 1995;1:644–648. doi: 10.1038/nm0795-644. [DOI] [PubMed] [Google Scholar]
- Kern JA, Schwartz DA, Nordberg JE, Weiner DB, Greene MI, Torney L, et al. p185neu expression in human lung adenocarcinomas predicts shortened survival. Cancer Res. 1990;50:5184–5187. [PubMed] [Google Scholar]
- Kokai Y, Myers JN, Wada T, Brown VI, LeVea CM, Davis JG, et al. Synergistic interaction of p185c-neu and the EGF receptor leads to transformation of rodent fibroblasts. Cell. 1989;58:287–292. doi: 10.1016/0092-8674(89)90843-x. [DOI] [PubMed] [Google Scholar]
- Kwak EL, Sordella R, Bell DW, Godin-Heymann N, Okimoto RA, Brannigan BW, et al. Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proc Natl Acad Sci USA. 2005;102:7665–7670. doi: 10.1073/pnas.0502860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodato RF, Maguire HC, Jr, Greene MI, Weiner DB, LiVolsi VA. Immunohistochemical evaluation of c-erbB-2 oncogene expression in ductal carcinoma in situ and atypical ductal hyperplasia of the breast. Mod Pathol. 1990;3:449–454. [PubMed] [Google Scholar]
- Mendoza N, Phillips GL, Silva J, Schwall R, Wickramasinghe D. Inhibition of ligand-mediated HER2 activation in androgen-independent prostate cancer. Cancer Res. 2002;62:5485–5488. [PubMed] [Google Scholar]
- Ogiso H, Ishitani R, Nureki O, Fukai S, Yamanaka M, Kim JH, et al. Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell. 2002;110:775–787. doi: 10.1016/s0092-8674(02)00963-7. [DOI] [PubMed] [Google Scholar]
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- Sanner MF. Python: a programming language for software integration and development. J Mol Graph Model. 1999;17:57–61. [PubMed] [Google Scholar]
- Sergina NV, Rausch M, Wang D, Blair J, Hann B, Shokat KM, et al. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445:437–441. doi: 10.1038/nature05474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Takai N, Jain A, Kawamata N, Popoviciu LM, Said JW, Whittaker S, et al. 2C4, a monoclonal antibody against HER2, disrupts the HER kinase signaling pathway and inhibits ovarian carcinoma cell growth. Cancer. 2005;104:2701–2708. doi: 10.1002/cncr.21533. [DOI] [PubMed] [Google Scholar]
- van de Vijver M, van de Bersselaar R, Devilee P, Cornelisse C, Peterse J, Nusse R. Amplification of the neu (c-erbB-2) oncogene in human mammary tumors is relatively frequent and is often accompanied by amplification of the linked c-erbA oncogene. Mol Cell Biol. 1987;7:2019–2023. doi: 10.1128/mcb.7.5.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T, Myers JN, Kokai Y, Brown VI, Hamuro J, LeVea CM, et al. Anti-receptor antibodies reverse the phenotype of cells transformed by two interacting proto-oncogene encoded receptor proteins. Oncogene. 1990a;5:489–495. [PubMed] [Google Scholar]
- Wada T, Qian XL, Greene MI. Intermolecular association of the p185neu protein and EGF receptor modulates EGF receptor function. Cell. 1990b;61:1339–1347. doi: 10.1016/0092-8674(90)90697-d. [DOI] [PubMed] [Google Scholar]
- Weiner DB, Liu J, Cohen JA, Williams WV, Greene MI. A point mutation in the neu oncogene mimics ligand induction of receptor aggregation. Nature. 1989;339:230–231. doi: 10.1038/339230a0. [DOI] [PubMed] [Google Scholar]
- Williams TM, Weiner DB, Greene MI, Maguire HC., Jr Expression of c-erbB-2 in human pancreatic adenocarcinomas. Pathobiology. 1991;59:46–52. doi: 10.1159/000163614. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.