Abstract
A recent study has shown that increased activity of matrix metalloproteinases-2 and metalloproteinases-9 (MMP-2 and MMP-9) has detrimental effect on the brain after neonatal hypoxia. The present study determined the effect of maternal hypoxia on neuronal survivability and the activity of MMP-2 and MMP-9, as well as the expression of tissue inhibitors of metalloproteinase 1 and 2 (TIMP-1 and TIMP-2) in the brain of neonatal rats. Pregnant rats were exposed to 10.5% oxygen for 6 days from the gestation day 15 to day 21. Pups were sacrificed at day 0, 4, 7, 14, and 21 after birth. Body weight and brain weight of the pups were measured at each time point. The activity of MMP-2 and MMP-9 and the protein abundance of TIMP-1 and TIMP-2 were determined by zymography and Western blotting, respectively. The tissue distribution of MMPs was examined by immunofluorescence staining. The neuronal death was detected by Nissl staining. Maternal hypoxia caused significant decreases in body and brain size, increased activity of MMP-2 at day 0, and increased MMP-9 at day 0 and 4. The increased activity of the MMPs was accompanied by an overall tendency towards a reduced expression of TIMPs at all ages with the significance observed for TIMPs at day 0, 4, and 7. Immunofluorescence analysis showed an increased expression of MMP-2, MMP-9 in the hippocampus at day 0 and 4. Nissl staining revealed significant cell death in the hippocampus at day 0, 4, and 7. Functional tests showed worse neurobehavioral outcomes in the hypoxic animals.
Keywords: maternal, chronic hypoxia, development, matrix metalloproteinases, neonate
INTRODUCTION
Chronic maternal hypoxia remains a primary contributor to intrauterine growth restriction and continues to be a major clinical problem for fetal development (Lueder et al., 1995). The occurrence of the maternal hypoxia can be a result of smoking, drug use, or living at high altitude during pregnancy. The insufficient oxygen supply from mother during pregnancy is one of the major causes for the fetal brain injury, mental retardation, and neurological deficits, and one of the most likely triggers of sudden infant death syndrome (Prandota, 2004). It may also delay the development of motor reflexes in the newborn (Golan et al., 2004). Mental retardation, cerebral palsy, and seizures all have been shown to be the consequences of the prenatal hypoxic insult (Hallak et al., 2000). Maternal hypoxia may have a devastating impact on the mother and fetus, and after parturition the health of the offspring is often permanently affected (Lueder et al., 1995; Schneider et al., 2008).
Matrix metalloproteinases (MMPs) comprise a family of zinc-binding proteolytic enzymes that degrade the extracellular matrix during physiological and pathological tissue remodeling (Badier-Commander et al., 2000; Buss et al., 2007; Sifringer et al., 2007; Tian et al., 2007). MMP-2 and MMP-9 (gelatinases) are secreted principally by astrocytes and are opposed by their endogenous inhibitors TIMP-2 and TIMP-1 (Vaillant et al., 1999). Gelatinases are the most investigated MMPs in the brain because numerous neurovascular matrix and cell surface proteins have been identified as their substrates (Tian et al., 2007; Sulik and Chyczewski, 2008). MMPs and TIMPs have been implicated in extracellular matrix (ECM) remodeling during brain development (Wojcik et al., 2009); however, MMPs may also have detrimental effects after brain injury. For example, MMPs trigger neuronal cell death after hypoxia-reoxygenation (Lee and Lo, 2004), and impair the blood brain barrier integrity after focal ischemia (Rosenberg et al., 1998).
Earlier authors reported that gestational hypoxia could alter neural development and one of the mechanism involved is a defect of brain energetic adaptation. The absence of response of Glut3 and hypoxia inducible factor-1α (HIF-1α) at E19 in the hypoxic fetal brains might attribute to insufficient energetic adaptation (Royer et al., 2000). On the other hand, extracellular matrix proteins such as MMP-2 and MMP-9 may play a critical role in the neonatal brain, as tissue remodeling is predominating and long-lasting process during brain maturation (Cockle et al., 2007). There is an indication that hypoxia can cause the increased levels of reactive oxygen species (ROS) that activate NF-κB transcription pathways, including that of genes encoding MMPs (Cockle et al., 2007). The resultant imbalance between MMP-9 and TIMP-1 (tissue inhibitor of metalloproteinase-1; the endogenous inhibitor of MMP-9) may exacerbate the neuronal loss (Rosenberg et al., 1996). Sunagawa and colleagues have demonstrated that the ratio of MMP-9 to TIMP-1 correlates with the severity of neurological sequelae after acute perinatal asphyxia (Sunagawa et al., 2009). However, whether chronic maternal hypoxia will have an effect on the brain MMPs and TIMPs in the neonatal offspring has not been determined. Thus, the present study was designed to investigate the effect of maternal hypoxia on neonatal brain injury and brain expression of MMP-2, MMP-9 and TIMP-1, TIMP-2 at postnatal ages from day 0 to day 21. We hypothesize that maternal hypoxia increases the activity of MMPs and decreases the expression of TIMPs in the brain of neonatal rats.
MATERIALS AND METHODS
Maternal Hypoxic Treatment
All animal research protocols have been approved by Loma Linda University Institutional Animal Care and Use Committee (IACUC). Time-dated Sprague-Dawley rats were purchased from Charles River Laboratories (Portage, MI) and housed in light and temperature controlled environment with food and water ad libitum for the duration of the study. Animals were assigned into the normoxic group and maternal hypoxic group (10.5% oxygen) from day 15 to day 21 of gestation. Hypoxia was induced with a mixture of nitrogen gas and air as described previously (Li et al., 2003). The normoxic control group was housed identically, except the room air was flowing through the chambers. Pups were delivered on gestational day 22.
Sample Collecting
Twelve pregnant rats were randomly assigned for normoxic and maternal hypoxic group. Eight–thirteen pups from each litter were delivered. A total of 12 unsexed rat pups were randomly chosen from normoxic or hypoxic litter and euthanized at postnatal day 0, 4, 7, 14, 21. Transcardial perfusion was performed as previously described (Hu et al., 1999). Briefly, under anesthesia with 3.0% isoflurane pups were thoracotomized. A catheter was placed in the apex of the left ventricle and an incision was made on the right atrium. The pups were perfused with 40 mL of ice-cold phosphate buffered saline (PBS). The brain tissue was then collected and stored at −80°C for zymography and Western blotting analysis. For immunohistochemical analysis, the pups were first perfused with 40 mL of PBS followed by 40 mL of 10% buffered formalin. Collected brains were post fixed in formalin at 4°C overnight followed by cryoprotection in 30% sucrose. Upon euthanization, both length and weight of the brain and body were measured, respectively.
Western Blotting
Protein was extracted from cerebral tissues of the right hemisphere by gentle homogenization in lysis buffer [20 mM Tris, pH 7.5, 150 mM NaCl, 1% NP40, 0.5% Na de-oxycholate, 1 mM EDTA, and 0.1% sodium dodecyl sulfate (SDS)], containing protease and phosphatase inhibitor cocktails (Sigma-Aldrich, St. Louis, MO), followed by centrifugation at 15,000g at 4°C for 20 min. The supernatant was used as a whole cell protein extract and the protein concentration was determined by using a detergent compatible assay (Bio-Rad). Equal amounts of protein (30 μg) were loaded on an SDS-PAGE gel. The protein samples were electrophoresed and transferred onto a nitrocellulose membrane, which was then blocked and incubated with the primary antibodies at 4°C overnight. The primary antibodies used were mouse monoclonal anti-TIMP-1 and anti-TIMP-2 (Millipore, MAB3300, MAB3310, 1:1000). Nitrocellulose membranes were incubated with secondary antibodies (Santa Cruz Biotechnology) for 1 h at room temperature. Immunoblots were then visualized with an ECL Plus chemiluminescence reagent kit (Amersham Biosciences, Arlington Heights, IL). The optical densities of the bands were calculated with Image J software, version 1.0 and normalized to β-actin.
Gel Zymography
The brain samples were homogenized in lysis buffer including protease and phosphatase inhibitor cocktails (Sigma-Aldrich, St. Louis, MO). After centrifugation, the supernatant was collected and the protein concentration was determined by Bradford assay (Bio-Rad, Hercules, CA). Equal amounts of protein (120 μg) were loaded and separated by 10% Tris-glycine gel with 0.1% gelatin as substrate. The gel was renatured and then incubated with a developing buffer at 37°C for 48 h. The gel was stained with 0.5% Coomassie blue R-250 for 1 h and then destained. Data were analyzed with the Image J software, version 1.0. The sample for positive control was collected in the severe HI neonatal injury group of an unrelated study, and was loaded with equal amount of the protein into different gels. When quantifying MMP-2 and MMP-9 activity, the density of each band in different gels was first normalized with respect to the positive control, and then all the normalized densities from different time points or different groups were normalized with the density from normoxic day 0, which was set as 100% OD baseline.
Nissl Staining
At postnatal day 0, 4, 7, 14, and 21, animals were anesthetized and transcardially perfused with ice-cold PBS followed by 10% buffered formalin. Brains were collected, postfixed overnight, and cryoprotected in 30% sucrose/PBS. Coronal brain sections of 10 μm thicknesses were cut in a cryostat and Nissl staining was performed as previously described (Ostrowski et al., 2006). Six animals per group were used for Nissl Staining.
Immunofluorescence Staining
The tissue expression of matrix metalloproteinases and glial fibrillary acidic protein (GFAP; astrocytic marker) were assessed in the hippocampus by using double immunofluorescence staining. First, the antigen retrieval was done by microwaving sections in a citrate buffer for 10 min. After blocking with 5% donkey serum (1 h at room temperature), the sections were incubated with the following primary antibodies (Millipore): rabbit anti MMP-9 or anti MMP-2, in combination with mouse anti GFAP antibody (1:100, 4°C, overnight). The sections were then incubated with the secondary antibodies—anti-mouse FITC-conjugated and anti-rabbit Texas Red-conjugated donkey antibodies (1:200, 2 h at room temperature). After three washes, the sections were cover slipped with Slow-Fade reagent (Invitrogen) and observed under the fluorescent microscope with a digital camera (OLYMPUS BX51). Microphotographs were taken separately for each stain and merged by means of the Magna Fire software.
Functional Tests
All neurobehavioral tests were performed in a blinded set-up.
T-Maze Test
The T-maze spontaneous alternation task has been used to test the impairment of cognitive ability and working memory caused by hippocampal dysfunction (Matchett et al., 2007). At 4-week-old, rats were tested for a spontaneous alternation on a T-shaped maze as previously described (Matchett et al., 2007). Rats were placed in the stem of T-maze and allowed to freely explore the two arms of the maze. After an arm of maze was chosen, animals were placed again in the stem, and the trial was repeated for a total of ten times. Data are expressed as the rate of spontaneous alteration.
Wire Hanging
The wire hanging test was conducted on day 10, 14, and 22 rats to evaluate the neuromuscular and locomotor development as previously described (Fan et al., 2005). Pups suspended by their forelimbs from a horizontal rod (5 mm × 5 mm area, 35 cm long, between two poles 50 cm high) tended to support themselves with their hind limbs, preventing from falling. Suspension latencies were recorded.
Statistical Analysis
Data are expressed as means ± standard deviation (SD). The statistical analysis of differences between individual groups was performed by using t-test or ANOVA test. Differences for which p < 0.05 were considered significant.
RESULTS
Body/Brain Weight and Length
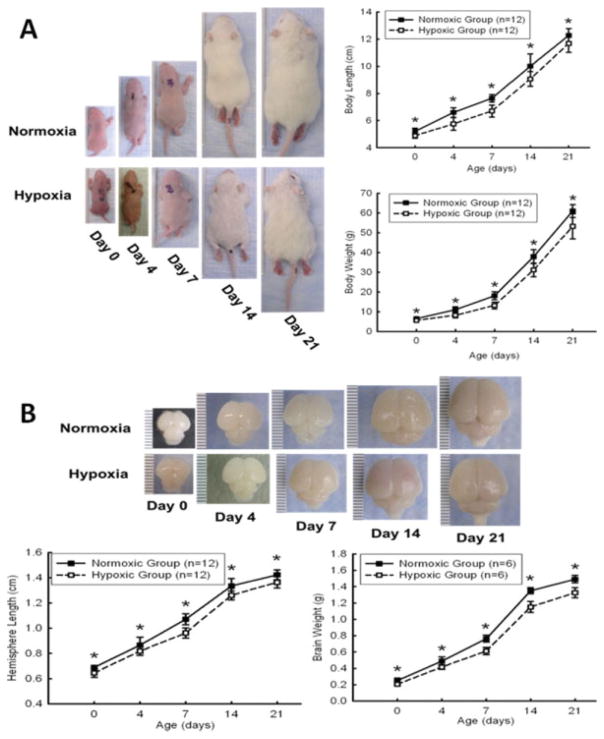
As shown in Figure 1, the treatment of maternal hypoxia in the rats for six days resulted in a significant decrease in the size and weight of body and brain [Fig. 1(A, B)], at postnatal ages of 0, 4, 7, 14, and 21 days.
Figure 1.
Chronic hypoxia on the body and brain weight and length in animals. Pups from maternal hypoxia dams were sacrificed at postnatal day 0, 4, 7, 14, and 21. Body and brain size and weight was measured at each time point. Chronic prenatal hypoxia significantly reduced body (A) and brain size (B) in maternal hypoxia offspring. *p < 0.05, N = 12 per group. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Profiles of MMPs and TIMPs
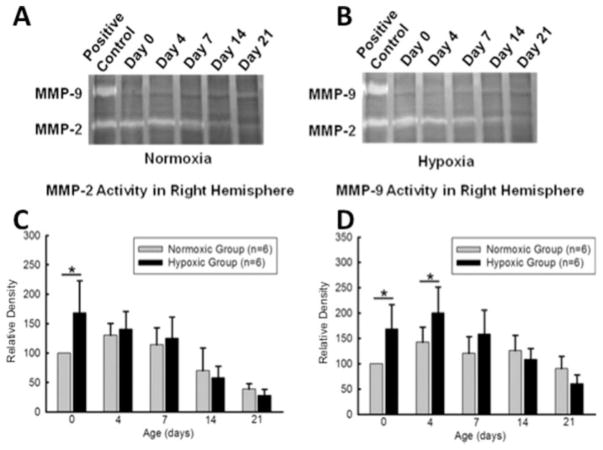
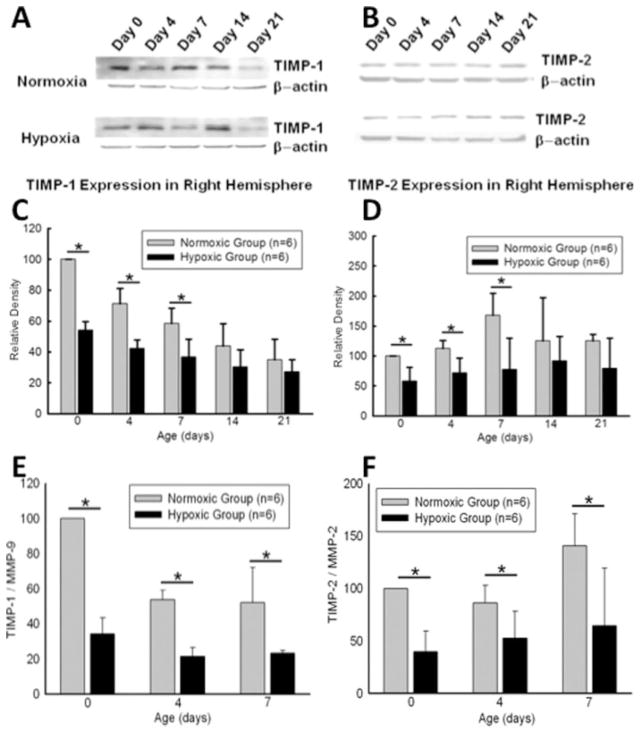
Profiles of MMP-2 and MMP-9 activity in neonates following 6 days of maternal hypoxia were determined at postnatal day 0 (n = 6), 4 (n = 6), 7 (n = 6), 14 (n = 6), and 21 (n = 6). Gel zymography in the brain extracts from right hemisphere showed that late maternal hypoxia triggered significant increase of MMP-2 activity at day 0. It also significantly increased MMP-9 activity at both day 0 and 4 (see Fig. 2). At day 0, 4, and 7, the protein abundance of TIMP-1 and TIMP-2 in the right hemisphere was significantly decreased in the hypoxic-treated pups, as compared with the normoxic controls [Fig. 3(A–D)], resulting in a significant increase in the ratio of TMIP-1/MMP-9 and TIMP-2/MMP-2 in the pups of hypoxic treatment [Fig. 3(E, F)].
Figure 2.
Gel zymography of right hemisphere homogenates at postnatal day 0, 4, 7, 14, and 21. Top photographs show representative zymogram gels (A, B). Bar graphs show quantified densitometry data. MMP-2 activity was increased in hypoxic animals compared to control animals at day 0 (C). MMP-9 activity was also increased in hypoxic animals at day 0 and 4 (D). No significant differences in MMP-2, MMP-9 activities were detected at other time points. *p < 0.05, N = 6 per group.
Figure 3.
A: Western blot analysis of right hemisphere homogenates at postnatal day 0, 4, 7, 14, and 21. Upper panel of A and B shows representative immunoblots. Lower panel of C and D shows quantified densitometry data. TIMP-2 and TIMP-9 expression was decreased in hypoxic animals compared to control animals at day 0, 4, and 7. No significant difference in TIMP-1, TIMP-2 expressions was detected at other time points. E, F: Ratios of TIMP-1/MMP-9 and TIMP-2/MMP-2 were analyzed. The significant decrease in both ratios was detected at day 0, 4, and 7 in the hypoxic group. *p < 0.05, N = 6 per group.
Morphological Changes
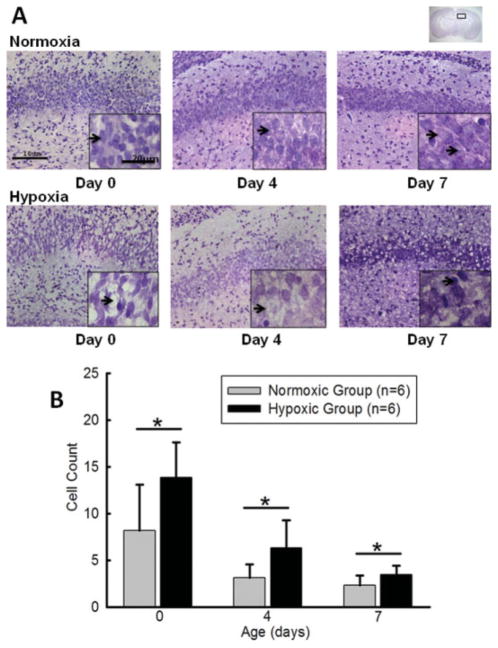
Morphological changes following prenatal hypoxia were exhibited by Nissl staining in the coronal brain sections [Fig. 4(A)]. Neurons in the CA1 hippocampal region of normoxic animals appeared normal morphologically on day 0, 4, and 7 after birth. In contrast, the pyramidal cells with condensation of cytoplasm and karyoplasms were found in CA1 following prenatal hypoxia. These neurons, as presented in the lower panels of the Figure 4(A), were dark stained and sometimes triangular in shape. Additionally, small foci of cell loss could occasionally be seen. As shown in Figure 4(B), the number of dead cells in the CA1 area of hypoxic hippocampus was significantly higher than in the normoxia group on day 0, 4, and 7 after birth (t-test, p < 0.05). The graph also demonstrates a tendency towards a decrease in numbers of dead cells as compared between day 0 and later time points.
Figure 4.
Effect of maternal hypoxia on morphological changes and cell death. A: Representative Nissl stained CA1 hippocampal area in coronal brain sections from normoxic and maternal hypoxic groups at day 0, 4, and 7 are shown. Scale bars indicate 10 μm (20 μm in the insets). At higher magnifications in hypoxia animals, the number of cells in hypoxia animals was markedly reduced and arrows indicate dying cells or empty spaces after cells died. Normal neurons are indicated by the arrows in the normoxia slides. B: Cell death counting revealed a significant cell loss after maternal hypoxia at day 0, 4, and 7. There were no detectable differences in the number of dead cells at day 14 and 21 (data not shown). *p < 0.05, N = 6 rats per group (three counting areas in each slide from each animal). The inserted brain slide photo indicates the areas that samples were taken. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Distribution of MMP-2 and MMP-9
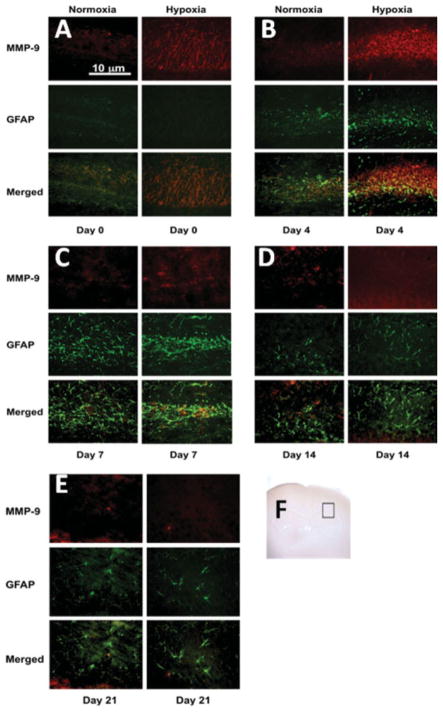
Double immunofluorescence analysis of the brain sections revealed a predominant distribution of MMP-2 and MMP-9 in the astroglial elements found within CA1 hippocampal region (Figs. 5 and 6). Only a weak MMP-9 immunoreactivity was observed in the normoxia group at days 0, 4, and 7. A mild tendency of increase in MMP-9 immunoreactivity was seen on day 14 and 21 after birth in the normoxia group. Interestingly, MMP-9 epitopes only partially colocalized with GFAP in the normoxic group (day 14 and 21). Following prenatal hypoxia, a tremendous induction of MMP-9 colocalizing with astrocytic marker GFAP could be seen in CA1 sector. This increased MMP-9 immunostaining was observed at day 0, reached its maximum on day 4, and started to decline on day 7.
Figure 5.
Double-labeling fluorescent immunohistochemistry for MMP-9 and GFAP in hypoxic pups. Red and green stain shows MMP-9s and GFAP expression, respectively. The photographs show that the expression of MMP-9 in astrocytes in CA1 region was increased in hypoxic animals at day 0 and 4. Scale bar = 10 μm. The inserted brain slide photo (F) indicates the areas that samples were taken. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Figure 6.
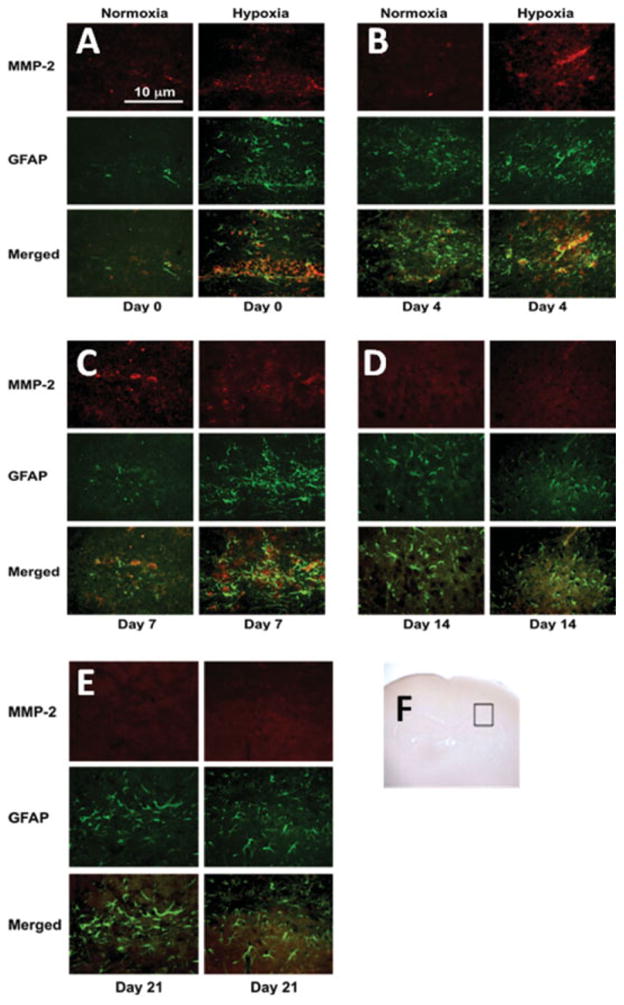
Double-labeling fluorescent immunohistochemistry for MMP-2 and GFAP in hypoxic pups. Red and green stain shows MMP-2 and GFAP expression, respectively. The photographs show that the expression of MMP-2 in astrocytes in CA1 region was increased in hypoxic animals at day 0 and 4. Scale bar = 10 μm. The inserted brain slide photo (F) indicates the areas that samples were taken. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The normoxic group exhibited a very low level of MMP-2 tissue expression in the brain region encompassing CA1 at all time points (see Fig. 6) with enhanced immunoreactivity observed on day 7. However, in the hypoxic animals, strong MMP-2 immunoreactivity was detected on the postnatal day 0, 4, and 7 (to a lesser degree) as compared with the normoxia group. Noticeably, MMP-2 stain was only partially colocalized with GFAP. MMP-2 immunostaining was reduced at day 14. On day 21, there was no detectable MMP-2 immunoreactivity in both hypoxia and normoxia groups.
Neurobehavioral Tests
To assess the effect of maternal hypoxia on postnatal neurological function, the T-maze test was performed at day 28 and the wire hanging test at day 10, 14, and 22. The results of T-maze testing for the spontaneous alternation and cognitive ability do not show significant difference between the normoxic and hypoxic groups. Moreover, hypoxic group has tendency to show more spontaneous alternation in the test [Fig. 7(A), n = 5 for each group]. However, the wire hanging test demonstrated that the duration of wire hanging in the hypoxic groups was significantly shorter than that in the control groups at all three ages determined [Fig. 7(B), n = 5 for each group, p < 0.05].
Figure 7.
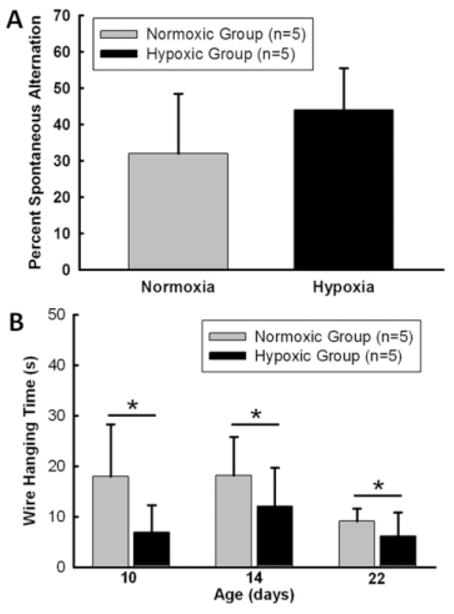
The effect of chronic maternal hypoxia on changes in functional performance. A: Two groups of animals did not show difference in percentage alternations when presented with two choices in the T-maze. B: Wire hanging analysis showed hypoxic animals performed worse on locomotors functional test than control animals. *p < 0.05, N = 5 per group.
DISCUSSION
The present study has examined the effect of chronic maternal hypoxia on neonatal growth and the neuronal survivability in the immature brain. Consistent with previous findings, chronic hypoxia during late pregnancy caused a significant reduction in the body and brain size of rat pups, indicating a developmental retardation. The previous study demonstrated that acute hypoxia down-regulated TIMP-2 mRNA levels and up-regulated MMP-2 mRNA and protein expression in vitro (Ben-Yosef et al., 2002). In acute asphyxiated neonates, the presence of neurological sequelae strongly correlated with a high serum MMP-9/TIMP-1 ratio (Sunagawa et al., 2008). The present study extends the investigations into the role of TIMPs and MMPs in the neonatal brain injury and growth retardation following chronic maternal hypoxia. We are unaware of other studies that demonstrate an imbalance of the TIMPs and MMPs in chronic maternal hypoxia.
The mechanism of brain damage caused by maternal hypoxia in offspring is not clear. However, the observed pattern of injury in the present study is consistent with the increased vulnerability of CA1 neurons to hypoxia (Hallak et al., 2000). The morphological changes detected in the CA1 included more cell death in maternal hypoxic rats than that in normoxic animals. Other authors reported that the acute prenatal hypoxia induced neuronal loss in the hippocampus, causing a reduction in CA1 cell layer width (Golan et al., 2004). This raises a possibility that neonatal growth retardation may occur due to cell death or inhibition of cell proliferation. This notion is in accordance with the results of a previous study demonstrating that acute perinatal hypoxia altered the structural and functional properties of cell membranes and initiated apoptotic genes transcription (Zhuravin et al., 2004).
Although the developmental retardation may also occur due to maternal nutrition restriction induced by hypoxic insult, Ream and colleagues have demonstrated that maternal nutrition is not a critical factor in prenatal hypoxia-induced growth retardation (Ream et al., 2008). Another study suggested that hypoxia-induced reduction of appetite did not contribute to ventricular hypertrophy in offspring (Xu et al., 2006). Taken together with our data it suggests that chronic or acute fetal hypoxia causes the brain developmental retardation via the activation of cell death pathways. Consistent with the present finding, previous studies in the same animal model demonstrated that maternal hypoxia resulted in the increased apoptosis in the heart of fetal and neonatal rats (Bae et al., 2003; Bae and Zhang, 2005).
To investigate the changes of TIMPs and MMPs in the development of brain injury caused by prenatal hypoxia, we compared the time course of the changes in the ratio of TIMPs and MMPs in hypoxic versus normoxic rats. Our data have shown (i) a significant decrease in this ratio in the brain of newborn and neonates up to 1 week old in hypoxic treated animals compared with controls, (ii) a consistent time course of the changes in the TIMPs expression and the decreased cell death between normoxic and hypoxic animals. Previous studies have reported that both TIMP-1 and TIMP-2 promote the cell division in vitro (Hayakawa et al., 1992, 1994), and that TIMP-1 has an anti-apoptosis effect in vitro (Guedez et al., 1998). The present findings suggest that maternal hypoxia alters the ratio of TIMPs and MMPs by suppressing the TIMPs expression, which is likely to contribute to the reduced cell growth and proliferation and the increased neuronal death in the developing brain. These findings also imply the possibility that raising TIMPs levels may restore the balance of TIMPs and MMPs and improve the neural cell survivability and proliferation, preventing the development impairment of the brain associated with fetal hypoxia. This strategy is, however, technically challenging to either directly and selectively inhibit MMPs or increase TIMPs in fetus in a pregnant mother rat. Our study has also implied that the extracellular matrix proteolysis dysfunction and disassociation of neurovascular unit might attribute to the pathophysiology of maternal hypoxia, which requires further investigations.
The present finding that maternal hypoxic-induced morphological changes in the CA1 region and the decreased TIMPs were not associated with a worsened performance in the T-Maze test is somewhat surprising, given that uncompensated maternal hypoxia has been shown to trigger significant alterations in fetal brain function (Pearce, 2006). In addition, a previous study demonstrated that short-term and long-term memory was impaired in offspring rats following the maternal hypoxic treatment (Golan et al., 2004). Several factors may explain this discrepancy. The first possible reason relates to the timing of hypoxia. In the present study, pregnant rats were subjected to chronic hypoxia during the late phase of pregnancy when neurula is fully-formed and encephalon is well-developed. Therefore, the brain is not as vulnerable as during the mid-phase of pregnancy, especially between embryonic days 6 and 13. This time period includes the events leading to neurulation and encephalization along with intense cell proliferation and glial lineage commitment (Gressens et al., 1997). The quantitative image analysis showed more dead cells in hypoxic than in normoxic group, however, there was no obvious cell loss in the CA1 area in both groups. Since the hippocampus is critical for the cognitive ability, it is possible that our chronic hypoxia model may still be too mild to cause severe functional deficits. The second possible reason relates to the maternal or fetal compensation. There is no difference for the ratio of brain to body size between normoxic and hypoxic animals at postnatal day 0 (data not shown), which may be due to a compensation of cardiac output redistribution to preserve the fetal brain in the hypoxic condition. This compensation protects the immature brain from severe damage (Williams et al., 2005).
In the present study, we have demonstrated that maternal hypoxia causes a decrease in neonatal brain TIMPs levels and developmental retardation through neuronal death, with no changes in the short-term cognitive ability. Future studies are needed to investigate (1) other than MMPs/TIMPs hypoxia-related factors involved in the neonatal brain injury following late maternal hypoxia and (2) the potential functional deficit of the brain in a long term in offspring after prenatal hypoxia. In addition, the question whether pharmacological intervention directed at the increased TIMPs levels selectively attenuates developmental retardation of the brain in pups needs to be addressed. Studies from our laboratory have shown that erythropoietin (EPO) indirectly increases TIMP-1 levels in vitro (unpublished data). However, there is a limitation for elevated TIMPs levels. Over-suppressing MMPs may be deleterious considering that MMPs play an important role in tissue modulation and organ development (Matrisian and Hogan, 1990; Talhouk et al., 1992). Our study has identified differences in MMPs and TIPMs immunoreactivity mainly in the hippocampus. However, since the significant changes of MMPs and TIPMs detected by Western blotting were sampled from right hemisphere, this sampling might dilute changes of those proteins in local tissue. In the future study, to determine brain region- and cell type-specific distribution of these molecules, in situ MMP zymography and immunostaining of specific cell type markers would be needed. We acknowledge that our study is correlational in nature and as such it does not allow us to establish causal relationship between alteration of MMPs, TIMPs, and hypoxic brain injury. However, the results of this study support the argument that hypoxia induced changes in MMPs and TIMPs may be involved in neonatal brain injury following late maternal hypoxia.
In the present study, the pups were not selected by their gender and the gender effect was not examined. Previous study, however, has indicated no gender differences following severe or moderate hypoxic-ischemic insult from postnatal day 5 to day 21. More extensive brain injury in males than females was found only at postnatal day 60 in moderate hypoxic-ischemic group (Zhu et al., 2006). Although it has been reported that the effects of circulating estrogens and progestins are likely involved in the greater neuroprotection afforded to adult females, Hurn and his colleagues have shown no differences in functional outcomes between male and female neonates, which indicates that gender differences may not be a major factor affecting brain injury after neonatal hypoxia (Hurn and Macrae, 2000; Hurn et al., 2005).
Overall, we conclude that chronic maternal hypoxia impairs the proteolytic balance by increasing the activity of MMPs and decreasing the expression of TIMPs in the brain of neonatal rats. Unopposed extracellular matrix degradation is likely to contribute to the brain injury and growth retardation observed in the present study. These results may provide a molecular basis for novel therapies targeting extracellular proteolysis in neonatal brain affected by maternal hypoxia in the future.
Acknowledgments
Contract grant sponsor: NIH; contract grant numbers: NS052492, NS060936, NS054685, HL83966.
References
- Bae S, Xiao Y, Li G, Casiano CA, Zhang L. Effect of Maternal chronic hypoxic exposure during gestation on apoptosis in fetal rat heart. Am J Physiol Heart Circ Physiol. 2003;285:H983–H990. doi: 10.1152/ajpheart.00005.2003. [DOI] [PubMed] [Google Scholar]
- Bae S, Zhang L. Prenatal cocaine exposure increases apoptosis of neonatal rat heart and heart susceptibility to ischemia-reperfusion injury in 1-month-old rat. Br J Pharmacol. 2005;144:900–907. doi: 10.1038/sj.bjp.0706129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badier-Commander C, Verbeuren T, Lebard C, Michel JB, Jacob MP. Increased TIMP/MMP ratio in varicose veins: A possible explanation for extracellular matrix accumulation. J Pathol. 2000;192:105–112. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH670>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Ben-Yosef Y, Lahat N, Shapiro S, Bitterman H, Miller A. Regulation of endothelial matrix metalloprotei-nase-2 by hypoxia/reoxygenation. Circ Res. 2002;90:784–791. doi: 10.1161/01.res.0000015588.70132.dc. [DOI] [PubMed] [Google Scholar]
- Buss A, Pech K, Kakulas BA, Martin D, Schoenen J, Noth J, Brook GA. Matrix metalloproteinases and their inhibitors in human traumatic spinal cord injury. BMC Neurol. 2007;7:17. doi: 10.1186/1471-2377-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockle JV, Gopichandran N, Walker JJ, Levene MI, Orsi NM. Matrix metalloproteinases and their tissue inhibitors in preterm perinatal complications. Reprod Sci. 2007;14:629–645. doi: 10.1177/1933719107304563. [DOI] [PubMed] [Google Scholar]
- Fan LW, Lin S, Pang Y, Lei M, Zhang F, Rhodes PG, Cai Z. Hypoxia-ischemia induced neurological dysfunction and brain injury in the neonatal rat. Behav Brain Res. 2005;165:80–90. doi: 10.1016/j.bbr.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Golan H, Kashtuzki I, Hallak M, Sorokin Y, Huleihel M. Maternal hypoxia during pregnancy induces fetal neurodevelopmental brain damage: Partial protection by magnesium sulfate. J Neurosci Res. 2004;78:430–441. doi: 10.1002/jnr.20269. [DOI] [PubMed] [Google Scholar]
- Gressens P, Muaku SM, Besse L, Nsegbe E, Gallego J, Delpech B, Gaultier C, et al. Maternal protein restriction early in rat pregnancy alters brain development in the progeny. Brain Res Dev Brain Res. 1997;103:21–35. doi: 10.1016/s0165-3806(97)00109-0. [DOI] [PubMed] [Google Scholar]
- Guedez L, Stetler-Stevenson WG, Wolff L, Wang J, Fukushima P, Mansoor A, Stetler-Stevenson M. In vitro suppression of programmed cell death of B cells by tissue inhibitor of metalloproteinases-1. J Clin Invest. 1998;102:2002–2010. doi: 10.1172/JCI2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallak M, Hotra JW, Kupsky WJ. Magnesium sulfate protection of fetal rat brain from severe maternal hypoxia. Obstet Gynecol. 2000;96:124–128. doi: 10.1016/s0029-7844(00)00844-9. [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Yamashita K, Ohuchi E, Shinagawa A. Cell growth-promoting activity of tissue inhibitor of metalloproteinases-2 (TIMP-2) J Cell Sci. 1994;107:2373–2379. doi: 10.1242/jcs.107.9.2373. [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Yamashita K, Tanzawa K, Uchijima E, Iwata K. Growth-promoting activity of tissue inhibitor of metalloproteinases-1 (TIMP-1) for a wide range of cells. A possible new growth factor in serum. FEBS Lett. 1992;298:29–32. doi: 10.1016/0014-5793(92)80015-9. [DOI] [PubMed] [Google Scholar]
- Hu X, Brännström T, Gu W, Wester P. A photothrombotic ring stroke model in rats with or without late spontaneous reperfusion in the region at risk. Brain Res. 1999;849:175–186. doi: 10.1016/s0006-8993(99)02152-6. [DOI] [PubMed] [Google Scholar]
- Hurn PD, Macrae IM. Estrogen as a neuroprotectant in stroke. J Cereb Blood Flow Metab. 2000;20:631–652. doi: 10.1097/00004647-200004000-00001. [DOI] [PubMed] [Google Scholar]
- Hurn PD, Vannucci SJ, Hagberg H. Adult or perinatal brain injury. Does sex matter? Stroke. 2005;36:193–195. doi: 10.1161/01.STR.0000153064.41332.f6. [DOI] [PubMed] [Google Scholar]
- Lee SR, Lo EH. Induction of caspase-mediated cell death by matrix metalloproteinases in cerebral endothelial cells after hypoxia-reoxygenation. J Cereb Blood Flow Metab. 2004;24:720–727. doi: 10.1097/01.WCB.0000122747.72175.47. [DOI] [PubMed] [Google Scholar]
- Li G, Xiao YH, Estrella JL, Ducsay CA, Gilbert RD, Zhang L. Effect of fetal hypoxia on heart susceptibility to ischemia and reperfusion injury in the adult rat. J Soc Gynecol Invest. 2003;10:265–274. doi: 10.1016/s1071-5576(03)00074-1. [DOI] [PubMed] [Google Scholar]
- Lueder FL, Kim SB, Buroker CA, Bangalore SA, Ogata ES. Chronic maternal hypoxia retards fetal growth and increases glucose utilization of select fetal tissues in the rat. Metabolism. 1995;44:532–537. doi: 10.1016/0026-0495(95)90063-2. [DOI] [PubMed] [Google Scholar]
- Matchett GA, Calinisan JB, Matchett GC, Martin RD, Zhang JH. The effect of granulocyte-colony stimulating factor in global cerebral ischemia in rats. Brain Res. 2007;1136:200–207. doi: 10.1016/j.brainres.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian LM, Hogan BL. Growth factor-regulated proteases and extracellular matrix remodeling during mammalian development. Curr Top Dev Biol. 1990;24:219–259. doi: 10.1016/s0070-2153(08)60089-7. [DOI] [PubMed] [Google Scholar]
- Ostrowski RP, Tang J, Zhang JH. Hyperbaric oxygen suppresses NADPH oxidase in a rat subarachnoid hemorrhage model. Stroke. 2006;37:1314–1318. doi: 10.1161/01.STR.0000217310.88450.c3. [DOI] [PubMed] [Google Scholar]
- Pearce W. Hypoxic regulation of the fetal cerebral circulation. J Appl Physiol. 2006;100:731–738. doi: 10.1152/japplphysiol.00990.2005. [DOI] [PubMed] [Google Scholar]
- Prandota J. Possible pathomechanisms of sudden infant death syndrome: Key role of chronic hypoxia, infection/inflammation states, cytokine irregularities, and metabolic trauma in genetically predisposed infants. Am J Ther. 2004;11:517–546. doi: 10.1097/01.mjt.0000140648.30948.bd. [DOI] [PubMed] [Google Scholar]
- Ream M, Ray AM, Chandra R, Chikaraishi DM. Early fetal hypoxia leads to growth restriction and myocardial thinning. Am J Physiol Regul Integr Comp Physiol. 2008;295:R583–R595. doi: 10.1152/ajpregu.00771.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg GA, Dencoff JE, Correa N, Jr, Reiners M, Ford CC. Effect of steroids on CSF matrix metalloproteinases in multiple sclerosis: Relation to blood-brain barrier injury. Neurology. 1996;46:1626–1632. doi: 10.1212/wnl.46.6.1626. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Estrada EY, Dencoff JE. Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke. 1998;29:2189–2195. doi: 10.1161/01.str.29.10.2189. [DOI] [PubMed] [Google Scholar]
- Royer C, Lachuer J, Crouzoulon G, Roux J, Peyronnet J, Mamet J, Pequignot J, et al. Effects of gestational hypoxia on mRNA levels of Glut3 and Glut4 transporters, hypoxia inducible factor-1 and thyroid hormone receptors in developing rat brain. Brain Res. 2000;21:119–128. doi: 10.1016/s0006-8993(99)02365-3. [DOI] [PubMed] [Google Scholar]
- Schneider J, Mitchell I, Singhal N, Kirk V, Hasan SU. Prenatal cigarette smoke exposure attenuates recovery from hypoxemic challenge in preterm infants. Am J Respir Crit Care Med. 2008;178:520–526. doi: 10.1164/rccm.200803-432OC. [DOI] [PubMed] [Google Scholar]
- Sifringer M, Stefovska V, Zentner I, Hansen B, Stepulak A, Knaute C, Marzahn J, et al. The role of matrix metalloproteinases in infant traumatic brain injury. Neurobiol Dis. 2007;25:526–535. doi: 10.1016/j.nbd.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Sulik A, Chyczewski L. Immunohistochemical analysis of MMP-9, MMP-2 and TIMP-1, TIMP-2 expression in the central nervous system following infection with viral and bacterial meningitis. Folia Histochem Cytobiol. 2008;46:437–442. doi: 10.2478/v10042-008-0058-8. [DOI] [PubMed] [Google Scholar]
- Sunagawa S, Ichiyama T, Honda R, Fukunaga S, Maeba S, Furukawa S. Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in perinatal asphyxia. Brain Dev. 2009;31:588–593. doi: 10.1016/j.braindev.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Talhouk RS, Bissell MJ, Werb Z. Coordinated expression of extracellular matrix-degrading proteinases and their inhibitors regulates mammary epithelial function during involution. J Cell Biol. 1992;118:1271–1282. doi: 10.1083/jcb.118.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Stefanidakis M, Ning L, Van Lint P, Nyman-Huttunen H, Libert C, Itohara S, et al. Activation of NMDA receptors promotes dendritic spine development through MMP-mediated ICAM-5 cleavage. J Cell Biol. 2007;178:687–700. doi: 10.1083/jcb.200612097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillant C, Didier-Bazès M, Hutter A, Belin MF, Thomasset N. Spatiotemporal expression patterns of metalloproteinases and their inhibitors in the postnatal developing rat cerebellum. J Neurosci. 1999;19:4994–5004. doi: 10.1523/JNEUROSCI.19-12-04994.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SJ, Campbell ME, McMillen IC, Davidge ST. Differential effects of maternal hypoxia or nutrient restriction on carotid and femoral vascular function in neonatal rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:360–367. doi: 10.1152/ajpregu.00178.2004. [DOI] [PubMed] [Google Scholar]
- Wojcik L, Sawicka A, Rivera S, Zalewska T. Neurogenesis in gerbil hippocampus following brain ischemia: Focus on the involvement of metalloproteinases. Acta Neurobiol Exp. 2009;69:52–61. doi: 10.55782/ane-2009-1729. [DOI] [PubMed] [Google Scholar]
- Xu Y, Williams SJ, O’Brien D, Davidge ST. Hypoxia or nutrient restriction during pregnancy in rats leads to progressive cardiac remodeling and impairs postischemic recovery in adult male offspring. FASEB J. 2006;20:1251–1253. doi: 10.1096/fj.05-4917fje. [DOI] [PubMed] [Google Scholar]
- Zhu C, Xu F, Wang X, Shibata M, Uchiyama Y, Blomgren K, Hagberg H. Different apoptotic mechanisms are activated in male and female brains after neonatal hypoxia-ischemia. J Neurochem. 2006;96:1016–1027. doi: 10.1111/j.1471-4159.2005.03639.x. [DOI] [PubMed] [Google Scholar]
- Zhuravin IA, Dubrovskaya NM, Tumanova NL. Post-natal physiological development of rats after acute prenatal hypoxia. Neurosci Behav Physiol. 2004;34:809–816. doi: 10.1023/b:neab.0000038132.08219.31. [DOI] [PubMed] [Google Scholar]