Abstract
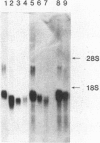
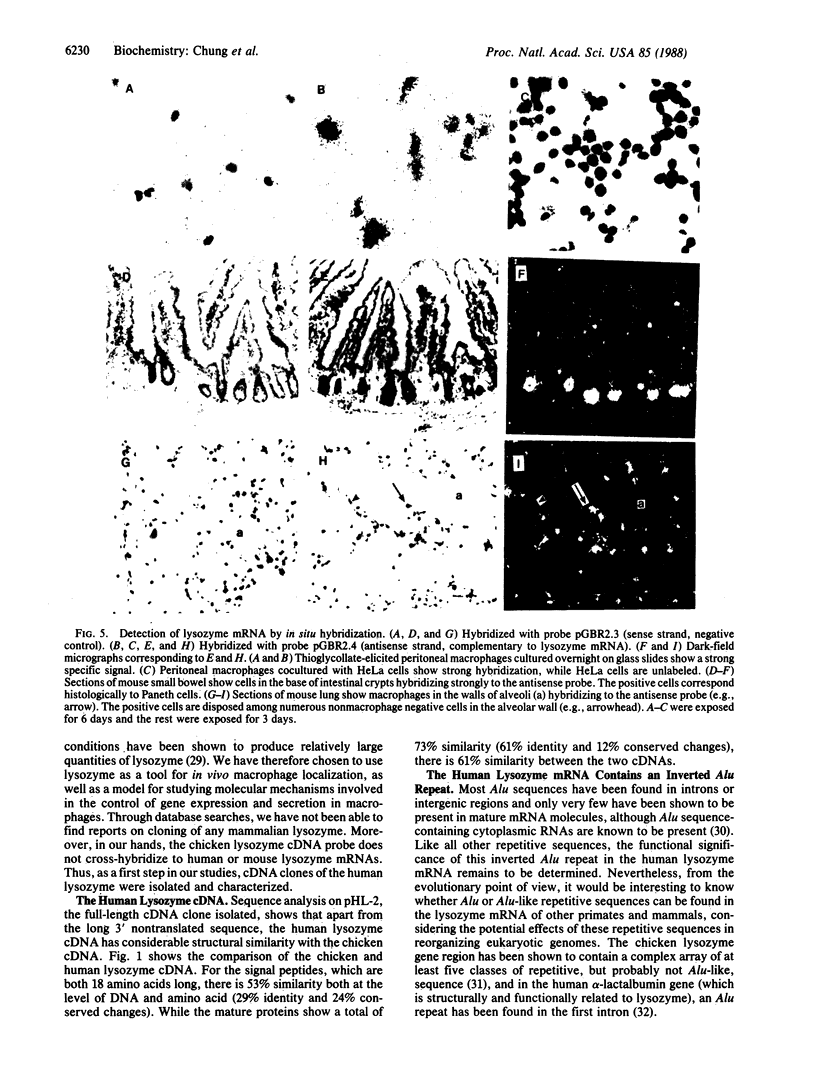
Lysozyme is a major secretory product of human and rodent macrophages and a useful marker for myelomonocytic cells. Based on the known human lysozyme amino acid sequence, oligonucleotides were synthesized and used as probes to screen a phorbol 12-myristate 13-acetate-treated U937 cDNA library. A full-length human lysozyme cDNA clone, pHL-2, was obtained and characterized. Sequence analysis shows that human lysozyme, like chicken lysozyme, has an 18-amino-acid-long signal peptide, but unlike the chicken lysozyme cDNA, the human lysozyme cDNA has a greater than 1-kilobase-long 3' nontranslated sequence. Interestingly, within this 3' region, an inverted repeat of the Alu family of repetitive sequences was discovered. In RNA blot analyses, DNA probes prepared from pHL-2 can be used to detect lysozyme mRNA not only from human but also from mouse and rat. Moreover, by in situ hybridization, complementary RNA transcripts have been used as probes to detect lysozyme mRNA in mouse macrophages and Paneth cells. This human lysozyme cDNA clone is therefore likely to be a useful molecular probe for studying macrophage distribution and gene expression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldacci P., Royal A., Brégégère F., Abastado J. P., Cami B., Daniel F., Kourilsky P. DNA organisation in the chicken lysozyme gene region. Nucleic Acids Res. 1981 Aug 11;9(15):3575–3588. doi: 10.1093/nar/9.15.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake C. C., Johnson L. N., Mair G. A., North A. C., Phillips D. C., Sarma V. R. Crystallographic studies of the activity of hen egg-white lysozyme. Proc R Soc Lond B Biol Sci. 1967 Apr 18;167(1009):378–388. doi: 10.1098/rspb.1967.0035. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Koenig D. F., Mair G. A., North A. C., Phillips D. C., Sarma V. R. Structure of hen egg-white lysozyme. A three-dimensional Fourier synthesis at 2 Angstrom resolution. Nature. 1965 May 22;206(4986):757–761. doi: 10.1038/206757a0. [DOI] [PubMed] [Google Scholar]
- Calabretta B., Robberson D. L., Maizel A. L., Saunders G. F. mRNA in human cells contains sequences complementary to the Alu family of repeated DNA. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6003–6007. doi: 10.1073/pnas.78.10.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chung L. P., Bentley D. R., Reid K. B. Molecular cloning and characterization of the cDNA coding for C4b-binding protein, a regulatory protein of the classical pathway of the human complement system. Biochem J. 1985 Aug 15;230(1):133–141. doi: 10.1042/bj2300133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth M. L., Gait M. J., Goelet P., Hong G. F., Singh M., Titmas R. C. Rapid synthesis of oligodeoxyribonucleotides VI. Efficient, mechanised synthesis of heptadecadeoxyribonucleotides by an improved solid phase phosphotriester route. Nucleic Acids Res. 1981 Apr 10;9(7):1691–1706. doi: 10.1093/nar/9.7.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström A., Xanthopoulos K. G., Boman H. G., Bennich H. Amino acid and cDNA sequences of lysozyme from Hyalophora cecropia. EMBO J. 1985 Aug;4(8):2119–2122. doi: 10.1002/j.1460-2075.1985.tb03901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlandsen S. L., Parsons J. A., Taylor T. D. Ultrastructural immunocytochemical localization of lysozyme in the Paneth cells of man. J Histochem Cytochem. 1974 Jun;22(6):401–413. doi: 10.1177/22.6.401. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gordon S. Biology of the macrophage. J Cell Sci Suppl. 1986;4:267–286. doi: 10.1242/jcs.1986.supplement_4.16. [DOI] [PubMed] [Google Scholar]
- Gordon S., Todd J., Cohn Z. A. In vitro synthesis and secretion of lysozyme by mononuclear phagocytes. J Exp Med. 1974 May 1;139(5):1228–1248. doi: 10.1084/jem.139.5.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall L., Emery D. C., Davies M. S., Parker D., Craig R. K. Organization and sequence of the human alpha-lactalbumin gene. Biochem J. 1987 Mar 15;242(3):735–742. doi: 10.1042/bj2420735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland P. W., Harper S. J., McVey J. H., Hogan B. L. In vivo expression of mRNA for the Ca++-binding protein SPARC (osteonectin) revealed by in situ hybridization. J Cell Biol. 1987 Jul;105(1):473–482. doi: 10.1083/jcb.105.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W. R., Schmid C. W. Repetitive sequences in eukaryotic DNA and their expression. Annu Rev Biochem. 1982;51:813–844. doi: 10.1146/annurev.bi.51.070182.004121. [DOI] [PubMed] [Google Scholar]
- Jollès J., Jollès P. Comparison between human and bird lysozymes: Note concerning the previously observed deletion. FEBS Lett. 1972 Apr 15;22(1):31–33. doi: 10.1016/0014-5793(72)80211-4. [DOI] [PubMed] [Google Scholar]
- Jollès P., Jollès J. What's new in lysozyme research? Always a model system, today as yesterday. Mol Cell Biochem. 1984 Sep;63(2):165–189. doi: 10.1007/BF00285225. [DOI] [PubMed] [Google Scholar]
- Jung A., Sippel A. E., Grez M., Schütz G. Exons encode functional and structural units of chicken lysozyme. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5759–5763. doi: 10.1073/pnas.77.10.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariya Y., Kato K., Hayashizaki Y., Himeno S., Tarui S., Matsubara K. Revision of consensus sequence of human Alu repeats--a review. Gene. 1987;53(1):1–10. doi: 10.1016/0378-1119(87)90087-4. [DOI] [PubMed] [Google Scholar]
- Krystosek A., Sachs L. Control of lysozyme induction in the differentiation of myeloid leukemic cells. Cell. 1976 Dec;9(4 Pt 2):675–684. doi: 10.1016/0092-8674(76)90131-8. [DOI] [PubMed] [Google Scholar]
- Law S. K., Gagnon J., Hildreth J. E., Wells C. E., Willis A. C., Wong A. J. The primary structure of the beta-subunit of the cell surface adhesion glycoproteins LFA-1, CR3 and p150,95 and its relationship to the fibronectin receptor. EMBO J. 1987 Apr;6(4):915–919. doi: 10.1002/j.1460-2075.1987.tb04838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Osserman E. F., Lawlor D. P. Serum and urinary lysozyme (muramidase) in monocytic and monomyelocytic leukemia. J Exp Med. 1966 Nov 1;124(5):921–952. doi: 10.1084/jem.124.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen J. E., Schultz D. W., Taylor A., Smith G. R. Nucleotide sequence of the lysozyme gene of bacteriophage T4. Analysis of mutations involving repeated sequences. J Mol Biol. 1983 Apr 5;165(2):229–248. doi: 10.1016/s0022-2836(83)80255-1. [DOI] [PubMed] [Google Scholar]
- Ralph P., Moore M. A., Nilsson K. Lysozyme synthesis by established human and murine histiocytic lymphoma cell lines. J Exp Med. 1976 Jun 1;143(6):1528–1533. doi: 10.1084/jem.143.6.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennell D., Poteete A. R. Phage P22 lysis genes: nucleotide sequences and functional relationships with T4 and lambda genes. Virology. 1985 May;143(1):280–289. doi: 10.1016/0042-6822(85)90115-1. [DOI] [PubMed] [Google Scholar]
- Saedi M. S., Garvey K. J., Ito J. Cloning and purification of a unique lysozyme produced by Bacillus phage phi 29. Proc Natl Acad Sci U S A. 1987 Feb;84(4):955–958. doi: 10.1073/pnas.84.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura R., Werb Z. Secretory products of macrophages and their physiological functions. Am J Physiol. 1984 Jan;246(1 Pt 1):C1–C9. doi: 10.1152/ajpcell.1984.246.1.C1. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]