Abstract
Graft-versus-host disease (GVHD), both acute and chronic, remains one of the major barriers to improving outcomes after allogeneic stem cell transplantation. The pathophysiology of GVHD is complex and incompletely understood. GVHD is believed to arise from the interaction of: tissue damage and proinflammatory cytokines causing activation of antigen-presenting cells (APCs, donor T-cell activation by APCs and cytokines and host tissue injury by effector T lymphocytes and proinflammatory cytokines. There is also a role for additional lymphocyte subtypes (naive and memory T cells, regulatory T cells, natural killer T cells and B cells) in GVHD pathogenesis. Strategies to improve donor–recipient HLA match, and to minimize conditioning toxicity, cytokine release and APC and effector T-lymphocyte activation, will likely improve prophylaxis of acute (and possibly chronic) GVHD. Therapy of established acute and chronic GVHD is still heavily dependent on corticosteroids, despite their limited efficacy and considerable toxicity. Novel agents (and/or combinations of agents) comprising pharmacologic, biologic and cellular therapies targeting specific steps or subsets involved in immune activation will likely comprise future advances in GVHD control. This article reviews the current state of knowledge regarding the prevention and treatment of acute and chronic GVHD. Novel approaches currently undergoing evaluation are also highlighted.
Keywords: allogeneic stem cell transplantation, graft-versus-host disease
Allogeneic stem cell transplantation (alloSCT) is often the only curative option for patients with various hematologic and/or immune disorders, particularly those with aggressive or advanced hematologic malignancies. However, the toxicity of alloSCT remains a significant barrier to its wider utilization. Graft-versus-host disease (GVHD) remains the most frequent complication after alloSCT.
Clinically, GVHD was categorized as ‘acute’ and ‘chronic’ based on time of presentation. Any GVHD before day 100 was known as ‘acute’, and after day 100 it was known as ‘chronic’. The severity of GVHD was graded: acute GVHD was categorized as grade I–IV by modified Glucksberg criteria (A–D by the International Bone Marrow Transplant Registry index) (Table 1) [1,2]; chronic GVHD was commonly categorized as limited or extensive [3]. Based on these criteria, grade II–IV acute GVHD is thought to occur in approximately 35% of recipients of matched, related donor transplants, and in up to 50% of unrelated or alternative donor transplant recipients. Chronic GVHD can affect up to 60% of recipients who survive beyond 100 days after matched donor alloSCT.
Table 1. Modified Glucksberg criteria for acute graft-versus-host disease grading.
| Organ stage | Skin* | Liver | Gut |
|---|---|---|---|
| 1 | Rash < 25% | Bilirubin 2-2.9 mg/dl | Diarrhea 500-1000cc/d or biopsy-proven upper GI involvement |
| 2 | Rash 25-50% | Bilirubin 3-6 mg/dl | Diarrhea 1000-1500cc/d |
| 3 | Rash > 50% | Bilirubin 6.1-15 mg/dl | Diarrhea 1500-2000cc/d |
| 4 | Generalized erythroderma with bullae | Bilirubin > 15 mg/dl | Diarrhea > 2000 cc/d or severe abdominal pain with or without ileus |
| Overall grade | |||
| I | Stage 1 or 2 | None | None |
| II | Stage 3 or | Stage 1 or | Stage 1 |
| III | - | Stage 2 or 3 or | Stage 2–4 |
| IV | Stage 4 or | Stage 4 | |
Use ‘rule of nines’ to determine body surface area.
Data from [1].
While the simplicity of the day 100 definition is appealing, it is irrelevant biologically and clinically. For instance, in patients receiving reduced intensity conditioning (RIC) alloSCT, or after donor lymphocyte infusion (DLI), clinical acute GVHD may develop months after the procedure [4]. Hence, there is a current attempt by the National Institutes of Health chronic GVHD consensus project working group to reclassify acute GVHD into classic acute and late-onset acute; and chronic GHVD into classic chronic and overlap syndrome [5]. Classic acute GVHD is characterized by a maculopapular erythematous skin rash, gastrointestinal symptoms or cholestatic hepatic abnormalities occurring within 100 days of alloSCT or DLI, while late acute GVHD presents similarly beyond 100 days after alloSCT or DLI. Classic chronic GVHD consists solely of manifestations ascribable to chronic GVHD (without acute GVHD features) (Table 2), while overlap syndrome has clinical features of both acute and chronic GVHD occurring together.
Table 2. Definite and probable manifestations of chronic graft-versus-host disease.
| Organ system | Definite manifestations of chronic GVHD | Possible manifestations of chronic GVHD |
|---|---|---|
| Skin | Scleroderma (superficial or fasciitis), lichen planus, vitiligo, scarring alopecia, hyperkeratosis pilaris, contractures from skin immobility, nail bed dysplasia | Eczematoid rash, dry skin, maculopapular rash, hair loss, hyperpigmentation |
| Mucous membranes | Lichen planus, noninfectious ulcers, corneal erosions/noninfectious conjunctivitis | Xerostomia, keratoconjunctivitis sicca |
| GI tract | Esophageal strictures, steatorrhea | Anorexia, malabsorption, weight loss, diarrhea, abdominal pain |
| Liver | None | Elevation of alkaline phosphatase, transaminitis, cholangitis, hyperbilirubinemia |
| GU tract | Vaginal stricture, lichen planus | Noninfectious vaginitis, vaginal atrophy |
| Musculoskeletal/serosa | Nonseptic arthritis, myositis, myasthenia, polyserositis, contractures from joint immobilization | Arthralgia |
| Hematologic | None | Thrombocytopenia, eosinophilia, autoimmune cytopenias |
| Lung | Bronchiolitis obliterans | Bronchiolitis obliterans with organizing pneumonia, interstitial pneumonitis |
GI: Gastrointestinal; GU: Genitourinary; GVHD: Graft-versus-host disease.
Risk factors for GVHD
The risk factors for GVHD include:
Donor–recipient match at the major histocompatibility complex (MHC) loci, for instance, HLA class I (HLA-A, -B and -C) and class II (HLA-DR, -DP and -DQ). Mismatches at HLA-A, -B, -C or -DRB1 (and possibly also -DQ and -DP) increase the risk of GVHD (nonpermissive donor–recipient HLA mismatches may particularly influence GVHD severity) and negatively impact survival [6–10];
Donor stem cell source: compared with bone marrow stem cells, peripheral blood stem cells (PBSCs) have a higher GVHD risk, while umbilical cord blood cells appear to have a lower risk [11–14];
T-cell dose: compared with T-cell replete grafts, 2–3 log depletion of CD3+ T lymphocytes of the graft can effectively reduce acute GVHD incidence (although the effect on chronic GVHD is less clear), while less-intensive log reductions of T cells have no significant impact [15,16]. However, the benefit of T-cell depletion is counteracted by increased risks of graft failure, opportunistic infection and disease relapse such that pan-T-cell depletion strategies are not currently favored [17];
Additional risk factors include donor and recipient age, donor–recipient sex mismatch (female donor to male recipient), donor parity and allosensitization, disease stage and intensity of conditioning (for acute GVHD). Acute GVHD is a powerful predictor of chronic GVHD risk [18].
Measures to reduce GVHD risk would therefore include improvements in donor selection, improved HLA matching, as well as reduced intensity conditioning where possible. However, other trends, such as the increased use of donor PBSCs as a source of stem cells, extending alloSCT to older/sicker patients and the use of alternative donors (haploidentical and HLA-mismatched donors), suggest that GVHD control will remain a significant issue for the foreseeable future.
Etiopathogenesis of GVHD
The etiology of GVHD is complex, but Billingham's criteria still apply [19]. First, the graft must contain immunologically competent cells (T lymphocytes and possibly B lymphocytes). Second, the recipient must be incapable of rejecting the transplanted cells (achieved by conditioning chemotherapy or radiation). Third, the recipient must express tissue antigens that are not present in the donor (major or minor histocompatibility mismatch).
Our current understanding of acute GVHD, although incomplete, is better than that of chronic GVHD. In part, this is due to the better availability of mouse models of acute GVHD. Broadly however, both forms of GVHD are believed to be caused by similar alloimmune responses that also underlie the beneficial graft-versus-leukemia (GVL) effect. Maintaining control of GVHD, while enabling the curative GVL response remains the holy grail of allotransplantation.
The development of acute GVHD is frequently divided into three phases (Figure 1):
Figure 1. Etiopathogenesis of acute graft-versus-host disease.
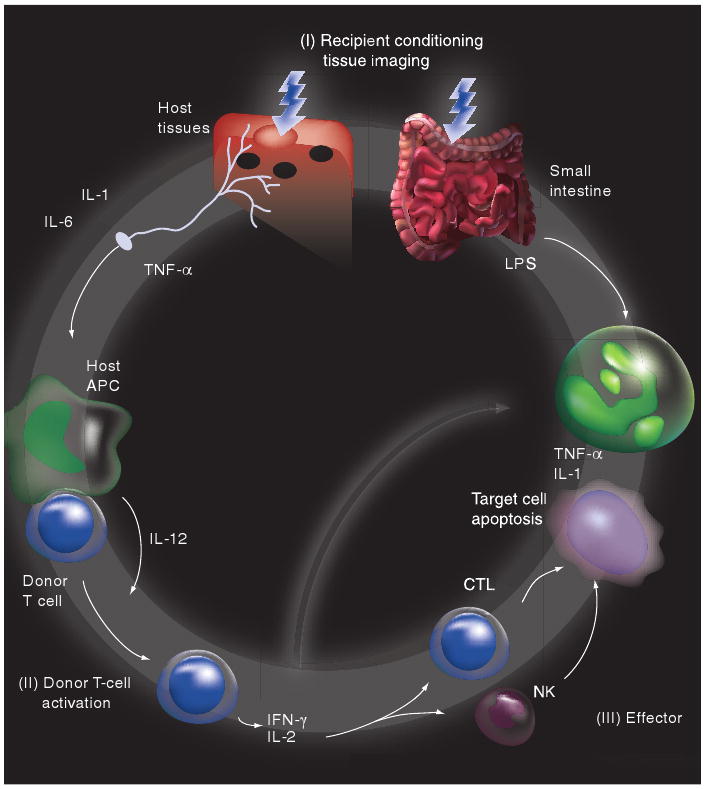
Modified with permission from [245].
Tissue damage, owing to underlying disease, infections and conditioning regimen toxicity, resulting in leakage of bacterial lipopolysaccharides across the damaged gut epithelium and a ‘cytokine storm’ with the production of inflammatory cytokines, such as TNF-α, and IL-1 by injured cells, resulting in secondary changes in expression of adhesion molecules, MHC antigens and chemokines, which can act as danger signals and activate residual host and donor antigen-presenting cells (APCs) [20–24]. APC activation can occur via both Toll-like receptor (TLR) and non-TLR (e.g., nucleotide-binding oligomerization domain [NOD]) pathways [25,26];
Donor T-cell activation, cytokine release, proliferation and tissue localization occurs in the context of the proinflammatory post-transplant milieu and after alloantigen presentation and costimulation by APCs (donor or host) [27–30];
The effector phase of GVHD target organ damage involves a complex interaction of cytokine and cellular effectors. Cytotoxic T lymphocytes (CTLs), both CD4+ and CD8+, are the major cellular effectors of GVHD and cause cell death by a variety of pathways, such as Fas–Fas ligand (FasL), TNF receptor (TNFR)-like death receptors (e.g., TRAIL and TWEAK) and perforin–granzyme [31–36]. Inflammatory cytokines, such as TNF-α and IL-1, synergize with CTLs and can also act directly to promote tissue injury and inflammation in GVHD target organs [37–40].
Based on their cytokine expression pattern, there are at least two types of T helper (Th) effector cells involved in GVHD: Th1 and Th2 cells. Th1 cells generate IL-2, TNF-α and IFN-γ, while Th2 cells produce IL-4 and IL-10. While the ‘cytokine storm’ phase of GVHD, which is amplified by Th1 cytokines, correlates with the development of acute GVHD, cytokines that polarize donor T cells to Th2 (e.g., granulocyte colony-stimulating factor [G-CSF], IL-4 and IL-18) can reduce acute GVHD [41–44]. However, this model may be an oversimplification, as Th1 and Th2 subsets can each cause injury to distinct GVHD target organs in some mouse models of acute GVHD [45]. Additional complexities involve possible roles for newly identified Th17 cells in GVHD and the interaction between Th17 effector cells and peripheral regulatory T cells (Tregs), given their alternate developmental fates from common naive precursor T cells [46–48].
Additionally, genetic polymorphisms that lead to altered cytokine expression levels (e.g., IL-6, IL-10 and TNF-α) have also been linked to differences in acute and chronic GVHD incidence [49–58]. Furthermore, polymorphisms involving natural killer (NK) cell receptor/ligand complex, collectively termed the killer immunoglobulin-like receptor family (KIR), have been linked to differences in both GVHD and relapse rates after alloSCT [59–61]. Similarly, polymorphisms in the non-TLR (NOD) pathway of adaptive immune activation can impact GVHD risk [62]. Genes involved in drug metabolism have also been linked to toxicity and GVHD after alloSCT [63,64]. Finally, genes with only indirect associations with immune activity have also been linked to GVHD [65–67]. Both donor and recipient polymorphisms are often relevant with regards to GVHD risk, as in the case of IL-10 [68].
There is increasing awareness of the role of additional cellular subsets in GVHD:
Naive and memory T cells: naive (CD62L+) T cells, but not memory (CD62L-) T cells, are often considered to have alloreactive potential that can result in acute GVHD [69,70]. However, contrasting recent data also suggest a role for alloreactive memory T cells and their precursor stem cells in the development of GVHD [71,72];
Tregs: CD4+CD25+ FoxP3+ Tregs from the donor have been shown to suppress the expansion of alloreactive donor T cells and the development of GVHD, without abrogating GVL in this MHC-mismatched murine model [73]. IL-2, initially identified as a lymphocyte growth factor and thought primarily to promote effector T-cell responses in vivo, is now identified as a cytokine critical for the development, expansion and activity of Tregs [74,75]. In humans, FoxP3 mRNA levels (considered a relatively specific marker for Tregs) was significantly decreased in patients with GVHD [76,77]. The expression of the cell surface marker CD62L was also found to be critical for the ability of donor Tregs to control GVHD [78,79];
NKT cells: host NKT cells also have immune suppressive effects that can control GVHD in an IL-4-dependent fashion [80,81]. Human clinical data suggest that enhancing recipient NKT cells by total lymphoid irradiation (TLI) in conjunction with anti-thymocyte globulin-based conditioning similarly promoted Th2 polarization and significantly reduced GVHD [82]. However, it is important to note that NKT cells are heterogeneous and their roles in GVHD are incompletely understood;
B cells: traditionally, a major role for B cells and humoral immunity in the development of GVHD has not been considered. However, recent work suggests that, in the context of matched sibling PBSC allotransplantation, the concentration of CD20+ B cells in the apheresis product may predict the development of acute GVHD [83]. Additionally, auto- and alloantibodies have been described in chronic GVHD, some of which may play a direct role in disease progression (e.g., activating PDGF receptor antibodies) [84–87]. High circulating levels of B-cell activation factor at 6-months post-transplant were a predictor of subsequent chronic GVHD, further supporting a role for B-cell dysfunction in chronic GVHD [88]. The role of humoral immunity in GVHD remains an area of controversy and further investigation.
Prophylaxis of GVHD
Pilot studies omitting GVHD prophylaxis indicated an acute GVHD incidence of nearly 100% [89]. Studies using methotrexate as a single agent for GVHD prophylaxis via inhibition of rapidly dividing alloreactive T cells, indicated an acute grade II–IV GVHD rate of over 50%, even in the setting of HLA-matched sibling donors [90]. The introduction of a calcineurin inhibitor, cyclosporine (and subsequently tacrolimus), represented the next advance in the prevention of GVHD, with improved efficacy in GVHD control compared with methotrexate [91–93]. Cyclosporine and tacrolimus bound to cyclophilin or FK-binding protein 12 (FKBP12), respectively, inhibit calcineurin (a protein phosphatase that is calcium- and calmodulin-dependent) and prevent the dephosphorylation and nuclear translocation of the transcription factor nuclear factor of activated T cells (NFAT). By blocking NFAT, one of the most important regulators of cytokine gene transcription following T-cell activation, calcineurin inhibitors block T-cell activation and proliferation [94,95]. The combination of calcineurin inhibitor (cyclosporine) and methotrexate was more effective than either agent alone, with grade II–IV acute GVHD rates of 20–56% after HLA-matched sibling alloSCT [96,97]. Compared with cyclosporine, tacrolimus has an improved toxicity profile and, more importantly, randomized data indicate improved acute GVHD prophylaxis in both HLA-matched siblings and unrelated donor allotransplants [98,99]. The length of immunosuppressive therapy appears to have no role in improving control of chronic GVHD. Patients with acute GVHD or biopsy evidence of subclinical acute GVHD were randomly assigned to 6 versus 24 months of cyclosporine therapy. The rates of clinical extensive chronic GVHD were 39 and 51%, respectively, a nonsignificant difference [100]. Similarly, the presence or absence of day 11 methotrexate does not likely impact chronic GVHD rates [101,102].
Corticosteroids, the mainstay of therapy for established acute GVHD, do not have a significant role in GVHD prophylaxis. Various trials compared prednisone and cyclosporine to the three-drug combination of methotrexate, cyclosporine and prednisone. In one large trial, the acute GVHD rate in the cyclosporine and prednisone control arm was 23%, compared with only 9% in the three-drug arm of methotrexate, cyclosporine and prednisone [103]. However, subsequent trials could not demonstrate similarly improved GVHD control, or improved long-term outcomes with the three-drug combination, and, currently, steroids are not routinely used in GVHD prophylaxis [104].
Cyclophosphamide has been used post-transplant since the 1980s for GVHD prevention and act via inhibition of rapidly dividing T cells (in a manner similar to methotrexate) [105]. Stem cells contain high levels of aldehyde dehydrogenase that converts the active metabolite 4-hydroxycyclophosphamide to an inactive nonalkylating metabolite, thus protecting the stem cell from the antiproliferative activity of the agent. Similarly, the gut epithelium has high levels of aldehyde dehydrogenase that is protective against excess mucosal toxicity despite prior intensive conditioning. Used as a single agent after myeloablative conditioning in related and unrelated allotransplants, the grade II–IV acute GVHD rate was 41%, with few late infections, attributed to the brief duration of immune suppressive therapy [106]. It is also currently being evaluated for alternative donor transplants (haploidentical donor) [107].
Mycophenolate mofetil (MMF) is a potent, selective, noncompetitive reversible inhibitor of inosine monophosphate dehydrogenase that inhibits the de novo pathway of guanosine nucleotide synthesis. It has potent cytostatic effects on lymphocytes (both T and B) whose proliferation is dependent on de novo purine synthesis. With good oral bioavailability, the optimal dosing interval remains uncertain, usually two- to three-times daily. It has been used for GVHD prophylaxis in various combinations (usually with a calcineurin inhibitor ± methotrexate). The incidence of grade II–IV acute GVHD has ranged between 38 and 62% [108,109]. In a single-center randomized study, the combination of cyclosporine plus MMF was associated with faster engraftment and reduced mucositis incidence, but with similar incidence of acute and chronic GVHD and survival comparable to cyclosporine plus methotrexate, possibly affected by limited sample size and follow-up duration for these secondary end points [110]. Longer-term use of cyclosporine in combination with MMF after RIC alloSCT with matched related donors did not impact the rates of acute grade II–IV or chronic GVHD [111].
Sirolimus (also called rapamycin) binds uniquely to FKBP12 and forms a complex with mammalian target of rapamycin (mTOR) that interacts with various upstream pathways including PTEN/PI3 kinase/Akt pathway and the Janus kinase pathway [112,113]. The sirolimus–mTOR complex inhibits several biochemical pathways, resulting in reduction of DNA transcription/translation, protein synthesis and cell cycle progression, which results in T-cell immunosuppression [114,115]. Interestingly, there is apparent differential inhibition of T-cell subsets, possibly involving selective inhibition of Th1 cell responses, and sparing of Th2 and Treg activity [116–120]. Despite theoretical concerns for competition for FKBP binding with calcineurin inhibitors, these agents appear to work synergistically, and sirolimus does not interact with calcineurin or its downstream effectors [112]. In contrast to calcineurin inhibitors, sirolimus may also exert its immunosuppressive effects through suppression of APC activity via a reduction in antigen uptake, cellular processing, intracellular signaling and induction of apoptosis [121–123]. The combination of sirolimus and tacrolimus appears more effective than sirolimus plus cyclosporine in reducing alloreactive memory T-cell production, abrogation of effector CTL induction and apoptosis induction [124]. Single-institution clinical studies of sirolimus and tacrolimus with and without low-dose methotrexate for GVHD prophylaxis after myeloablative conditioning with cyclophosphamide/total-body irradiation (TBI) indicate excellent efficacy and acceptable toxicity in the matched related and unrelated donor context, with grade II–IV acute GVHD rates of 19 and 23%, respectively [125]. The rates of chronic GVHD, however, were not significantly impacted. Similar efficacy in acute GVHD control was noted despite omitting low-dose methotrexate, and toxicity was further reduced [126]. Similar low-acute GVHD rates were also noted in the context of RIC. Other recent single-institution reports indicate concordant as well as variant estimates of sirolimus efficacy for GVHD prophylaxis in the myeloablative alloSCT context [127,128]. Sirolimus plus tacrolimus is currently being evaluated in a Phase III multi-institution context in comparison to methotrexate plus tacrolimus.
Biologic agents have also been evaluated for GVHD prophylaxis. In vivo T-cell depletion with horse- or rabbit-derived polyclonal antithymocyte globulin (ATG) has been evaluated for prevention of GVHD, as initially proposed by Ramsey et al. [129]. Such agents administered pre- and peritransplant can simultaneously target host and donor T cells to control both graft rejection and GVHD [130–132]. However, additional cellular components, such as B cells, NK cells and APCs, can also be affected by polyspecific antibodies. Their use does appear to reduce the incidence of chronic GVHD and chronic lung dysfunction, with improved late transplant-related mortality [133]. Whether the reduction in chronic GVHD is also associated with increased disease relapse remains to be determined. Higher doses of rabbit ATG (thymoglobulin) are associated with increased infections that can abrogate its positive impact on GVHD [134]. TLI in conjunction with ATG-based conditioning also significantly reduced GVHD [82].
Monoclonal antibodies, such as alemtuzumab (Campath-1H; anti-CD52), are widely used for in vivo GVHD prophylaxis. It has been found to reduce GVHD and nonrelapse mortality after related and unrelated transplants, and can also facilitate engraftment [135]. Monoclonal antibodies targeting the IL-2 receptor (CD25) may also show benefit [136]. However, IL-2 is also critical for Treg development, expansion and activity, hence IL-2 targeting in GVHD may have the unintended consequence of impairing Tregs that are important to control GVHD [74,75]. Low-dose IL-2 is currently being evaluated for GVHD prophylaxis. Some biologic agents that may have activity in established active GVHD, such as IL-1 antagonists and ricin-conjugated CD5 antibody, do not show benefit in the prophylactic setting [137–141]. Interestingly, rituximab, a monoclonal CD20 antibody that depletes B cells, may independently decrease acute GVHD risk [142]. It is also being evaluated for the prophylaxis of chronic GVHD.
In vitro T-cell depletion (TCD) has also been attempted to control GVHD, with some success in controlling acute (and possibly chronic) GVHD. However, in a randomized study comparing GVHD prophylaxis with approximately 1-log TCD (with monoclonal antibody T10B9 targeting the T-cell receptor) plus cyclosporine versus methotrexate and cyclosporine, improved acute GVHD control did not lead to improvement in long-term survival, and disease relapse and infection risk was significantly increased after T-cell depletion [16,143]. In an attempt to circumvent problems associated with global TCD, selective depletion strategies focused on T-cell subsets (e.g., CD4+, CD6+ and CD8+ T cells) have been utilized, with limited success [144–146]. Other studies have combined TCD and scheduled DLI post-alloSCT, to improve relapse rates and outcomes after TCD, with mixed results [147,148]. In an alternative strategy to induce anergy to donor alloantigens, a small trial utilized costimulation blockade of HLA haploidentical donor T cells by ex vivo incubation with CTLA4 antibody and donor APCs with some reported success [149].
Proteasome inhibition may have a role in GVHD control. The transcription factor NF-κB plays an important role in cytokine signaling and the generation of cell-mediated immune responses. In addition, the proteasome has been shown to play a critical role in T-cell activation, proliferation and apoptosis, largely through NF-κB activation [150–152]. In addition to direct cytotoxic effects, the proteasome inhibitor bortezomib demonstrates immunomodulatory effects through NF-κB [153]. It can attenuate TLR4-mediated APC activation, with reduced cytokine production and immunostimulatory activity [154]. Additionally, in the allogeneic setting, bortezomib preferentially and specifically depletes alloreactive T lymphocytes [155]. In murine models of GVHD, bortezomib early after stem cell infusion protected against GVHD without impairing engraftment [156,157]. Phase I and II trials for prevention and treatment of acute GVHD are ongoing, with interesting preliminary results [158].
Additional agents that have efficacy in the treatment of established acute GVHD are also being evaluated for primary GVHD prophylaxis. Examples include pentostatin and etanercept (discussed later). Novel approaches include blocking lymphocyte migration to GVHD target organs using chemokine blockade (although there is significant redundancy in this system, complicating targeting efforts) and the use of extracorporeal photopheresis, which may alter host antigen presentation and enhance Tregs for GVHD control [159–162]. Ursodiol, utilized for control of hepatotoxicity and treatment-related mortality (TRM) peritransplant, was reported to also control severe acute GVHD [163]. However, a meta-analysis confirmed the hepatotoxic and TRM benefit of ursodiol, but did not note improved GVHD control [164]. Thalidomide was evaluated for chronic GVHD prophylaxis in a Phase III trial, with negative impact on mortality and chronic GVHD incidence [165]. Revlimid® or newer congeners may be more useful. Attempts to prevent thymic atrophy and associated chronic GVHD with thymic tissue implants, thymic epithelial cells or thymic hormones have not had positive results [166,167].
Treatment of established GVHD
In patients with established acute GVHD, the goal of therapy is to achieve rapid control, since the probability of survival depends upon the initial stage of GVHD at presentation and response to therapy [168–170]. Long-term survival of patients with grade 0–I acute GVHD is 50%, while long-term survival of those with grade IV acute GVHD is as low as 11% [168]. Response to therapy is a key predictor of outcome, as mortality in acute grade II–IV GVHD is lowest in those with a complete response to initial therapy [169,171,172]. Corticosteroids are lympholytic and inhibit inflammatory cytokine cascades. Other agents have been utilized as first-line therapy, however none have proven superior to corticosteroids [169,173]. Corticosteroids also remain the primary front-line therapy for chronic GVHD, often in combination with a calcineurin inhibitor, as discussed later.
First-line therapy of GVHD
Corticosteroids (typically prednisone or methylprednisolone) dosed at approximately 2 mg/kg/day are the standard therapy for acute grade II–IV GVHD [174,175]. After single-agent steroid therapy, response rates were approximately 50% [169,171]. Higher doses have not been associated with improved response rates. In a prospective randomized study comparing methylprednisolone at 2 versus 10 mg/kg/day in patients with acute grade II–IV GVHD, response rates, TRM, GVHD progression and overall survival were similar [176]. TRM and long-term survival were significantly improved in patients with early acute GVHD response that permitted steroid taper by day 5 of therapy [172]. In order to reduce the toxicities of prolonged systemic steroids, adjuvant topical steroids have been evaluated. In one randomized study, prednisone with and without oral nonabsorbable steroids (enteric-coated beclomethasone) were evaluated for therapy of gastrointestinal acute GVHD. Prednisone taper was initiated on day 10 if clinical response occurred. Durable responses and day 200 mortality were improved in the beclomethasone plus prednisone arm [177].
The limited response to systemic steroids alone has prompted evaluation of additional immunosuppressive agents in the initial therapy of GVHD. This strategy has had only limited success, given the increased risks of infection and TRM. ATG is the most widely studied in this setting. Initial studies of upfront therapy with ATG plus steroids reported impressive response rates of 67–80% [178,179]. However, in a randomized study, initial therapy of acute grade II–IV GVHD with prednisone with and without ATG failed to demonstrate an improvement in response rates or survival in the ATG arm. Infectious complications were more common in the combination ATG arm [180].
Other biologic agents have been evaluated in combination with steroids for initial therapy of acute GVHD. One randomized study evaluated systemic steroids with and without the monoclonal antibody daclizumab that targets CD25 (the IL-2 receptor α-chain) present on activated T cells, dosed at 1 mg/kg on days 1 and 4 and weekly thereafter. Overall response rates were similar in the two groups, but survival at 100 days and 1 year was inferior in the daclizumab plus steroid group [181]. Similar lack of benefit was noted in a randomized study evaluating prednisone and cyclosporine with and without another monoclonal antibody targeting the IL-2 receptor (BT563) [182]. CD5, found on the majority of Tcells, acts as a costimulation molecule to regulate signaling via the T-cell receptor. A randomized trial utilizing a CD5-specific immunotoxin or placebo in combination with methylprednisolone found improved early response of acute GVHD in the immunoconjugate arm, but comparable long-term outcomes [140]. The TNF-α inhibitor etanercept in combination with corticosteroids plus tacrolimus demonstrated superior acute GVHD control in comparison to a historical cohort treated with steroids alone, in a small study that was recently updated [183,184].
Currently, none of these agents have displaced corticosteroids as upfront therapy for acute GVHD, but there is significant interest in finding better therapies for initial treatment of acute GVHD. Adjunctive agents that are currently being evaluated in an ongoing randomized trial by the Bone Marrow Transplant Clinical Trials Network include etanercept, denileukin diftitox, mycophenolate mofetil and pentostatin, each in combination with corticosteroids.
Corticosteroids are also the mainstay of therapy for chronic GVHD. Other single agents are associated with a low response rate. Currently, there is no standard second-line therapy for chronic GVHD and therapy typically consists of prolonged administration of a corticosteroid combined with other immunosuppressive medications, such as calcineurin inhibitors (cyclosporine or tacrolimus). In a randomized trial comparing single-agent prednisone versus prednisone plus cyclosporine in patients with extensive chronic GVHD, TRM, survival, relapse, need for salvage therapy and rate of discontinuation of immunosuppressive therapy were similar for the two arms. The median duration of therapy with corticosteroids and cyclosporine was 1.6 years. Owing to increased toxicity in the steroid-only arm, many favor combination therapy [185]. Despite dual immunosuppressive therapy, only 54% of patients were successfully weaned off corticosteroids at 5 years and the mortality directly attributable to chronic GVHD was 17% in the combination therapy arm. Similar lack of benefit with combination therapy was noted in a trial of cyclosporine, prednisone with and without thalidomide as initial therapy of chronic GVHD. Response rates were similar in the two arms, but the complication rate was greater in the thalidomide arm [186]. Additional agents for upfront therapy in chronic GVHD are urgently required. Ongoing studies evaluating the role of mycophenolate mofetil and the proteasome inhibitor bortezomib, each in combination with steroids, are of interest in this regard.
Salvage therapy for GVHD
Acute GVHD patients in whom initial therapy fails are commonly defined as:
Progression of GVHD after 3 days
No improvement after 7 days
Incomplete response after 14 days of systemic methylprednisolone therapy at 2 mg/kg/day or equivalent
These patients are considered for a variety of salvage regimens. There is currently no established second-line regimen for acute GVHD and long-term outcomes remain poor, despite various high initial response rates reported with some second-line agents.
Antithymocyte globulin (equine or rabbit polyclonal antibody) is still the most commonly used agent for steroid-refractory acute GVHD [170,187]. This popularity is despite the fact that randomized data did not identify a survival advantage to the ATG arm when patients with acute grade I–IV GVHD nonresponsive to methyprednisolone (2 mg/kg/day) were randomized to higher-dose methylprednisolone (5 mg/kg/day) with and without ATG [172].
Biologic therapies targeting CD25 have been evaluated in the steroid-refractory acute GVHD setting. Daclizumab, the humanized anti-IL2 receptor monoclonal antibody, has been evaluated in three Phase II studies. Overall response rates in the steroid-refractory setting have ranged from 29 to 50% [188–190]. A chimeric human/mouse monoclonal IL-2 receptor antibody, basiliximab, has also been evaluated in steroid-refractory acute GVHD. Phase II studies utilizing various dosing schedules report an overall response rate of 71–83% (complete responses of 18–53%) [191–193]. Denileukin diftitox (Ontak®), a conjugate of human IL-2 fused to the membrane translocation and catalytic domains of diphtheria toxin, targets the high-affinity IL-2 receptor. At the maximum-tolerated dose of 9 μg/kg on days 1, 3, 5, 15, 17 and 19, 71% of steroid-refractory acute GVHD patients had a response to therapy (complete responses of 46%) [194]. Dose-limiting toxicities were transient hepatic transaminitis, infusion reactions and a vascular leak syndrome. Another trial, with a different dose and schedule, reported an overall response rate of 41% with denileukin diftitox [195]. The negative impact on Tregs of targeting CD25 and the IL-2 pathway remains to be determined, but is a concern with regards to long-term GVHD control.
Cytokine blockade has been evaluated for steroid-refractory GVHD therapy. In addition to IL-2 receptor targeting via CD25, TNF-α is also an attractive target. Etanercept, a soluble human TNF receptor fusion protein, was used to treat 13 patients with steroid-refractory acute GVHD, dosed at 25 mg subcutaneously biweekly for 4 weeks. Clinical responses were seen in six patients (46%), including five complete responses [196]. Combination therapy with daclizumab plus etanercept has also been reported, with a 66% overall response rate [197]. However, long-term mortality due to infectious complications and chronic GVHD remained high. In a small retrospective analysis, etanercept in combination with ATG for steroid-refractory visceral acute GVHD may have improved response rate and survival compared with historical controls treated with ATG [198]. Infliximab, the chimeric TNF-α antibody, can neutralize circulating TNF-α and lyse TNF-α-producing cells. Various dose-escalation trials indicate the potential efficacy of the agent, and larger studies document response rates of 59–67%, with a predilection for response in gastrointestinal GVHD [199–201]. Invasive fungal infection rates have been high after infliximab therapy in this population [202].
The monoclonal anti-CD3 antibody OKT3 was initially reported to be an effective salvage therapy for acute GVHD [203]. The toxicity of OKT3 includes cytokine release syndrome (fever, chills, nausea and rash) in 60% of treated patients. In a recent randomized study of 80 patients with steroid-refractory GVHD, comparing OKT3 plus high-dose methylprednisolone (10 mg/kg) versus high-dose methylprednisolone alone, there was no significant difference in overall response rate at day 100 (53 vs 33%; p = 0.06) or overall survival at 1 year (45 vs 36%; p = 0.61) [204]. To minimize cytokine-release syndrome, a humanized anti-CD3 antibody (that does not bind human Fc receptor), visilizumab (HuM291), was evaluated in Phase I and II studies. In the Phase I study of 11 patients dosed once with visilizumab at 3 mg/m2, nine patients achieved a response (six with a complete response) [205]. The overall response in the Phase II cohort of 44 steroid-refractory patients (86% with acute grade III–IV GVHD), however was only 32% at 7 weeks (14% complete responses), and survival at 6 months was 32% [206]. The incidence of Epstein–Barr virus (EBV) reactivation in the studies was 40–50%.
In a Phase I trial of the anti-CD147 monoclonal antibody (ABX-CBL) that targets neurothelin, a glycoprotein weakly expressed on leukocytes that is upregulated on activated T and B lymphocytes, monocytes and dendritic cells (APCs), over half the patients with steroid-refractory acute GVHD responded, and overall survival was superior to a historical control group treated with ATG [207]. However, the Phase II/III study comparing ABX-CML versus ATG failed to show a benefit with ABX-CBL [208]. Targeting T cells and APCs with alemtuzumab, a monoclonal antibody against CD52, has had some reported success as therapy of steroid-refractory acute GVHD [209–211]. Owing to simultaneous depletion of B lymphocytes, EBV reactivation and post-transplant lymphoproliferative disorder rates are low, but there is substantial risk of cytomegalovirus (CMV) reactivation and other infections [130,212]. Alefacept (Amevive®) is a novel fusion protein of the extracellular CD2-binding domain of the human leukocyte function antigen-3 (LFA-3) fused to the Fc portion of IgG1, and has selective activity against memory T cells. It has activity in T-cell-mediated autoimmune diseases, and small case series' report on its utility in acute GVHD, with encouraging GVHD responses [213,214]. However, late complications from viral and fungal infections have been noted.
While chronic GVHD is primarily considered a T-cell disorder, humoral auto- and allo-immune activation has also been documented in this setting. There is evidence of B-cell activation, and alloantibodies (e.g., antibodies to minor histocompatibility Y chromosome antigens in gender-mismatched allotransplants: female donors to male recipients) correlate with chronic GVHD in this setting [85,87]. Rituximab, a humanized monoclonal antibody to CD20 that depletes B cells, has been evaluated in the setting of steroid-refractory chronic GVHD [215–218]. In the largest study, 70% of the 21 chronic GVHD patients treated with rituximab (375 mg/m2/week for four doses, repeated once if necessary) responded to therapy (primarily skin and musculoskeletal responses) [218]. Antibody titers also fell in the responders. Given the relatively limited toxicity of rituximab, it is an attractive agent for further evaluation in this context.
Chemotherapeutic agents have also been utilized for salvage therapy of steroid-refractory GVHD. Pentostatin, a nucleoside analog, kills lymphocytes by inhibiting adenosine deaminase, and the resulting accumulation of 2′-deoxyadenosine 5′-triphosphate causes apoptosis of dividing lymphocytes. It has modest myeloid toxicity and was well tolerated in GVHD patients with a suggestion of increased CMV and other viral infection risk. Dose adjustment for renal function was necessary [219]. The complete response rate was 63%, but only 26% of patients were alive at the 1-year follow-up. Pentostatin has also been evaluated as salvage therapy in chronic GVHD. In a Phase II study, 32 out of 58 heavily pretreated patients (55%) had an objective response. Infection was the most significant toxicity, with 11 grade 3–4 infectious events. Survival at 1 and 2 years was 78 and 70%, respectively [220].
Mycophenolate mofetil has a reported 65% response rate in steroid-refractory acute GVHD, and over 45% in chronic GVHD, with common toxicities of nausea, diarrhea, myelosuppression and opportunistic infections [221–224]. The largest study in the chronic GVHD setting reported retrospectively on the use of MMF for de novo or refractory chronic GVHD [225]. Response rates were 90% in the de novo setting (in conjunction with a calcineurin inhibitor or prednisone) and 75% in the refractory setting. The results of ongoing trials are awaited.
Sirolimus has had limited evaluation in the steroid-refractory acute GVHD setting. One study reported a response to sirolimus in 12 out of 21 steroid-refractory patients, with five complete responses [226]. Toxicity at the targeted serum level of 17–25 ng/ml was high, including thrombotic microangiopathy (TMA), cytopenias and hypertriglyceridemia. The impact of lower target doses of sirolimus remains to be determined in this setting. In steroid-refractory chronic GVHD, sirolimus in combination with tacrolimus and corticosteroids had a response in 22 out of 35 patients (63%), including six patients with a complete response, albeit at the expense of significant renal toxicity and TMA [227]. In another study, 15 out of 16 evaluable patients with steroid-refractory chronic GVHD had a response to sirolimus, but renal toxicity remained a concern [228].
Thalidomide and its analogs (e.g., lenalidomide) have potent immunomodulatory activity and inhibit TNF-α production by lipopolysaccharide-activated monocytes [229,230]. Cytokines such as IL-6, IL-1β and GM-CSF are also inhibited by thalidomide, while lenalidomide can stimulate IL-10 [231]. Thalidomide and its analogs can also reduce leukocyte recruitment and trafficking to inflamed tissues by blockade of TNF-α-induced adhesion molecules (e.g., intercellular adhesion molecule [ICAM]-1, vascular cell adhesion molecule [VCAM]-1 and E-selectin) [232]. Unfortunately, thalidomide did not show benefit in prophylaxis or initial therapy of chronic GVHD [165,186]. Lenalidomide is currently being evaluated in a Phase II setting for second-line therapy of chronic GVHD in patients who have failed a trial of steroids plus calcineurin inhibitors.
Phototherapy, primarily extracorporeal photopheresis (ECP), involving ex vivo therapy of T cells plus a psoralen sensitizer, exposed to ultraviolet light (UV-A) and subsequently reinfused, has been utilized to control refractory acute and chronic GVHD. Proposed mechanisms involve alteration of host antigen presentation, effector lymphocyte apoptosis and enhancement of regulatory T cells for improved GVHD control [159–162]. In a Phase I/II study of 59 patients with steroid-refractory acute grade II–IV GVHD, ECP therapy resulted in complete response rates in 70% of patients overall, with 86, 55 and 30% complete responses in grade II, III and IV acute GVHD, respectively, and 82, 61 and 61% complete responses for skin, intestinal and liver GVHD, respectively [233,234]. Complete responders had an overall survival benefit at 4 years of 59% compared with 11% for those who did not obtain a complete response. In the chronic GVHD setting, several retrospective studies have been reported [235–238]. In the largest study involving 71 patients, the overall response rate was 61% (19% complete), primarily responses involving the skin, liver and mucosal surfaces (mouth and eye), and some responses were sustained. However, the cumulative incidence of steroid withdrawal at 1 year was only 22%, with a 53% overall survival at 1 year following initiation of ECP [238]. In a small prospective study of 25 patients with chronic GVHD, skin and/or visceral improvement was noted in 71% of patients and, interestingly, there was no difference in response rates for twice-weekly versus once-weekly ECP [239]. Additional prospective evaluation of ECP in the Phase II and III setting is necessary to confirm these impressive results in both acute and chronic GVHD.
Mesenchymal stem cells (MSCs) are bone marrow progenitor cells capable of differentiating into various nonhematopoietic lineages that have immunomodulatory properties and can inhibit T-cell proliferation to mitogens and alloantigens in vitro and prolong skin allograft survival in vivo [240,241]. In the initial report, infusion of haploidentical (maternal) MSCs improved the severe treatment-refractory GVHD in a pediatric patient [242]. Subsequent case series have been reported. In the largest Phase II report, 39 out of 55 patients with steroid-refractory severe acute GVHD responded (30 complete responses) after one-to-five infusions of HLA identical, haploidentical or third party MSCs [243]. In a pilot, open-label, randomized trial of HLA-matched sibling allotransplantation with and without MSCs for prophylaxis of GVHD, promising reductions in grade II–IV acute GVHD were noted in the MSC arm (11 vs 53%), but a higher than expected relapse rate in the MSC arm raised concerns [244]. Additional clinical trials evaluating the safety and efficacy of MSCs in acute and chronic GVHD are ongoing.
Supportive care
Supportive care is critical for good outcomes after therapy for GVHD. In the setting of acute GVHD, organ-specific care includes topical emollients and wound care, including burn unit-type topical therapy if necessary. For gut GVHD, bowel rest, hyperalimentation, antimotility agents and antisecretagogues (e.g., octreotide, a somatostatin analog) are also used as necessary. Extensive gut epithelial denudation and bleeding may necessitate transfusion support, and biopsy evaluation is often necessary to confirm the diagnosis and rule out other confounding causes (e.g., viral or bacterial infections).
Infections remain a leading cause of mortality in patients with steroid-refractory acute GVHD, as well as in chronic GVHD. Standard prophylaxis includes trimethoprim–sulfamethoxazole (or equivalent agents) for Pneumocystis jiroveci, acyclovir for herpesvirus reactivation, and possibly antifungal agents (azoles or equivalent) for invasive fungal infection prophylaxis (e.g., Aspergillus spp.). Antibacterial prophylaxis with agents protective against encapsulated organisms may also be considered. Fungal monitoring with serum β-glucan and galactomannan is often utilized, particularly when prophylactic antifungal agents have not been initiated. Routine monitoring of viruses (e.g., CMV and EBV) with a plan for pre-emptive therapy if results are positive is a standard approach at many stem cell transplant centers. Immunoglobulin-replacement therapy can be useful, especially in patients with serum IgG levels lower than 400 mg/dl and a history of recurrent sinopulmonary infections. Improvements in rapid detection of incipient infections and new agents for therapy of viral, bacterial and fungal processes represent a major advance in ancillary care, and will likely improve clinical outcomes for patients with moderate or severe GVHD.
Expert commentary & five-year view
Despite significant improvements in allotransplantation methodology and supportive care, GVHD remains the major complication. Advances in chronic GVHD prevention and treatment have been especially incremental, owing at least partly to our limited understanding of its pathophysiology. Improvements in HLA typing, minimizing conditioning-related toxicity and advances in acute GVHD prophylaxis (that may supersede the combination of methotrexate plus a calcineurin inhibitor) will offer improved control of acute (and possibly chronic) GVHD, but this may be counterbalanced by the increased use of alternative (HLA-mismatched and haploidentical) donors and the treatment of older and sicker patients. Incorporating additional information predicting GVHD risk during donor and patient selection (e.g., genetic polymorphisms), if feasible in clinical practice, may be an additional way to reduce GVHD incidence.
Corticosteroids (with or without additional agents) remain the standard first-line therapy for both acute and chronic GVHD, even though response rates and patient long-term survival remains suboptimal. Improvements in supportive care can incrementally improve survival, but advances in understanding the basic biology of GVHD will also drive future improvements in therapy. While broadly immunosuppressive pharmacologic agents will continue to be utilized, it will be important to also look beyond this at approaches designed to sidestep the toxicity of increased nospecific immunosuppresion (i.e., infection and relapse risk).
More targeted therapies, pharmacologic, biologic or cellular agents that can selectively target immune response pathways, optimize the activity or localization of immune cellular subsets and/or modulate cytokine levels in the context of GVHD, are of considerable interest in this regard. The innovative combination of pharmacologic or biologic agents targeting specific immune processes (e.g., inhibiting localization of effector T cells, blockade of specific immune effector pathways and enhancement of Tregs), as well as cellular (e.g., MSCs) and phototherapy-based approaches will be critical to get beyond the limitations of current therapy.
Key issues
Acute and chronic graft-versus-host disease (GVHD) remain a major complication of allogneic transplantation.
The pathophysiology of GVHD is incompletely understood, but broadly involves the interplay of factors, including tissue injury from conditioning therapy, release of proinflammatory cytokines, activation of antigen-presenting cells and alloimmune sensitization of effector T lymphocytes, that result in damage to GVHD target organs.
GVHD prophylaxis involves improved HLA matching of donor and recipient, reduced conditioning regimen toxicity and the use of pharmacologic agents such as methotrexate and calcineurin inhibitors (that may be superseded by alternative agents such as sirolimus, a mammalian target of rapamycin inhibitor).
Initial therapy of established GVHD, both acute and chronic, remains dependent on the use of corticosteroids, despite their limited efficacy and significant toxicity.
Standard of care is not well established for therapy of steroid-refractory GVHD. Pharmacologic immunosuppressive agents (e.g., mycophenolate mofetil), biologic agents targeting effector immune cells (e.g., antithymocyte globulin) or proinflammatory cytokines (TNF-α blockade) have shown some efficacy, typically in early-phase studies.
Supportive care, including symptom control and prophylaxis, early detection and effective therapy of infections remain critical for good outcomes in GVHD patients.
Novel therapeutic approaches, such as targeting of B cells (e.g., rituximab), enhancement of regulatory T cells (e.g., extracorporeal photopheresis) and cellular therapies (e.g., mesenchymal stem cells) that avoid the toxicity of generalized immunosuppression, will likely play a prominent future role in GVHD therapy.
Clinical trials testing novel agents (or novel combinations of agents) are critical for future advances in GVHD control.
Footnotes
Financial & competing interests disclosure: The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Przepiorka D, Weisdorf D, Martin P, et al. Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1994;15(6):825–828. [PubMed] [Google Scholar]
- 2.Rowlings P, Przepiorka D, Klein J, et al. IBMTR severity index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97:855–864. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 3.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man: a long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 4.Mielcarek M, Martin PJ, Leisenring W, et al. Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood. 2003;102(2):756–762. doi: 10.1182/blood-2002-08-2628. [DOI] [PubMed] [Google Scholar]
- 5.Filipovich AH, Weisdorf D, Pavletic S, et al. Biol Blood Marrow Transplant. 12. Vol. 11. 2005. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and Staging Working Group Report; pp. 945–956. [DOI] [PubMed] [Google Scholar]; • The NIH consensus conference on chronic graft-versus-host disease (GVHD) took a critical step in organizing, codifying and refining how we approach chronic GVHD. The concepts presented should faciliate the development of clinical trials in this important area.
- 6.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor–recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110(13):4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 7.Shaw BE, Gooley TA, Malkki M, et al. The importance of HLA-DPB1 in unrelated donor hematopoietic cell transplantation. Blood. 2007;110(13):4560–4566. doi: 10.1182/blood-2007-06-095265. [DOI] [PubMed] [Google Scholar]
- 8.Petersdorf EW, Gooley T, Malkki M, Horowitz M. Clinical significance of donor–recipient HLA matching on survival after myeloablative hematopoietic cell transplantation from unrelated donors. Tissue Antigens. 2007;69(Suppl 1):25–30. doi: 10.1111/j.1399-0039.2006.759_2.x. [DOI] [PubMed] [Google Scholar]
- 9.Loiseau P, Esperou H, Busson M, et al. DPB1 disparities contribute to severe GVHD and reduced patient survival after unrelated donor bone marrow transplantation. Bone Marrow Transplant. 2002;30(8):497–502. doi: 10.1038/sj.bmt.1703658. [DOI] [PubMed] [Google Scholar]
- 10.Kawase T, Morishima Y, Matsuo K, et al. High-risk HLA allele mismatch combinations responsible for severe acute graft-versus-host disease and implication for its molecular mechanism. Blood. 2007;110(7):2235–2241. doi: 10.1182/blood-2007-02-072405. [DOI] [PubMed] [Google Scholar]
- 11.Cutler C, Giri S, Jeyapalan S, et al. Acute and chronic graft-versus-host disease after allogeneic peripheral-blood stem-cell and bone marrow transplantation: a meta-analysis. J Clin Oncol. 2001;19(16):3685–3691. doi: 10.1200/JCO.2001.19.16.3685. [DOI] [PubMed] [Google Scholar]; • While peripheral blood stem cells are the product of choice for allogeneic hematopoietic stem cell transplantation, there are concerns that the graft-versus-leukemia rate expected may be less than hoped. If so, and chronic GVHD is more prevalent, we may need to reconsider the role of bone marrow transplantation.
- 12.Wagner JE, Barker JN, DeFor TE, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100(5):1611–1618. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- 13.Rocha V, Labopin M, Sanz G, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004;351(22):2276–2285. doi: 10.1056/NEJMoa041469. [DOI] [PubMed] [Google Scholar]
- 14.Laughlin MJ, Eapen M, Rubinstein P, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351(22):2265–2275. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- 15.Przepiorka D, Huh YO, Khouri I, et al. Graft failure and graft-vs-host disease after subtotal T-cell-depleted marrow transplantation: correlations with marrow hematopoietic and lymphoid subsets. Prog Clin Biol Res. 1994;389:557–563. [PubMed] [Google Scholar]
- 16.Pavletic SZ, Carter SL, Kernan NA, et al. Influence of T-cell depletion on chronic graft-versus-host disease: results of a multicenter randomized trial in unrelated marrow donor transplantation. Blood. 2005;106(9):3308–3313. doi: 10.1182/blood-2005-04-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho VT, Soiffer RJ. The history and future of T-cell depletion as graft-versus-host disease prophylaxis for allogeneic hematopoietic stem cell transplantation. Blood. 2001;98(12):3192–3204. doi: 10.1182/blood.v98.12.3192. [DOI] [PubMed] [Google Scholar]
- 18.Atkinson K, Horowitz MM, Gale RP, et al. Risk factors for chronic graft-versus-host disease after HLA-identical bone marrow transplantation. Blood. 1990;75:2459–2464. [PubMed] [Google Scholar]
- 19.Billingham RE. The biology of graft-versus-host reactions. Harvey Lecture Series. 1966–1967;62:21–78. [PubMed] [Google Scholar]
- 20.Xun CQ, Thompson JC, Jennings CD, Brown SA, Widmer MB. Effect of total body irradiation, busulfan–cyclophosphamide, or cyclophosphamide conditioning on inflammatory cytokine release and development of acute and chronic graft-versus-host disease in H-2-incompatible transplanted SCID mice. Blood. 1994;83:2360–2367. [PubMed] [Google Scholar]
- 21.Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000;95(9):2754–2759. [PubMed] [Google Scholar]
- 22.Paris F, Fuks Z, Kang A, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293(5528):293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 23.Shlomchik WD, Couzens MS, Tang CB, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285(5426):412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]; •• First clear indication that host antigen-presenting cells are important mediators of GVHD. Subsequent work defined the role of donor antigen-presenting cells.
- 24.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296(5566):301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 25.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5(10):987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 26.Fritz JH, Ferrero RL, Philpott DJ, Girardin SE. Nod-like proteins in immunity, inflammation and disease. Nat Immunol. 2006;7(12):1250–1257. doi: 10.1038/ni1412. [DOI] [PubMed] [Google Scholar]
- 27.Bromley SK, Iaboni A, Davis SJ, et al. The immunological synapse and CD28–CD80 interactions. Nat Immunol. 2001;2(12):1159–1166. doi: 10.1038/ni737. [DOI] [PubMed] [Google Scholar]
- 28.Dustin ML. Role of adhesion molecules in activation signaling in T lymphocytes. J Clin Immunol. 2001;21(4):258–263. doi: 10.1023/a:1010927208180. [DOI] [PubMed] [Google Scholar]
- 29.Shlomchik WD. Antigen presentation in graft-vs-host disease. Exp Hematol. 2003;31(12):1187–1197. doi: 10.1016/j.exphem.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 30.Anderson BE, McNiff JM, Jain D, et al. Distinct roles for donor- and host-derived antigen-presenting cells and costimulatory molecules in murine chronic graft-versus-host disease: requirements depend on target organ. Blood. 2005;105(5):2227–2234. doi: 10.1182/blood-2004-08-3032. [DOI] [PubMed] [Google Scholar]
- 31.Korngold R, Sprent J. T-cell subsets and graft versus host disease. Transplantation. 1987;44:335. doi: 10.1097/00007890-198709000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Csencsits KL, Bishop DK. Contrasting alloreactive CD4+ and CD8+ T cells: there's more to it than MHC restriction. Am J Transplant. 2003;3(2):107–115. doi: 10.1034/j.1600-6143.2003.00036.x. [DOI] [PubMed] [Google Scholar]
- 33.Kagi D, Vignaux F, Ledermann B, et al. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994;265(5171):528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 34.van den Brink MR, Burakoff SJ. Cytolytic pathways in haematopoietic stem-cell transplantation. Nat Rev Immunol. 2002;2(4):273–281. doi: 10.1038/nri775. [DOI] [PubMed] [Google Scholar]; • Very useful review of effector mechanisms in acute GVHD.
- 35.Pan G, O'Rourke K, Chinnaiyan AM, et al. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276(5309):111–113. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- 36.Chicheportiche Y, Bourdon PR, Xu H, et al. TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J Biol Chem. 1997;272(51):32401–32410. doi: 10.1074/jbc.272.51.32401. [DOI] [PubMed] [Google Scholar]
- 37.Antin JH, Ferrara JL. Cytokine dysregulation and acute graft-versus-host disease. Blood. 1992;80:2964–2968. [PubMed] [Google Scholar]
- 38.Cooke KR, Hill GR, Crawford JM, et al. Tumor necrosis factor-α production to lipopolysaccharide stimulation by donor cells predicts the severity of experimental acute graft-versus-host disease. J Clin Invest. 1998;102(10):1882–1891. doi: 10.1172/JCI4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nestel FP, Price KS, Seemayer TA, Lapp WS. Macrophage priming and lipopolysaccharide-triggered release of tumor necrosis factor α during graft-versus-host disease. J Exp Med. 1992;175(2):405–413. doi: 10.1084/jem.175.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teshima T, Ordemann R, Reddy P, et al. Acute graft-versus-host disease does not require alloantigen expression on host epithelium. Nat Med. 2002;8(6):575–581. doi: 10.1038/nm0602-575. [DOI] [PubMed] [Google Scholar]
- 41.Fowler DH, Kurasawa K, Smith R, Eckhaus MA, Gress RE. Donor CD4-enriched cells of Th2 cytokine phenotype regulate graft-versus-host disease without impairing allogeneic engraftment in sublethally irradiated mice. Blood. 1994;84(10):3540–3549. [PubMed] [Google Scholar]
- 42.Pan L, Delmonte J, Jr, Jalonen CK, Ferrara JLM. Pretreatment of donor mice with granulocyte colony-stimulating factor polarizes donor T-lymphocytes toward type-2 cytokine production and reduces severity of experimental graft-versus-host disease. Blood. 1995;86:4422–4429. [PubMed] [Google Scholar]
- 43.Fowler DH, Gress RE. Th2 and Tc2 cells in the regulation of GVHD, GVL, and graft rejection: considerations for the allogeneic transplantation therapy of leukemia and lymphoma. Leuk Lymphoma. 2000;38(3–4):221–234. doi: 10.3109/10428190009087014. [DOI] [PubMed] [Google Scholar]
- 44.Reddy P, Teshima T, Hildebrandt G, et al. Pretreatment of donors with interleukin-18 attenuates acute graft-versus-host disease via STAT6 and preserves graft-versus-leukemia effects. Blood. 2003;101(7):2877–2885. doi: 10.1182/blood-2002-08-2566. [DOI] [PubMed] [Google Scholar]
- 45.Nikolic B, Lee S, Bronson RT, Grusby MJ, Sykes M. Th1 and Th2 mediate acute graft-versus-host disease, each with distinct end-organ targets. J Clin Invest. 2000;105(9):1289–1298. doi: 10.1172/JCI7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T-cell lineage with regulatory T-cell ties. Immunity. 2006;24(6):677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 47.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 48.Oukka M. Interplay between pathogenic Th17 and regulatory T cells. Ann Rheum Dis. 2007;66(Suppl 3):iii87–iii90. doi: 10.1136/ard.2007.078527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cavet J, Middleton PG, Segall M, et al. Recipient tumor necrosis factor-α and interleukin-10 gene polymorphisms associate with early mortality and acute graft-versus-host disease severity in HLA-matched sibling bone marrow transplants. Blood. 1999;94(11):3941–3946. [PubMed] [Google Scholar]
- 50.Cavet J, Dickinson AM, Norden J, et al. Interferon-γ and interleukin-6 gene polymorphisms associate with graft-versus-host disease in HLA-matched sibling bone marrow transplantation. Blood. 2001;98(5):1594–1600. doi: 10.1182/blood.v98.5.1594. [DOI] [PubMed] [Google Scholar]
- 51.Cullup H, Dickinson AM, Jackson GH, et al. Donor interleukin 1 receptor antagonist genotype associated with acute graft-versus-host disease in human leucocyte antigen-matched sibling allogeneic transplants. Br J Haematol. 2001;113(3):807–813. doi: 10.1046/j.1365-2141.2001.02811.x. [DOI] [PubMed] [Google Scholar]
- 52.Lin MT, Storer B, Martin PJ, et al. Genetic variation in the IL-10 pathway modulates severity of acute graft-versus-host disease following hematopoietic cell transplantation: synergism between IL-10 genotype of patient and IL-10 receptor β genotype of donor. Blood. 2005;106(12):3995–4001. doi: 10.1182/blood-2004-11-4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin MT, Storer B, Martin PJ, et al. Relation of an interleukin-10 promoter polymorphism to graft-versus-host disease and survival after hematopoietic-cell transplantation. N Engl J Med. 2003;349(23):2201–2210. doi: 10.1056/NEJMoa022060. [DOI] [PubMed] [Google Scholar]
- 54.Karabon L, Wysoczanska B, Bogunia-Kubik K, Suchnicki K, Lange A. IL-6 and IL-10 promoter gene polymorphisms of patients and donors of allogeneic sibling hematopoietic stem cell transplants associate with the risk of acute graft-versus-host disease. Hum Immunol. 2005;66(6):700–710. doi: 10.1016/j.humimm.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 55.Middleton PG, Taylor PR, Jackson G, Proctor SJ, Dickinson AM. Cytokine gene polymorphisms associating with severe acute graft-versus-host disease in HLA-identical sibling transplants. Blood. 1998;92(10):3943–3948. [PubMed] [Google Scholar]
- 56.Bogunia-Kubik K, Mlynarczewska A, Wysoczanska B, Lange A. Recipient interferon-γ 3/3 genotype contributes to the development of chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Haematologica. 2005;90(3):425–426. [PubMed] [Google Scholar]
- 57.Cullup H, Dickinson AM, Cavet J, Jackson GH, Middleton PG. Polymorphisms of interleukin-1α constitute independent risk factors for chronic graft-versus-host disease after allogeneic bone marrow transplantation. Br J Haematol. 2003;122(5):778–787. doi: 10.1046/j.1365-2141.2003.04510.x. [DOI] [PubMed] [Google Scholar]
- 58.Kim DH, Lee NY, Sohn SK, et al. IL-10 promoter gene polymorphism associated with the occurrence of chronic GVHD and its clinical course during systemic immunosuppressive treatment for chronic GVHD after allogeneic peripheral blood stem cell transplantation. Transplantation. 2005;79(11):1615–1622. doi: 10.1097/01.tp.0000159792.04757.d4. [DOI] [PubMed] [Google Scholar]
- 59.Gagne K, Brizard G, Gueglio B, et al. Relevance of KIR gene polymorphisms in bone marrow transplantation outcome. Hum Immunol. 2002;63(4):271–280. doi: 10.1016/s0198-8859(02)00373-7. [DOI] [PubMed] [Google Scholar]
- 60.Beelen DW, Ottinger HD, Ferencik S, et al. Genotypic inhibitory killer immunoglobulin-like receptor ligand incompatibility enhances the long-term antileukemic effect of unmodified allogeneic hematopoietic stem cell transplantation in patients with myeloid leukemias. Blood. 2005;105(6):2594–2600. doi: 10.1182/blood-2004-04-1441. [DOI] [PubMed] [Google Scholar]
- 61.Schaffer M, Malmberg KJ, Ringden O, Ljunggren HG, Remberger M. Increased infection-related mortality in KIR-ligand-mismatched unrelated allogeneic hematopoietic stem-cell transplantation. Transplantation. 2004;78(7):1081–1085. doi: 10.1097/01.tp.0000137103.19717.86. [DOI] [PubMed] [Google Scholar]
- 62.Holler E, Rogler G, Brenmoehl J, et al. Prognostic significance of NOD2/CARD15 variants in HLA-identical sibling hematopoietic stem cell transplantation: effect on long-term outcome is confirmed in 2 independent cohorts and may be modulated by the type of gastrointestinal decontamination. Blood. 2006;107(10):4189–4193. doi: 10.1182/blood-2005-09-3741. [DOI] [PubMed] [Google Scholar]
- 63.Kalayoglu-Besisik S, Caliskan Y, Sargin D, Gurses N, Ozbek U. Methylenetetrahydrofolate reductase C677T polymorphism and toxicity in allogeneic hematopoietic cell transplantation. Transplantation. 2003;76(12):1775–1777. doi: 10.1097/01.TP.0000093831.63661.DF. [DOI] [PubMed] [Google Scholar]
- 64.Robien K, Schubert MM, Bruemmer B, et al. Predictors of oral mucositis in patients receiving hematopoietic cell transplants for chronic myelogenous leukemia. J Clin Oncol. 2004;22(7):1268–1275. doi: 10.1200/JCO.2004.05.147. [DOI] [PubMed] [Google Scholar]
- 65.Middleton PG, Cullup H, Dickinson AM, et al. Vitamin D receptor gene polymorphism associates with graft-versus-host disease and survival in HLA-matched sibling allogeneic bone marrow transplantation. Bone Marrow Transplant. 2002;30(4):223–228. doi: 10.1038/sj.bmt.1703629. [DOI] [PubMed] [Google Scholar]
- 66.Middleton PG, Norden J, Cullup H, et al. Oestrogen receptor α gene polymorphism associates with occurrence of graft-versus-host disease and reduced survival in HLA-matched sib-allo BMT. Bone Marrow Transplant. 2003;32(1):41–47. doi: 10.1038/sj.bmt.1704090. [DOI] [PubMed] [Google Scholar]
- 67.Bogunia-Kubik K, Lange A. HSP70-hom gene polymorphism in allogeneic hematopoietic stem-cell transplant recipients correlates with the development of acute graft-versus-host disease. Transplantation. 2005;79(7):815–820. doi: 10.1097/01.tp.0000153157.97736.2c. [DOI] [PubMed] [Google Scholar]
- 68.Socie G, Loiseau P, Tamouza R, et al. Both genetic and clinical factors predict the development of graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Transplantation. 2001;72(4):699–706. doi: 10.1097/00007890-200108270-00024. [DOI] [PubMed] [Google Scholar]
- 69.Anderson BE, McNiff J, Yan J, et al. Memory CD4+ T cells do not induce graft-versus-host disease. J Clin Invest. 2003;112(1):101–108. doi: 10.1172/JCI17601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen BJ, Cui X, Sempowski GD, Liu C, Chao NJ. Transfer of allogeneic CD62L-memory T cells without graft-versus-host disease. Blood. 2004;103(4):1534–1541. doi: 10.1182/blood-2003-08-2987. [DOI] [PubMed] [Google Scholar]
- 71.Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nat Med. 2005;11(12):1299–1305. doi: 10.1038/nm1326. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Alloreactive memory T cells are responsible for the persistence of graft-versus-host disease. J Immunol. 2005;174(5):3051–3058. doi: 10.4049/jimmunol.174.5.3051. [DOI] [PubMed] [Google Scholar]
- 73.Edinger M, Hoffmann P, Ermann J, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9(9):1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]; •• Manipulations of regulatory T cells are likely to be important in the next generation of trials to prevent and treat GVHD.
- 74.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4(9):665–674. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 75.Nelson BH. IL-2, regulatory T cells, and tolerance. J Immunol. 2004;172(7):3983–3988. doi: 10.4049/jimmunol.172.7.3983. [DOI] [PubMed] [Google Scholar]
- 76.Miura Y, Thoburn CJ, Bright EC, et al. Association of Foxp3 regulatory gene expression with graft-versus-host disease. Blood. 2004;104(7):2187–2193. doi: 10.1182/blood-2004-03-1040. [DOI] [PubMed] [Google Scholar]
- 77.Zorn E, Kim HT, Lee SJ, et al. Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood. 2005;106:2903–2911. doi: 10.1182/blood-2005-03-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Taylor PA, Panoskaltsis-Mortari A, Swedin JM, et al. L-Selectin(hi) but not the L-selectin(lo) CD4+25+ T-regulatory cells are potent inhibitors of GVHD and BM graft rejection. Blood. 2004;104(12):3804–3812. doi: 10.1182/blood-2004-05-1850. [DOI] [PubMed] [Google Scholar]
- 79.Ermann J, Hoffmann P, Edinger M, et al. Only the CD62L+ subpopulation of CD4+CD25+ regulatory T cells protects from lethal acute GVHD. Blood. 2005;105(5):2220–2226. doi: 10.1182/blood-2004-05-2044. [DOI] [PubMed] [Google Scholar]
- 80.Zeng D, Lewis D, Dejbakhsh-Jones S, et al. Bone marrow NK1.1- and NK1.1+ T cells reciprocally regulate acute graft versus host disease. J Exp Med. 1999;189(7):1073–1081. doi: 10.1084/jem.189.7.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hashimoto D, Asakura S, Miyake S, et al. Stimulation of host NKT cells by synthetic glycolipid regulates acute graft-versus-host disease by inducing Th2 polarization of donor T cells. J Immunol. 2005;174(1):551–556. doi: 10.4049/jimmunol.174.1.551. [DOI] [PubMed] [Google Scholar]
- 82.Lowsky R, Takahashi T, Liu YP, et al. Protective conditioning for acute graft-versus-host disease. N Engl J Med. 2005;353(13):1321–1331. doi: 10.1056/NEJMoa050642. [DOI] [PubMed] [Google Scholar]
- 83.Iori AP, Torelli GF, De Propris MS, et al. B-cell concentration in the apheretic product predicts acute graft-versus-host disease and treatment-related mortality of allogeneic peripheral blood stem cell transplantation. Transplantation. 2008;85(3):386–390. doi: 10.1097/TP.0b013e3181622e36. [DOI] [PubMed] [Google Scholar]
- 84.Rouquette-Gally AM, Boyeldieu D, Prost AC, Gluckman E. Autoimmunity after allogeneic bone marrow transplantation. A study of 53 long-term-surviving patients. Transplantation. 1988;46(2):238–240. doi: 10.1097/00007890-198808000-00010. [DOI] [PubMed] [Google Scholar]
- 85.Miklos DB, Kim HT, Miller KH, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105(7):2973–2978. doi: 10.1182/blood-2004-09-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Antibodies and B-cell responses were recognized as altered in GVHD, but this paper confirms their roles as possible contributors to GVHD. This paper indicates a potentially critical role for humoral immunity and suggested new pathways for treatment and prevention of GVHD.
- 86.Baroni SS, Santillo M, Bevilacqua F, et al. Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N Engl J Med. 2006;354(25):2667–2676. doi: 10.1056/NEJMoa052955. [DOI] [PubMed] [Google Scholar]
- 87.Patriarca F, Skert C, Sperotto A, et al. The development of autoantibodies after allogeneic stem cell transplantation is related with chronic graft-vs-host disease and immune recovery. Exp Hematol. 2006;34(3):389–396. doi: 10.1016/j.exphem.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 88.Sarantopoulos S, Stevenson KE, Kim HT, et al. High levels of B-cell activating factor in patients with active chronic graft-versus-host disease. Clin Cancer Res. 2007;13(20):6107–6114. doi: 10.1158/1078-0432.CCR-07-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sullivan KM, Deeg HJ, Sanders J, et al. Hyperacute graft versus host disease in patients not given immunosuppression after allogeneic marrow transplantation. Blood. 1986;67:1172. [PubMed] [Google Scholar]
- 90.Storb R, Thomas ED, Buckner CD, et al. Allogeneic marrow grafting for treatment of aplastic anemia. Blood. 1974;43(2):157–180. [PubMed] [Google Scholar]
- 91.Powles RL, Clink HM, Spence D, et al. Cyclosporin A to prevent graft-versus-host disease in man after allogeneic bone-marrow transplantation. Lancet. 1980;1(8164):327–329. doi: 10.1016/s0140-6736(80)90881-8. [DOI] [PubMed] [Google Scholar]
- 92.Fay JW, Wingard JR, Antin JH, et al. FK506 (tacrolimus) monotherapy for prevention of graft-versus-host disease after histocompatible sibling allogeneic bone marrow transplantation. Blood. 1996;87:3514–3519. [PubMed] [Google Scholar]
- 93.Deeg HJ, Storb R, Thomas ED, et al. Cyclosporine as prophylaxis for graft versus host disease: a randomized study in patients undergoing marrow transplantation for acute nonlymphoblastic leukemia. Blood. 1985;65:1325. [PubMed] [Google Scholar]
- 94.Shaw JP, Utz PJ, Durand DB, et al. Identification of a putative regulator of early T-cell activation genes. Science. 1988;241(4862):202–205. doi: 10.1126/science.3260404. [DOI] [PubMed] [Google Scholar]
- 95.Crabtree GR. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989;243(4889):355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- 96.Storb R, Deeg HJ, Whitehead J, et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med. 1986;314:729–735. doi: 10.1056/NEJM198603203141201. [DOI] [PubMed] [Google Scholar]; •• Set the stage for the next 20 years of GVHD-prevention studies.
- 97.Storb R, Deeg HJ, Farewell V, et al. Marrow transplantation for severe aplastic anemia: methotrexate alone compared with a combination of methotrexate and cyclosporine for prevention of acute graft-versus-host disease. Blood. 1986;68:119–125. [PubMed] [Google Scholar]
- 98.Ratanatharathorn V, Nash RA, Przepiorka D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92(7):2303–2314. [PubMed] [Google Scholar]
- 99.Hiraoka A, Ohashi Y, Okamoto S, et al. Phase III study comparing tacrolimus (FK506) with cyclosporine for graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation. Bone Marrow Transplant. 2001;28(2):181–185. doi: 10.1038/sj.bmt.1703097. [DOI] [PubMed] [Google Scholar]
- 100.Kansu E, Gooley T, Flowers ME, et al. Administration of cyclosporine for 24 months compared with 6 months for prevention of chronic graft-versus-host disease: a prospective randomized clinical trial. Blood. 2001;98(13):3868–3870. doi: 10.1182/blood.v98.13.3868. [DOI] [PubMed] [Google Scholar]
- 101.Atkinson K, Downs K. Omission of day 11 methotrexate does not appear to influence the incidence of moderate to severe acute graft-versus-host disease, chronic graft-versus-host disease, relapse rate or survival after HLA-identical sibling bone marrow transplantation. Bone Marrow Transplant. 1995;16(6):755–758. [PubMed] [Google Scholar]
- 102.Bensinger W. Individual patient data meta-analysis of allogeneic peripheral blood stem cell transplant vs bone marrow transplant in the management of hematological malignancies: indirect assessment of the effect of day 11 methotrexate administration. Bone Marrow Transplant. 2006;38(8):539–546. doi: 10.1038/sj.bmt.1705488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chao NJ, Schmidt GM, Niland JC, et al. Cyclosporine, methotrexate, and prednisone compared with cyclosporine and prednisone for prophylaxis of acute graft-versus-host disease. N Engl J Med. 1993;329:1225–1230. doi: 10.1056/NEJM199310213291703. [DOI] [PubMed] [Google Scholar]
- 104.Chao NJ, Snyder DS, Jain M, et al. Equivalence of 2 effective graft-versus-host disease prophylaxis regimens: results of a prospective double-blind randomized trial. Biol Blood Marrow Transplant. 2000;6(3):254–261. doi: 10.1016/s1083-8791(00)70007-3. [DOI] [PubMed] [Google Scholar]
- 105.Santos G, Tutschka P, Brookmeyer R, et al. Cyclosporine plus methylprednisolone versus cyclophosphamide plus methylprednisolone for graft-versus-host disease: a randomized double blind study in patients undergoing allogeneic marrow transplantation. Clin Transplant. 1987;1:21–28. [Google Scholar]
- 106.Luznik L, Chen A, Kaup M, et al. Post-transplantation high dose cyclophosphamide (Cy) is effective single agent GVHD prophylaxis that permits prompt immune reconstitution after myeloablative HLA matched related and unrelated bone marrow transplantation (BMT) Blood. 2006;108:2891s. [Google Scholar]
- 107.Luznik L, O'Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nash RA, Johnston L, Parker P, et al. A Phase I/II study of mycophenolate mofetil in combination with cyclosporine for prophylaxis of acute graft-versus-host disease after myeloablative conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2005;11(7):495–505. doi: 10.1016/j.bbmt.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 109.Neumann F, Graef T, Tapprich C, et al. Cyclosporine A and mycophenolate mofetil vs cyclosporine A and methotrexate for graft-versus-host disease prophylaxis after stem cell transplantation from HLA-identical siblings. Bone Marrow Transplant. 2005;35(11):1089–1093. doi: 10.1038/sj.bmt.1704956. [DOI] [PubMed] [Google Scholar]
- 110.Bolwell B, Sobecks R, Pohlman B, et al. A prospective randomized trial comparing cyclosporine and short course methotrexate with cyclosporine and mycophenolate mofetil for GVHD prophylaxis in myeloablative allogeneic bone marrow transplantation. Bone Marrow Transplant. 2004;34(7):621–625. doi: 10.1038/sj.bmt.1704647. [DOI] [PubMed] [Google Scholar]
- 111.Burroughs L, Mielcarek M, Leisenring W, et al. Extending postgrafting cyclosporine decreases the risk of severe graft-versus-host disease after nonmyeloablative hematopoietic cell transplantation. Transplantation. 2006;81(6):818–825. doi: 10.1097/01.tp.0000203556.06145.5b. [DOI] [PubMed] [Google Scholar]
- 112.Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc. 2003;35(3 Suppl):7S–14S. doi: 10.1016/s0041-1345(03)00211-2. [DOI] [PubMed] [Google Scholar]
- 113.Sarbassov DD, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22(2):159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 114.Ali SM, Sabatini DM. Structure of S6 kinase 1 determines whether raptor-mTOR or rictor-mTOR phosphorylates its hydrophobic motif site. J Biol Chem. 2005;280(20):19445–19448. doi: 10.1074/jbc.C500125200. [DOI] [PubMed] [Google Scholar]
- 115.Sarbassov DD, Ali SM, Kim DH, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14(14):1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 116.Blazar BR, Taylor PA, Panoskaltsis-Mortari A, Vallera DA. Rapamycin inhibits the generation of graft-versus-host disease- and graft-versus-leukemia-causing T cells by interfering with the production of Th1 or Th1 cytotoxic cytokines. J Immunol. 1998;160(11):5355–5365. [PubMed] [Google Scholar]
- 117.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105(12):4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 118.Zeiser R, Nguyen VH, Beilhack A, et al. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood. 2006;108(1):390–399. doi: 10.1182/blood-2006-01-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mariotti J, Foley J, Jung U, et al. Ex vivo rapamycin generates apoptosis-resistant donor Th2 cells that persist in vivo and prevent hemopoietic stem cell graft rejection. J Immunol. 2008;180(1):89–105. doi: 10.4049/jimmunol.180.1.89. [DOI] [PubMed] [Google Scholar]
- 120.Jung U, Foley JE, Erdmann AA, et al. Ex vivo rapamycin generates Th1/Tc1 or Th2/Tc2 effector T cells with enhanced in vivo function and differential sensitivity to post-transplant rapamycin therapy. Biol Blood Marrow Transplant. 2006;12(9):905–918. doi: 10.1016/j.bbmt.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 121.Woltman AM, de Fijter JW, Kamerling SW, et al. Rapamycin induces apoptosis in monocyte- and CD34-derived dendritic cells but not in monocytes and macrophages. Blood. 2001;98(1):174–180. doi: 10.1182/blood.v98.1.174. [DOI] [PubMed] [Google Scholar]
- 122.Monti P, Mercalli A, Leone BE, et al. Rapamycin impairs antigen uptake of human dendritic cells. Transplantation. 2003;75(1):137–145. doi: 10.1097/00007890-200301150-00025. [DOI] [PubMed] [Google Scholar]
- 123.Hackstein H, Taner T, Zahorchak AF, et al. Rapamycin inhibits IL-4-induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo. Blood. 2003;101(11):4457–4463. doi: 10.1182/blood-2002-11-3370. [DOI] [PubMed] [Google Scholar]
- 124.Koenen HJ, Michielsen EC, Verstappen J, Fasse E, Joosten I. Superior T-cell suppression by rapamycin and FK506 over rapamycin and cyclosporine A because of abrogated cytotoxic T-lymphocyte induction, impaired memory responses, and persistent apoptosis. Transplantation. 2003;75(9):1581–1590. doi: 10.1097/01.TP.0000053752.87383.67. [DOI] [PubMed] [Google Scholar]
- 125.Antin JH, Kim HT, Cutler C, et al. Sirolimus, tacrolimus, and low-dose methotrexate for graft-versus-host disease prophylaxis in mismatched related donor or unrelated donor transplantation. Blood. 2003;102(5):1601–1605. doi: 10.1182/blood-2003-02-0489. [DOI] [PubMed] [Google Scholar]
- 126.Cutler C, Li S, Ho VT, et al. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood. 2007;109(7):3108–3114. doi: 10.1182/blood-2006-09-046219. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Methotrexate causes more toxicity than optimal in GVHD prophylaxis. Other drugs, such as sirolimus, promise better GVHD control with less intrinsic toxicity.
- 127.Furlong T, Kiem HP, Appelbaum FR, et al. Sirolimus in combination with cyclosporine or tacrolimus plus methotrexate for prevention of graft-versus-host disease following hematopoietic cell transplantation from unrelated donors. Biol Blood Marrow Transplant. 2008;14(5):531–537. doi: 10.1016/j.bbmt.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wolff D, Andree H, Hilgendorf I, et al. Sirolimus in combination with tacrolimus in allogeneic stem cell transplantation – timing and conditioning regimen may be crucial. Biol Blood Marrow Transplant. 2008;14(8):942–943. doi: 10.1016/j.bbmt.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 129.Ramsay NKC, Kersey JH, Robison LL, et al. A randomized study of the prevention of acute graft versus host disease. N Engl J Med. 1982;306:392. doi: 10.1056/NEJM198202183060703. [DOI] [PubMed] [Google Scholar]
- 130.Hale G, Jacobs P, Wood L, et al. CD52 antibodies for prevention of graft-versus-host disease and graft rejection following transplantation of allogeneic peripheral blood stem cells. Bone Marrow Transplant. 2000;26(1):69–76. doi: 10.1038/sj.bmt.1702477. [DOI] [PubMed] [Google Scholar]
- 131.Perez-Simon JA, Kottaridis PD, Martino R, et al. Nonmyeloablative transplantation with or without alemtuzumab: comparison between 2 prospective studies in patients with lymphoproliferative disorders. Blood. 2002;100(9):3121–3127. doi: 10.1182/blood-2002-03-0701. [DOI] [PubMed] [Google Scholar]
- 132.Russell JA, Turner AR, Larratt L, et al. Adult recipients of matched related donor blood cell transplants given myeloablative regimens including pretransplant antithymocyte globulin have lower mortality related to graft-versus-host disease: a matched pair analysis. Biol Blood Marrow Transplant. 2007;13(3):299–306. doi: 10.1016/j.bbmt.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 133.Bacigalupo A, Lamparelli T, Barisione G, et al. Thymoglobulin prevents chronic graft-versus-host disease, chronic lung dysfunction, and late transplant-related mortality: long-term follow-up of a randomized trial in patients undergoing unrelated donor transplantation. Biol Blood Marrow Transplant. 2006;12(5):560–565. doi: 10.1016/j.bbmt.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 134.Bacigalupo A, Lamparelli T, Bruzzi P, et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO) Blood. 2001;98(10):2942–2947. doi: 10.1182/blood.v98.10.2942. [DOI] [PubMed] [Google Scholar]
- 135.Chakrabarti S, Hale G, Waldmann H. Alemtuzumab (Campath-1H) in allogeneic stem cell transplantation: where do we go from here? Transplant Proc. 2004;36(5):1225–1227. doi: 10.1016/j.transproceed.2004.05.067. [DOI] [PubMed] [Google Scholar]
- 136.Blaise D, Olive D, Hirn M, et al. Prevention of acute GVHD by in vivo use of anti-interleukin-2 receptor monoclonal antibody (33B3.1): a feasibility trial in 15 patients. Bone Marrow Transplant. 1991;8(2):105–111. [PubMed] [Google Scholar]
- 137.Antin JH, Weinstein HJ, Guinan EC, et al. Recombinant human interleukin-1 receptor antagonist in the treatment of steroid-resistant graft-versus-host disease. Blood. 1994;84(4):1342–1348. [PubMed] [Google Scholar]
- 138.Antin JH, Weisdorf D, Neuberg D, et al. Interleukin-1 blockade does not prevent acute graft-versus-host disease: results of a randomized, double-blind, placebo-controlled trial of interleukin-1 receptor antagonist in allogeneic bone marrow transplantation. Blood. 2002;100(10):3479–3482. doi: 10.1182/blood-2002-03-0985. [DOI] [PubMed] [Google Scholar]
- 139.Koehler M, Hurwitz CA, Krance RA, et al. XomaZyme-CD5 immunotoxin in conjunction with partial T-cell depletion for prevention of graft rejection and graft-versus-host disease after bone marrow transplantation from matched unrelated donors. Bone Marrow Transplant. 1994;13(5):571–575. [PubMed] [Google Scholar]
- 140.Martin PJ, Nelson BJ, Appelbaum FR, et al. Evaluation of a CD5-specific immunotoxin for treatment of acute graft-versus-host disease after allogeneic marrow transplantation. Blood. 1996;88(3):824–830. [PubMed] [Google Scholar]
- 141.Weisdorf D, Filipovich A, McGlave P, et al. Combination graft-versus-host disease prophylaxis using immunotoxin (anti-CD5-RTA [Xomazyme-CD5]) plus methotrexate and cyclosporine or prednisone after unrelated donor marrow transplantation. Bone Marrow Transplant. 1993;12(5):531–536. [PubMed] [Google Scholar]
- 142.Ratanatharathorn V, Horowitz M, Logan B, et al. Prior therapy with rituximab correlates wtih less acute graft-versus-host disease and better survivial in B-cell lymphoma patients who received allogeneic peripheral blood stem cell transplantation (PBSCT) Blood. 2007;110a doi: 10.1111/j.1365-2141.2009.07674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wagner JE, Thompson JS, Carter SL, Kernan NA. Effect of graft-versus-host disease prophylaxis on 3-year disease-free survival in recipients of unrelated donor bone marrow (T-cell Depletion Trial): a multi-centre, randomised Phase II–III trial. Lancet. 2005;366(9487):733–741. doi: 10.1016/S0140-6736(05)66996-6. [DOI] [PubMed] [Google Scholar]
- 144.Martin PJ, Rowley SD, Anasetti C, et al. A phase I-II clinical trial to evaluate removal of CD4 cells and partial depletion of CD8 cells from donor marrow for HLA-mismatched unrelated recipients. Blood. 1999;94(7):2192–2199. [PubMed] [Google Scholar]
- 145.Soiffer RJ, Weller E, Alyea EP, et al. CD6+ donor marrow T-cell depletion as the sole form of graft-versus-host disease prophylaxis in patients undergoing allogeneic bone marrow transplant from unrelated donors. J Clin Oncol. 2001;19(4):1152–1159. doi: 10.1200/JCO.2001.19.4.1152. [DOI] [PubMed] [Google Scholar]
- 146.Ho VT, Kim HT, Li S, et al. Partial CD8+ T-cell depletion of allogeneic peripheral blood stem cell transplantation is insufficient to prevent graft-versus-host disease. Bone Marrow Transplant. 2004;34(11):987–994. doi: 10.1038/sj.bmt.1704690. [DOI] [PubMed] [Google Scholar]
- 147.Papadopoulos EB, Carabasi MH, Castro-Malaspina H, et al. T-cell-depleted allogeneic bone marrow transplantation as postremission therapy for acute myelogenous leukemia: freedom from relapse in the absence of graft-versus-host disease. Blood. 1998;91(3):1083–1090. [PubMed] [Google Scholar]
- 148.Alyea E, Weller E, Schlossman R, et al. T-cell-depleted allogeneic bone marrow transplantation followed by donor lymphocyte infusion in patients with multiple myeloma: induction of graft-versus-myeloma effect. Blood. 2001;98(4):934–939. doi: 10.1182/blood.v98.4.934. [DOI] [PubMed] [Google Scholar]
- 149.Guinan EC, Boussiotis VA, Neuberg D, et al. Transplantation of anergic histoincompatible bone marrow allografts. N Engl J Med. 1999;340(22):1704–1714. doi: 10.1056/NEJM199906033402202. see comments. [DOI] [PubMed] [Google Scholar]
- 150.Ghosh S, May MJ, Kopp EB. NF-κ B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 151.Wang X, Luo H, Chen H, Duguid W, Wu J. Role of proteasomes in T-cell activation and proliferation. J Immunol. 1998;160(2):788–801. [PubMed] [Google Scholar]
- 152.Finn PW, Stone JR, Boothby MR, Perkins DL. Inhibition of NF-κB-dependent T-cell activation abrogates acute allograft rejection. J Immunol. 2001;167(10):5994–6001. doi: 10.4049/jimmunol.167.10.5994. [DOI] [PubMed] [Google Scholar]
- 153.Sunwoo JB, Chen Z, Dong G, et al. Novel proteasome inhibitor PS-341 inhibits activation of nuclear factor-κ B, cell survival, tumor growth, and angiogenesis in squamous cell carcinoma. Clin Cancer Res. 2001;7(5):1419–1428. [PubMed] [Google Scholar]
- 154.Nencioni A, Schwarzenberg K, Brauer KM, et al. Proteasome inhibitor bortezomib modulates TLR4-induced dendritic cell activation. Blood. 2006;108(2):551–558. doi: 10.1182/blood-2005-08-3494. [DOI] [PubMed] [Google Scholar]
- 155.Blanco B, Perez-Simon JA, Sanchez-Abarca LI, et al. Bortezomib induces selective depletion of alloreactive T lymphocytes and decreases the production of Th1 cytokines. Blood. 2006;107(9):3575–3583. doi: 10.1182/blood-2005-05-2118. [DOI] [PubMed] [Google Scholar]
- 156.Vodanovic-Jankovic S, Hari P, Jacobs P, Komorowski R, Drobyski WR. NF-κB as a target for the prevention of graft versus host disease: comparative efficacy of bortezomib and PS-1145. Blood. 2006;107(2):827–834. doi: 10.1182/blood-2005-05-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sun K, Welniak LA, Panoskaltsis-Mortari A, et al. Inhibition of acute graft-versus-host disease with retention of graft-versus-tumor effects by the proteasome inhibitor bortezomib. Proc Natl Acad Sci USA. 2004;101(21):8120–8125. doi: 10.1073/pnas.0401563101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Koreth J, Kim HT, Ho VT, et al. Bortezomib plus tacrolimus/methotrexate for prophylaxis of acute GVHD after HLA mismatched allogeneic non-myeloablative transplantation: results of a dose finding Phase I study. Biol Blood Marrow Transplant. 2008;14(Suppl 2):53. 1. [Google Scholar]
- 159.Beilhack A, Schulz S, Baker J, et al. Prevention of acute graft-versus-host disease by blocking T-cell entry to secondary lymphoid organs. Blood. 2008;111(5):2919–2928. doi: 10.1182/blood-2007-09-112789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gatza E, Rogers CE, Clouthier SG, et al. Extracorporeal photopheresis reverses experimental graft-versus-host disease through regulatory T cells. Blood. 2008;112(4):1515–1521. doi: 10.1182/blood-2007-11-125542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Biagi E, Di Biaso I, Leoni V, et al. Extracorporeal photochemotherapy is accompanied by increasing levels of circulating CD4+CD25+GITR+Foxp3+CD62L+ functional regulatory T-cells in patients with graft-versus-host disease. Transplantation. 2007;84(1):31–39. doi: 10.1097/01.tp.0000267785.52567.9c. [DOI] [PubMed] [Google Scholar]
- 162.Heshmati F. Mechanisms of action of extracorporeal photochemotherapy. Transfus Apher Sci. 2003;29(1):61–70. doi: 10.1016/S1473-0502(03)00103-4. [DOI] [PubMed] [Google Scholar]
- 163.Ruutu T, Eriksson B, Remes K, et al. Ursodeoxycholic acid for the prevention of hepatic complications in allogeneic stem cell transplantation. Blood. 2002;100(6):1977–1983. doi: 10.1182/blood-2001-12-0159. [DOI] [PubMed] [Google Scholar]
- 164.Tay J, Tinmouth A, Fergusson D, Huebsch L, Allan DS. Systematic review of controlled clinical trials on the use of ursodeoxycholic acid for the prevention of hepatic veno-occlusive disease in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13(2):206–217. doi: 10.1016/j.bbmt.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 165.Chao NJ, Parker P, Niland JC, et al. Paradoxical effect of thalidomide prophylaxis on chronic graft-vs.-host disease. Biol Blood Marrow Transplant. 1996;2(2):86–92. [PubMed] [Google Scholar]
- 166.Witherspoon RP, Sullivan KM, Lum LG, et al. Use of thymic grafts or thymic factors to augment immunologic recovery after bone marrow transplantation: brief report with 2 to 12 years' follow-up. Bone Marrow Transplant. 1988;3(5):425–435. [PubMed] [Google Scholar]
- 167.Atkinson K, Storb R, Ochs HD, et al. Thymus transplantation after allogeneic bone marrow graft to prevent chronic graft-versus-host disease in humans. Transplantation. 1982;33(2):168–173. doi: 10.1097/00007890-198202000-00012. [DOI] [PubMed] [Google Scholar]
- 168.Weisdorf D, Haake R, Blazar B, et al. Treatment of moderate/severe acute graft-versus-host disease after allogeneic bone marrow transplantation: an analysis of clinical risk factors and outcome. Blood. 1990;75:1024–1030. [PubMed] [Google Scholar]
- 169.Martin PJ, Schoch G, Fisher L, et al. A retrospective analysis of therapy for acute graft-versus-host disease: Initial treatment. Blood. 1990;76:1464–1472. [PubMed] [Google Scholar]
- 170.Martin PJ, Schoch G, Fisher L, et al. A retrospective analysis of therapy for acute graft-versus-host disease: secondary treatment. Blood. 1991;77(8):1821–1828. [PubMed] [Google Scholar]
- 171.MacMillan ML, Weisdorf DJ, Wagner JE, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8(7):387–394. doi: 10.1053/bbmt.2002.v8.pm12171485. [DOI] [PubMed] [Google Scholar]
- 172.Van Lint MT, Milone G, Leotta S, et al. Treatment of acute graft-versus-host disease with prednisolone: significant survival advantage for day +5 responders and no advantage for nonresponders receiving anti-thymocyte globulin. Blood. 2006;107(10):4177–4181. doi: 10.1182/blood-2005-12-4851. [DOI] [PubMed] [Google Scholar]
- 173.Weiden PL, Doney K, Storb R, Thomas ED. Antihuman thymocyte globulin for prophylaxis of graft-versus-host disease. A randomized trial in patients with leukemia treated with HLA-identical sibling marrow grafts. Transplantation. 1979;27(4):227–230. doi: 10.1097/00007890-197904000-00003. [DOI] [PubMed] [Google Scholar]
- 174.Deeg HJ. How I treat refractory acute GVHD. Blood. 2007;109(10):4119–4126. doi: 10.1182/blood-2006-12-041889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bacigalupo A. Management of acute graft-versus-host disease. Br J Haematol. 2007;137(2):87–98. doi: 10.1111/j.1365-2141.2007.06533.x. [DOI] [PubMed] [Google Scholar]
- 176.Van Lint MT, Uderzo C, Locasciulli A, et al. Early treatment of acute graft-versus-host disease with high- or low-dose 6-methylprednisolone: a multicenter randomized trial from the Italian Group for Bone Marrow Transplantation. Blood. 1998;92(7):2288–2293. [PubMed] [Google Scholar]
- 177.Hockenbery DM, Cruickshank S, Rodell TC, et al. A randomized, placebo-controlled trial of oral beclomethasone dipropionate as a prednisone-sparing therapy for gastrointestinal graft-versus-host disease. Blood. 2007;109(10):4557–4563. doi: 10.1182/blood-2006-05-021139. [DOI] [PubMed] [Google Scholar]
- 178.Dugan MJ, DeFor TE, Steinbuch M, Filipovich AH, Weisdorf DJ. ATG plus corticosteroid therapy for acute graft-versus-host disease: predictors of response and survival. Ann Hematol. 1997;75(1–2):41–46. doi: 10.1007/s002770050310. [DOI] [PubMed] [Google Scholar]
- 179.Graziani F, Van Lint MT, Dominietto A, et al. Treatment of acute graft versus host disease with low dose-alternate day anti-thymocyte globulin. Haematologica. 2002;87(9):973–978. [PubMed] [Google Scholar]
- 180.Cragg L, Blazar BR, Defor T, et al. A randomized trial comparing prednisone with antithymocyte globulin/prednisone as an initial systemic therapy for moderately severe acute graft-versus-host disease. Biol Blood Marrow Transplant. 2000;6(4A):441–447. doi: 10.1016/s1083-8791(00)70036-x. [DOI] [PubMed] [Google Scholar]
- 181.Lee SJ, Zahrieh D, Agura E, et al. Effect of up-front daclizumab when combined with steroids for the treatment of acute graft-versus-host disease: results of a randomized trial. Blood. 2004;104(5):1559–1564. doi: 10.1182/blood-2004-03-0854. [DOI] [PubMed] [Google Scholar]
- 182.Cahn JY, Bordigoni P, Tiberghien P, et al. Treatment of acute graft-versus-host disease with methylprednisolone and cyclosporine with or without an anti-interleukin-2 receptor monoclonal antibody. A multicenter Phase III study. Transplantation. 1995;60(9):939–942. [PubMed] [Google Scholar]
- 183.Uberti JP, Ayash L, Ratanatharathorn V, et al. Pilot trial on the use of etanercept and methylprednisolone as primary treatment for acute graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11(9):680–687. doi: 10.1016/j.bbmt.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 184.Levine JE, Paczesny S, Mineishi S, et al. Etanercept plus methylprednisolone as initial therapy for acute graft-versus-host disease. Blood. 2008;111(4):2470–2475. doi: 10.1182/blood-2007-09-112987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Koc S, Leisenring W, Flowers ME, et al. Therapy for chronic graft-versus-host disease: a randomized trial comparing cyclosporine plus prednisone versus prednisone alone. Blood. 2002;100(1):48–51. doi: 10.1182/blood.v100.1.48. [DOI] [PubMed] [Google Scholar]
- 186.Arora M, Wagner JE, Davies SM, et al. Randomized clinical trial of thalidomide, cyclosporine, and prednisone versus cyclosporine and prednisone as initial therapy for chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2001;7(5):265–273. doi: 10.1053/bbmt.2001.v7.pm11400948. [DOI] [PubMed] [Google Scholar]
- 187.Hsu B, May R, Carrum G, Krance R, Przepiorka D. Use of antithymocyte globulin for treatment of steroid-refractory acute graft-versus-host disease: an international practice survey. Bone Marrow Transplant. 2001;28(10):945–950. doi: 10.1038/sj.bmt.1703269. [DOI] [PubMed] [Google Scholar]
- 188.Anasetti C, Hansen JA, Waldmann TA, et al. Treatment of acute graft-versus-host disease with humanized anti-Tac: an antibody that binds to the interleukin-2 receptor. Blood. 1994;84(4):1320–1327. [PubMed] [Google Scholar]
- 189.Przepiorka D, Kernan NA, Ippoliti C, et al. Daclizumab, a humanized anti-interleukin-2 receptor α chain antibody, for treatment of acute graft-versus-host disease. Blood. 2000;95(1):83–89. [PubMed] [Google Scholar]
- 190.Willenbacher W, Basara N, Blau IW, Fauser AA, Kiehl MG. Treatment of steroid refractory acute and chronic graft-versus-host disease with daclizumab. Br J Haematol. 2001;112(3):820–823. doi: 10.1046/j.1365-2141.2001.02582.x. [DOI] [PubMed] [Google Scholar]
- 191.Massenkeil G, Rackwitz S, Genvresse I, et al. Basiliximab is well tolerated and effective in the treatment of steroid-refractory acute graft-versus-host disease after allogeneic stem cell transplantation. Bone Marrow Transplant. 2002;30(12):899–903. doi: 10.1038/sj.bmt.1703737. [DOI] [PubMed] [Google Scholar]
- 192.Schmidt-Hieber M, Fietz T, Knauf W, et al. Efficacy of the interleukin-2 receptor antagonist basiliximab in steroid-refractory acute graft-versus-host disease. Br J Haematol. 2005;130(4):568–574. doi: 10.1111/j.1365-2141.2005.05631.x. [DOI] [PubMed] [Google Scholar]
- 193.Funke VA, de Medeiros CR, Setubal DC, et al. Therapy for severe refractory acute graft-versus-host disease with basiliximab, a selective interleukin-2 receptor antagonist. Bone Marrow Transplant. 2006;37(10):961–965. doi: 10.1038/sj.bmt.1705306. [DOI] [PubMed] [Google Scholar]
- 194.Ho VT, Zahrieh D, Hochberg E, et al. Safety and efficacy of denileukin diftitox in patients with steroid-refractory acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Blood. 2004;104(4):1224–1226. doi: 10.1182/blood-2004-01-0028. [DOI] [PubMed] [Google Scholar]
- 195.Shaughnessy PJ, Bachier C, Grimley M, et al. Denileukin diftitox for the treatment of steroid-resistant acute graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11(3):188–193. doi: 10.1016/j.bbmt.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 196.Busca A, Locatelli F, Marmont F, Ceretto C, Falda M. Recombinant human soluble tumor necrosis factor receptor fusion protein as treatment for steroid refractory graft-versus-host disease following allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2007;82(1):45–52. doi: 10.1002/ajh.20752. [DOI] [PubMed] [Google Scholar]
- 197.Wolff D, Roessler V, Steiner B, et al. Treatment of steroid-resistant acute graft-versus-host disease with daclizumab and etanercept. Bone Marrow Transplant. 2005;35(10):1003–1010. doi: 10.1038/sj.bmt.1704929. [DOI] [PubMed] [Google Scholar]
- 198.Kennedy GA, Butler J, Western R, et al. Combination antithymocyte globulin and soluble TNFα inhibitor (etanercept) +/- mycophenolate mofetil for treatment of steroid refractory acute graft-versus-host disease. Bone Marrow Transplant. 2006;37(12):1143–1147. doi: 10.1038/sj.bmt.1705380. [DOI] [PubMed] [Google Scholar]
- 199.Kobbe G, Schneider P, Rohr U, et al. Treatment of severe steroid refractory acute graft-versus-host disease with infliximab, a chimeric human/mouse antiTNFα antibody. Bone Marrow Transplant. 2001;28(1):47–49. doi: 10.1038/sj.bmt.1703094. [DOI] [PubMed] [Google Scholar]
- 200.Jacobsohn DA, Hallick J, Anders V, et al. Infliximab for steroid-refractory acute GVHD: a case series. Am J Hematol. 2003;74(2):119–124. doi: 10.1002/ajh.10392. [DOI] [PubMed] [Google Scholar]
- 201.Patriarca F, Sperotto A, Damiani D, et al. Infliximab treatment for steroid-refractory acute graft-versus-host disease. Haematologica. 2004;89(11):1352–1359. [PubMed] [Google Scholar]
- 202.Marty FM, Lee SJ, Fahey MM, et al. Infliximab use in patients with severe graft-versus-host disease and other emerging risk factors of non-Candida invasive fungal infections in allogeneic hematopoietic stem cell transplant recipients: a cohort study. Blood. 2003;102(8):2768–2776. doi: 10.1182/blood-2003-01-0267. [DOI] [PubMed] [Google Scholar]
- 203.Knop S, Hebart H, Gscheidle H, et al. OKT3 muromonab as second-line and subsequent treatment in recipients of stem cell allografts with steroid-resistant acute graft-versus-host disease. Bone Marrow Transplant. 2005;36(9):831–837. doi: 10.1038/sj.bmt.1705132. [DOI] [PubMed] [Google Scholar]
- 204.Knop S, Hebart H, Gratwohl A, et al. Treatment of steroid-resistant acute GVHD with OKT3 and high-dose steroids results in better disease control and lower incidence of infectious complications when compared with high-dose steroids alone: a randomized multicenter trial by the EBMT Chronic Leukemia Working Party. Leukemia. 2007;21(8):1830–1833. doi: 10.1038/sj.leu.2404731. [DOI] [PubMed] [Google Scholar]
- 205.Carpenter PA, Appelbaum FR, Corey L, et al. A humanized non-FcR-binding anti-CD3 antibody, visilizumab, for treatment of steroid-refractory acute graft-versus-host disease. Blood. 2002;99(8):2712–2719. doi: 10.1182/blood.v99.8.2712. [DOI] [PubMed] [Google Scholar]
- 206.Carpenter PA, Lowder J, Johnston L, et al. A Phase II multicenter study of visilizumab, humanized anti-CD3 antibody, to treat steroid-refractory acute graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11(6):465–471. doi: 10.1016/j.bbmt.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 207.Deeg HJ, Blazar BR, Bolwell BJ, et al. Treatment of steroid-refractory acute graft-versus-host disease with anti-CD147 monoclonal antibody ABX-CBL. Blood. 2001;98(7):2052–2058. doi: 10.1182/blood.v98.7.2052. [DOI] [PubMed] [Google Scholar]
- 208.Macmillan ML, Couriel D, Weisdorf DJ, et al. A Phase 2/3 multicenter randomized clinical trial of ABX-CBL versus ATG as secondary therapy for steroid-resistant acute graft-versus-host disease. Blood. 2007;109(6):2657–2662. doi: 10.1182/blood-2006-08-013995. [DOI] [PubMed] [Google Scholar]
- 209.Varadi G, Or R, Slavin S, Nagler A. In vivo CAMPATH-1 monoclonal antibodies: a novel mode of therapy for acute graft-versus-host disease. Am J Hematol. 1996;52(3):236–237. doi: 10.1002/(SICI)1096-8652(199607)52:3<236::AID-AJH23>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 210.Carella AM, Beltrami G, Scalzulli PR, Carella AM, Jr, Corsetti MT. Alemtuzumab can successfully treat steroid-refractory acute graft-versus-host disease (aGVHD) Bone Marrow Transplant. 2004;33(1):131–132. doi: 10.1038/sj.bmt.1704322. [DOI] [PubMed] [Google Scholar]
- 211.Busca A, Locatelli F, Lovisone E, et al. Treatment of severe refractory acute graft-versus-host disease of the gastrointestinal tract with Campath-1H. Biol Blood Marrow Transplant. 2005;11(9):734–736. doi: 10.1016/j.bbmt.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 212.Chakrabarti S, Mackinnon S, Chopra R, et al. High incidence of cytomegalovirus infection after nonmyeloablative stem cell transplantation: potential role of Campath-1H in delaying immune reconstitution. Blood. 2002;99(12):4357–4363. doi: 10.1182/blood.v99.12.4357. [DOI] [PubMed] [Google Scholar]
- 213.Shapira MY, Resnick IB, Bitan M, et al. Rapid response to alefacept given to patients with steroid resistant or steroid dependent acute graft-versus-host disease: a preliminary report. Bone Marrow Transplant. 2005;36(12):1097–1101. doi: 10.1038/sj.bmt.1705185. [DOI] [PubMed] [Google Scholar]
- 214.Toor AA, Stiff PJ, Nickoloff BJ, et al. Alefacept in corticosteroid refractory graft versus host disease: early results indicate promising activity. J Dermatolog Treat. 2007;18(1):13–18. doi: 10.1080/09546630601121045. [DOI] [PubMed] [Google Scholar]
- 215.Ratanatharathorn V, Carson E, Reynolds C, et al. Anti-CD20 chimeric monoclonal antibody treatment of refractory immune-mediated thrombocytopenia in a patient with chronic graft-versus-host disease. Ann Intern Med. 2000;133(4):275–279. doi: 10.7326/0003-4819-133-4-200008150-00011. [DOI] [PubMed] [Google Scholar]
- 216.Ratanatharathorn V, Ayash L, Reynolds C, et al. Treatment of chronic graft-versus-host disease with anti-CD20 chimeric monoclonal antibody. Biol Blood Marrow Transplant. 2003;9(8):505–511. doi: 10.1016/s1083-8791(03)00216-7. [DOI] [PubMed] [Google Scholar]
- 217.Canninga-van Dijk MR, van der Straaten HM, Fijnheer R, et al. Anti-CD20 monoclonal antibody treatment in 6 patients with therapy-refractory chronic graft-versus-host disease. Blood. 2004;104(8):2603–2606. doi: 10.1182/blood-2004-05-1855. [DOI] [PubMed] [Google Scholar]
- 218.Cutler C, Miklos D, Kim HT, et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006;108(2):756–762. doi: 10.1182/blood-2006-01-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Bolanos-Meade J, Jacobsohn DA, Margolis J, et al. Pentostatin in steroid-refractory acute graft-versus-host disease. J Clin Oncol. 2005;23(12):2661–2668. doi: 10.1200/JCO.2005.06.130. [DOI] [PubMed] [Google Scholar]
- 220.Jacobsohn DA, Chen AR, Zahurak M, et al. Phase II study of pentostatin in patients with corticosteroid-refractory chronic graft-versus-host disease. J Clin Oncol. 2007;25(27):4255–4261. doi: 10.1200/JCO.2007.10.8456. [DOI] [PubMed] [Google Scholar]
- 221.Basara N, Blau WI, Romer E, et al. Mycophenolate mofetil for the treatment of acute and chronic GVHD in bone marrow transplant patients. Bone Marrow Transplant. 1998;22(1):61–65. doi: 10.1038/sj.bmt.1701281. [DOI] [PubMed] [Google Scholar]
- 222.Krejci M, Doubek M, Buchler T, et al. Mycophenolate mofetil for the treatment of acute and chronic steroid-refractory graft-versus-host disease. Ann Hematol. 2005;84(10):681–685. doi: 10.1007/s00277-005-1070-0. [DOI] [PubMed] [Google Scholar]
- 223.Takami A, Mochizuki K, Okumura H, et al. Mycophenolate mofetil is effective and well tolerated in the treatment of refractory acute and chronic graft-versus-host disease. Int J Hematol. 2006;83(1):80–85. doi: 10.1532/IJH97.05111. [DOI] [PubMed] [Google Scholar]
- 224.Baudard M, Vincent A, Moreau P, et al. Mycophenolate mofetil for the treatment of acute and chronic GVHD is effective and well tolerated but induces a high risk of infectious complications: a series of 21 BM or PBSC transplant patients. Bone Marrow Transplant. 2002;30(5):287–295. doi: 10.1038/sj.bmt.1703633. [DOI] [PubMed] [Google Scholar]
- 225.Lopez F, Parker P, Nademanee A, et al. Efficacy of mycophenolate mofetil in the treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11(4):307–313. doi: 10.1016/j.bbmt.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 226.Benito AI, Furlong T, Martin PJ, et al. Sirolimus (rapamycin) for the treatment of steroid-refractory acute graft-versus-host disease. Transplantation. 2001;72(12):1924–1929. doi: 10.1097/00007890-200112270-00010. [DOI] [PubMed] [Google Scholar]
- 227.Couriel DR, Saliba R, Escalon MP, et al. Sirolimus in combination with tacrolimus and corticosteroids for the treatment of resistant chronic graft-versus-host disease. Br J Haematol. 2005;130(3):409–417. doi: 10.1111/j.1365-2141.2005.05616.x. [DOI] [PubMed] [Google Scholar]
- 228.Johnston LJ, Brown J, Shizuru JA, et al. Rapamycin (sirolimus) for treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11(1):47–55. doi: 10.1016/j.bbmt.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 229.Corral LG, Kaplan G. Immunomodulation by thalidomide and thalidomide analogues. Ann Rheum Dis. 1999;58(Suppl 1):I107–I113. doi: 10.1136/ard.58.2008.i107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Corral LG, Muller GW, Moreira AL, et al. Selection of novel analogs of thalidomide with enhanced tumor necrosis factor alpha inhibitory activity. Mol Med. 1996;2(4):506–515. [PMC free article] [PubMed] [Google Scholar]
- 231.Corral LG, Haslett PA, Muller GW, et al. Differential cytokine modulation and T-cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-α. J Immunol. 1999;163(1):380–386. [PubMed] [Google Scholar]
- 232.Geitz H, Handt S, Zwingenberger K. Thalidomide selectively modulates the density of cell surface molecules involved in the adhesion cascade. Immunopharmacology. 1996;31(2–3):213–221. doi: 10.1016/0162-3109(95)00050-x. [DOI] [PubMed] [Google Scholar]
- 233.Greinix HT, Volc-Platzer B, Kalhs P, et al. Extracorporeal photochemotherapy in the treatment of severe steroid-refractory acute graft-versus-host disease: a pilot study. Blood. 2000;96(7):2426–2431. [PubMed] [Google Scholar]
- 234.Greinix HT, Knobler RM, Worel N, et al. The effect of intensified extracorporeal photochemotherapy on long-term survival in patients with severe acute graft-versus-host disease. Haematologica. 2006;91(3):405–408. [PubMed] [Google Scholar]
- 235.Greinix HT, Volc-Platzer B, Rabitsch W, et al. Successful use of extracorporeal photochemotherapy in the treatment of severe acute and chronic graft-versus-host disease. Blood. 1998;92(9):3098–3104. [PubMed] [Google Scholar]
- 236.Apisarnthanarax N, Donato M, Korbling M, et al. Extracorporeal photopheresis therapy in the management of steroid-refractory or steroid-dependent cutaneous chronic graft-versus-host disease after allogeneic stem cell transplantation: feasibility and results. Bone Marrow Transplant. 2003;31(6):459–465. doi: 10.1038/sj.bmt.1703871. [DOI] [PubMed] [Google Scholar]
- 237.Ilhan O, Arat M, Arslan O, et al. Extracorporeal photoimmunotherapy for the treatment of steroid refractory progressive chronic graft-versus-host disease. Transfus Apher Sci. 2004;30(3):185–187. doi: 10.1016/j.transci.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 238.Couriel DR, Hosing C, Saliba R, et al. Extracorporeal photochemotherapy for the treatment of steroid-resistant chronic GVHD. Blood. 2006;107(8):3074–3080. doi: 10.1182/blood-2005-09-3907. [DOI] [PubMed] [Google Scholar]
- 239.Foss FM, DiVenuti GM, Chin K, et al. Prospective study of extracorporeal photopheresis in steroid-refractory or steroid-resistant extensive chronic graft-versus-host disease: analysis of response and survival incorporating prognostic factors. Bone Marrow Transplant. 2005;35(12):1187–1193. doi: 10.1038/sj.bmt.1704984. [DOI] [PubMed] [Google Scholar]
- 240.Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30(1):42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 241.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57(1):11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]; • Mesenchymal stem cells appear to be a promising form of cellular therapy for acute GVHD. Much is left to be determined about this novel approach.
- 242.Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363(9419):1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 243.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371(9624):1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 244.Ning H, Yang F, Jiang M, et al. The correlation between cotransplantation of mesenchymal stem cells and higher recurrence rate in hematologic malignancy patients: outcome of a pilot clinical study. Leukemia. 2008;22(3):593–599. doi: 10.1038/sj.leu.2405090. [DOI] [PubMed] [Google Scholar]
- 245.Reddy P, Ferrara JL. Pathophysiology of acute graft-versus-host disease. In: Soiffer RJ, editor. Stem Cell Transplantation for Hematologic Malignancies. 2nd. Humana Press; Totowa NJ, USA: In Press. [Google Scholar]


