Abstract
Epilepsy is one of the most common neurological disorders. Although epilepsy can be idiopathic, it is estimated that up to 50% of all epilepsy cases are initiated by neurological insults and are called acquired epilepsy (AE). AE develops in 3 phases: (1) the injury (central nervous system [CNS] insult), (2) epileptogenesis (latency), and (3) the chronic epileptic (spontaneous recurrent seizure) phases. Status epilepticus (SE), stroke, and traumatic brain injury (TBI) are 3 major examples of common brain injuries that can lead to the development of AE. It is especially important to understand the molecular mechanisms that cause AE because it may lead to innovative strategies to prevent or cure this common condition. Recent studies have offered new insights into the cause of AE and indicate that injury-induced alterations in intracellular calcium concentration levels [Ca2+]i and calcium homeostatic mechanisms play a role in the development and maintenance of AE. The injuries that cause AE are different, but they share a common molecular mechanism for producing brain damage—an increase in extracellular glutamate concentration that causes increased intracellular neuronal calcium, leading to neuronal injury and/or death. Neurons that survive the injury induced by glutamate and are exposed to increased [Ca2+]i are the cellular substrates to develop epilepsy because dead cells do not seize. The neurons that survive injury sustain permanent long-term plasticity changes in [Ca2+]i and calcium homeostatic mechanisms that are permanent and are a prominent feature of the epileptic phenotype. In the last several years, evidence has accumulated indicating that the prolonged alteration in neuronal calcium dynamics plays an important role in the induction and maintenance of the prolonged neuroplasticity changes underlying the epileptic phenotype. Understanding the role of calcium as a second messenger in the induction and maintenance of epilepsy may provide novel insights into therapeutic advances that will prevent and even cure AE.
Keywords: AE, Epileptogenesis, Calcium, Glutamate, Brain injury, Status epilepticus, Stroke, Traumatic brain injury, TBI
1. Introduction
1.1. Epilepsy
Epilepsy is a neurological disorder characterized by recurrent, unprovoked seizures. A seizure is the symptomatic, behavioral manifestation of abnormal, disordered, spontaneous, and synchronized, high-frequency firing of populations of neurons in the central nervous system (CNS; Lothman et al., 1991; McNamara, 1994, 1999). The overt behavioral signs and symptoms associated with a seizure are, in large part, attributed to the normal function of the involved neurons; therefore, seizure expression can be diverse (Hauser & Hesdorffer, 1990). Epilepsy can also vary in age of onset, cause, seizure type, and pattern of the electroencephalogram (DeLorenzo, 1989, 1991). This diversity of expression has led to the standard classification of epilepsy and the numerous different epilepsy syndromes (Frazen, 2000). Although epilepsy can manifest itself in a number of different ways, each type of epilepsy shares the common feature of persistently increased neuronal excitability that manifests sporadically as seizure generation (Lothman et al., 1991; McNamara, 1994, 1999).
Epilepsy is a common condition affecting ~1–2% of the population worldwide (Hauser & Hesdorffer, 1990; McNamara, 1999). Studies from the United States, Europe, China, and Africa report prevalence rates between 5 and 8 per 1000 (Dowzendo & Zielinski, 1971; Hauser & Kurland, 1975; Li et al., 1985; Haerer et al., 1986; Osuntokun et al., 1987; Hauser & Hesdorffer, 1990). The incidence of epilepsy is slighter higher in men than in women (Annegers, 1993) and appears to be higher in African-Americans than in Caucasians (Haerer et al., 1986). With exclusion of all other known factors, age alone constitutes a risk for epilepsy with a magnitude of 1.3 for every decade of life over the age of 30 (Ng et al., 1993). Interestingly, this age dependence of the incidence of epilepsy has shifted over recent years. Whereas the majority of epilepsies were once manifested in childhood and adolescence, today, the incidence is higher in persons over the age of 65 than during the first 2 decades of life (Kramer, 2001). In fact, epilepsy is the third most frequent neurological disorder encountered in the elderly after cerebrovascular disease and dementia (Kramer, 2001). In addition to epilepsy, population-based epidemiological studies demonstrate that status epilepticus (SE), a severe form of seizures, has a much greater incidence than previously reported and like epilepsy, also manifests the highest incidence in childhood and in the elderly, and is often present as the first seizure type in the development of epilepsy (DeLorenzo et al., 1995, 1996; DeLorenzo, 1997).
Epilepsy impacts society on multiple levels. From an economic standpoint, the total annual cost of epilepsy is estimated at nearly US$4 billion in direct medical expenses combined with indirect expenses such as lost wages, cost of home care, and premature death (Murray et al., 1996). The cost of SE is even higher (Penberthy et al., in press). Although advances have been made in the development of new anticonvulsant drugs and the surgical treatment of epilepsy, ~50% of epilepsy cases remain refractory to medical interventions and epilepsy greatly burdens the quality of life of 1–2 million Americans (Hauser & Hesdorffer, 1990). In daily life, both refractory epileptic patients, as well as epileptic patients dealing with constant management issues of treatment, can suffer from limitations in mental and physical functions, difficulties in employment status of both the individual and the family caregivers and altered interpersonal relationships at work, home, and school (Cramer et al., 1999; Buelow, 2001). Thus, the stigma associated with epilepsy, as well as functional disabilities of the disease, can greatly diminish the quality of life of persons with epilepsy (Beghi et al., 2004; Benavente-Aguilar et al., 2004; McEwan et al., 2004).
1.2. Idiopathic and acquired epilepsy
Although a significant number of epilepsy cases are idiopathic, it is estimated that up to 50% of epilepsy cases are associated with a previous neurological insult and are called acquired epilepsy (AE; DeLorenzo, 1989, 1991; Hauser & Hesdorffer, 1990). The other 50% of epilepsy cases occur in the absence of other brain abnormalities (Frazen, 2000). These epilepsies are called idiopathic, in that there is no known cause for the manifestation of epilepsy. Ongoing research in the field of medical genetics has led to the recent elucidation of an underlying cause for some of these idiopathic cases with the identification of cell migration abnormalities (Copp & Harding, 1999; Rakic, 2000; Lee et al., 2001; Haas et al., 2002; Sato et al., 2003) and numerous gene mutations in humans (Bertrand et al., 1998; Biervert et al., 1998; Wallace et al., 1998) and mouse models of epilepsy (Puranam & McNamara, 1999) that may underlie some of these idiopathic epilepsies. However, in the majority of idiopathic cases, the underlying cause of the epileptic phenotype is still not known.
In the remaining half of epilepsy cases, a known cause or injury produces a permanent plasticity change in a previously normal brain leading to the development of AE (Hauser & Hesdorffer, 1990; Lothman et al., 1991; McNamara, 1999). This transformation of healthy CNS tissue with a functional balance between excitation and inhibition to brain tissue having a hyperexcitable neuronal population of neurons is called epileptogenesis (Lothman et al., 1991; McNamara, 1999; DeLorenzo, 2004). Although genetic determinants may increase the risk that an insult to, or abnormality of, the CNS would trigger epileptogenesis (McNamara, 1999), this review will focus on the cellular and molecular events initiated by injury that culminate in AE. CNS injury is the major cause of AE (Hauser & Hesdorffer, 1990). A thorough understanding of the signaling cascades associated with the development of epileptogenesis and maintenance of the chronic epileptic state are required for understanding the development of AE and for developing novel interventional protocols to prevent or even cure AE (Stables et al., 2002). This review will focus on the basic mechanisms underlying injury-induced AE and the evidence that Ca2+ is a major second messenger that may play an important role in the development and maintenance of AE.
1.3. The role of Ca2+ in the induction and maintenance of acquired epilepsy
SE, stroke, and traumatic brain injury (TBI) are the 3 major examples of common brain injuries that can lead to the development of AE. These injuries, despite differences in the inciting event, share a common molecular mechanism for producing brain damage: an increase in extracellular glutamate concentration that has been associated with neuronal death and brain damage (Choi, 1988; Michaels & Rothman, 1990; Tymianski, 1996). The mechanisms of glutamate excitotoxicity have been well characterized and shown to associate excessive stimulation of glutamate receptors and a concomitant overwhelming increase in free intracellular calcium concentration levels ([Ca2+]i) with over stimulation of Ca2+ signaling pathways leading to neuronal death (Choi, 1988).
Calcium is a major signaling molecule in neurons, and as such, neuronal free [Ca2+]i is highly regulated. Brief, controlled elevations in Ca2+ occur during physiological processes such as neurotransmitter release and the plasticity changes of long-term potentiation in learning and memory (Malenka & Nicoll, 1999; Gnegy, 2000; West et al., 2001; Tzounopoulos & Stackman, 2003). In contrast, overwhelming, irreversible elevations in [Ca2+]i, as observed in glutamate excitotoxicity, have been implicated in mechanisms of delayed neuronal death secondary to SE, stroke, and TBI. The Ca2+ hypothesis of epileptogenesis postulates that the pathophysiological effects of Ca2+ on neuronal function may lie on a continuum with one extreme characterized by brief, controlled Ca2+ loads of normal function, another extreme characterized by irreversible Ca2+ loads and neuronal death, and a middle ground that is characterized by sublethal, prolonged, but reversible, elevations in [Ca2+]i that trigger pathological plasticity changes, leading to the development of epilepsy and the persistent elevations in [Ca2+]i that are associated with the epileptic phenotype play a role in maintaining chronic epilepsy. Thus, after a CNS injury, neurons via several mechanisms undergo elevations in [Ca2+]i. If the injury is sufficiently severe, the Ca2+ overload becomes irreversible leading to neuronal death. However, a less severe, epileptogenic CNS injury can lead to prolonged elevations in [Ca2+]i that are eventually buffered by neurons. The Ca2+ hypothesis of epileptogenesis proposes that these surviving neurons in the face of extended Ca2+ exposure undergo plasticity changes leading to epilepsy.
After a CNS insult, in the context of a relatively complicated set of variables including injury severity, anatomic location, physiological redundancy, and genetics, the complete spectrum of Ca2+ changes can occur at one time in different areas of the injured brain. Inherent to the Ca2+ hypothesis of epileptogenesis is the relatively, simple conception that dead neurons do not seize. Thus, neurons that survive a CNS injury are the potential substrate for the development of epilepsy. The purpose of this article is to review and summarize the evidence that long-term alterations in neuronal Ca2+ function in neurons that survive a brain insult underlie both the development and maintenance of the epileptic condition.
Furthermore, targeting alterations in Ca2+ homeostatic mechanisms induced in the development of AE may offer novel strategies for the development on new anticonvulsant, antiepileptogenic, and possibly agents that may cure AE (DeLorenzo, in press). Thus, understanding the role of Ca2+ in the development and maintenance of AE may offer important clinically relevant strategies to prevent or possibly cure this common neurological condition.
2. Acquired epilepsy and epileptogenesis— central nervous system insults lead to acquired epilepsy
2.1. Central nervous system injuries that produce acquired epilepsy
Epileptogenesis can be initiated by a number of types of brain lesions (Herman, 2002) and these numerous etiologies vary with age (Anderson et al., 1999). Illness in the form of tumors, infections, and degenerative diseases all increase the incidence of AE (Annegers, 1993). Developmental deficits, such as cerebral palsy, are the major risk factor for epileptogenesis in children and account for 18% of all AE cases (Hauser et al., 1991). The 3 major injuries to the brain produce the majority of AE and include SE, stroke, and TBI. SE is a common epileptogenic brain injury that has been extensively studied and shown to be a common cause of AE (Lothman & Bertram, 1993; DeLorenzo et al., 1995, 1996). Cerebral ischemia, or stroke, is also a common cause of AE accounting for ~40% of all AE cases (Hauser et al., 1991). In fact, up to 25% of stroke patients develop epilepsy (Witte & Freund, 1999). Stroke is the major risk factor for epilepsy in persons over the age of 45 (Hauser & Kurland, 1975). TBI, the major risk factor in young adults, is responsible for 13% of acquired epilepsies (Hauser & Hesdorffer, 1990; Hauser et al., 1991). Thus, brain injury is the major cause of AE and SE; stroke and TBI are the major common injuries that need to be studied to develop potential methods of prevention and cures for AE.
2.2. Three phases in the development of acquired epilepsy
Although epileptogenic CNS insults are sometimes associated with seizures in the acute setting, more often, a latent period exists before the manifestation of spontaneous recurrent seizures of epilepsy (Pitkanen, 2002; Pitkanen & Sutula, 2002). It is during this “silent” or latency period that epileptogenesis occurs. Traumatized, but surviving, neurons manifest the diverse neuronal plasticity changes in anatomic, biochemical, and physiological properties that lead to hyperexcitability. The injury causes a permanent change in neuronal processes that alters neuronal function through the induction and maintenance of long-term plasticity changes that underlie the development of AE. The current understanding of the development of AE is that it goes through 3 major phases of development: (1) the injury (acute), (2) epileptogenesis (latency), and (3) chronic epilepsy (spontaneous recurrent seizures) phases (Pitkanen, 2002; Pitkanen & Sutula, 2002). A major question in neuroscience research is how can the initial injury involved in causing AE produce these long-term changes in neuronal excitability. A major research effort in this laboratory has been to attempt to address this question. Emerging research has demonstrated that Ca2+, as a major second messenger system, underlies many of these injury-induced plasticity changes associated with the development of AE (DeLorenzo et al., 1998, Rice & DeLorenzo, 1998; Pal et al., 2000, 2001; Sun et al., 2002; Sun et al., 2004).
2.3. The role of Ca2+ in the development of acquired epilepsy
Several studies have led to the development of the Ca2+ hypothesis of epileptogenesis that implicates Ca2+ as a second messenger involved in the induction and maintenance of AE (DeLorenzo et al., 1998; Rice & DeLorenzo, 1998; Pal et al., 2000, 2001; Sun et al., 2002, 2004). The Ca2+ hypothesis of epileptogenesis has been developed from these studies and suggests that: (1) During the injury phase, Ca2+ reaches high levels in neurons, but not sufficient to induce cell death; (2) During the latency phase, Ca2+ remains elevated and initiates many second messenger effects that produce long-lasting plasticity changes in these neurons; and (3) During the chronic epilepsy phase, Ca2+ remains elevated in the epileptic neurons and plays a role in maintaining the spontaneous recurrent seizures. The prolonged changes in neuronal Ca2+ dynamics play an integral signaling role in initiating and maintaining AE. Studies from our laboratory have provided direct evidence that Ca2+ dynamics are altered in all 3 phases of the development of AE in both the hippocampal neuronal culture and pilocarpine models of AE (DeLorenzo et al., 1998; Rice & DeLorenzo, 1998; Pal et al., 2000, 2001; Sun et al., 2002, 2004).
The Ca2+ hypothesis of epileptogenesis suggests a role of Ca2+ in both the induction and maintenance of AE. In this hypothesis, we have emphasized the role of increased [Ca2+]i in altering γ-amino butyric acid (GABA) receptor recycling as a possible mechanism for the effect of Ca2+ on altering neuronal excitability (Blair et al., 2004). However, there are many other effects of altered Ca2+ dynamics that effect gene transcription, protein expression and turnover, neurogenesis, neuronal sprouting, and many other physiological processes (DeLorenzo & Morris, 1999). At the present time, it is not possible to determine which of these processes are individually or collectively involved in the effects of Ca2+. In addition, it is important to emphasize that epileptogenesis is a complex process, and there may be many other second messenger systems interacting with Ca2+ or acting independently in producing and maintaining AE. However, the evidence for the role of Ca2+ in this process and the close relationship of this second messenger to injury make it a likely important regulator of epileptogenesis. Thus, understanding the role of Ca2+ in this process may offer insights into preventing or reversing AE.
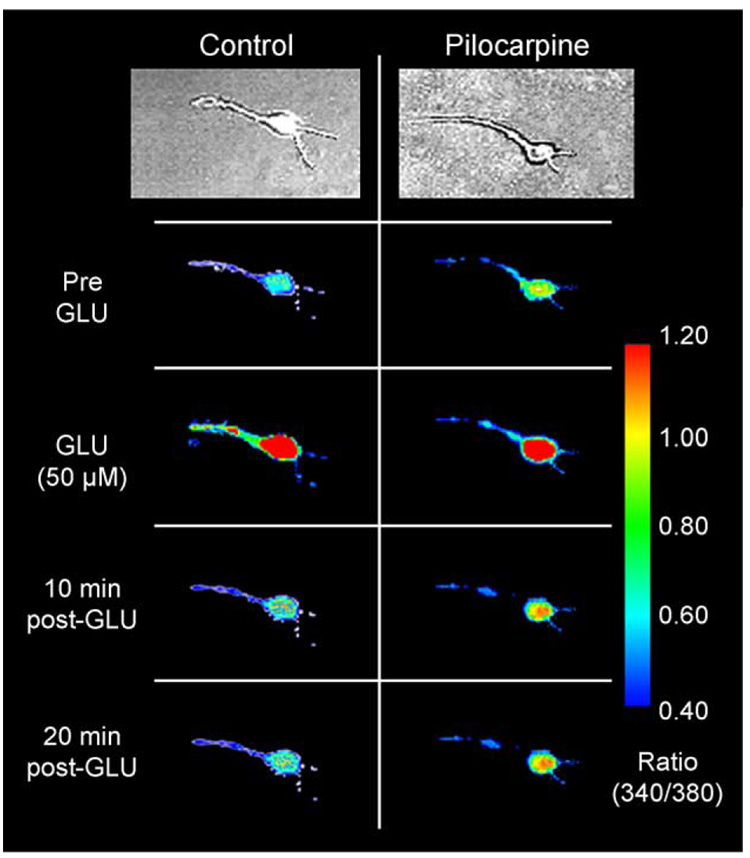
The silent period during the epileptogenesis phase represents a window of opportunity where targeted therapies could act to inhibit epileptogenesis, thereby preventing the development of AE. Recent studies have provided direct evidence in the SE- and stroke-induced hippocampal neuronal culture models of AE (Pal et al., 1999, 2000, 2001; Fig. 1 and Fig 2) and the pilocarpine model of epileptogenesis in the rat (Raza et al., 2001, 2004; Fig. 3); that [Ca2+]i is high in hippocampal CA1 pyramidal neurons during the injury phase remains significantly elevated in epileptogenesis and is chronically elevated in the chronic epilepsy phases of the development of AE. These results provide the first direct evidence that these changes in [Ca2+]i are actually altered during the injury and development of AE in both in vitro and intact animal models of AE and provide direct support for the Ca2+ hypothesis of epileptogenesis. This article will review the evidence for the role of Ca2+ in the pathophysiology of epilepsy.
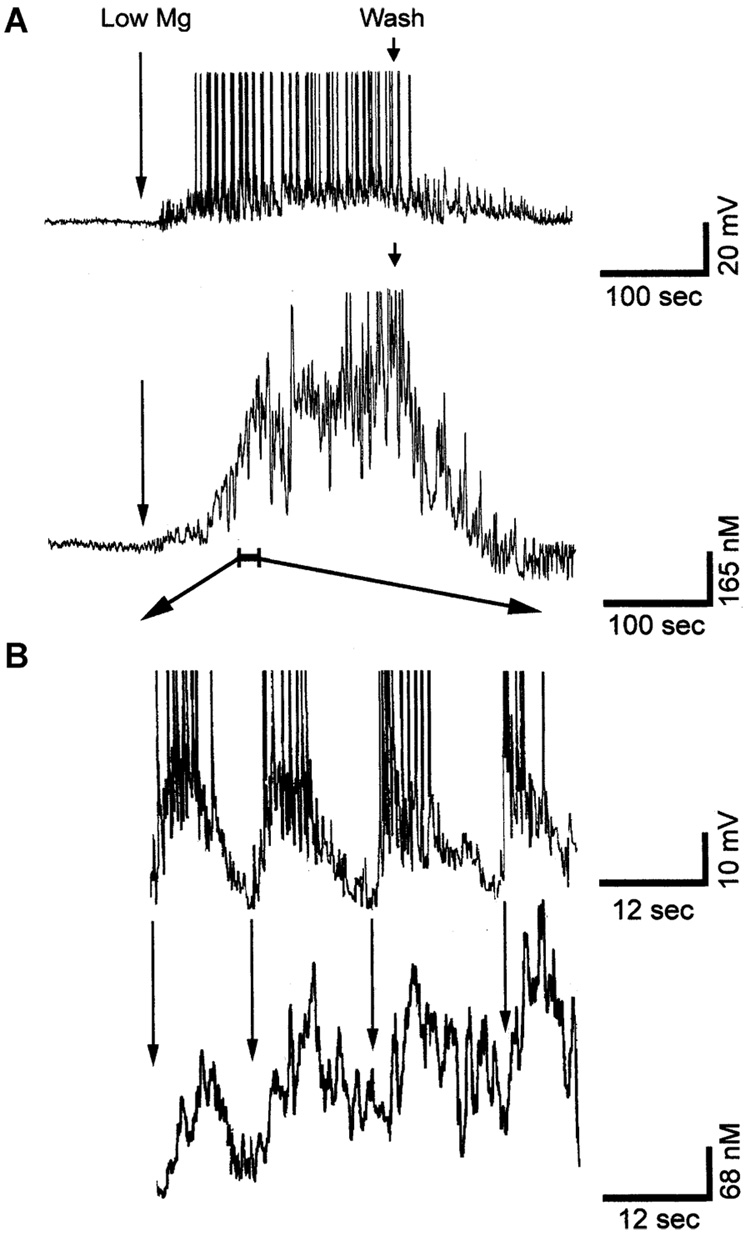
Fig. 1.
Simultaneous recording of epileptiform discharges and calcium dynamics during SE in the low-Mg2+-induced SE HNC model of AE. (A) Upper trace: a representative whole-cell current clamp recording from a neuron during SE. The neuron exhibited epileptiform-bursting activity consistent with continuous electrographic epileptiform activity. Each burst consisted of a large (30–40 mV) PDS with numerous superimposed spikes. Lower trace: simultaneous [Ca2+]i recording from the same neuron using ultra high-speed microfluorometry (5-msec resolution) demonstrating correlation between depolarization and elevations in [Ca2+]i. The [Ca2+]i level starts rising along with the subspike threshold waves of depolarization and level rises rapidly with the appearance of epileptiform bursts with numerous spikes. The comparison demonstrates that there is a direct correlation between elevated [Ca2+]i and continuous epileptiform discharges. (B) Expanded portion of a region indicated by the bar in panel A to demonstrate the relationship between each burst of action potential and the corresponding change in [Ca2+]i levels. The amplitude of spikes was truncated to emphasize the large PDS. The arrows denote the beginning of each PDS. Notice the brief time lag between the beginning of each epileptiform burst and the rise in calcium wave. Following each PDS, the [Ca2+]i could not recover to baseline levels before the next PDS occurred, and thus with each additional PDS, the [Ca2+]i gradually rose to a plateau level of ~600 nM (A). (Revised from Pal et al., 1999.)
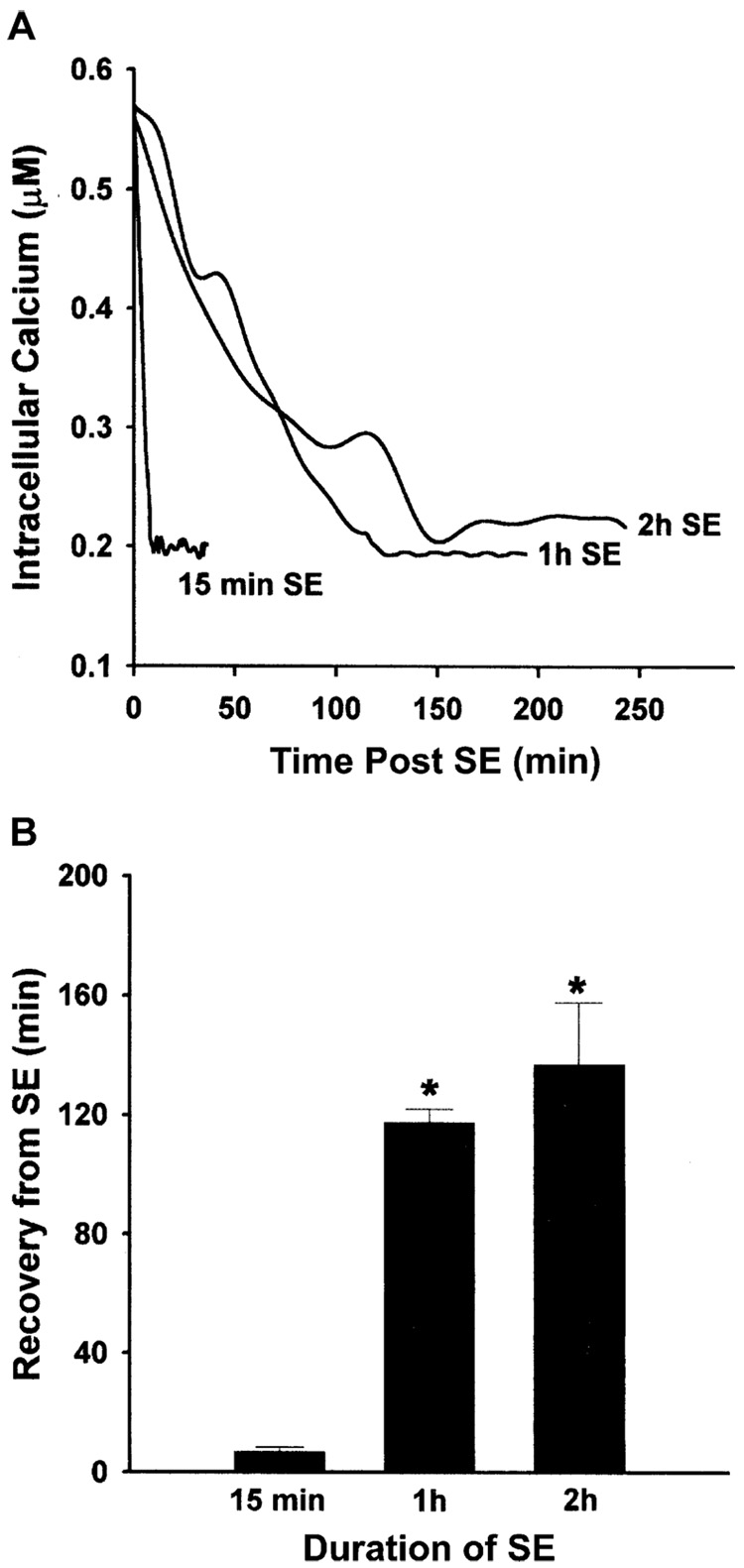
Fig. 2.
Recovery of [Ca2+]i after various durations of SE in the Mg2+-induced SE HNC model of AE. Cells were treated with low Mg2+ to produce 15 min, 1 h, and 2 h of SE. The average [Ca2+]i in representative pyramidal cells (n = 5) was measured as described in Section 2. (A) Line graph of recovery time of [Ca2+]i to basal levels from the SE durations of 15 min, 1 h, and 2 h. Each line graph represents the average [Ca2+]i at each time point for 5 cells. (B) Quantitation of [Ca2+]i recovery time after various durations of SE. The data represent the mean ± SEM for recovery time after 15 min, 1 h, and 2 h of SE. There was a significant effect of SE duration on the recovery time between 15 min and 1 and 2 h of SE (*p < 0.05, Student’s t-test with Bonferroni correction. Revised from Pal et al., 1999.)
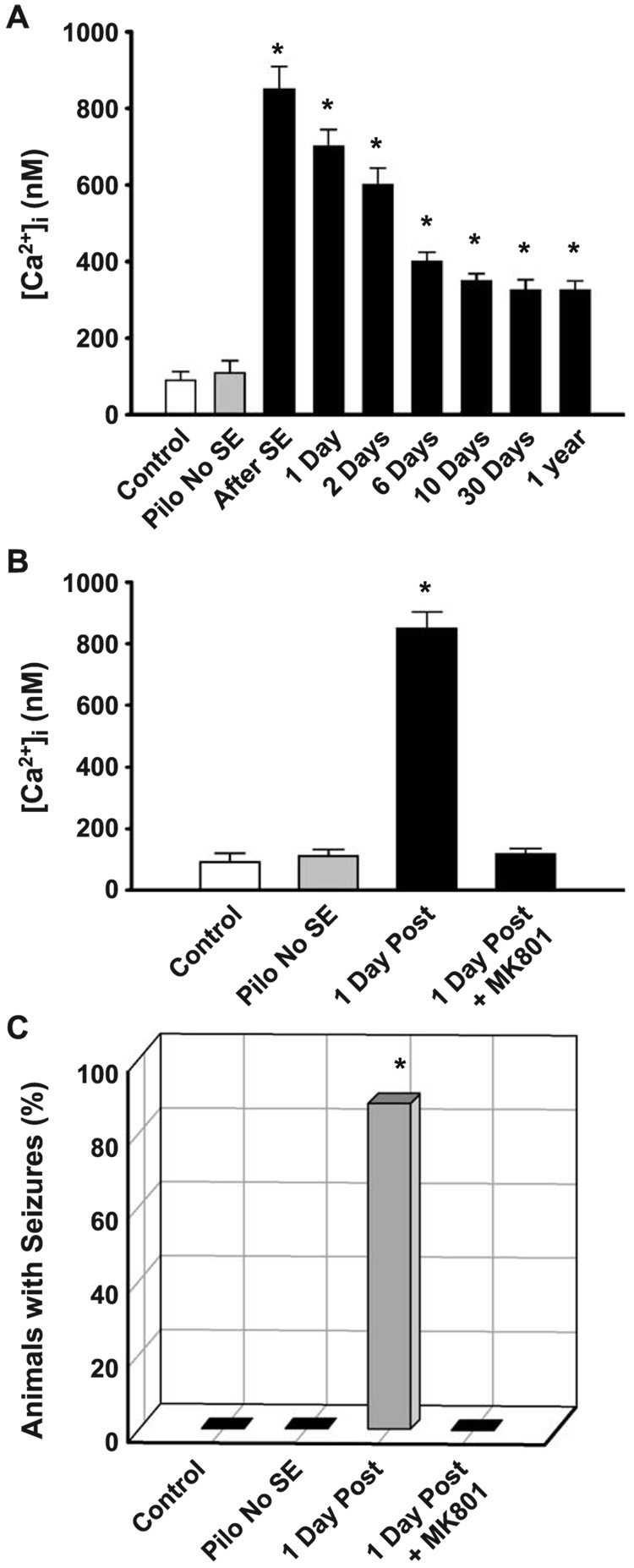
Fig. 3.
Direct evidence that increased [Ca2+]i occurs in the injury and epileptogenesis and chronic phases of AE in the pilocarpine model (A), and that inhibition of the NMDA receptor with MK801 during the injury induced by SE not only prevents the prolonged increase in [Ca2+]i (B) but also the development of AE (C). (A) Neurons were acutely isolated from hippocampal tissue during different times after the injury phase of AE. The data indicate that [Ca2+]i was significantly elevated immediately after SE and was still elevated as long as 1 year after the initial injury (*″p < 0.05). (B) The data present the mean ± SE [Ca2+]i for control (n = 35), pilo no SE (n = 21), 1 day after SE (n = 67), and 1 day after SE + MK801 (n = 29). MK801 blocked the development of increased [Ca2+]i after the SE injury (*p < 0.01, in comparison to control). (C) The data present, the percent animals that developed seizures at 60 days post-SE or control treatment in the control (n = 20), pilo no SE (n = 24), SE (n = 30), and SE + MK801 animals (n = 15). MK801 blocked the development of AE. (*p < 0.01, in comparison to control. Modified from Raza et al., 2004.)
3. Pathophysiology of epileptogenic central nervous system insults
As characterized by epidemiological studies, many types of injuries to the CNS can result in epileptogenesis (Herman, 2002). The most common causes of AE (SE, stroke, and TBI) are caused by different initial injuries, but they share a common mechanism for producing neuronal injury: the production of a pathological increase in the concentration of extracellular glutamate and an associated increase in [Ca2+]i. These injuries can result in a spectrum of brain damage: (1) in some circumstances leading to cell death with loss of neuronal and glial components and (2) in other circumstances, to injured tissue that survives. By understanding the pathophysiology of each of these injuries that induce epileptogenesis, it is possible to obtain insights into the basic mechanisms underlying the development of spontaneous recurrent seizures in previously normal brain tissue. The following discussion demonstrates that SE, stroke, and TBI all induce brain injury in part through elevations in extracellular glutamate concentrations that can then result in increased [Ca2+]i.
3.1. Status epilepticus: Causes neuronal injury and increased glutamate and Ca2+
Typically, a seizure is a short-lived event that terminates in a similarly spontaneous fashion as it started. As such, seizures do not usually cause brain damage. However, in some circumstances, seizures do not stop on their own. Seizures that progress in duration to greater than 30 min, or multiple seizures without regained consciousness, are classified as SE. The International League against Epilepsy defines SE as a seizure that “persists for a sufficient length of time or is repeated frequently enough that recovery between attacks does not occur” (DeLorenzo et al., 1995, 1996; DeLorenzo, 1997). Acute processes that cause SE include metabolic disturbances (e.g., electrolyte abnormalities, renal failure, and sepsis), CNS infection, stroke, head trauma, drug toxicity, and hypoxia. Chronic processes that cause SE include preexisting epilepsy or the discontinuation of antiepileptic drugs, chronic ethanol abuse, and remote processes such as CNS tumors or stroke (Lowenstein & Alldredge, 1998). The frequency of SE in the United States is ~102,000–152,000 per year, and roughly 55,000 deaths are associated with SE annually (DeLorenzo et al., 1995). Twelve percent to 30% of adult patients with a new diagnosis of epilepsy first present in SE. SE can be convulsive or nonconvulsive, and SE can cause neuronal damage under both situations (Fountain & Lothman, 1995; Drislane, 2000).
Prolonged seizures have been produced in numerous research models to replicate SE. Commonly, the systemic or intracerebral injections of chemical convulsants such as pilocarpine or kainic acid have been used (Ben-Ari et al., 1980; Cavalheiro et al., 1982; Turski et al., 1983). Also, prolonged stimulation of the hippocampus and other limbic regions with implanted electrodes has been used to produce self-sustaining SE (McIntyre et al., 1982; Brandt et al., 2003). With each protocol, animals acutely demonstrate behavioral and electrographic activity consistent with clinical SE and, if allowed to continue for a sufficient duration, develop some hippocampal neuronal damage that is also observed in humans. After sustained pilocarpine-induced SE, animals exhibit neuronal loss and damage to hippocampal and extrahippocampal structures, including amygdaloid nuclei, piriform cortex, entorhinal cortex, and other limbic sites, as well as the dorsomedial thalamic nucleus (Turski et al., 1984) and hippocampal synaptic reorganization in the form of mossy fiber sprouting (Mello et al., 1993). Kainic acid-treated rats demonstrate loss of CA3 pyramidal neurons and hilar interneurons of the dentate gyrus, and mossy fiber sprouting (de Lanerolle et al., 1989; Mathern et al., 1993; Mathern et al., 1995). However, controlling the duration of SE in the pilocarpine model to 1 hr of SE produces minimal neuronal loss that is limited to 10% of the CA1 neurons (Rice et al., 1996).
Neuronal injury secondary to SE is the consequence of excessive neuronal excitability with high-frequency discharges causing depolarizing shifts of neuronal membrane potential, free radical production secondary to an exhaustive metabolic load, and significant elevations in extracellular glutamate (Wasterlain et al., 1993; Heinemann et al., 2002a, 2002b). These variables all combine in a positive feed-forward cycle resulting in increased levels of intracellular Ca2+ that induce neuronal injury and death. Levels of extracellular glutamate have been shown to increase during seizures and SE (Sherwin, 1999). Using microdialysis in humans prior to neurological surgery, a clear association between potentially neurotoxic levels of glutamate and seizure progression has been demonstrated (During & Spencer, 1993; Wilson et al., 1996). Multiple studies in animals have similarly demonstrated an elevation in extracellular glutamate during SE (Liu et al., 1997; Smolders et al., 1997; Pena & Tapia, 1999; Ueda et al., 2002). This increase in glutamate has been implicated in the neuronal death observed after SE in these models, as antagonism of specific glutamate receptors during SE has shown to be neuroprotective (Meldrum, 1997; Rice & DeLorenzo, 1998; Hort et al., 1999; Ebert et al., 2002).
Thus, it is important to evaluate the effects of SE on [Ca2+]i. Using the low-magnesium (Mg2+) model of SE in vitro, it was possible to provide the first direct evidence that SE causes acute and prolonged increases in [Ca2+]i (Fig. 1 and Fig 2; Pal et al., 1999). These studies simultaneously measured in real time both [Ca2+]i and electrophysiological parameters to directly demonstrate the correlation between increased [Ca2+]i and seizure activity. In addition, this study also demonstrated that prolonged seizure activity causes prolonged elevations in [Ca2+]i levels that persisted for hours after the seizure activity stopped. Thus, this data provided a direct demonstration for the initial aspect of the Ca2+ hypothesis of epileptogenesis and showed that SE causes prolonged increases in [Ca2+]i in neurons that survived the SE and developed spontaneous recurrent seizures (epilepsy) in vitro. Using the intact pilocarpine model of AE, it was also possible using the acute neuronal isolation technique and Ca2+-imaging technology developed in this laboratory to demonstrate that SE caused acute and persistent increases in [Ca2+]i in hippocampal neurons in an intact model of AE (Raza et al., 2001, 2004; Fig. 3). Thus, it has been demonstrated that SE causes injury to neurons by increasing glutamate and by producing prolonged alterations in [Ca2+]i that underlie the basis for Ca2+ second messenger effects on developing neuronal plasticity in the surviving neurons.
3.2. Stroke: Causes neuronal injury and increased glutamate and Ca2+
Stroke refers to the brain damage caused by transient or permanent reduction of cerebral blood flow to a region of the CNS (Sharp et al., 1998). Stroke is the third leading cause of death in the United States (Wolf & D’Agostino, 1998), and 4 million survivors are coping with the debilitating consequences of this brain injury including epileptics (Taylor et al., 1996). As defined by the National Institutes of Neurological Disorders and Stroke, “stroke” includes the clinical sequelae of cerebral infarction, intra-cerebral hemorrhage, and subarachnoid hemorrhage (Vaughan & Bullock, 1999). Hemorrhagic strokes account for 15% of all strokes. Decreased cerebral blood perfusion in this case occurs because of bleeding from damaged blood vessels (Vaughan & Bullock, 1999). Secondary to the hemorrhage, this type of stroke invariably includes a secondary ischemic injury, in distant regions supplied by the affected vessel (Lee et al., 1999).
By far, the vast majority of strokes, 85%, occur via either thrombotic or embolic occlusion of a cerebral blood vessel (Wolf & D’Agostino, 1998). This vessel occlusion creates a region of severe focal ischemia in the brain area supplied by the affected vessel (Wolf & D’Agostino, 1998). Eventually, neurons and glia in this core region of ischemia die, creating a pan necrotic cerebral infarction (Dietrich, 1998). Surrounding this ischemic core of irreversible damage is a region that suffers only partially reduced blood supply. Designated the ischemic penumbra (Astrup et al., 1981), this brain tissue receives sufficient blood flow from collateral cerebral vasculature to prevent the development of a pan necrotic infarct core, but still undergoes a less severe, prolonged, transient ischemia (Vaughan & Bullock, 1999).
The mechanisms underlying ischemic brain injury are complex and diverse. Initially, the decreased flow of oxygenated blood to CNS tissue leads to impairment of the electron transport chain and decreased levels of the high-energy substrates, adenosine triphosphate (ATP) and phosphocreatine (Lipton, 1999). The depletion of high-energy substrates causes failure of the many active transport systems in neurons and glia. Inhibition of the sodium (Na+)/ potassium (K+) ATPase and the subsequent loss of exchangers dependent on normal Na+ and K+ gradients results in the loss of ionic homeostasis and membrane depolarization. This irreversible loss of membrane potential, called the anoxic depolarization (Katsura et al., 1994), is coupled to voltage-gated Ca2+ influx and the massive release of neurotransmitters including glutamate, GABA, and acetylcholine (Lipton, 1999).
Ischemia-induced elevations in extracellular glutamate concentration have been measured in numerous studies in humans (Bullock et al., 1995; Davalos et al., 1997) and animals (Benveniste et al., 1984; Globus et al., 1988; Takagi et al., 1993). In the ischemic core, extracellular glutamate has been estimated to increase from a physiological level of 5 µM to values ranging from 100 µM to 10 mM (Graham et al., 1990; Wahl et al., 1994; Rusakov & Kullmann, 1998), which leads to excessive glutamate receptor activation. Excessive activation of glutamate receptors further enhances the anoxic depolarization and Ca2+ influx. In combination with the loss of energy-dependent Ca2+ sequestration and extrusion mechanisms, Ca2+ influx leads to the irreversible overload of [Ca2+]i that initiates numerous cascades leading to neuronal cell death in the ischemic core (Kristian & Siesjo, 1998). Measurements of these ischemia-induced elevations of [Ca2+]i have been documented in the rat ranging from normal levels, below 200 nM, to as high as 30 µM (Lipton, 1999).
These pathophysiological events are dramatic and irreversible in the ischemic core causing all of the cells to die. However, these changes are much less severe in the ischemic penumbra (Lipton, 1999). Extracellular glutamate concentrations in penumbral tissue are elevated to a lesser extent than in the core (Wang et al., 2001), estimated at 30– 50 µM in different studies (Shimada et al., 1990; Wahl et al., 1994; Morimoto et al., 1996).
Because of the collateral blood supply to the penumbra, oxygenated blood flow is only partially reduced and energy metabolism is preserved (Hossmann, 1994). Therefore, this brain tissue does not undergo the irreversible anoxic depolarization of the ischemic core. Instead, the ischemic penumbra undergoes transient, episodic waves of depolarization, called ischemic depolarizations (Hossmann, 1996), that are mediated in part by episodic energy failure and in part by glutamate receptor activation (Gill et al., 1992; Dirnagl et al., 1999). In fact, antagonists of different glutamate receptor subtypes block ischemic depolarizations (Mies et al., 1993; Back et al., 1996; Dijkhuizen et al., 1999; Dirnagl et al., 1999).
Similar to the anoxic depolarization of the ischemic core, ischemic depolarizations of the penumbra are associated with increases in [Ca2+]i (Gill et al., 1992; Kristian & Siesjo, 1998). However, these [Ca2+]i elevations, such as ischemic depolarizations, are transient and reversible (Kristian & Siesjo, 1998). Inhibition of ischemic depolarizations by antagonism of the N-methyl-d-aspartic acid (NMDA) receptor has been shown to inhibit these changes in [Ca2+]i and reduce the infarct volume (Gill et al., 1992). Since ischemic depolarizations contribute to the extent of brain damage in the penumbra, the activation of glutamate receptors associated with the depolarization is critically related to the pathophysiology of ischemia.
Studies from this laboratory have developed an in vitro model of glutamate injury or stroke that causes a situation in cultured neurons that is similar to the penumbra effect in stroke (Sun et al., 2001). This model employed a concentration and glutamate exposure time that caused some of the neurons to die and the other neurons to undergo a reversible injured condition (Fig. 4). Fig. 4A demonstrates that glutamate exposure caused the neurons to depolarize, and Fig. 4B and C demonstrates that several of the neurons recovered from this glutamate injury as measured by membrane potential and membrane input resistance. Fig. 4D–F demonstrates that these injured neurons actually physically swell during the injury phase, but eventually recover. The ability to produce a reversible injury analogous to the core and penumbra model of stroke allowed us to utilize this new model to study the development of spontaneous recurrent seizures in injured but not killed neurons (Sun et al., 2001).
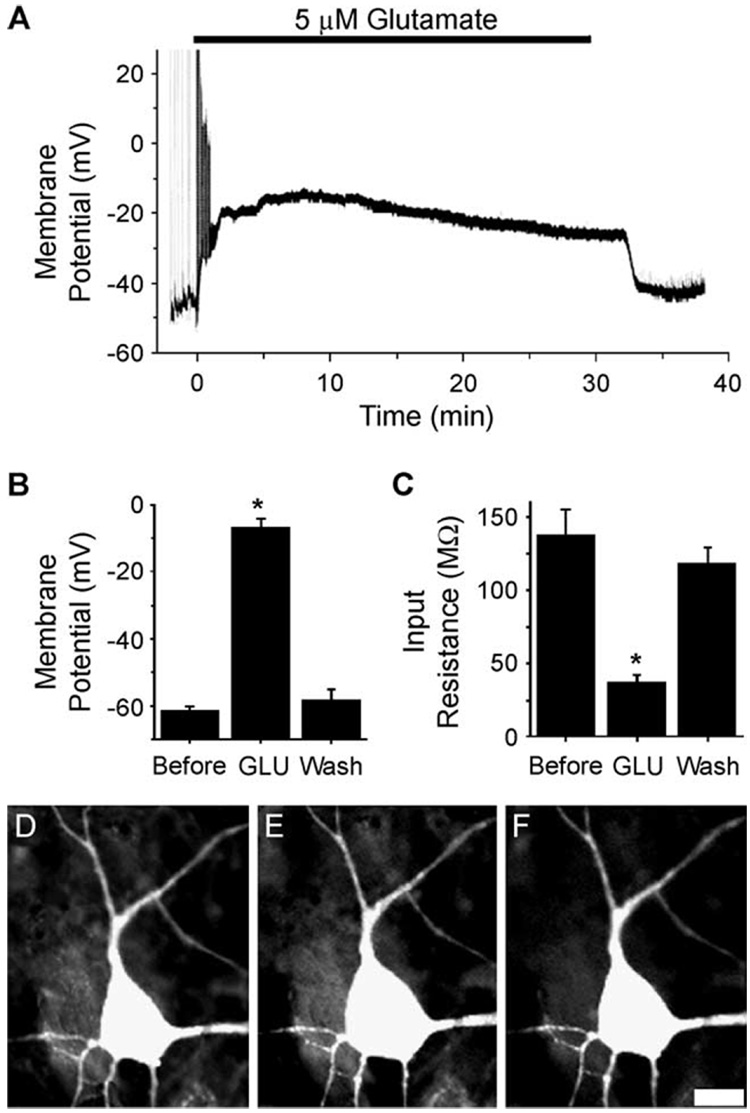
Fig. 4.
Effects of glutamate injury on hippocampal membrane potential (A and B), input resistance (C), and cell swelling (D–F). (A) Representative whole-cell current clamp recording of a hippocampal neuron before, during, and after glutamate application (5 µM, 30 min). In the presence of glutamate (black bar), this neuron depolarized from −52 to −17 mV, and synaptic potentials were lost. Upon washout, the neuron repolarized to −47 mV and EPSPs returned. (B) Effect of glutamate on neuronal membrane potential. Neuronal membrane potential before (Before, n = 12), during (GLU, n = 12), and 5 min or more after glutamate application (wash, n = 19). *p < 0.05 ANOVA, Tukey post hoc test. (C) Effect of glutamate on neuronal membrane input resistance. Neuronal membrane input resistance before (Before, n = 10), during (GLU, n = 10), and 5 min or more after glutamate application (wash, n = 10). *p < 0.05 RM ANOVA, Tukey post hoc test. Data are represented by mean ± SEM. (D–F) Glutamate-induced neuronal swelling. Digital images of a representative fluorescein-stained, hippocampal pyramidal shaped neuron before (D), during glutamate exposure (5 µM, 30 min; E), and within 1 hr of glutamate washout (F). In glutamate, this neuron swelled, increasing somatic area by 31%. The same neuron within an hour of washout restored preexposure morphology, only 4% greater than preexposure somatic area. Scale bar = 10 µM. (From Sun et al., 2001.)
3.3. Traumatic brain injury: Causes neuronal injury and increased glutamate and Ca2+
TBI is a common cause of mortality and morbidity (Ghajar, 2000). Survivors of TBI often suffer from diverse symptoms that, in addition to epilepsy, can include headaches, depression, and cognitive difficulties (Capruso & Levin, 1992; Shaw, 2002). Posttraumatic epilepsy is the most common cause of AE in young adults (Annegers, 1993). Posttraumatic epilepsy complicates up to 30% of severe head injuries (Asikainen et al., 1999). Many risk factors have been associated with the development of posttraumatic epilepsy (Angeleri et al., 1999). Penetrating injuries as seen with gun shot wounds are a well-characterized risk factor (Salazar et al., 1985; Walker, 1989). Studies of civilian populations have demonstrated strong association between TBI with brain contusion and subdural hematoma with epilepsy (Annegers & Coan, 2000). Prolonged unconsciousness and skull fractures also increase the risk for posttraumatic epilepsy (Herman, 2002). Interestingly, the occurrence of seizures during the first week after a head injury increases the probability of spontaneous recurrent seizures later on in life (Haltiner et al., 1997). Of special clinical importance, posttraumatic epilepsy is often refractory to current anticonvulsant therapeutics (Semah et al., 1998). Thus, the development of antiepileptogenic regimens is vital within the context of posttraumatic epilepsy.
The pathophysiology of TBI is a complex set of events initiated by the distribution of a mechanical load to the brain. Translation, rotational and shear stresses have all been characterized in models of head injury and areas of high stress are associated with neuronal and diffuse axonal injury (Misra & Chakravarty, 1984; Nishimoto & Murakami, 1998; Zhang et al., 2001). However, mechanical force is only the initial event in TBI. The primary injury to the brain that occurs at the moment of impact initiates a secondary or delayed injury that spreads via multiple molecular mechanisms including changes in cerebral blood flow, breakdown of blood brain barrier, local and systemic inflammation, alterations in oxygen delivery and metabolism, and both ischemic and apoptotic death of neural cells (Golding et al., 1999; Lenzlinger et al., 2001; Raghupathi, 2004). Intravascular clot formation is common in TBI and contributes to local ischemia; consumption of clotting factors can lead to systemic coagulopathy. Mussack et al. (2002) demonstrated that biochemical markers of both brain injury (S-100B) and systemic inflammation (interleukin-8) increase after TBI, and that this increase correlates with the patient’s degree of neurological dysfunction 12 months later (reviewed in Dutton & McCunn, 2003).
Similar to SE and stroke, increases in extracellular glutamate levels have been implicated in the pathophysiology of TBI. In human head injury, multiple studies have confirmed significant elevations in extracellular glutamate (Yamamoto et al., 1999; Gopinath et al., 2000). Further, these elevations have been correlated with poor outcomes 6-month postinjury (Koura et al., 1998). Posttraumatic seizures, which increase the risk of posttraumatic epilepsy, also cause glutamate surges in human patients (Vespa et al., 1998). Numerous animal models of TBI have been developed and (Laurer & McIntosh, 1999) have successfully replicated these human findings in multiple studies (Nilsson et al., 1990). Further, the mechanisms of glutamate release have been evaluated in animals of TBI. At the core of a concussive injury, extracellular glutamate release is most extreme and is likely secondary to the loss of ionic homeostasis and cellular depolarization associated with the primary mechanical disruption of neuronal and vascular element (Katayama et al., 1990). In contrast, the area surrounding the contusion core demonstrates significant but less dramatic increases in extracellular glutamate. These increases in glutamate can be inhibited by glutamate receptor antagonism, suggesting that glutamate from the core diffuses into noninjured tissue and triggers excessive presynaptic glutamate release (Maeda et al., 1998).
4. Glutamate excitotoxicity: a common mechanism underlying epileptogenic central nervous system insults that cause elevated Ca2+
Among a host of candidate mechanisms, one common thread underlying each of the epileptogenic CNS insults described above is an elevation in the extracellular concentration of glutamate and excessive activation of various subtypes of glutamate receptors. Olney and de Gubareff (1978) coined the term “excitotoxicity” to broadly describe the ability of excitatory amino acid neurotransmitters to cause neuronal death, presumably by prolonged excitation and energy depletion. Since the early associations between elevated extracellular glutamate and ischemia, a number of different in vitro brain slice and tissue culture preparations have been used extensively to study excitotoxicity (Choi, 1988; Michaels & Rothman, 1990; Tymianski, 1996). These in vitro systems offer a model of neuronal injury wherein discrete experimental variables can be studied in a controlled fashion at the level of networked neural populations down to the single neuron. Although these in vitro systems lack many pathophysiological factors of human brain including inflammation, temperature, and vasculature changes, these reductionist models manifest many of the phenomena observed in whole animal models and serve as a valuable research tool. Glutamate excitotoxicity was first studied within the context of ischemic stroke. However, as SE, stroke, and TBI are all associated with large increases in extracellular glutamate concentration, glutamate excitotoxicity and the associated increase in [Ca2+]i has become a well-accepted hypothesis of neuronal death secondary to these CNS insults. The following brief review summarizes how glutamate causes brain injury and the important role of NMDA receptors and Ca2+ in this process. It is essential to understand these mechanism in developing an insight into the causes of AE.
4.1. Mechanisms of glutamate excitotoxicity
The mechanisms of glutamate signal transduction and induction of brain injury have been extensively reviewed (Sattler & Tymianski, 2001; Aarts et al., 2003). Briefly, large increases in the concentration of the excitatory amino acid neurotransmitter glutamate causes excessive activation of glutamate receptors including the Ca2+-permeable NMDA subtype of glutamate receptors. This excessive activation leads to significant increases in [Ca2+]i, which then triggers a host of Ca2+-dependent degradative pathways leading to the neuronal death. Ca2+ is the key underlying second messenger mediating glutamate excitotoxicity (Choi, 1985, 1994; Choi et al., 1987, 1988, 1989; Choi & Rothman, 1990). Thus, understanding glutamate excitotoxicity and the role of Ca2+ in neuronal injury is essential in understanding the development of AE.
4.2. Glutamate
l-Glutamate is the most widespread amino acid in the brain and serves a number of functions in the CNS (Nicholls & Attwell, 1990). For example, this dicarboxylic amino acid is a precursor for the inhibitory amino acid neurotransmitter GABA, for the Krebs cycle intermediate α-ketoglutarate and for the amino acid glutamine. Glutamate also acts as a detoxification agent for ammonia products in the brain. In addition to the many metabolic functions of glutamate, the most significant role of glutamate in the brain is its function as the primary excitatory neurotransmitter (Mayer & Westbrook, 1987).
As a neurotransmitter, extracellular glutamate levels must be maintained at controlled levels. Under physiological conditions, extracellular glutamate has been measured in the range of 1–5 µM (Wahl et al., 1994). Although transporters exist to move glutamate into the brain across the blood-brain barrier, the vast majority of glutamate is synthesized de novo from glucose, glutamine, or aspartate (Laterra et al., 1999). Glutamate is stored in synaptic vesicles at concentrations in excess of 20 mM via a magnesium (Mg2+)/ATP-dependent transporter (Dingledine & McBain, 1999). The primary mechanism for uptake of extracellular glutamate is a class of high affinity, Na+-dependent glutamate transporters found on neurons and astrocytes. To date, 5 transporters have been characterized: glutamate-aspartate transporter (GLAST), glutamate transporter-1 (GLT-1), and excitatory amino acid carrier-1 (EAAC1), EAAC4, and EAAC5 (Arriza et al., 1997; Gegelashvili & Schousboe, 1997; Vandenberg, 1998). Astrocytes are primarily responsible for the uptake of glutamate at the synapse (Bergles & Jahr, 1998). Once inside the astrocyte, glutamate is converted to the nonpolar glutamine, which can pass freely from the astrocyte to the neuron. Within the neuron, glutamine is converted back to glutamate to replenish the transmitter pool (Pfrieger & Barres, 1996).
4.3. Glutamate receptors
The signaling actions of glutamate are mediated at the neuronal membrane through specialized receptor macromolecules. The binding of glutamate to specific sites on its receptor molecule causes a conformational change that initiates signal transduction cascades in the neuron. Glutamate receptors are broadly categorized based upon the signaling cascade that they trigger. Ionotropic glutamate receptors are coupled to ion permeant channels and under physiological conditions depolarize neurons. In contrast, metabotropic receptors are coupled to guanosine triphosphate-binding proteins (G proteins) and second messenger systems that modulate synaptic transmission (Dingledine et al., 1999). It is important to understand the effects of glutamate through its receptors in understanding injury-induced epilepsy. At present, glutamate activation of the NMDA type of glutamate receptor is that main mechanism mediating glutamate-induced neuronal injury and epileptogenesis.
4.3.1. Ionotropic glutamate receptors
The ionotropic glutamate receptors are postsynaptic, ligand-gated ion channels (Dingledine et al., 1999). Three types of ionotropic glutamate receptors have been categorized and named according to selective ability of NMDA, αamino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA), or kainate (KA) to activate them. These pharmacologically distinct glutamate receptor subtypes have been cloned and have distinct gene families (Dingledine et al., 1999). The AMPA receptor subtype of ionotropic glutamate receptors is comprised of various heteromeric configurations of the GluR1-GluR4 subunits (also known as GluRA-GluRD; Dingledine et al., 1999). Each of these protein subunits can exist as a “flip” splice variant or a “flop” splice variant, adding to the diversity of the AMPA receptor composition (Dingledine et al., 1999). The KA receptor subtype of ionotropic glutamate receptors contains combinations of subunits derived from 2 distinct gene families, the GluR5–GluR7 family and the KA1–KA2 family (Dingledine et al., 1999). The third subtype of ionotropic glutamate receptors, the NMDA receptor, is comprised of at least 1 subunit from the NR1 gene family and varied combinations of NR2A-NR2D subunits (Sucher et al., 1996). An NR3A subunit has also been described which inhibits channel activity (Das et al., 1998). Because of the diversity of subunit composition of the ionotropic glutamate receptor subtypes, the AMPA receptor, the KA receptor, and the NMDA receptor all contribute differently to the excitatory effects of glutamate in the CNS.
4.3.2. α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptors
The AMPA receptor contributes to the early, fast component of the excitatory postsynaptic potential (EPSP). The AMPA receptor is a low-affinity glutamate receptor that is typically permeable to the monovalent cations sodium and potassium and impermeable to the divalent cation, Ca2+ (Wisden & Seeburg, 1993). This ligand-gated channel demonstrates little voltage dependence, as evidenced by its linear current-voltage (I-V) relationship (Boulter et al., 1990). AMPA receptor currents are brief (a few milliseconds) due to the low-glutamate affinity and a high rate of desensitization (Dingledine et al., 1999).
The GluR2 subunit has special importance in AMPA receptor composition. GluR2, the most widely expressed subunit in AMPA receptors (Jonas & Burnashev, 1995), confers the Ca2+ impermeability of the great majority of AMPA receptors via a specific posttranscriptional modification of this subunit (Sommer et al., 1991). The modification arises when a specific adenosine of the ribonucleic acid (RNA) strand is changed to an inosine by adenine deaminase within the nucleus (Rueter et al., 1995). This RNA edit, which occurs with an approximate efficiency of 90% and high selectivity (Melcher et al., 1996), leads to the substitution of a glutamine with an arginine at the Q/R site (Michaelis, 1998). Since the majority of AMPA receptors contain at least 1 GluR2 subunit (Jonas & Burnashev, 1995), the vast majority of AMPA receptors are Ca2+-impermeable.
4.3.3. Kainate receptors
KA receptors are similar in function to AMPA receptors. Like AMPA receptors, KA receptors are voltage-independent, monovalent cation-permeable channels with low affinity and fast kinetics (Michaelis, 1998). KA receptor-mediated EPSPs have smaller peak amplitudes and slower decay kinetics than those derived from AMPA receptors (Frerking & Nicoll, 2000). Extensive RNA editing in these receptors also has implications on Ca2+ permeability (Dingledine et al., 1999) as well as anion permeability (Burnashev et al., 1996).
4.3.4. N-methyl-d-aspartic acid receptors
The NMDA receptor is quite different from the AMPA and KA subtypes of glutamate receptor. First, in addition to permeability to Na+ and K+, NMDA receptors have high permeability to Ca2+ (Dingledine et al., 1999). Also, NMDA receptors have slower kinetics attributed to a much higher affinity for glutamate (Conti & Weinberg, 1999). The conductance through NMDA receptors can last several hundred milliseconds and constitutes a slower, late phase of the EPSP (Conti & Weinberg, 1999).
Activation of the NMDA receptor is a more complicated process than activation of AMPA and KA receptors. The NMDA receptor requires the binding of a coagonist, glycine at a strychnine-insensitive glycine site. Originally thought to be a potentiator (Johnson & Ascher, 1987), glycine is actually required for the NMDA receptor to enter the open state (Ozawa, 1993). Another unique characteristic of the NMDA receptor is the voltage-dependent magnesium (Mg2+) blockade, which inhibits NMDA receptor conductance even when both glutamate and glycine are bound to the channel (Nowak et al., 1984).
The binding of Mg2+ within the pore is highly voltage-dependent and occurs at a site near or past the middle of the electric field (Ascher & Nowak, 1988). As such, large depolarizations, presumably mediated by activated AMPA and KA receptors, are required to expel Mg2+ from the pore. In fact, NMDA receptor inward currents are maximal when the neuron is depolarized to −20 to −30 mV despite the decrease in driving force (Mayer et al., 1984; Nowak et al., 1984). Thus, the NMDA receptors have a nonlinear I-V relationship, owing to this voltage-dependent Mg2+ blockade (Mayer et al., 1984).
Endogenous allosteric modulators finely regulate NMDA receptor function (Dingledine et al., 1999). Zinc, which is concentrated in the synaptic vesicles of some neurons, can inhibit NMDA receptor currents by both voltage-dependent and voltage-independent means (Christine & Choi, 1990). Extracellular cysteine residues on the NMDA receptor act as reduction/oxidation sites. Reduction of these residues enhances NMDA receptor currents, while oxidation inhibits them (McBain & Mayer, 1994). Extracellular pH also functions to modulate the NMDA receptor. NMDA receptors are inhibited by physiologically relevant concentrations of extracellular protons via a reduction in the single-channel opening frequency (Traynelis et al., 1995). Finally, endogenous polyamines such as spermidine and spermine modulate NMDA receptors. Polyamines can cause voltage-dependent inhibition, glycine-dependent potentiation, as well as voltage- and glycine-independent inhibitions (Rock & Macdonald, 1995).
4.3.5. Metabotropic glutamate receptors
As previously mentioned, G-protein-coupled metabotropic receptors are the other major category of glutamate receptors. There are 8 types of metabotropic glutamate receptors (mGluR1-mGluR8; Conn & Pin, 1997). These mGluRs are further classified according to the second messenger systems to which they are linked. Class I mGluRs consist of mGluR1 and mGluR5. Class I mGluRs are most potently activated by quisqualate and are coupled to phospholipase C (PLC) activation. Class II mGluRs, activated by 2R,4R-4-aminopyrrolidine-2-4-dicarboxylate (APDC), consist of mGluR2 and mGlur3, which inhibit adenylate cyclase activity. The Class III mGluRs (mGluR4 and mGluR6–mGluR8) also inhibit adenylate cyclase activity, but to a lesser extent, and are most potently activated by l-amino-4-phosphonobutyrate (l-AP4; Conn & Pin, 1997). Metabotropic glutamate receptors are found both on the presynaptic and post-synaptic membranes. Presynaptic mGluRs decrease neurotransmitter release (Conn & Pin, 1997; Fagni et al., 2000). mGluRs on the postsynaptic membrane regulate the function of ligand-gated ion channels, including all 3 subtypes of ionotropic glutamate receptors (Anwyl, 1999), as well as inhibit the function of voltage-gated Ca2+ channels (VGCCs) and some potassium channels (Dingledine & McBain, 1999). Thus, metabotropic glutamate receptors can act to modulate synaptic transmission in the CNS.
4.4. N-methyl-d-aspartic acid receptor-mediated excitotoxicity
The exogenous application of high concentrations (50–500 µM) of glutamate to cultured CNS neurons has been utilized to model the ischemic core in whole animal models of stroke. Neurons grown in culture respond to glutamate excitotoxicity in similar fashion to brain tissue injured by ischemia. For example, 100 µM glutamate exposure of a duration as short as 5 min leads to the death of large numbers of cultured cortical neurons, analogous to the infarct observed in the ischemic core (Choi et al., 1987). Approximately 30% of the glutamate-induced neuronal death in these cultures is mediated by acute neuronal swelling and lysis, which can be prevented by the replacement of extracellular Na+ or chloride (Cl−) with impermeant ions (Rothman, 1985; Olney et al., 1986). In contrast, delayed excitotoxic neuronal death occurs hours to days after the initial insult (Choi, 1985; Rothman et al., 1987). Delayed excitotoxic neuronal death is fundamentally dependent on the influx of extracellular Ca2+ ions (Choi, 1985, 1987) through the activated NMDA receptor (Dubinsky & Rothman, 1991; Hartley et al., 1993; Tymianski et al., 1993; Sattler & Tymianski, 2000). Just as NMDA receptor antagonists reduce the volume of the ischemic infarct in the whole animal (Gill et al., 1992), NMDA receptor antagonism protects cultured neurons from glutamate excitotoxicity (Choi et al., 1988; Moudy et al., 1994). Thus, the NMDA glutamate channel is the major injury causing mechanism involved in the development of AE.
5. Calcium as a common denominator in the injury phase of acquired epilepsy
Since Ca2+ entry through the NMDA receptor channel complex has been shown to be central to producing the initial insult associated with the injury phase of SE, stroke, and TBI, it is important to understand the role of Ca2+ in excitotoxic neuronal injury and death and how the neuron controls Ca2+ homeostasis. The following material reviews how Ca2+ is regulated in neurons and provides an insight into the potential alterations that occur in controlling [Ca2+]i levels during and after the injury phase of AE.
5.1. Neuronal calcium homeostasis
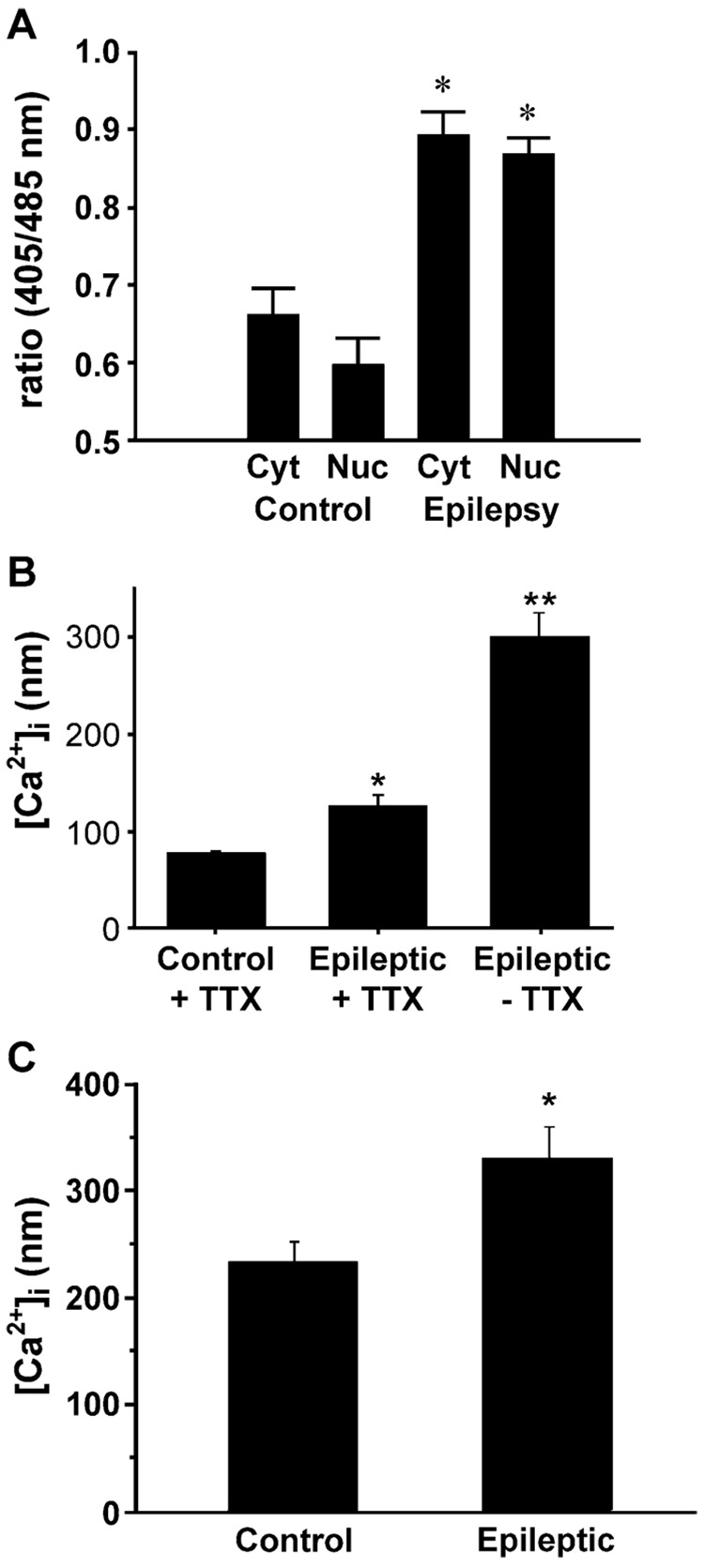
Calcium plays a pivotal role in normal neuronal function (DeCoster et al., 1992; Tymianski & Tator, 1996; Berridge, 1998). Calcium signaling in neurons is involved in processes as diverse as cell growth and differentiation (Spitzer, 1994), synaptic activity (Brose et al., 1992; Llinas et al., 1992), maintenance of the cytoskeleton (Trifaro & Vitale, 1993), and gene expression (Santella & Carafoli, 1997). Normal neuronal [Ca2+]i is maintained around 100 nM (Mody & MacDonald, 1995). This concentration is less than 1/10,000 of the free extracellular Ca2+ concentration ([Ca2+]e; Putney, 1999). In light of the important signaling function of Ca2+ and the excitotoxic implications of excess intracellular Ca2+, neurons have an intricate system to regulate [Ca2+]i. Evidence indicates that Ca2+ homeostasis is markedly altered acutely during the injury and epileptogenic phases of AE (Raza et al., 2004) and even in the chronic spontaneous recurrent seizures (epilepsy) phase (Pal et al., 2000, 2001; Raza et al., 2001).
5.1.1. Influx of extracellular Ca2+ across the plasma membrane
The neuronal plasma membrane is relatively impermeable to Ca2+ with exclusion of 3 fundamental mechanisms of Ca2+ entry: ligand-gated cation channels, VGCCs, and store-operated Ca2+ channels (SOCs). The NMDA receptor, a ligand-gated cation channel, mediates the vast majority of Ca2+ influx during excitatory neurotransmission (Ozawa, 1993). In addition, AMPA and KA receptors of certain subunit composition, as described previously, are permeable to Ca2+ (Jonas & Burnashev, 1995).
Multiple forms of VGCCs have been characterized and cloned. These receptor channels, designated as L-, N-, P-, Q-, and T-types (Tsien et al., 1995; Catterall, 1996; Catterall et al., 2003), are categorized according to their voltage sensitivities, voltage-dependent and intracellular Ca2+-dependent inactivation rates, and selective sensitivity to inhibiting drugs and toxins (Adams&Olivera, 1994). SOCs represent a third route of Ca2+ entry across the plasma membrane. These channels are activated upon depletion of intracellular Ca2+ stores (Petersen et al., 1999). Originally designated “capacitative Ca2+ entry” (Putney et al., 2001), store-operated Ca2+ entry serves to replenish intracellular Ca2+ pools such as the endoplasmic reticulum (ER). Alterations of Ca2+ entry into neurons in the development of AE represents target mechanism for altering Ca2+ homeostasis; however, at the present time, there are no direct studies demonstrating alterations of Ca2+ entry mechanisms in AE.
5.1.2. Calcium extrusion across the plasma membrane
Two major transport systems exist to pump free intracellular Ca2+ out of the neuron into the extracellular space. Because Ca2+ extrusion acts against a large Ca2+ concentration gradient, these systems are energy-dependent and are therefore highly susceptible to ischemic injury (Tymianski & Tator, 1996). The ATP-driven Ca2+ pump (Ca2+-ATPase) expends 1 ATP for each Ca2+ ion extruded and is modulated by calmodulin, fatty acids, and protein kinases (Carafoli, 1992). The second transport system, the Na+-Ca2+ exchanger, is indirectly coupled to ATP utilization in that it utilizes the Na+ concentration gradient maintained by the ATP-driven Na+-K+ exchanger. This electrogenic exchange system is triggered by increases in [Ca2+]i and extrudes 1 Ca2+ for every 2 or 3 Na+ that enters the neuron (Tymianski & Tator, 1996). At this time, there are no studies implicating these mechanisms in the development of AE. However, alteration of these Ca2+ extrusion mechanisms may play an important role and requires further investigation.
5.1.3. Calcium buffering, sequestration, and storage
Ca2+ buffering and sequestration can also reduce free intracellular Ca2+ levels. Ca2+-binding proteins in the cytoplasm, such as calbindin, calmodulin, and parvalbumin, buffer the vast majority of intracellular Ca2+ under physiological conditions (Baimbridge et al., 1992). Mitochondria sequester Ca2+ by way of a uniporter driven by the mitochondrial membrane potential (Bernardi, 1999). However, this mitochondrial accumulation only occurs when intracellular Ca2+ elevations are prolonged and high (Putney, 1999). Further, mitochondrial sequestration is a temporary buffering system, releasing Ca2+ back to the cytoplasm via mitochondrial Na+-Ca2+ and H+-Ca2+ exchangers (Bernardi, 1999).
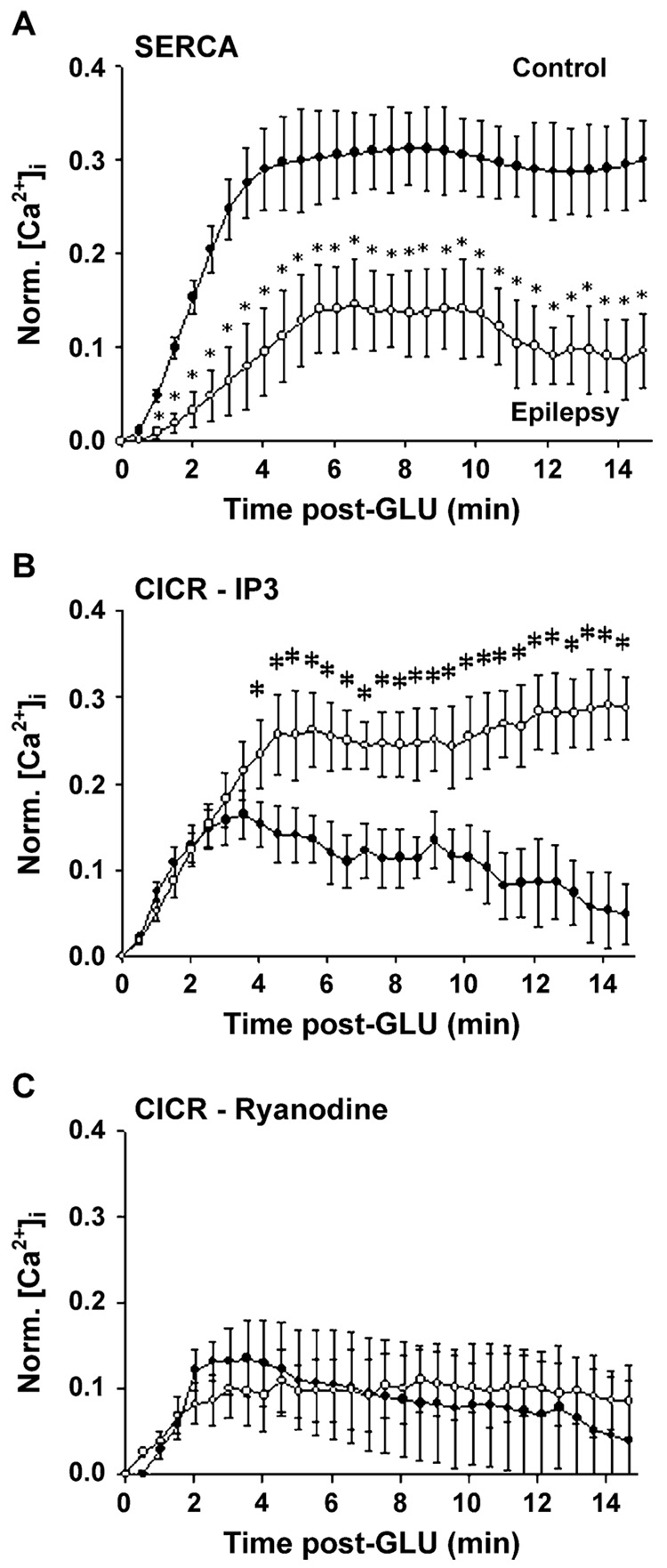
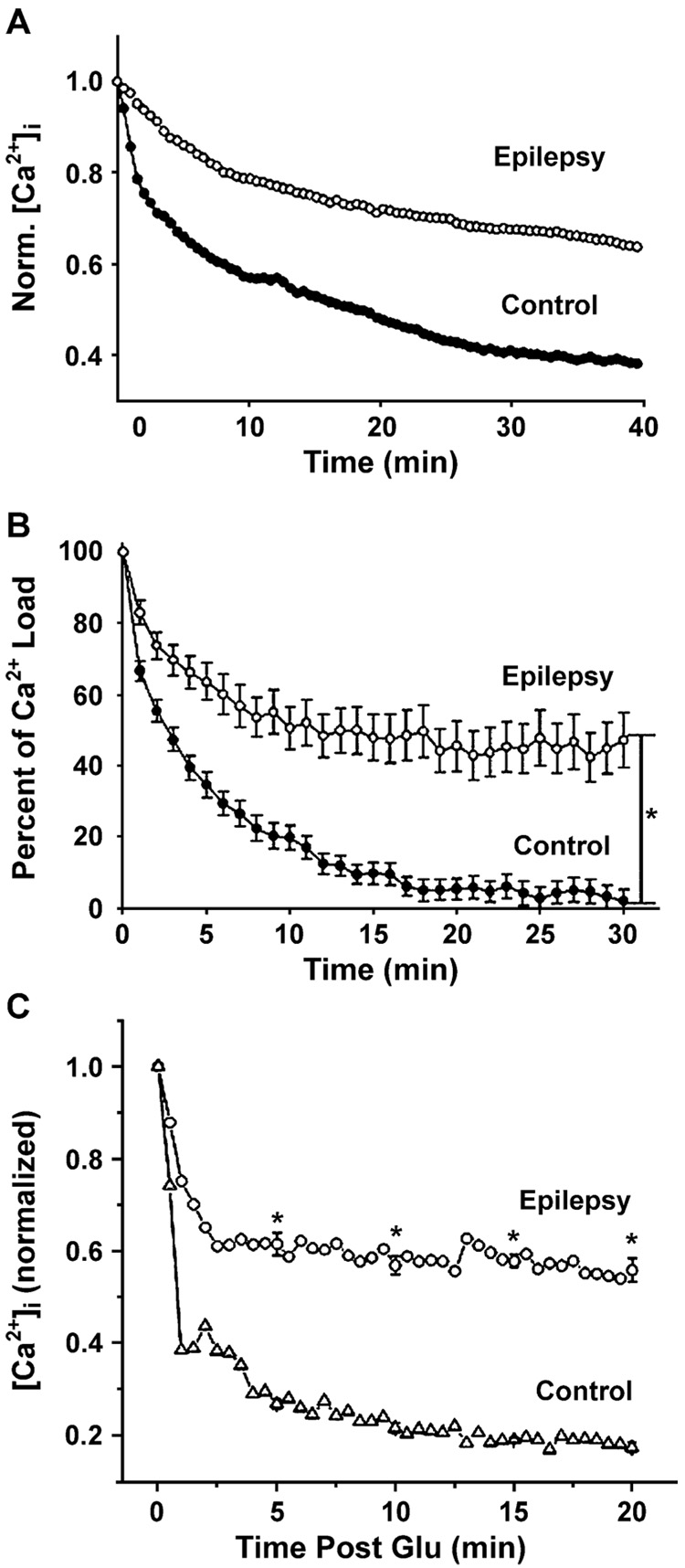
The ER also functions as a Ca2+ store. The ER accumulates Ca2+ via the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA). This enzyme is similar to that Ca2+-ATPase of the plasma membrane in that it requires ATP. Unlike the plasma membrane Ca2+-ATPase, SERCA function is independent of calmodulin and moves 2 Ca2+ ions into the ER for each ATP molecule utilized (Tymianski & Tator, 1996). Further, the activity of SERCA can be selectively inhibited by thapsigargin (Treiman et al., 1998). Direct evidence has implicated alterations in the function of SERCA in the development of AE (Pal et al., 2000).
5.1.4. Intracellular Ca2+ release
In addition to acting as a sink for intracellular Ca2+, the ER also serves as a dynamic Ca2+ source (Ogden & Khodakhah, 1996). Two classes of receptors on the ER membrane serve to release stored Ca2+ from the ER lumen to the cytoplasm. Calcium activates the ryanodine receptor (RyR) that results in Ca2+-induced Ca2+ release (Berridge, 1998). RYRs act to amplify Ca2+ signals in a regenerative, positive feedback loop (Putney, 1999). The inositol 1,4,5-tris-phosphate receptor (IP3R), activated by the second messenger IP3, is the other class of ER Ca2+ release receptors (Ogden & Khodakhah, 1996; Berridge, 1998). IP3 is produced by the cleavage of phosphatidyl 4,5-bisphosphate by PLC. IP3R-mediated Ca2+ release can be augmented by the simultaneous presence of Ca2+ and IP3 (Ehrlich et al., 1994). Together, Ca2+-induced Ca2+ release by RYRs and IP3Rs act to produce regenerative Ca2+ waves along the ER membrane analogous to sodium action potentials along the plasma membrane (Berridge, 1998; Rose & Konnerth, 2001). The ER is a continuous membrane system that makes close contact with the plasma membrane at specialized regions (Berridge, 1998). Since the ER membrane is continuous with the nuclear envelope, Ca2+ waves initiated by Ca2+ influx at the plasma membrane can travel to the nucleus, thus transducing external signals to the level of gene regulation (Jaffe & Brown, 1994). Direct evidence has shown that IP3R-mediated Ca2+ release is increased in epileptic neurons, but that no changes in the RyR Ca2+ release is seen in AE (Pal et al., 2000). These results demonstrate that selective Ca2+-regulating processes can be altered in the development of AE.
5.2. Nuclear Ca2+ signaling
The nuclear envelope is a continuous extension of the ER and contains RyRs, IP3Rs and SERCA (Santella & Carafoli, 1997). SERCA has been located on the outer leaflet of the nuclear envelope (Humbert et al., 1996); thus, the lumen of the nuclear envelope may be able to sequester Ca2+ (Carafoli et al., 1997). RyRs and IP3Rs have been identified on the both the outer and inner leaflets of the nuclear membrane (Payrastre et al., 1992; Santella & Carafoli, 1997). Thus, these 2 receptor systems allow the nuclear envelope to release Ca2+ into the cytoplasm or into the nucleus (Santella & Carafoli, 1997). Therefore, intracellular Ca2+ release initiated by extracellular Ca2+ influx at the ER can propagate to the nucleus and continue directly into the nucleoplasm (Berridge, 1998). As a second messenger, Ca2+ regulates gene transcription by modulating transcription factors directly or through Ca2+-dependent kinases and phosphatases (Santella & Carafoli, 1997; Mellstrom & Naranjo, 2001). Evaluating the effects of epileptogenesis on nuclear Ca2+ signaling represents an important area for further research.
5.3. Extended neuronal depolarization
Cultured hippocampal neurons exposed to excitotoxic levels of glutamate manifest a neuronal depolarization lasting hours after termination of the glutamate treatment (Sombati et al., 1991), resembling the anoxic depolarization observed in the ischemic core of a stroke (Katsura et al., 1994). This extended neuronal depolarization (END) is a persistent depolarizing shift of the resting membrane potential of more than 20 mV (Sombati et al., 1991; Coulter et al., 1992). While exhibiting END, neurons remain viable as evidenced by the ability to respond to glutamate and exclude trypan blue (Coulter et al., 1992). END requires NMDA receptor activation and the presence of extracellular Ca2+ (Coulter et al., 1992). The percentage of neurons in END correlates with extent of excitotoxic neuronal death in the cultures (Coulter et al., 1992). Other reports have confirmed that END occurs in different neuronal preparations and that it requires NMDA receptor activation and extracellular Ca2+ (Calabresi et al., 1995; Chen et al., 1997; Tanaka et al., 1997). Recent studies have demonstrated that a selective Ca2+ conductance, distinct from all other conventional routes of Ca2+ entry, is activated during excitotoxic glutamate exposure and maintains END (Limbrick et al., 2003). Since END is produced during the neuronal injury phases of AE, it is important to evaluate the role of END in the development of AE.
5.4. Inability to restore resting free intracellular Ca2+ concentration
Cultured neurons exposed to excitotoxic glutamate concentration also manifest changes in [Ca2+]i similar to that observed in neurons of the ischemic core. Cultured neurons demonstrate an inability to restore resting [Ca2+]i (IRRC; Limbrick et al., 1995) analogous to the irreversible neuronal Ca2+ overload observed after ischemic injury in the rat (Kristian & Siesjo, 1998). In these cultured neurons, Ca2+ levels do not return to normal levels even after glutamate removal (Connor et al., 1988; Glaum et al., 1990; Limbrick et al., 1995). Like END, IRRC is dependent on NMDA receptor activation and extracellular Ca2+, and a strong correlation exists between IRRC and excitotoxic neuronal death (Limbrick et al., 1995). It has recently been shown (Limbrick et al., 2003) that glutamate excitotoxicity is associated with the induction of a novel secondary Ca2+ conductance that is responsible for IRRC and the maintenance of END. It is suggested that this novel Ca2+ current that is responsible for indiscriminant Ca2+ entry during END determines the fate of the cell after an excitotoxic insult. It is therefore of pathophysiological importance during stroke, TBI, and other forms of neuronal injury.
5.5. Calcium-dependent mechanisms of neuronal death
Taken together, END, IRRC and the extensive neuronal death induced by excitotoxic glutamate exposure parallel the observed anoxic depolarization, irreversible Ca2+ overload and infarction of the ischemic core. The dependence of these phenomena on NMDA receptor activation and extracellular Ca2+ has led to the Ca2+ hypothesis of glutamate excitotoxicity, wherein NMDA receptor-mediated Ca2+ influx causes the disruption of Ca2+ homeostatic mechanisms and Ca2+ overload which initiate pathways leading to neuronal death (Choi, 1987; Rothman et al., 1987; Siesjo & Bengtsson, 1989; Dubinsky & Rothman, 1991; Randall & Thayer, 1992; Hartley et al., 1993; Tymianski et al., 1993; Sattler & Tymianski, 2000).
A number of pathological processes involving excessive NMDA receptor activation and intracellular Ca2+ overloads have been proposed as mediators of delayed excitotoxic neuronal death. The formation of free radical species has been implicated in excitotoxicity. The excitotoxic stimulation of the NMDA receptor leads to overactivation of phospholipase A2 (Lafon-Cazal et al., 1993; Dennis, 1994) and nitric oxide synthase (Dawson et al., 1991; Lipton et al., 1996), resulting in excess arachidonic acid and nitric oxide production, respectively. Arachidonic acid via its subsequent metabolism by cyclooxygenase and lipoxygenase leads to the production of highly reactive oxygen molecules such as superoxide and hydroxyl radicals (OH; Lafon-Cazal et al., 1993; Dennis, 1994). The reaction of nitric oxide with superoxide forms peroxynitrite (ONOO−), a highly reactive nitrogen species (Dawson et al., 1991; Lipton et al., 1996). Together, these free radicals destroy protein components of the cytoskeleton, nucleic acids, and membrane lipids (Hall, 1997; Hall et al., 1999).
Mitochondria play a role in the sequestration of the Ca2+ influx during glutamate excitotoxicity (White & Reynolds, 1997). However, as NMDA receptor-mediated Ca2+ accumulates in the mitochondria, superoxide is produced due to the inhibition of the electron transport chain (Dugan et al., 1995). Superoxide, in conjunction with pathological Ca2+ accumulation, initiates the opening of the mitochondrial permeability transition pore (Nicholls & Budd, 1998; Bernardi, 1999), which has been associated with the collapse of the mitochondrial proton gradient and subsequent loss of ATP generation. The opening of the mitochondrial permeability transition pore is also associated with the release of cytochrome C, a messenger that triggers apoptosis (Kluck et al., 1997).
A number of enzyme systems have also been associated with excitotoxic NMDA receptor-mediated Ca2+ influx. For example, caspases, a family of Ca2+-dependent endonucleases, trigger apoptosis during excitotoxicity (Du et al., 1997; Zipfel et al., 2000). Caspase inhibitors successfully inhibit apoptotic neuronal death after glutamate exposure (Du et al., 1997). Calcium overload also activates a family of Ca2+-dependent proteases, the calpains that degrade cytoskeletal components, receptors proteins, G proteins, and Ca2+-binding proteins (Emerich & Bartus, 1999).
In summary, glutamate excitotoxicity is an established in vitro model of neuronal injury (Choi & Rothman, 1990; Michaels & Rothman, 1990; Tymianski & Tator, 1996). Exposure of cultured neurons to high concentrations (50–500 µM) of glutamate causes END (Sombati et al., 1991) and IRRC (Limbrick et al., 1995), analogous to anoxic depolarization and irreversible Ca2+ overload initially observed in the ischemic core during a stroke. The irreversible loss of ionic homeostasis triggers a number of NMDA receptor-Ca2+-dependent processes leading to neuronal death.
As described earlier, SE, stroke, and TBI are all associated with significant increases in extracellular glutamate and [Ca2+]i. NMDA receptor antagonism is neuroprotective in multiple animal models of both SE and TBI. Thus, although the observations made in glutamate excitotoxicity were first hypothesized and correlated with findings in the context of ischemic stroke, these insights into mechanisms of neuronal death can clearly be related to brain damage observed in SE and TBI.
6. Dead cells do not seize: Surviving neurons are the substrate for epileptogenesis
Another concept original to stroke that has been extended to SE and TBI is the concept of the penumbra. The ischemic penumbra represents a region of injured brain tissue that is not necessarily destined to die. As described earlier, the ischemic penumbra undergoes a less severe, transient ischemia during stroke secondary to collateral cerebral vasculature (Vaughan & Bullock, 1999), which leads to less severe increases in extracellular glutamate in the penumbra. Thus, the damage in the penumbra is potentially, or at least partially, reversible, injured neurons in this region can recover and survive (Witte et al., 2000), and the ischemic penumbra has been the primary target for therapeutic intervention (Ginsberg, 1997). Similarly, glutamate elevations in the surrounding focus are smaller than in the core of a concussive TBI (Maeda et al., 1998). In SE, the duration of the seizure activity determines the degree of Ca2+ load and neuronal cell death. In addition, the hippocampus and other anatomical regions are more actively involved in the high production of glutamate produced during SE, and thus there are regional variations in the severity of injury in SE (Lothman & Bertram, 1993; Kulkarni & George, 1995). SE has been shown to also produce significant elevations in [Ca2+]i levels in both in vitro (Pal et al., 1999) and in vivo (Raza et al., 2004) modes of SE. Calcium levels can reach high levels (800 nM); however, if the SE is less than 1–3 hr in duration, the majority of neurons undergoing this prolonged exposure to elevated glutamate and elevated Ca2+ will survive (80%). However, if SE continues for longer durations, severe neuronal cell death can occur. Thus, the durations of the prolonged seizures in SE determine the magnitude of injury. The durations of most SE cases would thus result in the majority of effected neurons being injured but not killed. This is the ideal substrate for the development of AE.
Essential to the Ca2+ hypothesis of epileptogenesis is this concept of the penumbra or an area of reversibly injured neurons that survive the initial SE, stroke, or TBI. Although the penumbra represents a region of injured brain wherein many neurons recover and survive, chronic alterations in the physiology of these cells may contribute, in conjunction with neuronal cell loss, to the debilitating sequelae of CNS insults. Little is known regarding the function of surviving brain tissue in the penumbra (Witte et al., 2000). Since dead neurons cannot seize, we believe that the injured but surviving tissue of the penumbra or the injured brain tissue is the substrate for epileptogenesis, and that long-lasting alterations in the function of these injured, surviving neurons underlie mechanisms of epileptogenesis and the development of spontaneous recurrent seizures. Thus, the injury phase in the development of AE is essential, since it exposes neurons to abnormal Ca2+ exposure that are not lethal but are sufficient in the injury-surviving neurons to serve as the second messenger that initiates the long-term plasticity changes underlying the development of AE.
7. Experimental models of injury-induced spontaneous recurrent seizures
A number of research models have been utilized to demonstrate chronic neuronal hyperexcitability after stroke, SE, and TBI in both whole animal and in vitro systems. While some systems have demonstrated overt seizure expression, others have only demonstrated electrographic seizures. Still, others have only demonstrated a chronic shift towards enhanced excitability. These models are important in providing the substrate to evaluate the basic mechanisms underlying the role of Ca2+ in AE.
7.1. Status epilepticus-induced spontaneous recurrent seizures
SE models of AE were the first in vivo and in vitro models developed to study AE. Having these models is critical in understanding the molecular mechanisms underlying epileptogenesis. SE has been employed in a number of models to successfully model AE in both whole animal and in vitro systems.
7.1.1. In vivo models
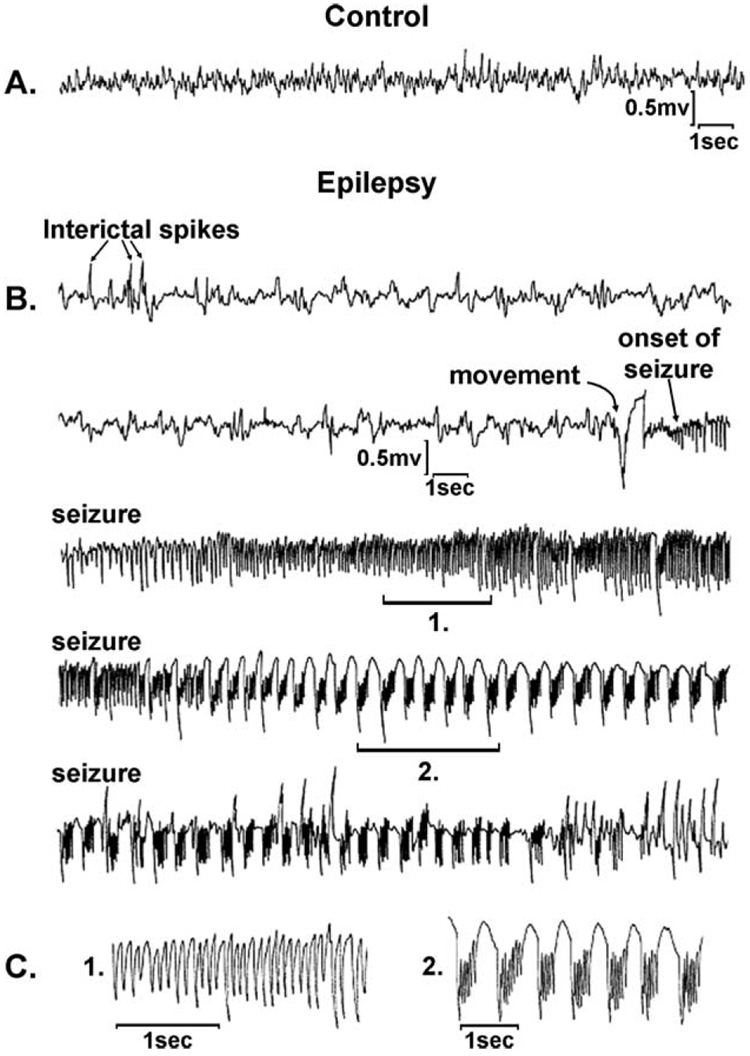
Intact animal models of SE described previously utilize the chemicals, pilocarpine, and kainic acid, or electrical stimulation to acutely induce a prolonged episode of seizure activity. After a latent period of 2–4 weeks, animals begin to manifest overt spontaneously recurring epileptic seizures (Leite et al., 2002; Loscher, 2002). These animal models of AE represent the most studied areas for the development of AE. Fig. 5 presents a representative spontaneous recurrent seizure in the pilocarpine model of AE. The clinical and electrographic parameters of these seizures in the rat are essentially identical to the epileptic seizures seen in man. The model employs SE as the injury to induce AE.
Fig. 5.
Hippocampal depth electrode EEG recordings from representative control (A) and epileptic (B and C) animals 2 months after vehicle or pilocarpine injections. (A) Control animals demonstrate normal background EEG rhythms. (B) Epileptic animals consistently manifested interictal spike discharges during EEG recordings; a typical seizure record is shown. Rapid spike discharges with frequencies between 10 and 20 Hz (region 1), followed by well-formed polyspike and slow wave discharges (region 2) were typically observed (C), and corresponded in time to the tonic and clonic phases of the clinical seizure activity that was observed on the video EEG. (From Rice et al., 1996.)
7.1.2. In vitro models
In vitro models of SE have also been developed over the past decade. The low-Mg2+ hippocampal neuronal culture model of epileptogenesis utilizes an episode of continuous seizure activity for 3 hr to induce epileptogenesis (Sombati & DeLorenzo, 1995). Upon return to normal Mg2+ solutions, networks of neurons manifest synchronized spontaneous recurrent epileptiform discharges (SREDs) for the life of the neurons in culture (Sombati & DeLorenzo, 1995). This model employs continuous electrographic epileptiform discharges for several hours as the injury phase to induce AE. After the restoration of physiological magnesium concentrations terminates the electrographic epileptiform discharges, the surviving neurons develop AE. In this model, ~80% of the neurons survive the SE-induced injury and develop AE.
7.1.3. Kindling models: prolonged kindling model
In contrast to the pilocarpine-induced and KA-induced SE models of epileptogenesis, the kindling models of epileptogenesis utilize repetitive, subconvulsive stimulation of particular brain regions to initiate epileptogenesis (Goddard et al., 1969; Bradford, 1995). Early in the protocol, electrical stimulation only elicits brief after discharges. However, after daily repetition of stimulations, after discharges increase in duration, eventually progressing to spontaneous seizure activity. Blockade of the NMDA receptor inhibits kindling epileptogenesis, demonstrating the necessity of NMDA receptor activation (Croucher et al., 1988, 1992; Morimoto et al., 1996).
Another kindling paradigm utilizes the daily repeated injections of glutamate to induce a hyperexcitable state (Croucher & Bradford, 1989, 1990). Although glutamate activates a number of receptor subtypes, antagonism of the NMDA receptor alone inhibits the development of hyperexcitability in this paradigm (Croucher & Bradford, 1989, 1990; Croucher et al., 1995). The role of NMDA receptors in kindling epileptogenesis is further supported by the ability of NMDA to substitute for glutamate as the kindling agent (Croucher et al., 1995).
Paradigms similar to those utilized in the electrical kindling model have been developed in hippocampal slice preparations wherein repeated trains of electrical stimulation result in the development of spontaneous bursting activity (Stasheff et al., 1989). This stimulus train-induced bursting (STIB) model of epileptogenesis has differentiated the role of NMDA receptors in epileptogenesis and seizure expression. NMDA receptor antagonists inhibit the induction of stimulus train-induced epileptogenesis, but produced no anticonvulsant effects on slices that were previously made epileptic (Anderson et al., 1987; Swartzwelder et al., 1988; Stasheff et al., 1989).
7.1.4. Direct electrical stimulation models
Another model of SE- or electrical stimulation-induced AE is the continuous electrical stimulation model. Several models have been developed to use direct electrical stimulation to produce AE (Bertram & Lothman, 1990; Lothman & Bertram, 1993; Shirasaka & Wasterlain, 1994; Lothman et al., 1996; Fountain et al., 1998; Hsieh, 1999; Mazarati et al., 2002). These models utilize the induction of electrical discharges in different regions of the brain to produce AE. They offer several advantages in their ability to control the degree of stimulation in comparison to the pilocarpine and kainic acid models.
7.2. Stroke-induced spontaneous recurrent seizures
Despite the high association of AE in strokes in man, it has been more difficult to develop animal models of stroke-induced spontaneous recurrent seizures. It has only been in the last few years that models have been developed to study AE in stroke.
7.2.1. In vivo models
The ischemic penumbra has been suggested as the substrate for epileptogenesis in stroke-induced epilepsy (Witte et al., 2000), and recent studies have demonstrated enhanced excitability in penumbral tissue. The most popular models of ischemia utilize middle cerebral artery occlusion (transient, permanent, or thrombotic) or complete forebrain ischemia (2-vessel occlusion, 4-vessel occlusion). Pronounced hyperexcitability has been observed in perilesional brain areas after focal ischemia produced by photothrombotic cortical infarct up to 4 months after injury (Neumann-Haefelin et al., 1995a, 1995b, 1998; Luhmann et al., 1996a, 1996b; Buchkremer-Ratzmann et al., 1998). For example, field potentials characterized by multiple discharges are produced in response to electrical stimulation in ischemia-injured animals. These multiple discharges are not observed in control neurons (Buchkremer-Ratzmann et al., 1998). Similar findings have been reported in other models of focal ischemia utilizing middle cerebral artery occlusion (Luhmann et al., 1996a, 1996b; Albensi, 2001). Stimulus-induced epileptiform discharges can be elicited up to 1 year after transient forebrain ischemia produced in the middle cerebral artery occlusion model. These epileptiform field potentials are NMDA receptor-dependent (Luhmann et al., 1996a, 1996b), and NMDA receptors are up-regulated in the penumbral region (Qu et al., 1998). Stimulus-induced field potentials are multiphasic and longer in duration than field recordings from controls, indicating that populations of neurons are bursting. Also, these discharges in ischemia-injured tissue are lower in amplitude, suggesting that fewer viable neurons are present and that some neuronal death occurred in the penumbra (Mittmann et al., 1998). In addition to producing electrical stimulus-induced epileptiform discharges, ischemia-injured tissue also has a lower seizure threshold (Congar et al., 2000). Both high extracellular K+ solutions and bath application of the convulsant KA induce epileptiform activity more often in postischemic tissue compared to control tissue (Congar et al., 2000).
Decreased GABA-mediated inhibition may contribute to the ischemia-induced hyperexcitability of penumbral tissue (Neumann-Haefelin et al., 1995a, 1995b, 1998; Luhmann et al., 1996a, 1996b; Congar et al., 2000). GABA-mediated inhibitory postsynaptic potentials are significantly decreased in ischemia-injured tissue (Neumann-Haefelin et al., 1995a, 1995b; Luhmann et al., 1996a, 1996b; Congar et al., 2000), and GABAA receptors are down-regulated in the same brain areas (Neumann-Haefelin et al., 1998; Qu et al., 1998). In addition, parvalbumin-positive, GABAergic interneurons in the penumbra have a pathologically altered appearance with shrunken cell bodies and reduced or pruned dendrites (Neumann-Haefelin et al., 1998; Arabadzisz & Freund, 1999).
At this time, no consensus has developed in regard to long-term ischemia-induced changes in the intrinsic membrane properties of neurons. While some laboratories have reported a persistent positive shift of membrane potential and increased input resistance that could enhance neuronal excitability after ischemia (Neumann-Haefelin et al., 1995a, 1995b; Luhmann et al., 1996a, 1996b; Congar et al., 2000), others have not observed these changes (Mittmann et al., 1998). In addition, changes in spike threshold, spike amplitude, accommodation, and fast and slow afterhyper-polarizations have not been observed (Congar et al., 2000). Modification of intrinsic membrane properties most likely depends on the mechanism by which ischemia is produced (Congar et al., 2000). These data demonstrate a chronic increase in neuronal excitability in the ischemic penumbra of whole animal models. Unfortunately, these studies do not demonstrate spontaneous epileptic seizure activity that more accurately represents the clinical scenario of stroke-induced epilepsy (Congar et al., 2000; Witte et al., 2000). Still, these models provide a system to study the potential mechanisms of hyperexcitability observed in the penumbra after an ischemic insult and the relationship between the pathophysiology of stroke and epileptogenesis.
7.2.2. In vitro models
Our laboratory recently developed a model in cultured hippocamapal neurons to study stroke-induced spontaneous recurrent seizures. As excitotoxic concentrations are applied to CNS neurons to model the ischemic core of a stroke, we utilized lower concentrations of glutamate to model the surrounding ischemic penumbra (Sun et al., 2001). This subexcitotoxic glutamate exposure induces transient depolarizations of neuronal membrane potential and prolonged, but reversible increases in [Ca2+]i similar to that seen in the ischemic penumbra in whole animal models of stroke (Sun et al., 2001). Further, a proportion of neurons in this in vitro system die secondary to glutamate exposure, while other injured neurons survive (Sun et al., 2001). These surviving neurons manifest SREDs analogous to seizures throughout the remainder the life of the cultures. Detailed analysis of these SREDs reveals that they are comprised of paroxysmal depolarizing shifts of membrane potential with high-frequency burst discharges in synchronized networks of neurons (Sun et al., 2001). These epileptiform discharges can also be controlled with anticonvulsant regimens consistent with temporal lobe epilepsy (Sun et al., 2001). This is, to our knowledge, the first ever report of an in vitro model of stroke-induced spontaneous recurrent seizures (Sun et al., 2001). Together, these models provide a system to study the potential mechanisms of hyperexcitability observed in the penumbra after an ischemic insult and the relationship between the pathophysiology of stroke and epileptogenesis.
7.3. Traumatic brain injury-induced spontaneous recurrent seizures
TBI models that cause AE have been extremely difficult to develop. It has only been in the last year that an in vivo TBI model of AE has been developed, and at the present time, there are no in vitro models of TBI-induced AE.
7.3.1. In vivo models
Until recently, animal models of TBI have had difficulty demonstrating overt epileptic seizures in similar fashion as models of stroke. Despite this impediment, many investigations have demonstrated potential mechanisms of hyperexcitability after TBI. Hyperexcitability of the hippocampal dentate gyrus has been described in the popular lateral fluid percussion injury model of closed head injury in the rat and potentially attributed to alterations in hippocampal circuitry (Lowenstein et al., 1992; Coulter et al., 1996; Toth et al., 1997; Santhakumar et al., 2000, 2001). Cytoarchitectual changes as demonstrated by selective hilar neuronal loss (Lowenstein et al., 1992; Coulter et al., 1996; Toth et al., 1997; Santhakumar et al., 2000, 2001) and mossy fiber sprouting (Santhakumar et al., 2001) have been shown and associated with dentate granule cell hyperexcitability, although some recent studies refute this hypothesis (Ratzliff et al., 2004). Larger, longer-lasting multiphasic field potential have been recorded from the hippocampus of animals after TBI compared to field potentials in controls (Santhakumar et al., 2000, 2001; Akasu et al., 2002; Coulter et al., 2002). Decreased seizure threshold has also been demonstrated in other models of TBI. A typically non-convulsant dose of pentylenetetrazol induced generalized tonic-clonic seizures in animals 15 weeks after weight drop contusion (Golarai et al., 2001).
Although the above studies demonstrated a decreased seizure threshold after TBI, only one study has demonstrated true posttraumatic epilepsy with the development of spontaneous chronic seizures after fluid percussion injury in the rat (D’Ambrosio et al., 2004). These animals demonstrated behavioral changes consistent with partial seizures as well as simultaneously observed electrographic epileptiform events. In addition, these animals manifested electrographic changes consistent with a postictal depression after seizures. This study further suggested that the penumbra of the traumatic focus was the focus of seizure generation as local field recordings from the injury site consistently manifested stimulus-induced after discharges in contrast to the contralateral side.
7.3.2. In vitro studies
An in vitro model of TBI has been utilized to examine electrophysiological characteristics of traumatized neurons. With this model, cultured neurons grown on flexible membrane substrate are stretched, imparting a direct mechanical injury to neuronal elements (Ellis et al., 1995). Studies in the acute setting of injury demonstrated hyperexcitability in the form of sodium pump-dependent membrane depolarization (Tavalin et al., 1995, 1997), reduced NMDA receptor Mg2+ blockade (Zhang et al., 1996), and enhanced AMPA receptor currents (Goforth et al., 1999) in the acute setting of stretch-induced injury. Some of these findings were reversed, however, with 24 hr of injury, and studies of chronic hyperexcitability and epileptiform activity in this model have yet to be fully investigated.
8. A calcium continuum from epileptogenesis to excitotoxicity
A major theory in developing the role of Ca2+ in the development of AE is that there is a continuum of severity in the effects of Ca2+ on neuronal tissue. Olney (1969) initially developed this concept in the discovery of excitotoxicity. This concept indicates that small changes in Ca2+ levels produced by glutamate receptor stimulation results in physiological activities related to synaptic transmission and normal physiological activity. However, excessive activation of the glutamate receptors can actually excite the neuron to death, leading to the term excitotoxicity. In between normal physiologic functions and cell death, there are other effects of prolonged activation of glutamate receptors and especially the NMDA receptors. Prolonged or increased activation of glutamate receptors have been implicated in the neuronal plasticity changes of memory and long-term potentiation (Abel & Lattal, 2001; Huang & Hsu, 2001; Davies et al., 2002; Lynch, 2004).
The development of AE is one of the most dramatic examples of long-term plasticity changes in neurons. Following an initial neuronal injury, permanent plasticity changes are developed, which lead to the induction and maintenance of AE. Since dead neurons do not seize, it is reasonable to assume that the injury phase of AE produces prolonged activation of glutamate receptors that exceed the effects developed in normal physiological function and memory, yet stop short of producing excitotoxicity and cell death. Thus, the severity of glutamate stimulation and its duration are essential in the development of AE.
As described above, Ca2+ entry and regulation by glutamate receptors play an important role in mediating the physiological, memory, and excitotoxic effects of glutamate on neuronal tissue. Growing evidence has been accumulating to indicate that Ca2+ alterations induced by excessive glutamate stimulation also play a role in the induction of AE. The following reviews the evidence for this conclusion.
9. Calcium and the induction of epileptogenesis
Ca2+ homeostasis is essential to normal neuronal function (DeCoster et al., 1992; Tymianski & Tator, 1996; Berridge, 1998), and irreversible Ca2+ overload leads to neuronal death after CNS injuries such as stroke (Choi & Rothman, 1990; Tymianski & Tator, 1996; Kristian & Siesjo, 1998; Lipton, 1999). The Ca2+ hypothesis of epileptogenesis suggests that a more moderate neuronal insult producing prolonged but reversible elevations in [Ca2+]i through the activated NMDA receptor contributes to the development of spontaneous recurrent seizures. Section 9.1 will review the evidence that has accumulated supporting the Ca2+ hypothesis of epileptogenesis that implicates a role for Ca2+ as a second messenger in the development of AE.
9.1. Evidence that the development of acquired epilepsy is dependent on N-methyl-d-aspartic acid receptor activation during the injury phase
A number of whole animal models of epilepsy have demonstrated a requirement for NMDA receptor activation during the injury phase of AE. While inhibition of the NMDA receptor with the noncompetitive NMDA receptor antagonist (+)5-methyl-10,11-dihydro-5H-dibenzo(a,b)cyclohepten-5,10-imine maleate (MK801) had no effect on the severity or duration of the injury caused by pilocarpineor kainic acid-induced SE (Stafstrom et al., 1993; Rice & DeLorenzo, 1998), MK801did block the development of spontaneous recurrent seizures, the histological characteristics of neuronal loss in the CA1 region, and the mossy fiber sprouting typical in these epileptic animal models (Stafstrom et al., 1993; McNamara & Routtenberg, 1995; Rice & DeLorenzo, 1998).
In contrast to the pilocarpine and KA models of epilepsy, which utilize convulsant drug exposure to trigger a single episode of SE, the kindling model of epileptogenesis utilizes repetitive, subconvulsive electrical stimulation of particular brain regions to initiate epileptogenesis (Goddard et al., 1969; Bradford, 1995). Early in the protocol, electrical stimulation only elicits brief after discharges. However, after daily repetition of stimulations, after discharges increase in duration, eventually progressing to spontaneous seizure activity. As seen in the SE-induced AE models, blockade of the NMDA receptor inhibits kindling epileptogenesis, demonstrating the necessity of NMDA receptor activation (Croucher et al., 1988, 1992; Morimoto et al., 1996).
Another kindling paradigm utilizes the daily repeated injections of glutamate to induce a hyperexcitable state (Croucher & Bradford, 1989, 1990). Although glutamate activates a number of receptor subtypes as described above, antagonism of the NMDA receptor alone inhibits the development of hyperexcitability in this paradigm (Croucher & Bradford, 1989, 1990; Croucher et al., 1995). The role of NMDA receptors in kindling epileptogenesis is further supported by the ability of NMDA alone to substitute for glutamate as the kindling agent (Croucher et al., 1995).
Analogous kindling paradigms have been studied using in vitro preparations wherein repeated trains of electrical stimulation of hippocampal slices result in the development of spontaneous bursting activity (Stasheff et al., 1989). This STIB model of epileptogenesis has further differentiated the role of NMDA receptors in epilepsy. NMDA receptor antagonists inhibit the induction of stimulus train-induced epileptogenesis, but produced no anticonvulsant effects on slices that were previously made epileptic (Anderson et al., 1987; Swartzwelder et al., 1988; Stasheff et al., 1989). Thus, in the STIB model, NMDA receptor activation is required for the development of the hyperexcitable state, but NMDA receptor activation is not mandatory for the initiation and expression of seizure activity once this state is achieved.
In another hippocampal slice model, the removal of extracellular Mg2+ results in the development of spontaneous and triggered epileptiform burst activity that persists even after the return of normal Mg2+-containing medium (Anderson et al., 1986). Epileptogenesis in this low-Mg2+ hippocampal slice model was abolished by application of the competitive NMDA receptor antagonist, 2-amino-5-phosphonovalerate (APV; Tancredi et al., 1990).
Similar results have been shown in the low-Mg2+ hippocampal neuronal culture model of SE-induced epileptogenesis as well as in the glutamate injury-induced epileptogenesis model. Blockade of the NMDA receptor with MK801 or APV in both of these primary culture models inhibited the future development of SREDs (Sombati & DeLorenzo, 1995; DeLorenzo et al., 1998; Sun et al., 2002; Fig. 6 and Fig 7). Inhibition of the AMPA, KA, and metabotropic glutamate receptors had no antiepileptogenic effects in either of these culture models (Fig. 6; DeLorenzo et al., 1998; Sun et al., 2002). This in vitro model of SE-induced AE is a powerful tool to evaluate the effects of agents on the development of AE. Fig. 6 demonstrates that test agents can be evaluated to determine their effects on SE. If an agent inhibited AE, it would inhibit the induction of AE not by a specific mechanism but rather by its effects on the injury induced by AE. MK801 and the other treatments studied in this model (DeLorenzo et al., 1998; Sun et al., 2002) were shown not to effect SE or the injury phase of AE development (Fig. 6 and Fig 7).
Fig. 6.
Induction of continuous seizure activity (status epilepticus) in cultured hippocampal neurons for 3 hr in the presence and the absence of several neuropharmacological agents. Intracellular recordings were obtained from hippocampal pyramidal neurons before, during, and after a 3-hr low-Mg2+ treatment employing established procedures. The recordings shown were representative of more than 10 independent experiments for each condition. (A) A representative intracellular recording from a control neuron showing occasional spontaneous action potentials. The resting potential of individual neurons ranged from 65 to 50 mV. Recording from more than 100 control neurons, no spontaneous seizure activity was observed. (B–J) Representative intracellular recordings during low-Mg2+ treatment in normal (2 mM; B) or low (0.2 mM) Ca2+ (C), or various neuropharmacological agents (see Fig. 2D–J). During the low-Mg2+ treatment, the cells developed longer-duration synaptic potentials and multiple-action potentials, evolving into continuous tonic high-frequency (4–20 Hz) burst discharges. The frequency of burst discharges varied from cell to cell. However, all the conditions shown in panels C–J manifested 3 hr of continuous seizure discharge. The low Ca2+ 0.2 mM (C), APV 25 µM (E), and MK801 10 µM (F) treatments consistently produced higher-frequency discharges. NBQX 10 µM (H) and 5 µM nifedipine (J) produced slightly lower-frequency discharges. After readdition of normal Mg2+ levels to the medium, all spontaneous continuous discharges attenuated. SE produced less than 10% neuronal cell death under these conditions. (From DeLorenzo et al., 1998.)
Fig. 7.
Induction of spontaneous recurrent seizures in cultured hippocampal neurons 2 days after low-Mg2+ treatment in the presence or the absence of low Ca2+ (C) or neuropharmacological agents (D–J) during the brief 3-hr exposure to low Mg2+. After low-Mg2+ treatment, cultures immediately were returned to normal Mg2+ levels in the media. Intracellular recordings were obtained from hippocampal pyramidal neurons 2 days after low-Mg2+ treatment. The recordings shown were representative of more than 10 independent experiments for each condition. SREDs (seizures) were observed under standard conditions, 2 mM Ca2+ (B), 10 µM CNQX (G), 10 µM NBQX (H), 250 µM MCPG (I), and 5 µM nifedipine (J). Low extracellular Ca2+ (0.2 mM; C) or intracellular chelation of Ca2+ with 100 µM BAPTA (D) during low-Mg2+ treatment completely blocked the development of spontaneous recurrent seizures despite causing even a higher-frequency seizure discharge during the low-Mg2+ treatment. Expansion of a 5-sec interval of seizure activity in panel B demonstrated the numerous spikes associated with each epileptiform burst. More than 80 spike discharges occurred during this 5-sec segment, giving an average spike frequency discharge of 16 Hz during this segment of the seizure shown in (B). The electrophysiological patterns for conditions A–J were representative of recordings taken earlier or later than the 2-day post-SE sample time. Conditions that blocked the induction of spontaneous recurrent seizures never manifested seizure discharges. (From DeLorenzo et al., 1998.)
Fig. 7 presents the results of these same agents on the development of AE in this model. It is evident in this model that inhibition of the NMDA receptor system with MK801 and APV inhibited the development of AE (Fig. 7). Other receptor blocking agents were not effective in preventing the development of AE in this model (Fig. 7). Essentially identical results were obtained with these techniques in the stroke-induced HNC model of AE (Sun et al., 2002).
This evidence demonstrates that NMDA receptor activation during the injury phase of AE is essential for the development of AE in both in vitro and in vivo models. In addition, the role of NMDA receptor activation in the development of AE has been shown in numerous laboratories and in several different models including kindling.
9.2. Evidence that the development of acquired epilepsy is dependent on elevated [Ca2+]i during the injury phase of acquired epilepsy
The effects of these agents on [Ca2+]i can also be simultaneously evaluated in this model using Ca2+ imaging (Fig. 8). This data demonstrates that agents that inhibit VGCCs, such as nifedipine, were able to lower [Ca2+]i during SE without decreasing the severity of SE (Fig. 6), but they were not able to inhibit the induction of AE (Fig. 7). CNQX, NBQX, and MCPG that inhibit AMPA, KA, and metabatropic glutamate receptors were also not effective in preventing the development of AE. Thus, employing the SE-induced HNC model of AE provided a powerful tool combining Ca2+ imaging and electrophysiological techniques to demonstrate that the development of AE in this model was dependent on activation of the NMDA receptor and the resulting increase in [Ca2+]i.
Fig. 8.
[Ca2+]i imaging of hippocampal neurons in culture before, during, and after low-Mg2+ treatment in the presence or the absence of low Ca2+ or various neuropharmacological agents. (A) A representative hippocampal neuron under conditions with basal [Ca2+]i levels of 150 nM. (B) The same neuron shown in A during low-Mg2+ treatment. [Ca2+]i rose to 570 nM. (C) The same neuron shown in panels A and B after return to normal Mg2+ conditions after 3 hr of treatment. [Ca2+]i returned to near-basal conditions. Neurons remained viable for as long as 8–12 hr during Ca2+-imaging studies. Panels D–I represent groups of neurons during low-Mg2+ treatment with normal (2 mM; D) or low Ca2+ (0.2 mM; E), or the presence of 100 µM BAPTA (F), 10 µM MK801 (G), 10 µM CNQX (H), and 5 µM nifedipine (I). The calibration bar provides a direct comparison of color intensity and [Ca2+]i levels in nM. (From DeLorenzo et al., 1998.)
9.3. Elevated levels of free [Ca2+]i in the acute and epileptogenesis phases of acquired epilepsy
The development of primary hippocampal culture models of epileptogenesis has allowed direct investigation of [Ca2+]i during epileptogenic injury. Experiments using the low-Mg2+ hippocampal neuronal culture model of epileptogenesis have directly implicated Ca2+ influx through the activated NMDA receptor in the development of epilepsy (DeLorenzo et al., 1998). Although MK801 and APV have no effect on the low-Mg2+-induced continuous spike firing of this model, these NMDA antagonists do block the development of SREDs (Sombati & DeLorenzo, 1995; DeLorenzo et al., 1998). These drugs also significantly reduce the sustained elevations in [Ca2+]i that occur during epileptogenic low-Mg2+-induced spike firing (DeLorenzo et al., 1998; Pal et al., 1999; Fig. 8). Similarly, when the driving force for Ca2+ influx is decreased by reducing [Ca2+]e from 2.0 to 0.2 mM, neurons in low Mg2+ continue to undergo spike firing with significantly lower elevation in [Ca2+]i compared to parallel experiments in normal [Ca2+]e (DeLorenzo et al., 1998; Fig. 6 and Fig 7). Lowering [Ca2+]e produced a decrease in [Ca2+]i during SE that did not stop the electrographic seizure discharges due to low Mg2+ (Fig. 6), but could be directly and simultaneously observed by Ca2+ imaging to have decreased [Ca2+]i (Fig. 8). These neurons did not exhibit epileptiform activity (Fig. 11). Lowering [Ca2+]i during SE with [1,2-bis(2)-aminophenoxy] ethane-N,N-N′,N′-tetraacetic acid (BAPTA) also lowered [Ca2+]i during SE (Fig. 12) and blocked the development of AE (Fig. 7). Blockade of AMPA receptors, KA receptors, and L-type VGCCs had no effect on low-Mg2+-induced continuous spike firing, the associated increase in [Ca2+]i, or the induction of epileptogenesis (DeLorenzo et al., 1998; Fig. 6–Fig 8). These studies directly relate Ca2+ elevations through the NMDA receptor as an initiating factor in the development of epilepsy.
Fig. 11.
Pseudocolor fluorescent optical images of representative control and epileptic acutely isolated neurons from the pilocarpine model of AE before, during, and after the 50 µM glutamate exposure for 1 min utilizing the low-affinity Ca2+ indicator Fura-FF. (Modified from Raza et al., 2001.) As seen for the other treatments, basal [Ca2+]i levels were low in both the control and the epileptic neurons and rose rapidly during the glutamate exposure to high levels (~5 µM [Ca2+]i). While the control CA1 neurons were near preglutamate levels 10 min after glutamate exposure, the epileptic neurons exhibited high [Ca2+]i levels at that time point. Twenty minutes after the initial [Ca2+]i load, control cells had completely returned to basal levels of [Ca2+]i, while the [Ca2+]i levels in the epileptic cells did not reach baseline levels. The epileptic cells did not recover even after 40-min postglutamate exposure (data not shown). The top 2 panels display the phase micrographs of the representative control and epileptic neurons. The data shown were representative of 15 experiments.
Fig. 12.
Activity of SERCA (A), CICR-IP3 (B), and CICR-Ryanodine (C) in control and epileptic neurons in the chronic epilepsy phase of AE in the SE-induced HNC model of AE. (Modified from Pal et al., 2001.) (A) SERCA activity in control and epileptic neurons. The results indicate epileptogenesis-inhibited SERCA activity. The data represent the means ± SE of the mean differences between the curves. These differences in means between the control and the “epileptic” curves were statistically significant (*p < 0.05, Hoteling’s T2-test). (B) Contribution of IP3 CICR activity to the delayed recovery of [Ca2+]i levels in normal and “epileptic” cells following a 50 µM glutamate [Ca2+]i load. IP3 CICR activity estimated for control and epileptic neurons. The curves indicate a much more augmented 2APB-inhibited release mechanism in the “epileptic” neurons compared to the control neurons. This release is an estimate of the IP3 receptor-activated CICR from the ER. The data represent the means ± SE of the mean differences between the curves. These differences in means between the control and the “epileptic” curves were statistically significant (*p < 0.05, Hoteling’s T2-test). (C) Contribution of ryanodine CICR activity to the delayed recovery of [Ca2+]i levels in normal and “epileptic” cells following a 50 µM glutamate [Ca2+]i load. Ryanodine CICR activity estimated for control and epileptic neurons. The curves indicate similar dantrolene-inhibited release in both control and “epileptic” neurons. This release represents the ryanodine receptor-activated CICR from the ER. The data represent the means ± SE of the mean differences between the curves. These differences in means between the control and the “epileptic” curves were not statistically significant (Hoteling’s T2-test).
In the glutamate injury-induced epileptogenesis model of stroke-induced spontaneous recurrent seizures, the subexcitotoxic glutamate exposure used to initiate epileptogenesis causes a prolonged but reversible elevation in neuronal [Ca2+]i (Sun et al., 2002). As in the low-Mg2+ model, this prolonged elevation is significantly decreased when glutamate injury is performed in the presence of NMDA receptor antagonists or in low [Ca2+]e, and these same experimental conditions inhibit epileptogenesis (Sun et al., 2002). Prolonged, reversible increases in [Ca2+]i can be produced in cultured neurons by increasing the extracellular potassium concentration of the bathing media. However, this elevation in [Ca2+]i does not induce epileptogenesis (Sun et al., 2002). Treatment of cultures with antagonists of non-NMDA receptor subtypes had neither antiepileptogenic effects nor significant effects on glutamate-induced changes in [Ca2+]i. Statistical analysis in this model further revealed that the duration of statistically elevated [Ca2+]i correlated with epileptogenesis, whereas total or percent increase in [Ca2+]i did not (Sun et al., 2002). Thus, Ca2+ influx through the activated NMDA receptor and prolonged elevations of neuronal free intracellular Ca2+ appear to be required in glutamate injury-induced epileptogenesis.
The role of Ca2+ in epileptogenesis is not simply limited to its role as a charge carrier through the NMDA receptor but also as a second messenger molecule within the cytoplasm. Preloading cultured hippocampal neurons with the intracellular Ca2+ chelator, BAPTA, prevents epileptogenesis in normal Ca2+ environments (DeLorenzo et al., 1998; Sun et al., 2002). In this case, Ca2+ still crosses the neuronal membrane through the NMDA receptor, but is prevented from initiating Ca2+-sensitive pathways because of its rapid binding to BAPTA (DeLorenzo et al., 1998; Sun et al., 2002). In experiments where barium is substituted for extracellular Ca2+, epileptogenesis does not occur (DeLorenzo et al., 1998; Sun et al., 2002). Since barium does not support Ca2+-dependent second messenger pathways, these data suggest that a specific Ca2+ signaling cascade is involved in the epileptogenesis in primary hippocampal culture models of SE- and stroke-induced spontaneous recurrent seizures (DeLorenzo et al., 1998; Sun et al., 2002).
Recently, acute neuronal isolation techniques have been utilized to study [Ca2+]i in whole animal models of epileptogenesis (Raza et al., 2004). Acutely isolated neurons were isolated from the hippocampus of rats during the acute (injury) and epileptogenic (latency) phases of the development of AE. Fig. 3A provides data on the hippocampal neuronal [Ca2+]i during the acute, epileptogenic, and chronic phases of AE. This data provides the first direct evidence that [Ca2+]i is significantly elevated immediately after SE and remains elevated during the time window of epileptogenesis in the pilocarpine model (Fig. 3A and D). Sham and pilocarpine without SE control animals did not develop elevated [Ca2+]i or AE. Furthermore, some animals that were given pilocarpine developed SE, but did not develop AE. SE without AE animals did not manifest elevations in [Ca2+]i (Fig. 3A). These results provide evidence for a close relationship between elevated [Ca2+]i and the development of AE (Fig. 3A and D). The effects of SE on elevations in [Ca2+]i were shown not to be due to injury-induced increases in [Ca2+]i due to apoptosis or necrosis, since it was possible to demonstrate that acutely isolated neurons used for [Ca2+]i measurements did not manifest more that 10% apoptosis or necrosis under conditions where more than 70% of the neurons had elevated [Ca2+]i (Fig. 3C). In addition, employing MK801 during the SE-induced injury blocked this rise in [Ca2+]i during the acute, epileptogenic, and chronic phases of AE and also prevented the development of AE (Raza et al., 2004). These results provide strong evidence that elevations of [Ca2+]i play a role in the development of AE.
9.4. Increased [Ca2+]i during the injury phase of acquired epilepsy in other brain regions and hippocampal neurons
Since the hippocampus is the focus of many of the long-term plasticity changes in AE, it is important to determine if changes in CA1 neurons are observed in other types of hippocampal neurons and in other brain regions. In the pilocarpine model, it was shown that the acute changes in [Ca2+]i following SE in hippocampal neurons were not occurring in frontal or occipital cortical neurons (Raza et al., 2004; Fig. 3B). These results indicate that there may be some selectivity to the effects of SE on [Ca2+]i in different brain regions. Since the hippocampus is at the center of many of the electrophysiological and molecular changes observed in the pilocarpine model of AE (Sanabria et al., 2002), it is not surprising that the [Ca2+]i changes would also be most pronounced in the hippocampus in comparison to frontal and occipital cortical regions. Further studies are needed to evaluate the anatomic distribution of the SE-induced alterations in [Ca2+]i levels and to determine if they are seen throughout the limbic system or in other relevant structures.
Initial studies from our laboratory focused on SE-induced changes in CA1 hippocampal [Ca2+]i levels (Raza et al., 2001, 2004). However, additional studies are needed to evaluate [Ca2+]i changes in other neurons in the dentate gyrus and CA1-CA3 regions and the hilus. It was found that interneurons in the CA1 region also manifested alterations in [Ca2+]i after SE (Raza et al., in press). Although acutely isolated neurons were prepared from tissue punches dissected from the pyramidal neuronenriched stratum pyramidale of the CA1 and layers 3–5 of the frontal and occipital cortex, it was necessary to evaluate possible minimal contamination from nonprinciple neurons or interneurons. Pyramidal neurons were identified morphologically and by the absence of immunoreactivity for specific protein markers for interneurons that include parvalbumin, cholecystokinin, vasoactive intestinal peptide, somatostatin, and neuropeptide Y (Scharfman et al., 2000; Matyas et al., 2004). Employing these markers that stain interneurons, we are characterizing neurons studied by calcium imaging to more clearly identify the neuronal types that undergo SE-induced changes in [Ca2+]i. This technique can be employed in both in vitro and acutely isolated in vivo experiments. These studies will allow us to more definitively identify the characteristics of neurons that undergo altered Ca2+ dynamics after injury in different brain regions and areas of the hippocampus.
Epilepsy is an excellent model of neuronal plasticity (Heinemann, 2004; Scharfman, 2002a). Since numerous changes in Ca2+ dynamics in the interneurons as well as pyramidal neurons have been observed in the pilocarpine and in the SE and GET in vitro models of AE, it is possible that these changes in Ca2+ dynamics may play a role in some of the many plasticity changes observed in pyramidal neurons, dentate granule neurons and interneurons that occur with the development of AE. These changes include changes in GABA receptor function (Coulter, 2001), the initiation of mossy fiber sprouting (Ben-Ari, 2001, Nadler, 2003), alterations in peptide expression (Wasterlain et al., 2002), neuronal reorganization (Sanabria et al., 2002), reorganization of cannabinoid receptor expression (Wallace et al., 2003), changes in the expression of BDNF (Scharfman, 1999; Scharfman et al., 1999; Binder et al., 2001; Scharfman, 2002b), the onset of neurogenesis (Parent et al., 1997, 2002), and other cellular and functional changes (Scharfman, 1992, 1995). It is important to understand the molecular basis for these long-term alterations. The possible role of Ca2+ as a second messenger mediating some of these changes in hippocampal CA neurons, hilar neurons, dentate granule neurons, and interneurons is an important area of further investigation. It is possible that some but not all of these plasticity changes associated with SE are mediated by calcium.
10. Calcium and the maintenance of acquired epilepsy
The Ca2+ hypothesis of epileptogenesis proposes that persistent alterations in Ca2+ homeostasis also underlie the maintenance of the epileptic condition. Whereas Ca2+ overload and irreversible loss of Ca2+ homeostasis have been implicated in excitotoxic neuronal death (Limbrick et al., 1995), long-lasting changes in normal Ca2+ handling, below threshold for excitotoxic cascade activation, may sustain the pathophysiological changes associated with the expression of spontaneous recurrent seizures. The Ca2+ hypothesis of epileptogenesis proposes that long-term alterations in Ca2+ homeostasis underlie the chronic nature of the epileptic condition.
10.1. Permanent elevations of hippocampal neuronal [Ca2+]i are associated with the chronic phase of acquired epilepsy
Experiments using chronically epileptic brain tissue and HNC neuronal networks in the SE and stroke HNC and the pilocarpine models of AE have revealed permanent alterations in basal levels of free [Ca2+]i (Fig. 9). Basal [Ca2+]i in both the cytoplasm and the nucleus of cultured epileptic neurons was significantly higher than in control neurons (Pal et al., 2000; Sun et al., 2004) using the SE induce HNC AE model (Fig. 9A). The stroke-induced HNC model of AE produced the same permanent increase in [Ca2+]i (Fig. 9B). This elevation in [Ca2+]i was maintained in comparison to control conditions, when epileptiform discharges were blocked by tetrodotoxin in established control and epileptic cultures (Fig. 9B), suggesting that Ca2+ entry during seizure activity contributed in part to the elevated Ca2+ levels (Sun et al., 2004), but that the chronic epileptic phase of AE was still associated with a chronic increase in [Ca2+]i.
Fig. 9.
Effect of epileptogenesis on hippocampal neuronal [Ca2+]i levels in control and epileptic neurons in the chronic epilepsy phase of AE in the SE-induced HNC (A), stroke-induced HNC (B), and the pilocarpine (C) models of AE. (A) Induction of SREDs caused increased [Ca2+]i levels in the nucleus and cytosol of hippocampal neurons. Ratio values (405/485 nm) were obtained with Indo-1 using the point scan mode of the ACAS confocal fluorescence microscope. The ratio values shown are representative of 100-msec scans for a 2-min period. Baseline [Ca2+]i levels in both the nucleus and cytosol of control neurons were low. Neurons undergoing SREDs exhibited a statistically significant increase in the basal [Ca2+]i in both the nucleus (*p < 0.001, Student’s t-test) and cytosol (*p < 0.001, Student’s t-test) for the life of the neurons in culture. (From Pal et al., 1999.) (B) Epileptiform activity contributed, in part, to the chronic elevations in basal [Ca2+]i measured 5 days after glutamate injury-induced epileptogenesis. Blockade of epileptiform activity by 600 nM TTX significantly reduced the long-lasting elevations in basal [Ca2+]i in epileptic neurons (n = 99) in comparison to epileptic neurons (n = 94) exhibiting SREDs. Basal [Ca2+]i in epileptic neurons (n = 99) was still significantly elevated compared to control neurons (n = 71) in the presence of 600 nM TTX (*p < 0.05, Student’s t-test). Data are represented by mean ± SEM. (Modified from Sun et al., 2002.) (C) Induction of epileptogenesis caused increased basal [Ca2+]i levels in neurons acutely isolated from animals subjected to pilocarpine-induced temporal lobe epilepsy. Absolute [Ca2+]i levels in control and epileptic neurons were obtained after using a calibration curve for the Fura-2 Ca2+ indicator. Baseline [Ca2+]i levels in both control and epileptic CA1 hippocampal neurons were low. Epileptic neurons exhibited a statistically significant increase (*p < 0.005, n = 171 for control and n = 164 for the epileptic neurons) in the basal [Ca2+]i at 1 year after induction of epileptogenesis. (Modified from Raza et al., 2001.)
To further evaluate whether [Ca2+]i levels were elevated in hippocampal neurons in epileptic animals, our laboratory developed the acute isolation procedure for Ca2+ imaging of intact hippocampal neurons acutely isolated from control and epileptic animals (Raza et al., 2001). This technique allowed us to determine if the chronic phase of AE produced the same effect on hippocampal [Ca2+]i levels as observed in the HNC culture models of AE. Resting [Ca2+]i measured in acutely isolated CA1 hippocampal neurons 1 year after the induction of spontaneous recurrent seizures in the chronic phase of AE were significantly elevated compared to control neurons in the pilocarpine model of SE-induced AE (Raza et al., 2001, 2004; Fig. 9C). Thus, the development of AE is associated with essentially permanent elevations of [Ca2+]i. in hippocampal neurons in the epileptic neurons in both in vitro and animal models of AE.
10.2. Altered calcium homeostatic mechanisms in the chronic phase of acquired epilepsy
In addition to essentially permanent elevations of [Ca2+]i in hippocampal neurons in the epileptic neurons, chronically epileptic neurons also manifest chronically altered Ca2+ homeostatic mechanisms. Alterations in Ca2+ homeostatic mechanisms have been demonstrated in the chronic phase of AE in the SE- and stroke-induced HNC models of AE (Pal et al., 2000, 2001; Sun et al., 2004) and in the pilocarpine model of AE (Raza et al., 2001, 2004; Fig. 10 and Fig 11).
Fig. 10.
Comparison of [Ca2+]i recovery after a glutamate-induced calcium load in control and epileptic neurons in the SE-induced HNC (A), stroke-induced HNC (B), and the pilocarpine (C) models of AE. (A) Delayed recovery of [Ca2+]i levels in “epileptic” neurons in the SE HNC model of AE compared to control neurons in culture after a [Ca2+]i load produced by a 2-min exposure of 50 µM glutamate using low-affinity (Fura-FF) calcium indicators. (Modified from Pal et al., 2001.) Control and “epileptic” neurons exposed to glutamate under standard conditions. Figure represents the Fura-FF normalized average decay curves for control (n = 45) and “epileptic” (n = 45) neurons, respectively. The inability to restore resting [Ca2+]i levels in the “epileptic” neurons compared to control neurons was evident in these combined plots. Individual curves were smoothed and averaged, and the resultant decay curves from the highest point after 2 min of glutamate exposure are displayed. The “epileptic” cells showed statistically significantly delayed decline in [Ca2+]i compared to the control cells at 0.5 min fixed time points with both high- and low-affinity indicators ( p < 0.05, ANOVA, n = 45). (B) Impaired handling of glutamate-induced Ca2+ loads in epileptic neurons from the stroke HNC model of AE demonstrated by Ca2+ decay curves normalized as percent of the Ca2+ load induced during the glutamate exposure (Sun et al., 2004). The percent of Ca2+ load remaining at all time points after glutamate washout (time post-GLU) was significantly higher in epileptic neurons (n = 44; white) compared to control neurons (n = 43; black). *p < 0.01, multivariate ANOVA. Data are represented by mean ± SEM. (C) [Ca2+]i decay curves for the control and epileptic neurons acutely isolated from the hippocampus in the pilocarpine model of AE after a high[Ca2+]i load (50 µM glutamate for 1 min) using Fura-FF. (Modified from Raza et al., 2001.) The epileptic cells demonstrated a delayed recovery, which was statistically significantly higher than in the controls (*p < 0.002, n = 12 for controls and n = 9 for epileptics). Moreover, this delayed recovery was pronounced compared to the recovery with either the 5 or the 10 M glutamate stimuli. Recovery of [Ca2+]i levels was not evident until about 40-min postglutamate exposure (data not shown).
When challenged with moderate levels of glutamate (50 µM, 1 min), acutely isolated epileptic CA1 hippocampal neurons from control and epileptic animals in the chronic epilepsy phase of AE in the pilocarpine model of AE manifest a delayed recovery of resting Ca2+ levels compared to controls (Raza et al., 2001; Fig. 10 and Fig 11). This impaired ability to restore resting [Ca2+]i in epileptic neurons was not caused by enhanced Ca2+ influx during glutamate exposure (Raza et al., 2001). Instead, experiments suggest that this delayed restoration of resting Ca2+ levels may be caused by impairment in neuronal Ca2+ sequestration mechanisms.
Similar results were demonstrated in the SE- and stroke-induced HNC models of AE (Pal et al., 2000, 2001; Sun et al., 2004; Fig. 10). Using glutamate (50 µM, 1 min) to expose control and epileptic neuronal populations with a Ca2+ load, the rate of removal of [Ca2+]i can be evaluated. Epileptic neurons manifested altered Ca2+ homeostatic mechanisms, since they could not remove [Ca2+]i as effectively as control neurons (Fig. 10). These results demonstrate that neurons in the 2 HNC models of AE demonstrate the same alterations in Ca2+ homeostatic mechanisms observed in the hippocampal neurons in the pilocarpine model during the chronic phase of AE. Further studies have demonstrated that these alterations are mediated in part by chronic changes in the activity of SERCA- and IP3-stimulated CICR (Pal et al., 2001).
10.3. Inhibition of SERCA associated with the chronic phase of acquired epilepsy
Microsomes prepared from epileptic tissue demonstrate impaired SERCA activity immediately after pilocarpine-induced SE as well as 1 year after pilocarpine-induced epilepsy (Parsons et al., 2000, 2001). In the kindling model of epileptogenesis, Ca2+-binding proteins which rapidly buffer increases in [Ca2+]i (Baimbridge et al., 1992) are progressively lost (Baimbridge et al., 1985; Kohr et al., 1991). Measurements of [Ca2+]i in networks of cultured hippocampal neurons in the low-Mg2+ and glutamate-injury models of epileptogenesis demonstrate similar alterations in Ca2+ homeostasis to that seen in pilocarpine- induced epileptic animals (Pal et al., 2000, 2001).
Impaired SERCA-mediated Ca2+ sequestration similar to that of pilocarpine animals also contribute to chronically increased basal [Ca2+]i in the SE HNC model of AE (Pal et al., 2000, 2001). These studies demonstrate in intact neurons that SERCA is chronically inhibited in the chronic phase of AE (Fig. 12). The association of decreased SERCA activity in the epileptic state indicates that epileptic neurons are less able to remove elevated [Ca2+]i due to a decreased ability of SERCA to pump calcium out of the neuronal cytoplasm into the ER. These results provide a direct demonstration of a permanent alteration in SERCA activity in the chronic epileptic state that could be contributing to the altered Ca2+ homeostatic mechanisms observed in the chronic epileptic phase of AE.
10.4. Increased IP3 CICR activity associated with the chronic phase of acquired epilepsy
Recent studies were initiated to study the activity of RyR- and IP3-stimulated CICR enzyme systems in control and epileptic neurons in the SE HNC model of AE (Pal et al., 2001). Both of these CICR systems mediate Ca2+-stimulated release of Ca2+ from the ER during an increase in [Ca2+]i. It was possible to evaluate the activity of both the RyR- and IP3-stimulated CICR enzyme systems in control and epileptic neurons in the SE-induced HNC model of AE (Pal et al., 2001). The results demonstrate that the activity of IP3-stimulated CICR was significantly increased in the epileptic neurons in comparison to controls, while no effect of epileptogenesis was observed on the level of activity of the of RyR-stimulated CICR (Fig. 12).
These results demonstrate that the chronic epilepsy phase of AE is associated with a selective stimulation of the IP3-stimulated CICR but not of the RyR-stimulated CICR. The increased activity of IP3-stimulated CICR could also account for the results shown in Fig. 10, demonstrating the altered Ca2+ homeostatic mechanisms in the epileptic neurons. These studies provide direct evidence of long-term plasticity changes in both SERCA- and IP3-stimulated CIRC activity in the chronic epilepsy phase of AE and provide specific molecular targets to inhibit or stimulate to alter the abnormal changes induced by epileptogenesis in Ca2+ homeostatic mechanisms.
11. Effects of elevated [Ca2+]i and altered calcium homeostatic mechanisms in the chronic phase of acquired epilepsy on calcium-dependent signaling cascades
It has now been established in multiple models of AE that the epileptic phenotype is associated with elevated [Ca2+]i and altered Ca2+ homeostatic mechanisms in neurons in the hippocampus and possibly in other areas of the brain. Since Ca2+ is such a major second messenger system affecting many aspects of neuronal function, it is reasonable to assume that this underlying change in [Ca2+]i plays a major role in altering neuronal activity. Although it is still not possible to clearly define which second messenger systems are causing AE, it is possible to document several long-term changes in Ca2+-regulated neuronal processes. These long-term changes are suggested to be responsible for some of the long-lasting plasticity changes associated with AE in the calcium hypothesis of epileptogenesis and AE (DeLorenzo, in press). Epilepsy is an excellent model of neuronal plasticity (Scharfman, 2002a; Heinemann, 2004). The development of epilepsy is associated with many changes in the hippocampus, including initiation of mossy fiber sprouting (Ben-Ari, 2001; Nadler, 2003), changes in GABA receptor function (Coulter, 2001), neuronal reorganization (Sanabria et al., 2002), alterations in peptide expression (Wasterlain et al., 2002), changes in the expression of BDNF (Scharfman et al., 1999; Binder et al., 2001; Scharfman, 2002b), the onset of neurogenesis (Parent et al., 1997, 2002), reorganization of cannabinoid receptor expression (Blair et al., 2003), and other cellular and functional changes (Scharfman, 1992, 1995). It is important to understand the molecular basis for these long-term alterations. The possible role of Ca2+ as a second messenger mediating some of these changes is an important area of further investigation. It is possible that some but not all of these plasticity changes associated with SE are mediated by calcium.
It is important to identify the Ca2+ systems altered in AE, as a first initial step in understanding the molecular processes regulating the development of AE. Some of these plasticity changes may regulate neuronal excitability and seizure activity, others may offer protection against seizure activity, and still others may not play any role in the altered neuronal excitability underlying AE. Identifying these processes and understanding their role in the development of AE is important in novel developing treatments and strategies to prevent and reverse AE. Several [Ca2+]i-regulated enzyme systems and cellular processes have already been shown to be associated with AE. The following reviews these studies.
11.1. Effects of altered [Ca2+]i on Ca2+-regulated enzyme systems
Because of the significant regulatory and signaling role of Ca2+ in neurons (Ghosh & Greenberg, 1995; Berridge, 1998), basal elevations in [Ca2+]i, although slight in magnitude, may sustain alterations in Ca2+-dependent enzyme systems that contribute to the chronic manifestation of epileptiform activity. Levels of protein kinase C (α) isozyme are decreased after kindling epileptogenesis (Buzsaki et al., 1992) and the activity of the Ca2+/calmodulin-dependent phosphatase calcineurin is increased after pilocarpine-induced SE (Kurz et al., 2001). Ca2+/calmodulin kinase II (CaMKII) activity is depressed in human epileptic tissue (Battaglia et al., 2002) and in numerous whole animal models of epilepsy (Bronstein et al., 1990, 1992; Perlin & DeLorenzo, 1992; Wasterlain et al., 1992; Kochan et al., 2000). Epileptic neurons in culture also manifest some of the biochemical changes observed in whole animal models. For example, decreased CaMKII activity and α subunit expression are observed in the low-Mg2+ hippocampal neuronal culture model (Blair et al., 1999). Like epileptogenesis, these changes are dependent on NMDA receptor activation (Blair et al., 1999; Kochan et al., 2000).Decreased CaMKII activity has been further implicated in epileptogenesis by experiments using transgenic mice with a null mutation for the α subunit of CaMKII. These mice, lacking active CaMKII, manifest epileptiform activity after subconvulsive stimulation (Butler et al., 1995). Further, naRve hippocampal neuronal cultures treated with antisense oligonucleotides to the CaMKII α subunit, express decreased levels of CaMKII and exhibit SREDs as seen in the low-Mg2+ and glutamate injury-induced epileptogenesis models (Churn et al., 2000).
11.2. Calcium-dependent gene regulation in the development and maintenance of epilepsy
Alterations in messenger RNA and protein levels have been characterized in many models of epileptogenesis (DeLorenzo & Morris, 1999) and growing evidence suggests that Ca2+-dependent transcription factor regulation may be involved in epileptogenesis (Bading, 1999). NMDA receptor activation, cytoplasmic Ca2+ signals and nuclear Ca2+ signals can regulate gene transcription through multiple Ca2+-activated enzyme and transcription factor pathways (Ghosh & Greenberg, 1995; Hardingham et al., 1997; Platenik et al., 2000). Thus, activation of the NMDA receptor-Ca2+ transduction pathway during epileptogenesis and the chronic elevations of cytoplasmic and nuclear Ca2+ in epileptic neurons may underlie many of the alterations in gene expression associated with the development of epilepsy (DeLorenzo, 1991).
In the pilocarpine model of limbic epilepsy, increases in the binding and expression of the transcription factors, serum response factor (SRF) and ΔfosB, have been observed during epileptogenesis, as well as in chronically, epileptic animals (Morris et al., 1999, 2000). This increased expression required activation of the NMDA receptor-Ca2+ transduction pathway (Morris et al., 1999). Further, the expression of activator protein-1 (AP-1) transcription factor binding is increased in the pilocarpine and KA models (Bing et al., 1997; Morris et al., 1999). SRF phosphorylation by Ca2+-activated MAP kinase (MAPK) and Ca2+/calmodulin kinase IV (CaMKIV) pathways autoregulates its own expression via a positive feedback loop in addition to up-regulating the transcription of the FosB gene producing ΔFosB (Sheng & Greenberg, 1990; Morris et al., 1999, 2000). When dimerized with jun, ΔFosB may interact with the AP-1 DNA consensus promoter sequence on the GABAA receptor α5 subunit (and perhaps α2) to inhibit transcription of this gene(s) (Nakabeppu & Nathans, 1991; Lazo et al., 1992; Gizang-Ginsberg & Ziff, 1994). These pathways ultimately result in reduced GABAA receptor α5 and α2 subunit mRNAs, observed in some epileptogenesis models, which can lead to altered GABAA receptor function, decreased inhibition and the spontaneous recurrent seizure activity of epilepsy (Rice et al., 1996; Vick et al., 1996; Gibbs et al., 1997).
11.3. Calcium-dependent changes in neuronal inhibitory function
Hyperexcitability can manifest secondary to enhanced neuronal excitation or decreased inhibition. Alterations in the inhibitory function of neuronal networks have been established in many models of spontaneous recurrent seizures (Treiman, 2001). There exist many potential mechanisms for Ca2+ modulation of CNS inhibition. The GABAA receptor is responsible for fast inhibitory neurotransmission in the CNS (Kittler et al., 2002; Steiger & Russek, 2004) and in both human epileptic and pilocarpine-induced epileptic tissue, decreased GABAA receptor function has been observed to be both dependent on NMDA receptor activation and increased [Ca2+]i (Isokawa, 1998). As referred above, altered levels of GABAA receptor subunits (Rice et al., 1996; Vick et al., 1996; Brooks-Kayal et al., 1998) and Ca2+-dependent transcription factors (Morris et al., 1999, 2000) have been demonstrated in models of AE. Since CaMKII phosphorylation of the GABAA receptor enhances agonist binding (Churn & DeLorenzo, 1998) and GABAergic function (Wang et al., 1995), the decreased activity of CaMKII in epileptic neurons may contribute to decreased inhibitory neurotransmission in the epileptic brain (Gibbs et al., 1997; Naylor, 2002). Altered Ca2+ may also contribute to changes in GABAA receptor isoform gene expression (DeLorenzo & Morris, 1999).
11.4. Calcium and γ-amino butyric acidA receptor recycling in acquired epilepsy
Recent data suggests that increased receptor trafficking and a net decrease in the total number of available membrane GABAA receptors may contribute to decreased inhibition in epilepsy (Naylor, 2002; Blair et al., 2004). GABAA receptor trafficking at the neuronal synapse is dependent on clathrin-mediated endocytosis (Kneussel, 2002) and rates of clathrin-based endocytosis are thought to mediate in part by [Ca2+]i. The effects of elevated [Ca2+]i on the rate of GABAA receptor endocytosis may play an important role in the induction and maintenance of AE. Recent studies have demonstrated that decreased GABAA receptor binding and function in the SE HNC model of AE is in part regulated by increased endocytosis of the receptor in the epileptic condition (Blair et al., 2004). This process does not change the total protein expression of the receptor, but it decreased the available or functional GABAA receptor at the membrane surface. Using this model of AE is was possible to demonstrate that inhibition of Our preliminary results indicate that increased [Ca2+]i produced during the chronic epileptic phase of AE is sufficient to cause an increased rate of GABAA receptor endocytosis causing decreased GABAA receptor expression on the neuronal membrane and decreased response to GABA. Understanding the effects of increased [Ca2+]i on receptor recycling is a promising area for developing novel therapies and interventions for AE. Thus, there are many different pathways by which the NMDA receptor-mediated Ca2+ influx during epileptogenesis and the chronic changes in epileptic neuronal Ca2+ homeostasis can culminate in decreased CNS inhibition.
12. Implications for the development of novel anticonvulsant drugs, antiepileptogenic agents and potential therapies to possibly reverse acquired epilepsy
Understanding the role of Ca2+ as a second messenger in AE, may provide important insights into the molecular basis of the development of epileptogenesis. Although changes in Ca2+ second messenger effects in the development of AE are unlikely to explain all of the complex changes associated with the development of AE, the demonstration of alterations in Ca2+ systems in all 3 phases of the development of AE offer an important starting point for further exploration of the role of Ca2+ second messenger systems in AE.
12.1. Calcium systems as a target for anticonvulsant agents
Agents that alter Ca2+ homeostasis or regulate Ca2+ systems may provide important new anticonvulsant drugs. Several anticonvulsants have been shown to alter Ca2+ homeostasis (DeLorenzo, 1986; Stefani et al., 1997). Understanding the role of Ca2+ in the induction and maintenance of AE may provide targets for novel anticonvulsant compounds. Inhibiting Ca2+ entry or activity in the neuron may offer novel strategies for the development of anticonvulsant agents.
12.2. Calcium systems as a target for antiepileptogenic agents and the prevention of acquired epilepsy
A potentially exciting area suggested by these studies is the development of antiepileptogenic agents. At the present time there are no clinically demonstrated antiepileptogenic drugs (Anderson et al., 2003; Temkin, 2003; Watson et al., 2004).
Since it has been shown that Ca2+ remains significantly elevated for prolonged periods in the injury and epileptogenic phases in the development of AE, it may be possible to develop strategies to restore Ca2+ to control levels during these critical times in the development of AE. It has been shown that the inhibition of the NMDA receptor during SE can prevent the development of AE (Rice & DeLorenzo, 1998). More recent studies (Raza et al., 2004) have demonstrated that elevations of Ca2+ are also prevented by this same treatment. Thus, these studies provide a link between the prolonged second messenger effects produced by elevated Ca2+ during epileptogenesis and the development of AE.
Using this knowledge, it should be possible to target the reduction of Ca2+ in the injury and epileptogenesis phase of AE to prevent the development of seizures. Studies in our laboratory have demonstrated that reduction of [Ca2+]i during the injury and epileptogenesis phase of AE can prevent the development of seizures in the hippocampal neuronal culture model of AE (DeLorenzo, in press). These results underscore the potential importance of developing methods for restoring normal Ca2+ homeostasis in the prevention of AE. Novel antiepileptogenic drugs that target the restoration of physiological [Ca2+]i during these critical time periods in the injury and epileptogenesis phases of AE offer a unique opportunity to prevent AE and significantly reduce the incidence of this common disorder. Administration of these “Ca2+ restoring” antiepileptogeneic agents as soon as possible after SE, stroke, or TBI may offer new frontiers for preventing the development of AE.
12.3. Calcium systems as a target for a possible cure of acquired epilepsy
The most exciting possible are for future research opened by the Ca2+ hypothesis of AE is the role of Ca2+ in the maintenance phase of AE. Experimental evidence presented in this paper indicates that altered Ca2+ homeostatic mechanisms may play an important role in the maintenance of the long-term plasticity changes induces during epileptogenesis and perpetuate the chronic epilepsy phase of AE. Although it is unlikely that restoring Ca2+ homeostatic mechanisms to normal in epileptic brain tissue will completely reverse all of the complex changes associated with AE (Scharfman, 2002a, 2002b), it is possible that it may restore enough normal physiological function to the epileptic neuron to decrease or even terminate seizure discharge. Several in vitro models have yielded insights in to the possible reversal or cure of AE by using agents that restore Ca2+ dynamics in the chronic epileptic phenotype (DeLorenzo, in press). Thus, targeting the long-term alterations in Ca2+ homeostatic mechanisms offers a potential site to begin our quest for the ultimate agents that may eventually lead to a cure of AE. Since Ca2+ is such a major second messenger system in neurons, it is not inconceivable to speculate that many of the long-term plasticity changes induced by epileptogenesis are mediated by changes in this second messenger in the epileptic phenotype. This are of research offers an exciting frontier for epilepsy research.
Acknowledgments
This research was supported by NIH grants RO1-NS and P50-NS. We would also like to thank our research colleagues, Dr. Robert Blair, Dr. Sompong Sombati, Dr. S.B. Churn, Dr. Douglas Coulter, Dr. David Limbrick, Dr. Michael Miles, and Dr. Steven Shapiro for their encouragement and suggestions over the years in developing this work. The encouragement and support of Dr. Michael Rogawski were greatly appreciated.
Abbreviations
- AE
acquired epilepsy
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate
- APV
2-amino-5-phosphonovalerate
- ATP
adenosine triphosphate
- BAPTA
[1,2-bis(2)-aminophenoxy] ethane-N,N-N′,N′-tetraacetic acid
- [Ca2+]i
intracellular calcium concentration levels
- CNS
centralt nervous system
- END
extended neuronal depolarization
- EPSP
excitatory postsynaptic potential
- ER
endoplasmic reticulum
- GABA
γ-amino butyric acid
- IP3R
inositol 1,4,5-tris-phosphate (IP3) receptor
- IRRC
inability to restore resting [Ca2+]i
- KA
kainate
- MK801
(+)5-methyl-10,11-dihydro-5H-dibenzo(a,b)cyclohepten-5,10-imine maleate
- NMDA
N-methyl-d-aspartic acid
- PLC
phospholipase C
- RNA
ribonucleic acid
- RyR
ryanodine receptor
- SE
status epilepticus
- SOCs
store-operated Ca2+ channels
- SREDs
spontaneous recurrent epileptiform discharges
- STIB
stimulus train-induced bursting
- TBI
traumatic brain injury
- VGCC
voltage-gated Ca2+ channel
References
- Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, et al. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115(7):863–877. doi: 10.1016/s0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- Abel T, Lattal KM. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol. 2001;11(2):180–187. doi: 10.1016/s0959-4388(00)00194-x. [DOI] [PubMed] [Google Scholar]
- Adams ME, Olivera BM. Neurotoxins: overview of an emerging research technology. Trends Neurosci. 1994;17(4):151–155. doi: 10.1016/0166-2236(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Akasu T, Muraoka N, Hasuo H. Hyperexcitability of hippocampal CA1 neurons after fluid percussion injury of the rat cerebral cortex. Neurosci Lett. 2002;329(3):305–308. doi: 10.1016/s0304-3940(02)00707-3. [DOI] [PubMed] [Google Scholar]
- Albensi BC. Models of brain injury and alterations in synaptic plasticity. J Neurosci Res. 2001;65(4):279–283. doi: 10.1002/jnr.1151. [DOI] [PubMed] [Google Scholar]
- Anderson WW, Lewis DV, Swartzwelder HS, Wilson WA. Magnesium-free medium activates seizure-like events in the rat hippocampal slice. Brain Res. 1986;398(1):215–219. doi: 10.1016/0006-8993(86)91274-6. [DOI] [PubMed] [Google Scholar]
- Anderson WW, Swartzwelder HS, Wilson WA. The NMDA receptor antagonist 2-amino-5-phosphonovalerate blocks stimulus train-induced epileptogenesis but not epileptiform bursting in the rat hippocampal slice. J Neurophysiol. 1987;57(1):1–21. doi: 10.1152/jn.1987.57.1.1. [DOI] [PubMed] [Google Scholar]
- Anderson VE, Hauser WA, Rich SS. Genetic heterogeneity and epidemiology of the epilepsies. In: Delgado-Escueta AV, Porter RJ, editors. Jasper’s Basic Mechanisms of the Epilepsies vol. 79. Philadephia: Lippincott Williams and Wilkins; 1999. pp. 59–73. [PubMed] [Google Scholar]
- Anderson GD, Temkin NR, Chandler WL, Winn HR. Effect of valproate on hemostatic function in patients with traumatic brain injury. Epilepsy Res. 2003;57(2–3):111–119. doi: 10.1016/j.eplepsyres.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Angeleri F, Majkowski J, Cacchio G, Sobieszek A, D’Acunto S, Gesuita R, et al. Posttraumatic epilepsy risk factors: one-year prospective study after head injury. Epilepsia. 1999;40(9):1222–1230. doi: 10.1111/j.1528-1157.1999.tb00850.x. [DOI] [PubMed] [Google Scholar]
- Annegers JF. The epidemiology of epilepsy. In: Wyllie E, editor. The Treatment of Epilepsy. Philadelphia: Lea and Febiger; 1993. pp. 157–164. [Google Scholar]
- Annegers JF, Coan SP. The risks of epilepsy after traumatic brain injury. Seizure. 2000;9(7):453–457. doi: 10.1053/seiz.2000.0458. [DOI] [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Brain Res Rev. 1999;29(1):83–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- Arabadzisz D, Freund TF. Changes in excitatory and inhibitory circuits of the rat hippocampus 12–14 months after complete forebrain ischemia. Neuroscience. 1999;92(1):27–45. doi: 10.1016/s0306-4522(98)00736-2. [DOI] [PubMed] [Google Scholar]
- Arriza JL, Eliasof S, Kavanaugh MP, Amara SG. Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proc Natl Acad Sci U S A. 1997;94(8):4155–4160. doi: 10.1073/pnas.94.8.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P, Nowak L. The role of divalent cations in the N-methyl-d-aspartate responses of mouse central neurones in culture. J Physiol (Lond) 1988;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asikainen I, Kaste M, Sarna S. Early and late posttraumatic seizures in traumatic brain injury rehabilitation patients: brain injury factors causing late seizures and influence of seizures on long-term outcome. Epilepsia. 1999;40(5):584–589. doi: 10.1111/j.1528-1157.1999.tb05560.x. [DOI] [PubMed] [Google Scholar]
- Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia—the ischemic penumbra. Stroke. 1981;12(6):723–725. doi: 10.1161/01.str.12.6.723. [DOI] [PubMed] [Google Scholar]
- Back T, Ginsberg MD, Dietrich WD, Watson BD. Induction of spreading depression in the ischemic hemisphere following experimental middle cerebral artery occlusion: effect on infarct morphology. J Cereb Blood Flow Metab. 1996;16(2):202–213. doi: 10.1097/00004647-199603000-00004. [DOI] [PubMed] [Google Scholar]
- Bading H. Nuclear calcium-activated gene expression: possible roles in neuronal plasticity and epileptogenesis. Epilepsy Res. 1999;36(2–3):225–231. doi: 10.1016/s0920-1211(99)00053-4. [DOI] [PubMed] [Google Scholar]
- Baimbridge KG, Mody I, Miller JJ. Reduction of rat hippocampal calcium-binding protein following commissural, amygdala, septal, perforant path, and olfactory bulb kindling. Epilepsia. 1985;26(5):460–465. doi: 10.1111/j.1528-1157.1985.tb05681.x. [DOI] [PubMed] [Google Scholar]
- Baimbridge KG, Celio MR, Rogers JH. Calcium-binding proteins in the nervous system. Trends Neurosci. 1992;15(8):303–308. doi: 10.1016/0166-2236(92)90081-i. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Pagliardini S, Ferrario A, Gardoni F, Tassi L, Setola V, et al. AlphaCaMKII and NMDA-receptor subunit expression in epileptogenic cortex from human periventricular nodular heterotopia. Epilepsia. 2002;43 Suppl 5:209–216. doi: 10.1046/j.1528-1157.43.s.5.38.x. [DOI] [PubMed] [Google Scholar]
- Beghi E, Roncolato M, Visona G. Depression and altered quality of life in women with epilepsy of childbearing age. Epilepsia. 2004;45(1):64–70. doi: 10.1111/j.0013-9580.2004.56502.x. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Cell death and synaptic reorganizations produced by seizures. Epilepsia. 2001;42 Suppl 3:5–7. doi: 10.1046/j.1528-1157.2001.042suppl.3005.x. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Tremblay E, Ottersen OP. Injections of kainic acid into the amygdaloid complex of the rat: an electrographic, clinical and histological study in relation to the pathology of epilepsy. Neuroscience. 1980;5(3):515–528. doi: 10.1016/0306-4522(80)90049-4. [DOI] [PubMed] [Google Scholar]
- Benavente-Aguilar I, Morales-Blanquez C, Rubio EA, Rey JM. Quality of life of adolescents suffering from epilepsy living in the community. J Paediatr Child Health. 2004;40(3):110–113. doi: 10.1111/j.1440-1754.2004.00308.x. [DOI] [PubMed] [Google Scholar]
- Benveniste H, Drejer J, Schousboe A, Diemer NH. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J Neurochem. 1984;43(5):1369–1374. doi: 10.1111/j.1471-4159.1984.tb05396.x. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Jahr CE. Glial contribution to glutamate uptake at Schaffer collateral-commissural synapses in the hippocampus. J Neurosci. 1998;18(19):7709–7716. doi: 10.1523/JNEUROSCI.18-19-07709.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999;79(4):1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21(1):13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- Bertram EH, Lothman EW. NMDA receptor antagonists and limbic status epilepticus: a comparison with standard anticonvulsants. Epilepsy Res. 1990;5(3):177–184. doi: 10.1016/0920-1211(90)90036-u. [DOI] [PubMed] [Google Scholar]
- Bertrand S, Weiland S, Berkovic SF, Steinlein OK, Bertrand D. Properties of neuronal nicotinic acetylcholine receptor mutants from humans suffering from autosomal dominant nocturnal frontal lobe epilepsy. Br J Pharmacol. 1998;125(4):751–760. doi: 10.1038/sj.bjp.0702154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biervert C, Schroeder BC, Kubisch C, Berkovic SF, Propping P, Jentsch TJ, et al. A potassium channel mutation in neonatal human epilepsy. Science. 1998;279(5349):403–406. doi: 10.1126/science.279.5349.403. [DOI] [PubMed] [Google Scholar]
- Binder DK, Croll SD, Gall CM, Scharfman HE. BDNF and epilepsy: too much of a good thing? Trends Neurosci. 2001;24(1):47–53. doi: 10.1016/s0166-2236(00)01682-9. [DOI] [PubMed] [Google Scholar]
- Bing G, Wilson B, Hudson P, Jin L, Feng Z, Zhang W, et al. A single dose of kainic acid elevates the levels of enkephalins and activator protein-1 transcription factors in the hippocampus for up to 1 year. Proc Natl Acad Sci U S A. 1997;94(17):9422–9427. doi: 10.1073/pnas.94.17.9422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RE, Churn SB, Sombati S, Lou JK, DeLorenzo RJ. Long-lasting decrease in neuronal Ca2+/calmodulin-dependent protein kinase II activity in a hippocampal neuronal culture model of spontaneous recurrent seizures. Brain Res. 1999;851(1–2):54–65. doi: 10.1016/s0006-8993(99)02100-9. [DOI] [PubMed] [Google Scholar]
- Blair RE, Sombati S, Lawrence DC, McCay BD, DeLorenzo RJ. Epileptogenesis causes acute and chronic increases in GABAA receptor endocytosis that contributes to the induction and maintenance of seizures in the hippocampal culture model of AE. J Pharmacol Exp Ther. 2004;310(3):871–880. doi: 10.1124/jpet.104.068478. [DOI] [PubMed] [Google Scholar]
- Boulter J, Hollmann M, O’Shea-Greenfield A, Hartley M, Deneris E, Maron C, et al. Molecular cloning and functional expression of glutamate receptor subunit genes. Science. 1990;249(4972):1033–1037. doi: 10.1126/science.2168579. [DOI] [PubMed] [Google Scholar]
- Bradford HF. Glutamate, GABA and epilepsy. Prog Neurobiol. 1995;47(6):477–511. doi: 10.1016/0301-0082(95)00030-5. [DOI] [PubMed] [Google Scholar]
- Brandt C, Glien M, Potschka H, Volk H, Loscher W. Epileptogenesis and neuropathology after different types of status epilepticus induced by prolonged electrical stimulation of the basolateral amygdala in rats. Epilepsy Res. 2003;55(1–2):83–103. doi: 10.1016/s0920-1211(03)00114-1. [DOI] [PubMed] [Google Scholar]
- Bronstein JM, Farber DB, Micevych PE, Lasher R, Wasterlain CG. Kindling induced changes in calmodulin kinase II immunoreactivity. Brain Res. 1990;524(1):49–53. doi: 10.1016/0006-8993(90)90490-3. [DOI] [PubMed] [Google Scholar]
- Bronstein JM, Micevych P, Popper P, Huez G, Farber DB, Wasterlain CG. Long-lasting decreases of type II calmodulin kinase expression in kindled rat brains. Brain Res. 1992;584(1–2):257–260. doi: 10.1016/0006-8993(92)90903-m. [DOI] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABA(A) receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4(10):1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- Brose N, Petrenko AG, Sudhof TC, Jahn R. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science. 1992;256(5059):1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- Buchkremer-Ratzmann I, August M, Hagemann G, Witte OW. Epileptiform discharges to extracellular stimuli in rat neocortical slices after photothrombotic infarction. J Neurol Sci. 1998;156(2):133–137. doi: 10.1016/s0022-510x(98)00034-3. [DOI] [PubMed] [Google Scholar]
- Buelow JM. Epilepsy management issues and techniques. J Neurosci Nurs. 2001;33(5):260–269. doi: 10.1097/01376517-200110000-00006. [DOI] [PubMed] [Google Scholar]
- Bullock R, Zauner A, Woodward J, Young HF. Massive persistent release of excitatory amino acids following human occlusive stroke. Stroke. 1995;26(11):2187–2189. doi: 10.1161/01.str.26.11.2187. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Villarroel A, Sakmann B. Dimensions and ion selectivity of recombinant AMPA and kainate receptor channels and their dependence on Q/R site residues. J Physiol. 1996;496(Pt 1):165–173. doi: 10.1113/jphysiol.1996.sp021674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler LS, Silva AJ, Abeliovich A, Watanabe Y, Tonegawa S, McNamara JO. Limbic epilepsy in transgenic mice carrying a Ca2+/calmodulin-dependent kinase II alpha-subunit mutation. Proc Natl Acad Sci U S A. 1995;92(15):6852–6855. doi: 10.1073/pnas.92.15.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Hsu M, Horvath Z, Horsburgh K, Sundsmo M, Masliah E, et al. Kindling-induced changes of protein kinase C levels in hippocampus and neocortex. Epilepsy Res Suppl. 1992;9:279–283. [PubMed] [Google Scholar]
- Calabresi P, Pisani A, Mercuri NB, Bernardi G. On the mechanisms underlying hypoxia-induced membrane depolarization in striatal neurons. Brain. 1995;118(Pt 4):1027–1038. doi: 10.1093/brain/118.4.1027. [DOI] [PubMed] [Google Scholar]
- Capruso DX, Levin HS. Cognitive impairment following closed head injury. Neurol Clin. 1992;10(4):879–893. [PubMed] [Google Scholar]
- Carafoli E. The plasma membrane calcium pump. Structure, function, regulation. Biochim Biophys Acta. 1992;1101(2):266–267. [PubMed] [Google Scholar]
- Carafoli E, Nicotera P, Santella L. Calcium signalling in the cell nucleus. Cell Calcium. 1997;22(5):313–319. doi: 10.1016/s0143-4160(97)90016-6. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Molecular properties of sodium and calcium channels. J Bioenerg Biomembr. 1996;28(3):219–230. doi: 10.1007/BF02110697. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Striessnig J, Snutch TP. International union of pharmacology: XL. Compendium of voltage-gated ion channels: calcium channels. Pharmacol Rev. 2003;55(4):579–581. doi: 10.1124/pr.55.4.8. [DOI] [PubMed] [Google Scholar]
- Cavalheiro EA, Riche DA, Le Gal La Salle G. Long-term effects of intrahippocampal kainic acid injection in rats: a method for inducing spontaneous recurrent seizures. Electroencephalogr Clin Neurophysiol. 1982;53(6):581–589. doi: 10.1016/0013-4694(82)90134-1. [DOI] [PubMed] [Google Scholar]
- Chen QX, Perkins KL, Choi DW, Wong RKS. Secondary activation of a cation conductance is responsible for NMDA toxicity in acutely isolated hippocampal neurons. J Neurosci. 1997;17(11):4032–4036. doi: 10.1523/JNEUROSCI.17-11-04032.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW. Glutamate neurotoxicity in cortical cell culture is calcium dependent. Neurosci Lett. 1985;58(3):293–297. doi: 10.1016/0304-3940(85)90069-2. [DOI] [PubMed] [Google Scholar]
- Choi DW. Ionic dependence of glutamate neurotoxicity. J Neurosci. 1987;7(2):369–379. doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1(8):623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Choi DW. Glutamate receptors and the induction of excitotoxic neuronal death. Prog Brain Res. 1994;100:47–51. doi: 10.1016/s0079-6123(08)60767-0. [DOI] [PubMed] [Google Scholar]
- Choi DW, Rothman SM. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- Choi DW, Maulucci-Gedde M, Kriegstein AR. Glutamate neurotoxicity in cortical cell culture. J Neurosci. 1987;7(2):357–368. doi: 10.1523/JNEUROSCI.07-02-00357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW, Koh JY, Peters S. Pharmacology of glutamate neurotoxicity in cortical cell culture: attenuation by NMDA antagonists. J Neurosci. 1988;8(1):185–196. doi: 10.1523/JNEUROSCI.08-01-00185.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW, Weiss JH, Koh JY, Christine CW, Kurth MC. Glutamate neurotoxicity, calcium, and zinc. Ann NY Acad Sci. 1989;568:219–224. doi: 10.1111/j.1749-6632.1989.tb12511.x. [DOI] [PubMed] [Google Scholar]
- Christine CW, Choi DW. Effect of zinc on NMDA receptor-mediated channel currents in cortical neurons. J Neurosci. 1990;10(1):108–116. doi: 10.1523/JNEUROSCI.10-01-00108.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churn SB, DeLorenzo RJ. Modulation of GABAergic receptor binding by activation of calcium and calmodulin-dependent kinase II membrane phosphorylation. Brain Res. 1998;809(1):68–76. doi: 10.1016/s0006-8993(98)00834-8. [DOI] [PubMed] [Google Scholar]
- Churn SB, Kochan LD, DeLorenzo RJ. Chronic inhibition of Ca(2+)/calmodulin kinase II activity in the pilocarpine model of epilepsy [in process citation] Brain Res. 2000;875(1–2):66–77. doi: 10.1016/s0006-8993(00)02623-8. [DOI] [PubMed] [Google Scholar]
- Congar P, Gaiarsa JL, Popovici T, Ben-Ari Y, Crepel V. Permanent reduction of seizure threshold in post-ischemic CA3 pyramidal neurons. J Neurophysiol. 2000;83(4):2040–2046. doi: 10.1152/jn.2000.83.4.2040. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Connor JA, Wadman WJ, Hockberger PE, Wong RK. Sustained dendritic gradients of Ca2+ induced by excitatory amino acids in CA1 hippocampal neurons. Science. 1988;240(4852):649–653. doi: 10.1126/science.2452481. [DOI] [PubMed] [Google Scholar]
- Conti F, Weinberg RJ. Shaping excitation at glutamatergic synapses. Trends Neurosci. 1999;22(10):451–458. doi: 10.1016/s0166-2236(99)01445-9. [DOI] [PubMed] [Google Scholar]
- Copp AJ, Harding BN. Neuronal migration disorders in humans and in mouse models—an overview. Epilepsy Res. 1999;36(2–3):133–141. doi: 10.1016/s0920-1211(99)00047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter DA. Epilepsy-associated plasticity in gamma-amino-butyric acid receptor expression, function, and inhibitory synaptic properties. Int Rev Neurobiol. 2001;45:237–252. doi: 10.1016/s0074-7742(01)45013-6. [DOI] [PubMed] [Google Scholar]
- Coulter DA, Sombati S, DeLorenzo RJ. Electrophysiology of glutamate neurotoxicity in vitro: induction of a calcium-dependent extended neuronal depolarization. J Neurophysiol. 1992;68(2):362–373. doi: 10.1152/jn.1992.68.2.362. [DOI] [PubMed] [Google Scholar]
- Coulter DA, Rafiq A, Shumate M, Gong QZ, DeLorenzo RJ, Lyeth BG. Brain injury-induced enhanced limbic epilepto-genesis: anatomical and physiological parallels to an animal model of temporal lobe epilepsy. Epilepsy Res. 1996;26(1):81–91. doi: 10.1016/s0920-1211(96)00044-7. [DOI] [PubMed] [Google Scholar]
- Coulter DA, McIntyre DC, Loscher W. Animal models of limbic epilepsies: what can they tell us? Brain Pathol. 2002;12(2):240–256. doi: 10.1111/j.1750-3639.2002.tb00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer JA, Westbrook LE, Devinsky O, Perrine K, Glassman MB, Camfield C. Development of the quality of life in epilepsy inventory for adolescents: the QOLIE-AD-48. Epilepsia. 1999;40(8):1114–1121. doi: 10.1111/j.1528-1157.1999.tb00828.x. [DOI] [PubMed] [Google Scholar]
- Croucher MJ, Bradford HF. Kindling of full limbic seizures by repeated microinjections of excitatory amino acids into the rat amygdala. Brain Res. 1989;501(1):58–65. doi: 10.1016/0006-8993(89)91026-3. [DOI] [PubMed] [Google Scholar]
- Croucher MJ, Bradford HF. NMDA receptor blockade inhibits glutamate-induced kindling of the rat amygdala. Brain Res. 1990;506(2):349–352. doi: 10.1016/0006-8993(90)91279-p. [DOI] [PubMed] [Google Scholar]
- Croucher MJ, Bradford HF, Sunter DC, Watkins JC. Inhibition of the development of electrical kindling of the prepyriform cortex by daily focal injections of excitatory amino acid antagonists. Eur J Pharmacol. 1988;152(1–2):29–38. doi: 10.1016/0014-2999(88)90832-1. [DOI] [PubMed] [Google Scholar]
- Croucher MJ, Cotterell KL, Bradford HF. Competitive NMDA receptor antagonists raise electrically kindled generalized seizure thresholds. Neurochem Res. 1992;17(5):409–413. doi: 10.1007/BF00969885. [DOI] [PubMed] [Google Scholar]
- Croucher MJ, Cotterell KL, Bradford HF. Amygdaloid kindling by repeated focal N-methyl-d-aspartate administration: comparison with electrical kindling. Eur J Pharmacol. 1995;286(3):265–271. doi: 10.1016/0014-2999(95)00459-6. [DOI] [PubMed] [Google Scholar]
- D’Ambrosio R, Fairbanks JP, Fender JS, Born DE, Doyle DL, Miller JW. Post-traumatic epilepsy following fluid percussion injury in the rat. Brain. 2004;127(Pt 2):304–314. doi: 10.1093/brain/awh038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Sasaki YF, Rothe T, Premkumar LS, Takasu M, Crandall JE, et al. Increased NMDA current and spine density in mice lacking the NMDA receptor subunit NR3A. Nature. 1998;393(6683):377–381. doi: 10.1038/30748. [DOI] [PubMed] [Google Scholar]
- Davalos A, Castillo J, Serena J, Noya M. Duration of glutamate release after acute ischemic stroke. Stroke. 1997;28(4):708–710. doi: 10.1161/01.str.28.4.708. [DOI] [PubMed] [Google Scholar]
- Davies SN, Pertwee RG, Riedel G. Functions of cannabinoid receptors in the hippocampus. Neuropharmacology. 2002;42(8):993–1007. doi: 10.1016/s0028-3908(02)00060-6. [DOI] [PubMed] [Google Scholar]
- Dawson VL, Dawson TM, London ED, Bredt DS, Snyder SH. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci U S A. 1991;88(14):6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoster MA, Koenig ML, Hunter JC, Tortella FC. Calcium dynamics in neurons treated with toxic and non-toxic concentrations of glutamate. Neuroreport. 1992;3(9):773–776. doi: 10.1097/00001756-199209000-00013. [DOI] [PubMed] [Google Scholar]
- de Lanerolle NC, Kim JH, Robbins RJ, Spencer DD. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res. 1989;495(2):387–395. doi: 10.1016/0006-8993(89)90234-5. [DOI] [PubMed] [Google Scholar]
- DeLorenzo RJ. A molecular approach to the calcium signal in brain: relationship to synaptic modulation and seizure discharge. Adv Neurol. 1986;44:435–464. [PubMed] [Google Scholar]
- DeLorenzo RJ. The Epilepsies. In: Bradley WG, Daroff RB, Fenichel GM, Marsden CD, editors. Neurology in Clinical Practice. Stoneham, MA: Butterworth Publishers; 1989. [Google Scholar]
- DeLorenzo RJ. The challenging genetics of epilepsy. Epilepsy Res Suppl. 1991;4:3–17. [PubMed] [Google Scholar]
- DeLorenzo RJ. Clinical and Epidemiological Study of Status Epilepticus in the Elderly. Newton, MA: Butterworth-Heinemann; 1997. [Google Scholar]
- DeLorenzo RJ. New drugs for the prevention of epilepsy and how they work: the role of calcium in epileptogenesis. Epilepsy Res. 2004 (in press). [Google Scholar]
- DeLorenzo RJ, Morris TA. Long-term modulation of gene expression in epilepsy. Neuroscientist. 1999;5:86–99. [Google Scholar]
- DeLorenzo RJ, Pellock JM, Towne AR, Boggs JG. Epidemiology of status epilepticus. J Clin Neurophysiol. 1995;12(4):316–325. [PubMed] [Google Scholar]
- DeLorenzo RJ, Hauser WA, Towne AR, Boggs JG, Pellock JM, Penberthy L, et al. A prospective, population-based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology. 1996;46(4):1029–1035. doi: 10.1212/wnl.46.4.1029. [DOI] [PubMed] [Google Scholar]
- DeLorenzo RJ, Pal S, Sombati S. Prolonged activation of the N-methyl-d-aspartate receptor-Ca2+ transduction pathway causes spontaneous recurrent epileptiform discharges in hippocampal neurons in culture. Proc Natl Acad Sci U S A. 1998;95(24):14482–14487. doi: 10.1073/pnas.95.24.14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EA. Diversity of group types, regulation, and function of phospholipase A2. J Biol Chem. 1994;269(18):13057–13060. [PubMed] [Google Scholar]
- Dietrich WD. Neurobiology of stroke. Int Rev Neurobiol. 1998;42:55–101. doi: 10.1016/s0074-7742(08)60608-x. [DOI] [PubMed] [Google Scholar]
- Dijkhuizen RM, Beekwilder JP, van der Worp HB, BvdSJW, Tulleken KA, Nicolay K. Correlation between tissue depolarizations and damage in focal ischemic rat brain. Brain Res. 1999;840(1–2):194–205. doi: 10.1016/s0006-8993(99)01769-2. [DOI] [PubMed] [Google Scholar]
- Dingledine R, McBain CJ. Glutamate and aspartate. In: Siegel GJ, editor. Basic Neurochemistry: Molecular, Cellular, and Medical Aspects. Philadelphia: Lippincott-Raven; 1999. pp. 315–333. [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51(1):7–61. [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22(9):391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Dowzendo A, Zielinski JJ. Problems of epilepsy in Poland. Epilepsia. 1971;12(2):173–177. doi: 10.1111/j.1528-1157.1971.tb03930.x. [DOI] [PubMed] [Google Scholar]
- Drislane FW. Presentation, evaluation, and treatment of non-convulsive status epilepticus. Epilepsy Behav. 2000;1(5):301–314. doi: 10.1006/ebeh.2000.0100. [DOI] [PubMed] [Google Scholar]
- Du Y, Dodel RC, Bales KR, Jemmerson R, Hamilton-Byrd E, Paul SM. Involvement of a caspase-3-like cysteine protease in 1-methyl-4-phenylpyridinium-mediated apoptosis of cultured cerebellar granule neurons. J Neurochem. 1997;69(4):1382–1388. doi: 10.1046/j.1471-4159.1997.69041382.x. [DOI] [PubMed] [Google Scholar]
- Dubinsky JM, Rothman SM. Intracellular calcium concentrations during “chemical hypoxia” and excitotoxic neuronal injury. J Neurosci. 1991;11(8):2545–2551. doi: 10.1523/JNEUROSCI.11-08-02545.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugan LL, Sensi SL, Canzoniero LM, Handran SD, Rothman SM, Lin TS, et al. Mitochondrial production of reactive oxygen species in cortical neurons following exposure to N-methyl-d-aspartate. J Neurosci. 1995;15(10):6377–6388. doi: 10.1523/JNEUROSCI.15-10-06377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- During MJ, Spencer DD. Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet. 1993;341(8861):1607–1610. doi: 10.1016/0140-6736(93)90754-5. [DOI] [PubMed] [Google Scholar]
- Dutton R, McCunn M. Traumatic brain injury. Curr Opin Crit Care. 2003;9(6):503–509. doi: 10.1097/00075198-200312000-00007. [DOI] [PubMed] [Google Scholar]
- Ebert U, Brandt C, Loscher W. Delayed sclerosis, neuro-protection, and limbic epileptogenesis after status epilepticus in the rat. Epilepsia. 2002;43 Suppl 5:86–95. doi: 10.1046/j.1528-1157.43.s.5.39.x. [DOI] [PubMed] [Google Scholar]
- Ehrlich BE, Kaftan E, Bezprozvannaya S, Bezprozvanny I. The pharmacology of intracellular Ca(2+)-release channels. Trends Pharmacol Sci. 1994;15(5):145–149. doi: 10.1016/0165-6147(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Ellis EF, McKinney JS, Willoughby KA, Liang S, Povlishock JT. A new model for rapid stretch-induced injury of cells in culture: characterization of the model using astrocytes. J Neurotrauma. 1995;12(3):325–339. doi: 10.1089/neu.1995.12.325. [DOI] [PubMed] [Google Scholar]
- Emerich D, Bartus RT. Intracellular events associated with cerebral ischemia. In: Miller L, editor. Stroke Therapy: Basic, Preclinical, and Clinical Directions. New York: Wiley-Liss; 1999. pp. 195–218. [Google Scholar]
- Fagni L, Chavis P, Ango F, Bockaert J. Complex interactions between mGluRs, intracellular Ca2+ stores and ion channels in neurons. Trends Neurosci. 2000;23(2):80–88. doi: 10.1016/s0166-2236(99)01492-7. [DOI] [PubMed] [Google Scholar]
- Fountain NB, Lothman EW. Pathophysiology of status epilepticus. J Clin Neurophysiol. 1995;12(4):326–342. [PubMed] [Google Scholar]
- Fountain NB, Bear J, Bertram EH, III, Lothman EW. Responses of deep entorhinal cortex are epileptiform in an electrogenic rat model of chronic temporal lobe epilepsy. J Neurophysiol. 1998;80(1):230–240. doi: 10.1152/jn.1998.80.1.230. [DOI] [PubMed] [Google Scholar]
- Frazen N. Seizures and Epilepsy: Hope Through Research. Bethesda, MD: NINDS; 2000. [Google Scholar]
- Frerking M, Nicoll RA. Synaptic kainate receptors. Curr Opin Neurobiol. 2000;10(3):342–351. doi: 10.1016/s0959-4388(00)00094-5. [DOI] [PubMed] [Google Scholar]
- Gegelashvili G, Schousboe A. High affinity glutamate transporters: regulation of expression and activity. Mol Pharmacol. 1997;52(1):6–15. doi: 10.1124/mol.52.1.6. [DOI] [PubMed] [Google Scholar]
- Ghajar J. Traumatic brain injury. Lancet. 2000;356(9233):923–929. doi: 10.1016/S0140-6736(00)02689-1. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Greenberg ME. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science. 1995;268(5208):239–247. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- Gibbs JW, III, Sombati S, DeLorenzo RJ, Coulter DA. Physiological and pharmacological alterations in postsynaptic GABA(A) receptor function in a hippocampal culture model of chronic spontaneous seizures. J Neurophysiol. 1997;77(4):2139–2152. doi: 10.1152/jn.1997.77.4.2139. [DOI] [PubMed] [Google Scholar]
- Gill R, Andine P, Hillered L, Persson L, Hagberg H. The effect of MK-801 on cortical spreading depression in the penumbral zone following focal ischaemia in the rat. J Cereb Blood Flow Metab. 1992;12(3):371–379. doi: 10.1038/jcbfm.1992.54. [DOI] [PubMed] [Google Scholar]
- Ginsberg MD. The new language of cerebral ischemia. AJNR Am J Neuroradiol. 1997;18(8):1435–1445. [PMC free article] [PubMed] [Google Scholar]
- Gizang-Ginsberg E, Ziff EB. Fos family members successively occupy the tyrosine hydroxylase gene AP-1 site after nerve growth factor or epidermal growth factor stimulation and can repress transcription. Mol Endocrinol. 1994;8(2):249–262. doi: 10.1210/mend.8.2.7909583. [DOI] [PubMed] [Google Scholar]
- Glaum SR, Scholz WK, Miller RJ. Acute- and long-term glutamate-mediated regulation of [Ca2+]i in rat hippocampal pyramidal neurons in vitro. J Pharmacol Exp Ther. 1990;253(3):1293–1302. [PubMed] [Google Scholar]
- Globus MY, Busto R, Dietrich WD, Martinez E, Valdes I, Ginsberg MD. Effect of ischemia on the in vivo release of striatal dopamine, glutamate, and gamma-aminobutyric acid studied by intracerebral microdialysis. J Neurochem. 1988;51(5):1455–1464. doi: 10.1111/j.1471-4159.1988.tb01111.x. [DOI] [PubMed] [Google Scholar]
- Gnegy ME. Ca2+/calmodulin signaling in NMDA-induced synaptic plasticity. Crit Rev Neurobiol. 2000;14(2):91–129. [PubMed] [Google Scholar]
- Goddard GV, McIntyre DC, Leech CK. A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol. 1969;25(3):295–330. doi: 10.1016/0014-4886(69)90128-9. [DOI] [PubMed] [Google Scholar]
- Goforth PB, Ellis EF, Satin LS. Enhancement of AMPA-mediated current after traumatic injury in cortical neurons. J Neurosci. 1999;19(17):7367–7374. doi: 10.1523/JNEUROSCI.19-17-07367.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golarai G, Greenwood AC, Feeney DM, Connor JA. Physiological and structural evidence for hippocampal involvement in persistent seizure susceptibility after traumatic brain injury. J Neurosci. 2001;21(21):8523–8537. doi: 10.1523/JNEUROSCI.21-21-08523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding EM, Robertson CS, Bryan RMJ. The consequences of traumatic brain injury on cerebral blood flow and autoregulation: a review. Clin Exp Hypertens. 1999;21(4):299–332. doi: 10.3109/10641969909068668. [DOI] [PubMed] [Google Scholar]
- Gopinath SP, Valadka AB, Goodman JC, Robertson CS. Extracellular glutamate and aspartate in head injured patients. Acta Neurochir Suppl. 2000;76:437–438. doi: 10.1007/978-3-7091-6346-7_90. [DOI] [PubMed] [Google Scholar]
- Graham SH, Shiraishi K, Panter SS, Simon RP, Faden AI. Changes in extracellular amino acid neurotransmitters produced by focal cerebral ischemia. Neurosci Lett. 1990;110(1–2):124–130. doi: 10.1016/0304-3940(90)90799-f. [DOI] [PubMed] [Google Scholar]
- Haas CA, Dudeck O, Kirsch M, Huszka C, Kann G, Pollak S, et al. Role for reelin in the development of granule cell dispersion in temporal lobe epilepsy. J Neurosci. 2002;22(14):5797–5802. doi: 10.1523/JNEUROSCI.22-14-05797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haerer AF, Anderson DW, Schoenberg BS. Prevalence and clinical features of epilepsy in a biracial United States population. Epilepsia. 1986;27(1):66–75. doi: 10.1111/j.1528-1157.1986.tb03503.x. [DOI] [PubMed] [Google Scholar]
- Hall ED. Brain attack. Acute therapeutic interventions. Free radical scavengers and antioxidants. Neurosurg Clin N Am. 1997;8(2):195–206. [PubMed] [Google Scholar]
- Hall ED, Kupina NC, Althaus JS. Peroxynitrite scavengers for the acute treatment of traumatic brain injury. Ann NY Acad Sci. 1999;890:462–468. doi: 10.1111/j.1749-6632.1999.tb08025.x. [DOI] [PubMed] [Google Scholar]
- Haltiner AM, Temkin NR, Dikmen SS. Risk of seizure recurrence after the first late posttraumatic seizure. Arch Phys Med Rehabil. 1997;78(8):835–840. doi: 10.1016/s0003-9993(97)90196-9. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Chawla S, Johnson CM, Bading H. Distinct functions of nuclear and cytoplasmic calcium in the control of gene expression. Nature. 1997;385(6613):260–265. doi: 10.1038/385260a0. [DOI] [PubMed] [Google Scholar]
- Hartley DM, Kurth MC, Bjerkness L, Weiss JH, Choi DW. Glutamate receptor-induced 45Ca2+ accumulation in cortical cell culture correlates with subsequent neuronal degeneration. J Neurosci. 1993;13(5):1993–2000. doi: 10.1523/JNEUROSCI.13-05-01993.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser WA, Hesdorffer DC. Epilepsy: Frequency, Causes and Consequences. New York: Demos; 1990. [Google Scholar]
- Hauser WA, Kurland LT. The epidemiology of epilepsy in Rochester, Minnesota, 1935 through 1967. Epilepsia. 1975;16(1):1–66. doi: 10.1111/j.1528-1157.1975.tb04721.x. [DOI] [PubMed] [Google Scholar]
- Hauser WA, Annegers JF, Kurland LT. Prevalence of epilepsy in Rochester, Minnesota: 1940–1980. Epilepsia. 1991;32(4):429–445. doi: 10.1111/j.1528-1157.1991.tb04675.x. [DOI] [PubMed] [Google Scholar]
- Heinemann U. Basic mechanisms of partial epilepsies. Curr Opin Neurol. 2004;17(2):155–159. doi: 10.1097/00019052-200404000-00012. [DOI] [PubMed] [Google Scholar]
- Heinemann U, Buchheim K, Gabriel S, Kann O, Kovacs R, Schuchmann S. Cell death and metabolic activity during epileptiform discharges and status epilepticus in the hippocampus. Prog Brain Res. 2002a;135:197–210. doi: 10.1016/S0079-6123(02)35019-2. [DOI] [PubMed] [Google Scholar]
- Heinemann U, Buchheim K, Gabriel S, Kann O, Kovacs R, Schuchmann S. Coupling of electrical and metabolic activity during epileptiform discharges. Epilepsia. 2002b;43 Suppl 5:168–173. doi: 10.1046/j.1528-1157.43.s.5.15.x. [DOI] [PubMed] [Google Scholar]
- Herman ST. Epilepsy after brain insult: targeting epileptogenesis. Neurology. 2002;59(9 Suppl 5):S21–S26. doi: 10.1212/wnl.59.9_suppl_5.s21. [DOI] [PubMed] [Google Scholar]
- Hort J, Brozek G, Mares P, Langmeier M, Komarek V. Cognitive functions after pilocarpine-induced status epilepticus: changes during silent period precede appearance of spontaneous recurrent seizures. Epilepsia. 1999;40(9):1177–1183. doi: 10.1111/j.1528-1157.1999.tb00845.x. [DOI] [PubMed] [Google Scholar]
- Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol. 1994;36(4):557–565. doi: 10.1002/ana.410360404. [see comments]. [DOI] [PubMed] [Google Scholar]
- Hossmann KA. Excitotoxic mechanisms in focal ischemia. Adv Neurol. 1996;71:69–74. [PubMed] [Google Scholar]
- Hsieh PF. Neuropathology of limbic status epilepticus induced by electrical stimulation of naive rats. Neurol Res. 1999;21(4):399–403. doi: 10.1080/01616412.1999.11740950. [DOI] [PubMed] [Google Scholar]
- Huang CC, Hsu KS. Progress in understanding the factors regulating reversibility of long-term potentiation. Rev Neurosci. 2001;12(1):51–68. doi: 10.1515/revneuro.2001.12.1.51. [DOI] [PubMed] [Google Scholar]
- Humbert JP, Matter N, Artault JC, Koppler P, Malviya AN. Inositol 1,4,5-trisphosphate receptor is located to the inner nuclear membrane vindicating regulation of nuclear calcium signaling by inositol 1,4,5-trisphosphate. Discrete distribution of inositol phosphate receptors to inner and outer nuclear membranes. J Biol Chem. 1996;271(1):478–485. doi: 10.1074/jbc.271.1.478. [DOI] [PubMed] [Google Scholar]
- Isokawa M. Modulation of GABAA receptor-mediated inhibition by postsynaptic calcium in epileptic hippocampal neurons. Brain Res. 1998;810(1–2):241–250. doi: 10.1016/s0006-8993(98)00922-6. [DOI] [PubMed] [Google Scholar]
- Jaffe DB, Brown TH. Metabotropic glutamate receptor activation induces calcium waves within hippocampal dendrites. J Neurophysiol. 1994;72(1):471–474. doi: 10.1152/jn.1994.72.1.471. [DOI] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Jonas P, Burnashev N. Molecular mechanisms controlling calcium entry through AMPA-type glutamate receptor channels. Neuron. 1995;15(5):987–990. doi: 10.1016/0896-6273(95)90087-x. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Becker DP, Tamura T, Hovda DA. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J Neurosurg. 1990;73(6):889–900. doi: 10.3171/jns.1990.73.6.0889. [DOI] [PubMed] [Google Scholar]
- Katsura K, Kristian T, Siesjo BK. Energy metabolism, ion homeostasis, and cell damage in the brain. Biochem Soc Trans. 1994;22(4):991–996. doi: 10.1042/bst0220991. [DOI] [PubMed] [Google Scholar]
- Kittler JT, McAinsh K, Moss SJ. Mechanisms of GABAA receptor assembly and trafficking: implications for the modulation of inhibitory neurotransmission. Mol Neurobiol. 2002;26(2–3):251–268. doi: 10.1385/MN:26:2-3:251. [DOI] [PubMed] [Google Scholar]
- Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275(5303):1132–1136. doi: 10.1126/science.275.5303.1132. [see comments]. [DOI] [PubMed] [Google Scholar]
- Kneussel M. Dynamic regulation of GABA(A) receptors at synaptic sites. Brain Res Brain Res Rev. 2002;39(1):74–83. doi: 10.1016/s0165-0173(02)00159-5. [DOI] [PubMed] [Google Scholar]
- Kochan LD, Churn SB, Omojokun O, Rice A, DeLorenzo RJ. Status epilepticus results in an N-methyl-d-aspartate receptor-dependent inhibition of Ca2+/calmodulin-dependent kinase II activity in the rat. Neuroscience. 2000;95(3):735–743. doi: 10.1016/s0306-4522(99)00462-5. [DOI] [PubMed] [Google Scholar]
- Kohr G, Lambert CE, Mody I. Calbindin-D28K (CaBP) levels and calcium currents in acutely dissociated epileptic neurons. Exp Brain Res. 1991;85(3):543–551. doi: 10.1007/BF00231738. [DOI] [PubMed] [Google Scholar]
- Koura SS, Doppenberg EM, Marmarou A, Choi S, Young HF, Bullock R. Relationship between excitatory amino acid release and outcome after severe human head injury. Acta Neurochir Suppl (Wien) 1998;71:244–246. doi: 10.1007/978-3-7091-6475-4_70. [DOI] [PubMed] [Google Scholar]
- Kramer G. Epilepsy in the elderly: some clinical and pharmaco-therapeutic aspects. Epilepsia. 2001;42 Suppl 3:55–59. doi: 10.1046/j.1528-1157.2001.042suppl.3055.x. [DOI] [PubMed] [Google Scholar]
- Kristian T, Siesjo BK. Calcium in ischemic cell death. Stroke. 1998;29(3):705–718. doi: 10.1161/01.str.29.3.705. [DOI] [PubMed] [Google Scholar]
- Kulkarni SK, George B. Lithium-pilocarpine neurotoxicity: a potential model of status epilepticus. Methods Find Exp Clin Pharmacol. 1995;17(8):551–567. [PubMed] [Google Scholar]
- Kurz JE, Sheets D, Parsons JT, Rana A, Delorenzo RJ, Churn SB. A significant increase in both basal and maximal calcineurin activity in the rat pilocarpine model of status epilepticus. J Neurochem. 2001;78(2):304–315. doi: 10.1046/j.1471-4159.2001.00426.x. [DOI] [PubMed] [Google Scholar]
- Lafon-Cazal M, Pietri S, Culcasi M, Bockaert J. NMDA-dependent superoxide production and neurotoxicity. Nature. 1993;364(6437):535–537. doi: 10.1038/364535a0. [DOI] [PubMed] [Google Scholar]
- Laterra J, Keep R, Betz AL, Goldstein GW. Blood-brain-cerebrospinal fluid barriers. In: Siegel GJ, editor. Basic Neurochemistry: Molecular, Cellular, and Medical Aspects. Philadphia: Lippincott-Raven; 1999. pp. 671–689. [Google Scholar]
- Laurer HL, McIntosh TK. Experimental models of brain trauma. Curr Opin Neurol. 1999;12(6):715–721. doi: 10.1097/00019052-199912000-00010. [DOI] [PubMed] [Google Scholar]
- Lazo PS, Dorfman K, Noguchi T, Mattei MG, Bravo R. Structure and mapping of the fosB gene. FosB downregulates the activity of the fosB promoter. Nucleic Acids Res. 1992;20(2):343–350. doi: 10.1093/nar/20.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Zipfel GJ, Choi DW. The changing landscape of ischaemic brain injury mechanisms. Nature. 1999;399(6738 Suppl):A7–A14. doi: 10.1038/399a007. [DOI] [PubMed] [Google Scholar]
- Lee MC, Kim GM, Woo YJ, Kim MK, Kim JH, Nam SC, et al. Pathogenic significance of neuronal migration disorders in temporal lobe epilepsy. Hum Pathol. 2001;32(6):643–648. doi: 10.1053/hupa.2001.24997. [DOI] [PubMed] [Google Scholar]
- Leite JP, Garcia-Cairasco N, Cavalheiro EA. New insights from the use of pilocarpine and kainate models. Epilepsy Res. 2002;50(1–2):93–103. doi: 10.1016/s0920-1211(02)00072-4. [DOI] [PubMed] [Google Scholar]
- Lenzlinger PM, Morganti-Kossmann MC, Laurer HL, McIntosh TK. The duality of the inflammatory response to traumatic brain injury. Mol Neurobiol. 2001;24(1–3):169–181. doi: 10.1385/MN:24:1-3:169. [DOI] [PubMed] [Google Scholar]
- Li SC, Schoenberg BS, Wang CC, Cheng XM, Zhou SS, Bolis CL. Epidemiology of epilepsy in urban areas of the People’s Republic of China. Epilepsia. 1985;26(5):391–394. doi: 10.1111/j.1528-1157.1985.tb05669.x. [DOI] [PubMed] [Google Scholar]
- Limbrick DD, Jr, Churn SB, Sombati S, DeLorenzo RJ. Inability to restore resting intracellular calcium levels as an early indicator of delayed neuronal cell death. Brain Res. 1995;690(2):145–156. doi: 10.1016/0006-8993(95)00552-2. [DOI] [PubMed] [Google Scholar]
- Limbrick DD, Jr, Sombati S, DeLorenzo RJ. Calcium influx constitutes the ionic basis for the maintenance of glutamate-induced extended neuronal depolarization associated with hippocampal neuronal death. Cell Calcium. 2003;33(2):69–81. doi: 10.1016/s0143-4160(02)00054-4. [DOI] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79(4):1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Choi YB, Sucher NJ, Pan ZH, Stamler JS. Redox state, NMDA receptors and NO-related species [comment] Trends Pharmacol Sci. 1996;17(5):186–187. doi: 10.1016/0165-6147(96)20028-8. [DOI] [PubMed] [Google Scholar]
- Liu Z, Stafstrom CE, Sarkisian MR, Yang Y, Hori A, Tandon P, et al. Seizure-induced glutamate release in mature and immature animals: an in vivo microdialysis study. Neuroreport. 1997;8(8):2019–2023. doi: 10.1097/00001756-199705260-00043. [DOI] [PubMed] [Google Scholar]
- Llinas R, Sugimori M, Silver RB. Microdomains of high calcium concentration in a presynaptic terminal. Science. 1992;256(5057):677–679. doi: 10.1126/science.1350109. [DOI] [PubMed] [Google Scholar]
- Loscher W. Animal models of epilepsy for the development of antiepileptogenic and disease-modifying drugs. A comparison of the pharmacology of kindling and post-status epilepticus models of temporal lobe epilepsy. Epilepsy Res. 2002;50(1–2):105–123. doi: 10.1016/s0920-1211(02)00073-6. [DOI] [PubMed] [Google Scholar]
- Lothman EW, Bertram EH., III Epileptogenic effects of status epilepticus. Epilepsia. 1993;34 Suppl 1:S59–S70. doi: 10.1111/j.1528-1157.1993.tb05907.x. [DOI] [PubMed] [Google Scholar]
- Lothman EW, Bertram EH, III, Stringer JL. Functional anatomy of hippocampal seizures. Prog Neurobiol. 1991;37(1):1–82. doi: 10.1016/0301-0082(91)90011-o. [DOI] [PubMed] [Google Scholar]
- Lothman EW, Bertram EH, III, Kapur J, Bekenstein JW. Temporal lobe epilepsy: studies in a rat model showing dormancy of GABAergic inhibitory interneurons. Epilepsy Res Suppl. 1996;12:145–156. [PubMed] [Google Scholar]
- Lowenstein DH, Alldredge BK. Status epilepticus. N Engl J Med. 1998;338(14):970–976. doi: 10.1056/NEJM199804023381407. [DOI] [PubMed] [Google Scholar]
- Lowenstein DH, Thomas MJ, Smith DH. Selective vulnerability of dentate hilar neurons following traumatic brain injury: a potential mechanistic link between head trauma and disorders of the hippocampus. J Neurosci. 1992;12(12):4846–4853. doi: 10.1523/JNEUROSCI.12-12-04846.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhmann HJ, Mittmann T, Schmidt-Kastner R, Eysel UT, Mudrick-Donnon LA, Heinemann U. Hyperexcitability after focal lesions and transient ischemia in rat neocortex. Epilepsy Res Suppl. 1996a;12:119–128. [PubMed] [Google Scholar]
- Luhmann HJ, Mittmann T, Schmidt-Kastner R, Eysel UT, Mudrick-Donnon LA, Heinemann U. Hyperexcitability after focal lesions and transient ischemia in rat neocortex. Epilepsy Res Suppl. 1996b;12:119–128. [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84(1):87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Maeda T, Katayama Y, Kawamata T, Yamamoto T. Mechanisms of excitatory amino acid release in contused brain tissue: effects of hypothermia and in situ administration of Co2+ on extracellular levels of glutamate. J Neurotrauma. 1998;15(9):655–664. doi: 10.1089/neu.1998.15.655. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation—a decade of progress? Science. 1999;285(5435):1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Cifuentes F, Leite JP, Pretorius JK, Babb TL. Hippocampal EEG excitability and chronic spontaneous seizures are associated with aberrant synaptic reorganization in the rat intrahippocampal kainate model. Electroencephalogr Clin Neurophysiol. 1993;87(5):326–339. doi: 10.1016/0013-4694(93)90186-y. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Pretorius JK, Babb TL, Quinn B. Unilateral hippocampal mossy fiber sprouting and bilateral asymmetric neuron loss with episodic postictal psychosis. J Neurosurg. 1995;82(2):228–233. doi: 10.3171/jns.1995.82.2.0228. [DOI] [PubMed] [Google Scholar]
- Matyas F, Freund TF, Gulyas AI. Hippocampus. 2004;14:460–481. doi: 10.1002/hipo.10191. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL. Permeation and block of N-methyl-d-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. J Physiol. 1987;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Mazarati A, Bragin A, Baldwin R, Shin D, Wilson C, Sankar R, et al. Epileptogenesis after self-sustaining status epilepticus. Epilepsia. 2002;43 Suppl 5:74–80. doi: 10.1046/j.1528-1157.43.s.5.25.x. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Mayer ML. N-methyl-d-aspartic acid receptor structure and function. Physiol Rev. 1994;74(3):723–760. doi: 10.1152/physrev.1994.74.3.723. [DOI] [PubMed] [Google Scholar]
- McEwan MJ, Espie CA, Metcalfe J, Brodie MJ, Wilson MT. Quality of life and psychosocial development in adolescents with epilepsy: a qualitative investigation using focus group methods. Seizure. 2004;13(1):15–31. doi: 10.1016/s1059-1311(03)00080-3. [DOI] [PubMed] [Google Scholar]
- McIntyre DC, Nathanson D, Edson N. A new model of partial status epilepticus based on kindling. Brain Res. 1982;250(1):53–63. doi: 10.1016/0006-8993(82)90952-0. [DOI] [PubMed] [Google Scholar]
- McNamara JO. Cellular and molecular basis of epilepsy. J Neurosci. 1994;14(6):3413–3425. doi: 10.1523/JNEUROSCI.14-06-03413.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara JO. Emerging insights into the genesis of epilepsy. Nature. 1999;399(6738 Suppl):A15–A22. doi: 10.1038/399a015. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Routtenberg A. NMDA receptor blockade prevents kainate induction of protein F1/GAP-43 mRNA in hippocampal granule cells and subsequent mossy fiber sprouting in the rat. Brain Res Mol Brain Res. 1995;33(1):22–28. doi: 10.1016/0169-328x(95)00083-5. [DOI] [PubMed] [Google Scholar]
- Melcher T, Maas S, Herb A, Sprengel R, Seeburg PH, Higuchi M. A mammalian RNA editing enzyme. Nature. 1996;379(6564):460–464. doi: 10.1038/379460a0. [DOI] [PubMed] [Google Scholar]
- Meldrum BS. First Alfred Meyer Memorial Lecture. Epileptic brain damage: a consequence and a cause of seizures. Neuropathol Appl Neurobiol. 1997;23(3):185–201. [PubMed] [Google Scholar]
- Mello LE, Cavalheiro EA, Tan AM, Kupfer WR, Pretorius JK, Babb TL, et al. Circuit mechanisms of seizures in the pilocarpine model of chronic epilepsy: cell loss and mossy fiber sprouting. Epilepsia. 1993;34(6):985–995. doi: 10.1111/j.1528-1157.1993.tb02123.x. [DOI] [PubMed] [Google Scholar]
- Mellstrom B, Naranjo JR. Mechanisms of Ca(2+)-dependent transcription. Curr Opin Neurobiol. 2001;11(3):312–319. doi: 10.1016/s0959-4388(00)00213-0. [DOI] [PubMed] [Google Scholar]
- Michaelis EK. Molecular biology of glutamate receptors in the central nervous system and their role in excitotoxicity, oxidative stress and aging. Prog Neurobiol. 1998;54(4):369–415. doi: 10.1016/s0301-0082(97)00055-5. [DOI] [PubMed] [Google Scholar]
- Michaels RL, Rothman SM. Glutamate neurotoxicity in vitro: antagonist pharmacology and intracellular calcium concentrations. J Neurosci. 1990;10(1):283–292. doi: 10.1523/JNEUROSCI.10-01-00283.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mies G, Kohno K, Hossmann KA. MK-801, a glutamate antagonist, lowers flow threshold for inhibition of protein synthesis after middle cerebral artery occlusion of rat. Neurosci Lett. 1993;155(1):65–68. doi: 10.1016/0304-3940(93)90674-a. [DOI] [PubMed] [Google Scholar]
- Misra JC, Chakravarty S. A study of rotational brain injury. J Biomech. 1984;17(7):459–466. doi: 10.1016/0021-9290(84)90014-9. [DOI] [PubMed] [Google Scholar]
- Mittmann T, Qu M, Zilles K, Luhmann HJ. Long-term cellular dysfunction after focal cerebral ischemia: in vitro analyses. Neuroscience. 1998;85(1):15–27. doi: 10.1016/s0306-4522(97)00638-6. [DOI] [PubMed] [Google Scholar]
- Mody I, MacDonald JF. NMDA receptor-dependent excitotoxicity: the role of intracellular Ca2+ release. Trends Pharmacol Sci. 1995;16(10):356–359. doi: 10.1016/s0165-6147(00)89070-7. [DOI] [PubMed] [Google Scholar]
- Morimoto T, Globus MY, Busto R, Martinez E, Ginsberg MD. Simultaneous measurement of salicylate hydroxylation and glutamate release in the penumbral cortex following transient middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 1996;16(1):92–99. doi: 10.1097/00004647-199601000-00011. [DOI] [PubMed] [Google Scholar]
- Morris TA, Jafari N, Rice AC, Vasconcelos O, DeLorenzo RJ. Persistent increased DNA-binding and expression of serum response factor occur with epilepsy-associated long-term plasticity changes. J Neurosci. 1999;19(19):8234–8243. doi: 10.1523/JNEUROSCI.19-19-08234.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris TA, Jafari N, DeLorenzo RJ. Chronic DeltaFosB expression and increased AP-1 transcription factor binding are associated with the long term plasticity changes in epilepsy. Brain Res Mol Brain Res. 2000;79(1–2):138–149. doi: 10.1016/s0169-328x(00)00112-1. [DOI] [PubMed] [Google Scholar]
- Moudy AM, Yamada KA, Rothman SM. Rapid desensitization determines the pharmacology of glutamate neurotoxicity. Neuropharmacology. 1994;33(8):953–962. doi: 10.1016/0028-3908(94)90153-8. [DOI] [PubMed] [Google Scholar]
- Murray MI, Halpern MT, Leppik IE. Cost of refractory epilepsy in adults in the USA. Epilepsy Res. 1996;23(2):139–148. doi: 10.1016/0920-1211(95)00090-9. [DOI] [PubMed] [Google Scholar]
- Mussack T, Biberthaler P, Kanz KG, Wiedemann E, Gippner-Steppert C, Mutschler W, et al. Serum S-100B and interleukin-8 as predictive markers for comparative neurologic outcome analysis of patients after cardiac arrest and severe traumatic brain injury. Crit Care Med. 2002;30(12):2669–2674. doi: 10.1097/00003246-200212000-00010. [DOI] [PubMed] [Google Scholar]
- Nadler JV. The recurrent mossy fiber pathway of the epileptic brain. Neurochem Res. 2003;28(11):1649–1658. doi: 10.1023/a:1026004904199. [DOI] [PubMed] [Google Scholar]
- Nakabeppu Y, Nathans D. A naturally occurring truncated form of FosB that inhibits Fos/Jun transcriptional activity. Cell. 1991;64(4):751–759. doi: 10.1016/0092-8674(91)90504-r. [DOI] [PubMed] [Google Scholar]
- Naylor D. Changes in nonlinear signal processing in rat hippocampus associated with loss of paired-pulse inhibition or epileptogenesis. Epilepsia. 2002;43 Suppl 5:188–193. doi: 10.1046/j.1528-1157.43.s.5.37.x. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin T, Hagemann G, Witte OW. Cellular correlates of neuronal hyperexcitability in the vicinity of photochemically induced cortical infarcts in rats in vitro. Neurosci Lett. 1995a;193(2):101–104. doi: 10.1016/0304-3940(95)11677-o. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin T, Hagemann G, Witte OW. Cellular correlates of neuronal hyperexcitability in the vicinity of photochemically induced cortical infarcts in rats in vitro. Neurosci Lett. 1995b;193(2):101–104. doi: 10.1016/0304-3940(95)11677-o. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin T, Staiger JF, Redecker C, Zilles K, Fritschy JM, Mohler H, et al. Immunohistochemical evidence for dysregulation of the GABAergic system ipsilateral to photochemically induced cortical infarcts in rats. Neuroscience. 1998;87(4):871–879. doi: 10.1016/s0306-4522(98)00124-9. [DOI] [PubMed] [Google Scholar]
- Ng SK, Hauser WA, Brust JC, Susser M. Hypertension and the risk of new-onset unprovoked seizures. Neurology. 1993;43(2):425–428. doi: 10.1212/wnl.43.2.425. [DOI] [PubMed] [Google Scholar]
- Nicholls D, Attwell D. The release and uptake of excitatory amino acids. Trends Pharmacol Sci. 1990;11(11):462–468. doi: 10.1016/0165-6147(90)90129-v. [see comments]. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Budd SL. Neuronal excitotoxicity: the role of mitochondria. Biofactors. 1998;8(3–4):287–299. doi: 10.1002/biof.5520080317. [DOI] [PubMed] [Google Scholar]
- Nilsson P, Hillered L, Ponten U, Ungerstedt U. Changes in cortical extracellular levels of energy-related metabolites and amino acids following concussive brain injury in rats. J Cereb Blood Flow Metab. 1990;10(5):631–637. doi: 10.1038/jcbfm.1990.115. [DOI] [PubMed] [Google Scholar]
- Nishimoto T, Murakami S. Relation between diffuse axonal injury and internal head structures on blunt impact. J Biomech Eng. 1998;120(1):140–147. doi: 10.1115/1.2834294. [DOI] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Ogden D, Khodakhah K. Intracellular Ca2+ release by InsP3 in cerebellar Purkinje neurones. Acta Physiol Scand. 1996;157(3):381–394. doi: 10.1046/j.1365-201X.1996.28252000.x. [DOI] [PubMed] [Google Scholar]
- Olney JW. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science. 1969;164(880):719–721. doi: 10.1126/science.164.3880.719. [DOI] [PubMed] [Google Scholar]
- Olney JW, de Gubareff T. Glutamate neurotoxicity and Huntington’s chorea. Nature. 1978;271(5645):557–559. doi: 10.1038/271557a0. [DOI] [PubMed] [Google Scholar]
- Olney JW, Price MT, Samson L, Labruyere J. The role of specific ions in glutamate neurotoxicity. Neurosci Lett. 1986;65(1):65–71. doi: 10.1016/0304-3940(86)90121-7. [DOI] [PubMed] [Google Scholar]
- Osuntokun BO, Adeuja AO, Nottidge VA, Bademosi O, Olumide A, Ige O, et al. Prevalence of the epilepsies in Nigerian Africans: a community-based study. Epilepsia. 1987;28(3):272–279. doi: 10.1111/j.1528-1157.1987.tb04218.x. [DOI] [PubMed] [Google Scholar]
- Ozawa S. Glutamate receptor channels in hippocampal neurons. Jpn J Physiol. 1993;43(2):141–159. doi: 10.2170/jjphysiol.43.141. [DOI] [PubMed] [Google Scholar]
- Pal S, Sombati S, Limbrick DD, Jr, DeLorenzo RJ. In vitro status epilepticus causes sustained elevation of intracellular calcium levels in hippocampal neurons. Brain Res. 1999;851(1–2):20–31. doi: 10.1016/s0006-8993(99)02035-1. [DOI] [PubMed] [Google Scholar]
- Pal S, Limbrick DD, Jr, Rafiq A, DeLorenzo RJ. Induction of spontaneous recurrent epileptiform discharges causes longterm changes in intracellular calcium homeostatic mechanisms. Cell Calcium. 2000;28(3):181–193. doi: 10.1054/ceca.2000.0146. [DOI] [PubMed] [Google Scholar]
- Pal S, Sun D, Limbrick D, Rafiq A, DeLorenzo RJ. Epileptogenesis induces long-term alterations in intracellular calcium release and sequestration mechanisms in the hippocampal neuronal culture model of epilepsy. Cell Calcium. 2001;30(4):285–296. doi: 10.1054/ceca.2001.0236. [DOI] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neuro-genesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;15(1710):3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Valentin VV, Lowenstein DH. Prolonged seizures increase proliferating neuroblasts in the adult rat subventricular zone-olfactory bulb pathway. J Neurosci. 2002;15(228):3174–3188. doi: 10.1523/JNEUROSCI.22-08-03174.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT, Churn SB, Kochan LD, DeLorenzo RJ. Pilocarpine-induced status epilepticus causes N-methyl-d-aspartate receptor-dependent inhibition of microsomal Mg(2+)/Ca(2+) ATPase-mediated Ca(2+) uptake. J Neurochem. 2000;75(3):1209–1218. doi: 10.1046/j.1471-4159.2000.0751209.x. [DOI] [PubMed] [Google Scholar]
- Parsons JT, Churn SB, DeLorenzo RJ. Chronic inhibition of cortex microsomal Mg(2+)/Ca(2+) ATPase-mediated Ca(2+) uptake in the rat pilocarpine model following epileptogenesis. J Neurochem. 2001;79(2):319–327. doi: 10.1046/j.1471-4159.2001.00576.x. [DOI] [PubMed] [Google Scholar]
- Payrastre B, Nievers M, Boonstra J, Breton M, Verkleij AJ, Van Bergen en Menegouwen PM. A differential location of phosphoinositide kinases, diacylglycerol kinase, and phospholipase C in the nuclear matrix. J Biol Chem. 1992;267(8):5078–5084. [PubMed] [Google Scholar]
- Pena F, Tapia R. Relationships among seizures, extracellular amino acid changes, and neurodegeneration induced by 4-amino-pyridine in rat hippocampus: a microdialysis and electroencephalo-graphic study. J Neurochem. 1999;72(5):2006–2014. doi: 10.1046/j.1471-4159.1999.0722006.x. [DOI] [PubMed] [Google Scholar]
- Penberthy LT, Towne AR, Garnett LK, Perlin JB, DeLorenzo RJ. Estimating the economic burden of status epilepticus to the health care system. Seizure: Eur J Epilepsy. 2004 doi: 10.1016/j.seizure.2004.06.001. (in press). [DOI] [PubMed] [Google Scholar]
- Perlin JB, DeLorenzo RJ. Calcium and epilepsy. In: Pedley TA, Meldrum BS, editors. Recent Advances in Epilepsy vol. 5. Edinburgh: Churchill Livingstone; 1992. pp. 15–36. [Google Scholar]
- Petersen OH, Burdakov D, Tepikin AV. Regulation of store-operated calcium entry: lessons from a polarized cell. Eur J Cell Biol. 1999;78(4):221–223. doi: 10.1016/s0171-9335(99)80054-5. [DOI] [PubMed] [Google Scholar]
- Pfrieger FW, Barres BA. New views on synapse-glia interactions. Curr Opin Neurobiol. 1996;6(5):615–621. doi: 10.1016/s0959-4388(96)80093-6. [DOI] [PubMed] [Google Scholar]
- Pitkanen A. Drug-mediated neuroprotection and antiepilepto-genesis: animal data. Neurology. 2002;59(9 Suppl 5):S27–S33. doi: 10.1212/wnl.59.9_suppl_5.s27. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Sutula TP. Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancet Neurol. 2002;1(3):173–181. doi: 10.1016/s1474-4422(02)00073-x. [DOI] [PubMed] [Google Scholar]
- Platenik J, Kuramoto N, Yoneda Y. Molecular mechanisms associated with long-term consolidation of the NMDA signals. Life Sci. 2000;67(4):335–364. doi: 10.1016/s0024-3205(00)00632-9. [DOI] [PubMed] [Google Scholar]
- Puranam RS, McNamara JO. Seizure disorders in mutant mice: relevance to human epilepsies. Curr Opin Neurobiol. 1999;9(3):281–287. doi: 10.1016/s0959-4388(99)80041-5. [DOI] [PubMed] [Google Scholar]
- Putney J., Jr . Calcium. In: Siegel GJ, editor. Basic Neurochemistry: Molecular, Cellular, and Medical Aspects. Philadelphia: Lippincott-Raven Publishers; 1999. pp. 453–469. [Google Scholar]
- Putney JW, Jr, Broad LM, Braun FJ, Lievremont JP, Bird GS. Mechanisms of capacitative calcium entry. J Cell Sci. 2001;114(Pt 12):2223–2229. doi: 10.1242/jcs.114.12.2223. [DOI] [PubMed] [Google Scholar]
- Qu M, Mittmann T, Luhmann HJ, Schleicher A, Zilles K. Long-term changes of ionotropic glutamate and GABA receptors after unilateral permanent focal cerebral ischemia in the mouse brain. Neuroscience. 1998;85(1):29–43. doi: 10.1016/s0306-4522(97)00656-8. [DOI] [PubMed] [Google Scholar]
- Raghupathi R. Cell death mechanisms following traumatic brain injury. Brain Pathol. 2004;14(2):215–222. doi: 10.1111/j.1750-3639.2004.tb00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Molecular and cellular mechanisms of neuronal migration: relevance to cortical epilepsies. Adv Neurol. 2000;84:1–14. [PubMed] [Google Scholar]
- Randall RD, Thayer SA. Glutamate-induced calcium transient triggers delayed calcium overload and neurotoxicity in rat hippocampal neurons. J Neurosci. 1992;12(5):1882–1895. doi: 10.1523/JNEUROSCI.12-05-01882.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzliff AdH, Howard AL, Santhakumar V, Osapay I, Soltesz I. Rapid deletion of mossy cells does not result in a hyperexcitable dentate gyrus: implications for epileptogenesis. J Neurosci. 2004;24(9):2259–2269. doi: 10.1523/JNEUROSCI.5191-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza M, Pal S, Rafiq A, DeLorenzo RJ. Long-term alteration of calcium homeostatic mechanisms in the pilocarpine model of temporal lobe epilepsy. Brain Res. 2001;903(1–2):1–12. doi: 10.1016/s0006-8993(01)02127-8. [DOI] [PubMed] [Google Scholar]
- Raza M, Blair RE, Sombati S, Carter DS, Deshpande LS, DeLorenzo RJ. Evidence that injury-induced changes in hippocampal neuronal calcium dynamics during epileptogenesis cause acquired epilepsy. Proc Natl Acad Sci. 2004;101(50):17522–17527. doi: 10.1073/pnas.0408155101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice AC, DeLorenzo RJ. NMDA receptor activation during status epilepticus is required for the development of epilepsy. Brain Res. 1998;782(1–2):240–247. doi: 10.1016/s0006-8993(97)01285-7. [DOI] [PubMed] [Google Scholar]
- Rice A, Rafiq A, Shapiro SM, Jakoi ER, Coulter DA. Long-lasting reduction of inhibitory function and gamma-aminobutyric acid type A receptor subunit mRNA expression in a model of temporal lobe epilepsy. Proc Natl Acad Sci U S A. 1996;93(18):9665–9669. doi: 10.1073/pnas.93.18.9665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock DM, Macdonald RL. Polyamine regulation of N-methyl-d-aspartate receptor channels. Annu Rev Pharmacol Toxicol. 1995;35:463–482. doi: 10.1146/annurev.pa.35.040195.002335. [DOI] [PubMed] [Google Scholar]
- Rose CR, Konnerth A. Stores not just for storage. Intracellular calcium release and synaptic plasticity. Neuron. 2001;31(4):519–522. doi: 10.1016/s0896-6273(01)00402-0. [DOI] [PubMed] [Google Scholar]
- Rothman SM. The neurotoxicity of excitatory amino acids is produced by passive chloride influx. J Neurosci. 1985;5(6):1483–1489. doi: 10.1523/JNEUROSCI.05-06-01483.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman SM, Thurston JH, Hauhart RE. Delayed neurotoxicity of excitatory amino acids in vitro. Neuroscience. 1987;22(2):471–480. doi: 10.1016/0306-4522(87)90347-2. [DOI] [PubMed] [Google Scholar]
- Rueter SM, Burns CM, Coode SA, Mookherjee P, Emeson RB. Glutamate receptor RNA editing in vitro by enzymatic conversion of adenosine to inosine. Science. 1995;267(5203):1491–1494. doi: 10.1126/science.7878468. [DOI] [PubMed] [Google Scholar]
- Rusakov DA, Kullmann DM. Extrasynaptic glutamate diffusion in the hippocampus: ultrastructural constraints, uptake, and receptor activation. J Neurosci. 1998;18(9):3158–3170. doi: 10.1523/JNEUROSCI.18-09-03158.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar AM, Jabbari B, Vance SC, Grafman J, Amin D, Dillon JD. Epilepsy after penetrating head injury: I. Clinical correlates: a report of the Vietnam head injury study. Neurology. 1985;35(10):1406–1414. doi: 10.1212/wnl.35.10.1406. [DOI] [PubMed] [Google Scholar]
- Sanabria ER, da Silva AV, Spreafico R, Cavalheiro EA. Damage, reorganization, and abnormal neocortical hyperexcitability in the pilocarpine model of temporal lobe epilepsy. Epilepsia. 2002;43 Suppl 5:96–106. doi: 10.1046/j.1528-1157.43.s.5.31.x. [DOI] [PubMed] [Google Scholar]
- Santella L, Carafoli E. Calcium signaling in the cell nucleus. FASEB J. 1997;11(13):1091–1109. [PubMed] [Google Scholar]
- Santhakumar V, Bender R, Frotscher M, Ross ST, Hollrigel GS, Toth Z, et al. Granule cell hyperexcitability in the early post-traumatic rat dentate gyrus: the “irritable mossy cell” hypothesis. J Physiol. 2000;524(Pt 1):117–134. doi: 10.1111/j.1469-7793.2000.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhakumar V, Ratzliff AD, Jeng J, Toth Z, Soltesz I. Long-term hyperexcitability in the hippocampus after experimental head trauma. Ann Neurol. 2001;50(6):708–717. doi: 10.1002/ana.1230. [DOI] [PubMed] [Google Scholar]
- Sato K, Iwai M, Zhang WR, Kamada H, Ohta K, Omori N, et al. Highly polysialylated neural cell adhesion molecule (PSA-NCAM) positive cells are increased and change localization in rat hippocampus by exposure to repeated kindled seizures. Acta Neurochir Suppl. 2003;86:575–579. doi: 10.1007/978-3-7091-0651-8_117. [DOI] [PubMed] [Google Scholar]
- Sattler R, Tymianski M. Molecular mechanisms of calcium-dependent excitotoxicity. J Mol Med. 2000;78(1):3–13. doi: 10.1007/s001090000077. [DOI] [PubMed] [Google Scholar]
- Sattler R, Tymianski M. Molecular mechanisms of glutamate receptor-mediated excitotoxic neuronal cell death. Mol Neurobiol. 2001;24(1–3):107–129. doi: 10.1385/MN:24:1-3:107. [DOI] [PubMed] [Google Scholar]
- Scharfman HE. Differentiation of rat dentate neurons by morphology and electrophysiology in hippocampal slices: granule cells, spiny hilar cells and aspiny ‘fast-spiking’ cells. Epilepsy Res Suppl. 1992;7:93–109. [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE. Electrophysiological diversity of pyramidal-shaped neurons at the granule cell layer/hilus border of the rat dentate gyrus recorded in vitro. Hippocampus. 1995;5(4):287–305. doi: 10.1002/hipo.450050403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE. The role of nonprincipal cells in dentate gyrus excitability and its relevance to animal models of epilepsy and temporal lobe epilepsy. Adv Neurol. 1999;79:805–820. [PubMed] [Google Scholar]
- Scharfman HE. Epilepsy as an example or neuronal plasticity. Neuroscientist. 2002a;8(2):154–173. doi: 10.1177/107385840200800211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman H. Does BDNF contribute to temporal lobe epilepsy? Epilepsy Curr. 2002b;2(3):92–94. doi: 10.1046/j.1535-7597.2002.t01-1-00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Goodman JH, Sollas AL. Actions of brain-derived neurotrophic factor in slices from rats with spontaneous seizures and mossy fiber sprouting in the dentate gyrus. J Neurosci. 1999;19(13):5619–5631. doi: 10.1523/JNEUROSCI.19-13-05619.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Goodman JH, Sollas AL. J Neurosci. 2000;20:6144–6158. doi: 10.1523/JNEUROSCI.20-16-06144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semah F, Picot MC, Adam C, Broglin D, Arzimanoglou A, Bazin B, et al. Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology. 1998;51(5):1256–1262. doi: 10.1212/wnl.51.5.1256. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Swanson RA, Honkaniemi J, Kogure K, Massa SM. Neurochemistry and molecular biology. In: Barnett HJM, Mohr JP, Stein BM, Yatsu FM, editors. Stroke: Pathophysiology, Diagnosis, and Management. Philadelphia: Churchill Livingstone; 1998. pp. 51–83. [Google Scholar]
- Shaw NA. The neurophysiology of concussion. Prog Neurobiol. 2002;67(4):281–344. doi: 10.1016/s0301-0082(02)00018-7. [DOI] [PubMed] [Google Scholar]
- Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4(4):477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- Sherwin AL. Neuroactive amino acids in focally epileptic human brain: a review. Neurochem Res. 1999;24(11):1387–1395. doi: 10.1023/a:1022580506443. [DOI] [PubMed] [Google Scholar]
- Shimada N, Graf R, Rosner G, Heiss WD. Differences in ischemia-induced accumulation of amino acids in the cat cortex. Stroke. 1990;21(10):1445–1451. doi: 10.1161/01.str.21.10.1445. [DOI] [PubMed] [Google Scholar]
- Shirasaka Y, Wasterlain CG. Chronic epileptogenicity following focal status epilepticus. Brain Res. 1994;655(1–2):33–44. doi: 10.1016/0006-8993(94)91594-6. [DOI] [PubMed] [Google Scholar]
- Siesjo BK, Bengtsson F. Calcium fluxes, calcium antagonists, and calcium-related pathology in brain ischemia, hypoglycemia, and spreading depression: a unifying hypothesis. J Cereb Blood Flow Metab. 1989;9(2):127–140. doi: 10.1038/jcbfm.1989.20. [DOI] [PubMed] [Google Scholar]
- Smolders I, Khan GM, Manil J, Ebinger G, Michotte Y. NMDA receptor-mediated pilocarpine-induced seizures: characterization in freely moving rats by microdialysis. Br J Pharmacol. 1997;121(6):1171–1179. doi: 10.1038/sj.bjp.0701231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sombati S, DeLorenzo RJ. Recurrent spontaneous seizure activity in hippocampal neuronal networks in culture. J Neurophysiol. 1995;73(4):1706–1711. doi: 10.1152/jn.1995.73.4.1706. [DOI] [PubMed] [Google Scholar]
- Sombati S, Coulter DA. Neurotoxic activation of glutamate receptors induces an extended neuronal depolarization in cultured hippocampal neurons. Brain Res. 1991;566(1–2):316–319. doi: 10.1016/0006-8993(91)91716-e. [DOI] [PubMed] [Google Scholar]
- Sommer B, Kohler M, Sprengel R, Seeburg PH. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell. 1991;67(1):11–19. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- Spitzer NC. Spontaneous Ca2+ spikes and waves in embryonic neurons: signaling systems for differentiation. Trends Neurosci. 1994;17(3):115–118. doi: 10.1016/0166-2236(94)90120-1. [DOI] [PubMed] [Google Scholar]
- Stables JP, Bertram EH, White HS, Coulter DA, Dichter MA, Jacobs MP, et al. Models for epilepsy and epileptogenesis: report from the NIH Workshop, Bethesda, Maryland. Epilepsia. 2002;43(11):1410–1420. doi: 10.1046/j.1528-1157.2002.06702.x. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE, Holmes GL, Thompson JL. MK801 pretreatment reduces kainic acid-induced spontaneous seizures in prepubescent rats. Epilepsy Res. 1993;14(1):41–48. doi: 10.1016/0920-1211(93)90073-g. [DOI] [PubMed] [Google Scholar]
- Stasheff SF, Anderson WW, Clark S, Wilson WA. NMDA antagonists differentiate epileptogenesis from seizure expression in an in vitro model. Science. 1989;245(4918):648–651. doi: 10.1126/science.2569762. [DOI] [PubMed] [Google Scholar]
- Stefani A, Spadoni F, Bernardi G. Voltage-activated calcium channels: targets of antiepileptic drug therapy? Epilepsia. 1997;38(9):959–965. doi: 10.1111/j.1528-1157.1997.tb01477.x. [DOI] [PubMed] [Google Scholar]
- Steiger JL, Russek SJ. GABAA receptors: building the bridge between subunit mRNAs, their promoters, and cognate transcription factors. Pharmacol Ther. 2004;101(3):259–281. doi: 10.1016/j.pharmthera.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Sucher NJ, Awobuluyi M, Choi YB, Lipton SA. NMDA receptors: from genes to channels. Trends Pharmacol Sci. 1996;17(10):348–355. [PubMed] [Google Scholar]
- Sun DA, Sombati S. Glutamate injury-induced epilepto-genesis in hippocampal neurons: an in vitro model of stroke-induced “epilepsy”. Stroke. 2001;32(10):2344–2350. doi: 10.1161/hs1001.097242. [DOI] [PubMed] [Google Scholar]
- Sun DA, Sombati S, Blair RE, DeLorenzo RJ. Calcium-dependent pileptogenesis in an in vitro model of stroke-induced “epilepsy”. Epilepsia. 2002;43(11):1296–1305. doi: 10.1046/j.1528-1157.2002.09702.x. [DOI] [PubMed] [Google Scholar]
- Sun DA, Sombati S, Blair RE, DeLorenzo RJ. Long-lasting alterations in neuronal calcium homeostasis in an in vitro model of stroke-induced epilepsy. Cell Calcium. 2004;35(2):155–163. doi: 10.1016/j.ceca.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Anderson WW, Wilson WA. Mechanism of electrographic seizure generation in the hippocampal slice in Mg2+-free medium: the role of GABAa inhibition. Epilepsy Res. 1988;2(4):239–245. doi: 10.1016/0920-1211(88)90014-9. [DOI] [PubMed] [Google Scholar]
- Takagi K, Ginsberg MD, Globus MY, Dietrich WD, Martinez E, Kraydieh S, et al. Changes in amino acid neurotransmitters and cerebral blood flow in the ischemic penumbral region following middle cerebral artery occlusion in the rat: correlation with histopathology. J Cereb Blood Flow Metab. 1993;13(4):575–585. doi: 10.1038/jcbfm.1993.75. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Yamamoto S, Kudo Y, Mihara S, Higashi H. Mechanisms underlying the rapid depolarization produced by deprivation of oxygen and glucose in rat hippocampal CA1 neurons in vitro. J Neurophysiol. 1997;78(2):891–902. doi: 10.1152/jn.1997.78.2.891. [DOI] [PubMed] [Google Scholar]
- Tancredi V, Hwa GG, Zona C, Brancati A, Avoli M. Low magnesium epileptogenesis in the rat hippocampal slice: electrophysiological and pharmacological features. Brain Res. 1990;511(2):280–290. doi: 10.1016/0006-8993(90)90173-9. [DOI] [PubMed] [Google Scholar]
- Tavalin SJ, Ellis EF, Satin LS. Mechanical perturbation of cultured cortical neurons reveals a stretch-induced delayed depolarization. J Neurophysiol. 1995;74(6):2767–2773. doi: 10.1152/jn.1995.74.6.2767. [DOI] [PubMed] [Google Scholar]
- Tavalin SJ, Ellis EF, Satin LS. Inhibition of the electrogenic Na pump underlies delayed depolarization of cortical neurons after mechanical injury or glutamate. J Neurophysiol. 1997;77(2):632–638. doi: 10.1152/jn.1997.77.2.632. [DOI] [PubMed] [Google Scholar]
- Taylor TN, Davis PH, Torner JC, Holmes J, Meyer JW, Jacobson MF. Lifetime cost of stroke in the United States. Stroke. 1996;27(9):1459–1466. doi: 10.1161/01.str.27.9.1459. [DOI] [PubMed] [Google Scholar]
- Temkin NR. Risk factors for posttraumatic seizures in adults. Epilepsia. 2003;44 Suppl 10:18–20. doi: 10.1046/j.1528-1157.44.s10.6.x. [DOI] [PubMed] [Google Scholar]
- Toth Z, Hollrigel GS, Gorcs T, Soltesz I. Instantaneous perturbation of dentate interneuronal networks by a pressure wave-transient delivered to the neocortex. J Neurosci. 1997;17(21):8106–8117. doi: 10.1523/JNEUROSCI.17-21-08106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Hartley M, Heinemann SF. Control of proton sensitivity of the NMDA receptor by RNA splicing and polyamines. Science. 1995;268(5212):873–876. doi: 10.1126/science.7754371. [DOI] [PubMed] [Google Scholar]
- Treiman DM. GABAergic mechanisms in epilepsy. Epilepsia. 2001;42 Suppl 3:8–12. doi: 10.1046/j.1528-1157.2001.042suppl.3008.x. [DOI] [PubMed] [Google Scholar]
- Treiman M, Caspersen C, Christensen SB. A tool coming of age: thapsigargin as an inhibitor of sarco-endoplasmic reticulum Ca(2+)-ATPases. Trends Pharmacol Sci. 1998;19(4):131–135. doi: 10.1016/s0165-6147(98)01184-5. [DOI] [PubMed] [Google Scholar]
- Trifaro JM, Vitale ML. Cytoskeleton dynamics during neurotransmitter release. Trends Neurosci. 1993;16(11):466–472. doi: 10.1016/0166-2236(93)90079-2. [DOI] [PubMed] [Google Scholar]
- Tsien RW, Lipscombe D, Madison D, Bley K, Fox A. Reflections on Ca(2+)-channel diversity, 1988–1994. Trends Neurosci. 1995;18(2):52–54. [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Schwarz M, Czuczwar SJ, Kleinrok Z, Turski L. Limbic seizures produced by pilocarpine in rats: behavioural, electroencephalographic and neuropathological study. Behav Brain Res. 1983;9(3):315–335. doi: 10.1016/0166-4328(83)90136-5. [DOI] [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Bortolotto ZA, Mello LM, Schwarz M, Turski L. Seizures produced by pilocarpine in mice: a behavioral, electroencephalographic and morphological analysis. Brain Res. 1984;321(2):237–253. doi: 10.1016/0006-8993(84)90177-x. [DOI] [PubMed] [Google Scholar]
- Tymianski M. Cytosolic calcium concentrations and cell death in vitro. Adv Neurol. 1996;71:85–105. [PubMed] [Google Scholar]
- Tymianski M, Tator CH. Normal and abnormal calcium homeostasis in neurons: a basis for the pathophysiology of traumatic and ischemic central nervous system injury. Neurosurgery. 1996;38(6):1176–1195. doi: 10.1097/00006123-199606000-00028. [DOI] [PubMed] [Google Scholar]
- Tymianski M, Charlton MP, Carlen PL, Tator CH. Source specificity of early calcium neurotoxicity in cultured embryonic spinal neurons. J Neurosci. 1993;13(5):2085–2104. doi: 10.1523/JNEUROSCI.13-05-02085.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzounopoulos T, Stackman R. Enhancing synaptic plasticity and memory: a role for small-conductance Ca(2+)-activated K+ channels. Neuroscientist. 2003;9(6):434–439. doi: 10.1177/1073858403259282. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Yokoyama H, Nakajima A, Tokumaru J, Doi T, Mitsuyama Y. Glutamate excess and free radical formation during and following kainic acid-induced status epilepticus. Exp Brain Res. 2002;147(2):219–226. doi: 10.1007/s00221-002-1224-4. [DOI] [PubMed] [Google Scholar]
- Vandenberg RJ. Molecular pharmacology and physiology of glutamate transporters in the central nervous system. Clin Exp Pharmacol Physiol. 1998;25(6):393–400. doi: 10.1111/j.1440-1681.1998.tb02221.x. [DOI] [PubMed] [Google Scholar]
- Vaughan J, Bullock R. Cellular and vascular pathophysiology of stroke. In: Miller LP, editor. Stroke Therapy: Basic, Preclinical, and Clinical Directions. New York: Wiley-Liss; 1999. pp. 3–38. [Google Scholar]
- Vespa P, Prins M, Ronne-Engstrom E, Caron M, Shalmon E, Hovda DA, et al. Increase in extracellular glutamate caused by reduced cerebral perfusion pressure and seizures after human traumatic brain injury: a microdialysis study. J Neurosurg. 1998;89(6):971–982. doi: 10.3171/jns.1998.89.6.0971. [DOI] [PubMed] [Google Scholar]
- Vick RS, Rafiq A, Coulter DA, Jakoi ER. GABAA alpha 2 mRNA levels are decreased following induction of spontaneous epileptiform discharges in hippocampal-entorhinal cortical slices. Brain Res. 1996;721(1–2):111–119. doi: 10.1016/0006-8993(96)00060-1. [DOI] [PubMed] [Google Scholar]
- Wahl F, Obrenovitch TP, Hardy AM, Plotkine M, Boulu R, Symon L. Extracellular glutamate during focal cerebral ischaemia in rats: time course and calcium dependency. J Neurochem. 1994;63(3):1003–1011. doi: 10.1046/j.1471-4159.1994.63031003.x. [DOI] [PubMed] [Google Scholar]
- Walker AE. Posttraumatic epilepsy in World War II veterans. Surg Neurol. 1989;32(3):235–236. doi: 10.1016/0090-3019(89)90185-7. [DOI] [PubMed] [Google Scholar]
- Wallace RH, Wang DW, Singh R, Scheffer IE, George AL, Jr, Phillips HA, et al. Febrile seizures and generalized epilepsy associated with a mutation in the Na+-channel beta1 subunit gene SCN1B. Nat Genet. 1998;19(4):366–370. doi: 10.1038/1252. [DOI] [PubMed] [Google Scholar]
- Wallace MJ, Blair RE, Falenski KW, Martin BR, DeLorenzo RJ. The endogenous cannabinoid system regulates seizure frequency and duration in a model of temporal lobe epilepsy. J Pharmacol Exp Ther. 2003;307(1):129–137. doi: 10.1124/jpet.103.051920. [DOI] [PubMed] [Google Scholar]
- Wang RA, Cheng G, Kolaj M, Randic M. Alpha-subunit of calcium/calmodulin-dependent protein kinase II enhances gamma-aminobutyric acid and inhibitory synaptic responses of rat neurons in vitro. J Neurophysiol. 1995;73(5):2099–2106. doi: 10.1152/jn.1995.73.5.2099. [DOI] [PubMed] [Google Scholar]
- Wang X, Shimizu-Sasamata M, Moskowitz MA, Newcomb R, Lo EH. Profiles of glutamate and GABA efflux in core versus peripheral zones of focal cerebral ischemia in mice. Neurosci Lett. 2001;313(3):121–124. doi: 10.1016/s0304-3940(01)02262-5. [DOI] [PubMed] [Google Scholar]
- Wasterlain CG, Bronstein JM, Morin AM, Dwyer BE, Sankar R. Translocation and autophosphorylation of brain calmodulin kinase II in status epilepticus. Epilepsy Res Suppl. 1992;9:231–238. [PubMed] [Google Scholar]
- Wasterlain CG, Fujikawa DG, Penix L, Sankar R. Pathophysiological mechanisms of brain damage from status epilepticus. Epilepsia. 1993;34 Suppl 1:S37–S53. doi: 10.1111/j.1528-1157.1993.tb05905.x. [DOI] [PubMed] [Google Scholar]
- Wasterlain CG, Mazarati AM, Naylor D, Niquet J, Liu H, Suchomelova L, et al. Short-term plasticity of hippocampal neuropeptides and neuronal circuitry in experimental status epilepticus. Epilepsia. 2002;43 Suppl 5:20–29. doi: 10.1046/j.1528-1157.43.s.5.1.x. [DOI] [PubMed] [Google Scholar]
- Watson NF, Barber JK, Doherty MJ, Miller JW, Temkin NR. Does glucocorticoid administration prevent late seizures after head injury? Epilepsia. 2004;45(6):690–694. doi: 10.1111/j.0013-9580.2004.59403.x. [DOI] [PubMed] [Google Scholar]
- West AE, Chen WG, Dalva MB, Dolmetsch RE, Kornhauser JM, Shaywitz AJ, et al. Calcium regulation of neuronal gene expression. Proc Natl Acad Sci U S A. 2001;98(20):11024–11031. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RJ, Reynolds IJ. Mitochondria accumulate Ca2+ following intense glutamate stimulation of cultured rat forebrain neurones. J Physiol. 1997;498(Pt 1):31–47. doi: 10.1113/jphysiol.1997.sp021839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CL, Maidment NT, Shomer MH, Behnke EJ, Ackerson L, Fried I, et al. Comparison of seizure related amino acid release in human epileptic hippocampus versus a chronic, kainate rat model of hippocampal epilepsy. Epilepsy Res. 1996;26(1):245–254. doi: 10.1016/s0920-1211(96)00057-5. [DOI] [PubMed] [Google Scholar]
- Wisden W, Seeburg PH. Mammalian ionotropic glutamate receptors. Curr Opin Neurobiol. 1993;3(3):291–298. doi: 10.1016/0959-4388(93)90120-n. [DOI] [PubMed] [Google Scholar]
- Witte OW, Freund HJ. Neuronal dysfunction, epilepsy, and postlesional brain plasticity. In: Stefan H, Andermann F, Chauvel P, Shorvon S, editors. Plasticity in Epilepsy: Dynamic Aspects of Brain Function vol. 81. Philadeplhia: Lippincott Williams and Wilkins; 1999. pp. 25–36. [Google Scholar]
- Witte OW, Bidmon HJ, Schiene K, Redecker C, Hagemann G. Functional differentiation of multiple perilesional zones after focal cerebral ischemia. J Cereb Blood Flow Metab. 2000;20(8):1149–1165. doi: 10.1097/00004647-200008000-00001. [DOI] [PubMed] [Google Scholar]
- Wolf PA, D’Agostino RB. Epidemiology of stroke. In: Barnett HJM, Mohr JP, Stein BM, Yatsu FM, editors. Stroke: Pathophysiology, Diagnosis, and Management. Philadelphia: Churchill Livingstone; 1998. pp. 3–28. [Google Scholar]
- Yamamoto T, Rossi S, Stiefel M, Doppenberg E, Zauner A, Bullock R, et al. CSF and ECF glutamate concentrations in head injured patients. Acta Neurochir Suppl (Wien) 1999;75:17–19. doi: 10.1007/978-3-7091-6415-0_4. [DOI] [PubMed] [Google Scholar]
- Zhang L, Rzigalinski BA, Ellis EF, Satin LS. Reduction of voltage-dependent Mg2+ blockade of NMDA current in mechanically injured neurons. Science. 1996;274(5294):1921–1923. doi: 10.1126/science.274.5294.1921. [DOI] [PubMed] [Google Scholar]
- Zhang L, Yang KH, King AI. Biomechanics of neurotrauma. Neurol Res. 2001;23(2–3):144–156. doi: 10.1179/016164101101198488. [DOI] [PubMed] [Google Scholar]
- Zipfel GJ, Babcock DJ, Lee JM, Choi DW. Neuronal apoptosis after CNS injury: the roles of glutamate and calcium. J Neurotrauma. 2000;17(10):857–869. doi: 10.1089/neu.2000.17.857. [DOI] [PubMed] [Google Scholar]