Abstract
In plants, cell signaling connects the environmental input to the intracellular responses in plants. Exogenous signals play an important role in cell metabolism leading to growth and defense responses. Some of these stimuli induce anatomical and physiological modifications that are generally modulated by gene expression. SERK belongs to a small family of genes that code for a transmembrane protein involved in signal transduction and that have been strongly associated with somatic embryogenesis and apomixis in a number of plant species. Recent studies corroborate its role in somatic embryogenesis and suggest a broader range of functions in plant response to biotic and abiotic stimuli. This mini-review aims to present new data on SERK and discuss its involvement in plant development as well as in response to environmental stress.
Key words: SERK, fungus tolerance, environmental stress, brassinosteroids, SAR
Role of SERK in Plant Tissue Organogenesis and Somatic Embryogenesis
During somatic embryogenesis, biochemical and morphological changes occur throughout the development of induced tissues, which is closely related to alterations in gene expression. Several genes are differentially expressed during somatic embryogenesis induction, while others are expressed during differentiation from embryo maturation up to full plant development. Among the genes involved in the induction of somatic embryogenesis, the Somatic Embryogenesis Receptor Kinase (SERK) gene is claimed to have an important role. SERK gene was first isolated from carrot embryogenic cells, hailed as a molecular marker for somatic embryogenesis1,2 and associated with somatic embryogenesis (SE) in a number of species including Dactylis glomerata,2 Arabidopsis thaliana,3 Medicago truncatula,4 Helianthus annuus,5 Ocotea catharinensi,6 Citrus unshiu7 and Theobroma cacao8 and lately for apomixes.9,10 SERK is a leucine-rich repeat (LRR) transmembrane protein kinase that enhances the ability of the apical meristem in Arabidopsis to form somatic embryos.3 LRR kinases transmit their signal by forming homodimers or heterodimers with other RLKs, in response to binding by a ligand. This ligand-induced dimerization causes phosphorylation of the intracellular kinase domains of the receptor-like kinases (RLKs), which activates the next stages of the signal transduction pathway (Fig. 1). There is potential for different levels of complexity in the signaling through variation in the binding partners of different RLKs.11
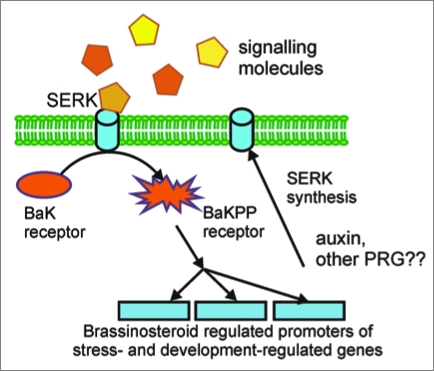
Figure 1.
Somatic embryogenic receptor kinase (SERK) signaling. The SERK gene is expressed under auxin or other Plant Growth Regulator (PRG) stimuli. The SERK protein, a membrane receptor, phosphorylates the brassinosteroid receptor under appropriate external signal, to upregulate pathogen-related (PR) genes or disease response genes, increasing plant pathogen tolerance. SERK is involved in the expression of stress-response genes, and developmental pathways such as somatic embryogenesis or shoot organogenesis.
In sunflower, SERK expression is correlated to induction of two different developmental pathways, somatic embryogenesis and shoot organogenesis.5 In spite of its role in plant development, different genes from the family showed a different expression pattern during somatic or zygotic embryogenesis12 and the expression of these genes was differentially detected in different tissues in several plant species, such as in the apical meristem, root and leaves,5,8,11,13,14 suggesting additional roles for this gene. T-DNA mutational insertions showed the redundancy of two SERK homologs and its role in male sporogenesis in Arabidopsis.15,16
Somatic embryogenesis is an artificial simulation of natural embryogenesis and may be induced by different signalling stresses such as osmotic pressure, ABA induction and SA pathway, which are generally activated by auxin, mainly 2,4-D, which promotes oxidative stress in plants or similar molecules. The SERK pattern of expression in cultures suggests that it is part of a signaling pathway that mediates developmental changes in cells in response to culture conditions. These developmental changes involve the initiation of cell division and cellular reprogramming. Cell fate involving organogenesis or somatic embryogenesis might be conditioned by the status of SERK gene expression, and it is supposed to be a key factor in differentiation.5,11 SERK gene expression was strongly related to auxin elicitation, a known somatic embryogenesis inducer.4,17,18
Brassinosteroid is an essential class of plant growth regulator involved in signal transduction during different plant growth events19 that include salinity stress,20 osmotic stress during seed germination21 or disease resistance.22 It was shown that BRASSINOSTEROID-INSENSITIVE1 (BRI1) receptor coprecipitates and interacts in vivo with SERK1.23 SERK autophosphorylates in the threonine serine site24 and is involved in transphosphorylation of BRI, which is enhanced under brassinosteroid treatment.25 Although SERK gene belongs to a family that may show redundant roles and differential expression, some evolutionary changes in protein function were observed and differences in spatial expression may not be the only way to control its role.26
SERK as a Broader Signal in Response to Biotic and Abiotic Stress
In addition to the study of plant development and totipotency of cells, studies have been carried out with plants exposed to different sources of stress, involving plant growth regulators, elicitors and signal transduction (Fig. 1). These plant stresses can be separated into two main classes: biotic stresses including herbivores, fungal and bacterial attack or virus infection, and a second class that includes abiotic stresses such as drought, extreme temperature, salinity, light etc. Environmental interactions are mediated by elicitors signaling molecules through the transduction pathway.27
Recent advances in research on gene regulation have led to the emergence of post-transcriptional gene silencing (PTGS) or epigenetic variation as additional levels of gene regulation.28 In recent work we have produced a stable PTGS silencing state in transgenic lettuce carrying a SERK gene antisense cassette.29 These lettuce plants exhibited LsSERK gene silencing, showed reduced somatic embryogenesis ability and became more susceptible to Sclerotinia attack. Thus, the elimination of LsSERK via RNA silencing corroborates the hypothesis that SERK is involved in somatic embryogenesis and also in plant defence, since these plants had less ability to form somatic embryos or to resist fungus attack.29
Similarly, increased somatic embryogenesis ability was induced by overexpressing OsSERK1, controlled by the constitutive CaMV35S promoter, in rice plants.13 These transgenic rice plants showed an increase resistance to Magnapothe grisea.13 Reinforcing the idea that SERK is involved in response to biotic stress, transgenic rice expressing the bacterial flagellin (N1141) gene triggered the activation of innate immune response and increased resistance to M. grisea.30 The OsBISERK gene showed upregulation under benzothiadiazole (BTH) treatment, which also leads to an increase in rice resistance to M. grisea.31 It has been shown that BTH induces systemic acquired resistance (SAR) and activates the gene involved in disease resistance.32 In Vitis vinifera differential expression of SERK genes was observed concomitantly to some PR proteins upregulated by 2,4-D, a known somatic embryogenesis inducer.14
On the other hand, it was demonstrated that the auxin naphthalene acetic acid (NAA) was able to upregulate SERK gene in Medicago trucatula.4 In rice some defense signaling molecules, such as SA and JA, induced SERK upregulation and a slight induction by ABA.13 SERK3/BAK1 proteins act on BR signaling and were associated as a component of pathogen-associated molecular pattern (PAMP)-triggered immunity by flagellin elicitation (PTI).33,34
Concluding Remarks
Current data collectively lead to the idea that SERK may be involved in plant signaling and multiple other functions, and not only in plant development. SERK is highly expressed in embryogenic tissues and some undifferentiated cells,35 preceding and coinciding with early somatic embryogenesis.36 Plant cell pluripotency may be associated with chromatin folding37 and the pattern of DNA methylation, which determine plant cell differentiation.38,39 Additional investigation of SERK gene methylation patterns during plant development and under biotic and abiotic stress response would increase knowledge on its role in plant environmental response.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/9900
References
- 1.Schmidt ED, Guzzo F, Toonen MA, de Vries SC. A leucine-rich repeat containing receptor-like kinase marks somatic plant cells competent to form embryos. Development. 1997;124:2049–2062. doi: 10.1242/dev.124.10.2049. [DOI] [PubMed] [Google Scholar]
- 2.Somleva MN, Schmidt EDL, de Vries SC. Embryogenic cells in Dactylis glomerata L. (Poaceae) explants identified by cell tracking and by SERK expression. Plant Cell Rep. 2000;19:718–726. doi: 10.1007/s002999900169. [DOI] [PubMed] [Google Scholar]
- 3.Hecht V, Vielle-Calzada JP, Hartog MV, Schmidt ED, Boutilier K, Grossniklaus U, et al. The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE 1 gene is expressed in developing ovules and embryos and enhances embryogenic competence in culture. Plant Physiol. 2001;127:803–816. [PMC free article] [PubMed] [Google Scholar]
- 4.Nolan KE, Irwanto RR, Rose RJ. Auxin upregulates MtSERK1 expression in both Medicago truncatula root-forming and embryogenic cultures. Plant Physiol. 2003;133:218–230. doi: 10.1104/pp.103.020917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas C, Meyer D, Himber C, Steinmetz A. Spatial expression of a sunflower SERK gene during induction of somatic embryogenesis and shoot organogenesis. Plant Physiol Biochem. 2004;42:35–42. doi: 10.1016/j.plaphy.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Santa-Catarina C, Hanai LR, Dornelas MC, Viana AM, Floh EIS. SERK gene homolog expression, polyamines and amino acids associated with somatic embryogenic competence of Ocotea catharinensis Mez. (Lauraceae) Plant Cell Tiss Org Cult. 2004;79:53–61. [Google Scholar]
- 7.Shimada T, Hirabayashi T, Endo T, Fujii H, Kita M, Omura M. Isolation and characterization of the somatic embryogenesis receptor-like kinase gene homologue (CitSERK1) from Citrus unshiu Marc. Sci Hortic. 2005;103:233–238. [Google Scholar]
- 8.Santos MO, Romano E, Yotoko KSC, Tinoco MLP, Dias BBA, Aragào FJL. Characterisation of the cacao somatic embryogenesis receptor-like kinase (SERK) gene expressed during somatic embryogenesis. Plant Sci. 2005;168:723–729. [Google Scholar]
- 9.Tucker MR, Araujo AC, Paech NA, Hecht V, Schmidt ED, Rossell JB, et al. Sexual and apomictic reproduction in Hieracium subgenus pilosella are closely interrelated developmental pathways. Plant Cell. 2003;15:1524–1537. doi: 10.1105/tpc.011742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albertini E, Marconi G, Reale L, Barcaccia G, Porceddu A, Ferranti F, et al. SERK and APOSTART. Candidate genes for apomixis in Poa pratensis. Plant Physiol. 2005;138:2185–2199. doi: 10.1104/pp.105.062059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nolan KE, Kurdyukov S, Rose RJ. Expression of the SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE1 (SERK1) gene is associated with developmental change in the life cycle of the model legume Medicago truncatula. J Exp Bot. 2009;60:1759–1771. doi: 10.1093/jxb/erp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baudino S, Hansen S, Brettschneider R, Hecht VF, Dresselhaus T, Lörz H, et al. Molecular characterisation of two novel maize LRR receptor-like kinases, which belong to the SERK gene family. Planta. 2001;213:1–10. doi: 10.1007/s004250000471. [DOI] [PubMed] [Google Scholar]
- 13.Hu H, Xiong L, Yang Y. Rice SERK1 gene positively regulates somatic embryogenesis of cultured cell and host defense response against fungal infection. Planta. 2005;222:107–117. doi: 10.1007/s00425-005-1534-4. [DOI] [PubMed] [Google Scholar]
- 14.Maillot P, Lebel S, Schellenbaum P, Jacques A, Walter B. Differential regulation of SERK, LEC1-like and pathogenesis-related genes during indirect secondary somatic embryogenesis in grapevine. Plant Physiol Biochem. 2009;47:743–752. doi: 10.1016/j.plaphy.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Albrecht C, Russinova E, Hecht V, Baaijens E, de Vries S. The Arabidopsis thaliana SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASES1 and 2 control male sporogenesis. Plant Cell. 2005;17:3337–3349. doi: 10.1105/tpc.105.036814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colcombet J, Boisson-Dernier A, Ros-Palau R, Vera CE, Schroeder JI. Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASES1 and 2 are essential for tapetum development and microspore maturation. Plant Cell. 2005;17:3350–3361. doi: 10.1105/tpc.105.036731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma SK, Millam S, Hein I, Bryan GJ. Cloning and molecular characterisation of a potato SERK gene transcriptionally induced during initiation of somatic embryogenesis. Planta. 2008;228:319–330. doi: 10.1007/s00425-008-0739-8. [DOI] [PubMed] [Google Scholar]
- 18.Singla B, Khurana JP, Khurana P. Characterization of three somatic embryogenesis receptor kinase genes from wheat, Triticum aestivum. Plant Cell Rep. 2008;27:833–843. doi: 10.1007/s00299-008-0505-1. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Chory J. Brassinosteroid action in plants. J Exp Bot. 1999;50:275–282. [Google Scholar]
- 20.Anuradha S, Rao SSR. Effect of brassinosteroids on salinity stress induced inhibition of seed germination and seedling growth of rice (Oryza sativa L.) Plant Growth Regul. 2001;33:151–153. [Google Scholar]
- 21.Vardhini VB, Rao SSR. Amelioration of osmotic stress by brassinosteroids on seed germination and seedling growth of three varieties of sorghum. Plant Growth Regul. 2003;41:25–31. [Google Scholar]
- 22.Nakashita H, Yasuda M, Nitta T, Asami T, Fujioka S, Arai Y, et al. Brassinosteroid functions in a broad range of disease resistance in tobacco and rice. Plant J. 2003;33:887–898. doi: 10.1046/j.1365-313x.2003.01675.x. [DOI] [PubMed] [Google Scholar]
- 23.Karlova R, Boeren S, Russinova E, Aker J, Vervoort J, de Vries S. The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE1 Protein Complex Includes BRASSINOSTEROIDINSENSITIVE1. The Plant Cell. 2006;18:626–638. doi: 10.1105/tpc.105.039412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah K, Schmidt ED, Vlak JM, de Vries SC. Expression of the Daucus carota somatic embryogenesis receptor kinase (DcSERK) protein in insect cells. Biochimie. 2001;83:415–421. doi: 10.1016/s0300-9084(01)01257-3. [DOI] [PubMed] [Google Scholar]
- 25.Karlova R, Boeren S, van Dongen W, Kwaaitaal M, Aker J, Vervoort J, et al. Identification of in vitro phosphorylation sites in the Arabidopsis thaliana somatic embryogenesis receptor-like kinases. Proteomics. 2009;9:368–379. doi: 10.1002/pmic.200701059. [DOI] [PubMed] [Google Scholar]
- 26.Albrecht C, Russinova E, Kemmerling B, Kwaaitaal M, de Vries SC. Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE proteins serve brassinosteroid-dependent and -independent signaling pathways. Plant Physiol. 2008;148:611–619. doi: 10.1104/pp.108.123216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.deWit PJ. How plants recognize pathogens and defend themselves. Cell Mol Life Sci. 2007;64:2726–2732. doi: 10.1007/s00018-007-7284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hollick JB. Sensing the epigenome. Trends Plant Sci. 2008;13:398–404. doi: 10.1016/j.tplants.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Santos MO, Romano E, Vieira LS, Baldoni AB, Aragão FJL. Suppression of SERK gene expression affects fungus tolerance and somatic embryogenesis in transgenic lettuce. Plant Biol 2. 2009;11:83–89. doi: 10.1111/j.1438-8677.2008.00103.x. [DOI] [PubMed] [Google Scholar]
- 30.Takakura Y, Che FS, Ishida Y, Tsutsumi F, Kurotani K, Usami S, et al. Expression of a bacterial flagellin gene triggers plant immune responses and confers disease resistance in transgenic rice plants. Mol Plant Pathol. 2008;9:525–529. doi: 10.1111/j.1364-3703.2008.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song D, Li G, Song F, Zheng Z. Molecular characterization and expression analysis of OsBISERK1, a gene encoding a leucine-rich repeat receptor-like kinase, during disease resistance responses in rice. Mol Biol Rep. 2009;35:275–2783. doi: 10.1007/s11033-007-9080-8. [DOI] [PubMed] [Google Scholar]
- 32.Gorlach J, Volrath S, Knauf-Beite G, Hengy G, Beckhove U, Kogel K, et al. Benzothiadiazole, a novel class of inducers of systemic acquired resistance, activates gene expression and disease resistance in wheat. Plant Cell. 1996;8:629–643. doi: 10.1105/tpc.8.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nürnberger T, Jones JD, et al. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature. 2007;448:497–500. doi: 10.1038/nature05999. [DOI] [PubMed] [Google Scholar]
- 34.Heese A, Hann DR, Gimenez-Ibanez S, Jones AME, He K, Li J, et al. The receptor-like kinase SERK3/ BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci USA. 2007;104:12217–12222. doi: 10.1073/pnas.0705306104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwaaitaal MA, de Vries SC, Russinova E. Arabidopsis thaliana Somatic Embryogenesis Receptor Kinase 1 protein is present in sporophytic and gametophytic cells and undergoes endocytosis. Protoplasma. 2005;226:55–65. doi: 10.1007/s00709-005-0111-9. [DOI] [PubMed] [Google Scholar]
- 36.Salaj J, von Recklinghausen IR, Hecht V, de Vries SC, Schel JH, van Lammeren AA. AtSERK1 expression precedes and coincides with early somatic embryogenesis in Arabidopsis thaliana. Plant Physiol Biochem. 2008;46:709–7014. doi: 10.1016/j.plaphy.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 37.Verdeil JL, Alemanno L, Niemenak N, Tranbarger TJ. Pluripotent versus totipotent plant stem cells: dependence versus autonomy? Trends Plant Sci. 2007;12:245–252. doi: 10.1016/j.tplants.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Gehring M, Henikoff S. DNA methylation dynamics in plant genomes. Biochim Biophys Acta. 2007;1769:276–286. doi: 10.1016/j.bbaexp.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Gehring M, Reik W, Henikoff S. DNA demethylation by DNA repair. Trends Genet. 2009;25:82–890. doi: 10.1016/j.tig.2008.12.001. [DOI] [PubMed] [Google Scholar]