Abstract
The establishment of localized auxin gradients plays a central role in developmental patterning in plants. Auxin levels and responses have been shown to increase with temperature although developmental patterning is not affected. This suggests the existence of a homeostatic mechanism that ensures that patterning occurs normally over a range of temperatures. We recently described the cloning and characterization of BOBBER1 (BOB1), an Arabidopsis gene which encodes a small heat shock protein. BOB1 is required for the establishment of auxin gradients and for normal developmental patterning. BOB1 is also required for organismal thermotolerance and localizes to heat shock granules at elevated temperatures. Since BOB1 functions in both temperature responses and developmental patterning we propose that BOB1 may encode a component of a developmental temperature compensation mechanism.
Key words: BOBBER1, BOB1, temperature, development, homeostasis, heat shock granule, sHSP
Polar auxin transport and the resulting establishment of local auxin gradients is a central theme in plant developmental patterning. Auxin acts as a morphogen which establishes patterning and cell identity in the female gametophyte and is subsequently required for the establishment of polarity and patterning during embryogenesis.1,2 During vegetative plant development the correct establishment of auxin gradients is required for the establishment of phyllotaxy and for sculpting the shape of leaf margins among many other patterning functions.3 These patterning events occur in a stereotypical manner regardless of changes in temperature.
Given that these important developmental functions proceed normally over a range of temperatures it is perhaps surprising that auxin levels and responses are significantly influenced by changing temperatures. Gray et al. showed that there is a ∼1.75x increase in free IAA levels when Arabidopsis plants are grown at 29°C instead of at 20°C.4 Increased IAA levels at elevated temperatures are accompanied by auxin dependent hypocotyl elongation as well as changes in the patterns of auxin responsive gene expression. However, changes in developmental patterning were not reported. This suggests the existence of a robust homeostatic mechanism which normalizes auxin mediated developmental patterning events in response to changing temperatures.
We recently characterized BOBBER1 (BOB1) which encodes a non-canonical small heat shock protein (sHSP) with functions both in developmental patterning and in high temperature responses.5,6 BOB1 is an essential gene and bob1-1 null mutants arrest as globular embryos. In bob1-1 mutants a DR5:GFP reporter was used to show that there is a failure to establish auxin gradients during embryogenesis with resulting patterning abnormalities. A similar lack of auxin gradients is observed in embryos treated with 2,4-D, which, like bob1-1 embryos, arrest at the globular stage.7 In the apical half of bob1-1 embryos the central meristematic domain is expanded to encompass the lateral domains where cotyledons would normally form. Similarly, in the basal half of bob1-1 embryos auxin maxima are not established and the expression of SCARECROW (SCR) at the root pole is never observed. This observation is consistent with a requirement for a localized auxin maxima in the formation of the root meristem.8
During post-embryonic development BOB1 is required for normal growth as well as for leaf and floral patterning. When bob1 activity is genetically reduced pin-formed inflorescence meristems are observed. Pinformed meristems reflect a lack of organ primordium initiation. This phenotype is specifically observed in mutants including pinoid (pid), pin-formed1 (pin1) and yucca1,4,6 triple mutants in which the ability to establish auxin maxima has been disrupted.9–11 While the presence of phenotypes that phenocopy auxin deficiencies is not evidence that BOB1 directly modulates auxin mediated patterning, these observation are certainly suggestive. Given the extensive overlap between bob1 phenotypes and auxin phenotypes a plausible hypothesis is that BOB1 may be required for normal auxin transport or signal transduction.
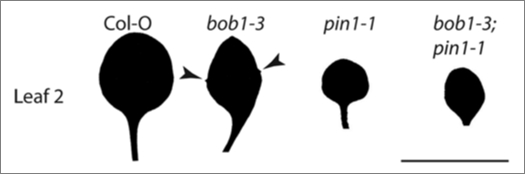
bob1-3 is a partial loss of function allele which has, among other phenotypes, serrated leaf margins on early leaves.6 Normal (Col-O) plants do not produce serrations on the margins of their first two leaves, however bob1-3 mutant plants have one or two leaf serrations on leaves one and two. The formation of leaf margin serrations has been shown to require the activity of the auxin efflux carrier PIN1.12 In order to investigate whether the leaf margin phenotype observed in bob1-3 mutants requires normal PIN1 activity we crossed bob1-3 plants to pin1-1 mutant plants to generate bob1-3; pin1-1 double mutants. The margins of the first leaves of double mutant plants are smooth. This demonstrates that the serrated leaf phenotype of bob1-3 mutants depends on, and modulates, normal PIN1 activity (Fig. 1).
Figure 1.
Leaf morphology of col-O, bob1-3, pin1-1 and bob1-3; pin1-1 plants. the margins of leaf 2 of col-O plants but are completely smooth, while serrations are present on the margins of bob1-3 leaves (arrow heads). no serrations are present on pin1-1 leaves, and pin1-1 is epistatic to bob1-3 in bob1-3; pin1-1 double mutants. Scale bar is 1 cm.
In addition to characterizing BOB1’s developmental functions we also demonstrated that BOB1 encodes a small heat shock protein (sHSP) with protein chaperone activity. BOB1 protein can prevent the thermal denaturation of a model substrate in vitro and co-localizes to heat shock granules with canonical sHSPs. bob1-3 mutants also have thermotolerance defects.6 sHSP chaperones function by binding to partially unfolded substrates and preventing their irreversible aggregation.13 Assuming that bob1 developmental defects are due to a change in chaperone activity this would suggest a model where BOB1 modulates the function of developmental pathways at normal temperatures by ensuring the correct folding of target proteins. One possible target of BOB1 activity could be signal transduction molecules, perhaps in the auxin signaling pathway, which require stabilization in the absence of ligands or interaction partners, similar to Hsp90 targets.14
Developmental homeostasis of auxin mediated events over a range of temperatures requires a mechanism which responds to temperature and also modulates auxin responses. We propose that BOB1 might be a component of this homeostatic mechanism as it has both of these characteristics. sHSPs like BOB1 are known to undergo temperature dependent conformational changes that result in altered substrate interactions.13 In addition to a role in temperature responses we have shown that BOB1 is required for the establishment of auxin gradients and appears to modulate auxin mediated developmental events throughout the plant life cycle. We are currently investigating this hypothesis in order to obtain a better understanding of how plants develop normally over a wide range of temperatures.
Addendum to: Perez DE, Hoyer JS, Johnson AI, Moody ZR, Lopez J, Kaplinsky NJ, et al. BOBBER1 is a non-canonical Arabidopsis small heat shock protein required for both development and thermotolerance. Plant Physiol. 2009;151:241–252. doi: 10.1104/pp.109.142125. and Jurkuta RJ, Kaplinsky NJ, Spindel JE, Barton MK, et al. Partitioning the apical domain of the Arabidopsis embryo requires the BOBBER1 NudC domain protein. Plant Cell. 2009;21:1957–1971. doi: 10.1105/tpc.108.065284.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/9949
References
- 1.Pagnussat GC, Alandete-Saez M, Bowman JL, Sundaresan V. Auxin-dependent patterning and gamete specification in the Arabidopsis female gametophyte. Science. 2009;324:1684–1689. doi: 10.1126/science.1167324. [DOI] [PubMed] [Google Scholar]
- 2.Jenik PD, Barton MK. Surge and destroy: the role of auxin in plant embryogenesis. Development. 2005;132:3577–3585. doi: 10.1242/dev.01952. [DOI] [PubMed] [Google Scholar]
- 3.Teale WD, Paponov IA, Palme K. Auxin in action: signalling, transport and the control of plant growth and development. Nature Rev. 2006;7:847–859. doi: 10.1038/nrm2020. [DOI] [PubMed] [Google Scholar]
- 4.Gray WM, Ostin A, Sandberg G, Romano CP, Estelle M. High temperature promotes auxin-mediated hypocotyl elongation in Arabidopsis. Proc Natl Acad Sci USA. 1998;95:7197–7202. doi: 10.1073/pnas.95.12.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jurkuta RJ, Kaplinsky NJ, Spindel JE, Barton MK. Partitioning the apical domain of the Arabidopsis embryo requires the BOBBER1 NudC domain protein. Plant Cell. 2009;21:1957–1971. doi: 10.1105/tpc.108.065284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez DE, Hoyer JS, Johnson AI, Moody ZR, Lopez J, Kaplinsky NJ. BOBBER1 is a non-canonical Arabidopsis small heat shock protein required for both development and thermotolerance. Plant Physiol. 2009;151:241–252. doi: 10.1104/pp.109.142125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, et al. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature. 2003;426:147–153. doi: 10.1038/nature02085. [DOI] [PubMed] [Google Scholar]
- 8.Costa S, Dolan L. Development of the root pole and cell patterning in Arabidopsis roots. Curr Opin Genet Dev. 2000;10:405–409. doi: 10.1016/s0959-437x(00)00104-0. [DOI] [PubMed] [Google Scholar]
- 9.Cheng Y, Dai X, Zhao Y. Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Gen Dev. 2006;20:1790–1799. doi: 10.1101/gad.1415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y. Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell. 1991;3:677–684. doi: 10.1105/tpc.3.7.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett SRM, Alvarez J, Bossinger G, Smyth DR. Morphogenesis in pinoid mutants of Arabidopsis thaliana. Plant J. 1995;8:505–520. [Google Scholar]
- 12.Hay A, Barkoulas M, Tsiantis M. ASYMMETRIC LEAVES1 and auxin activities converge to repress BREVIPEDICELLUS expression and promote leaf development in Arabidopsis. Development. 2006;133:3955–3961. doi: 10.1242/dev.02545. [DOI] [PubMed] [Google Scholar]
- 13.Haslbeck M, Franzmann T, Weinfurtner D, Buchner J. Some like it hot: the structure and function of small heat-shock proteins. Nat Struct Mol Biol. 2005;12:842–846. doi: 10.1038/nsmb993. [DOI] [PubMed] [Google Scholar]
- 14.Young JC, Moarefi I, Hartl FU. Hsp90: a specialized but essential protein-folding tool. J Cell Biol. 2001;154:267–274. doi: 10.1083/jcb.200104079. [DOI] [PMC free article] [PubMed] [Google Scholar]