Abstract
Plastids and mitochondria are organelles in plant cells, which are considered to have evolved from endosymbiotic associations of bacteria. These organelles have their own genomes descended from their ancestors, and the organelle DNA replications (ODR) of plant cells are coordinated with the nuclear DNA replication (NDR) as ODR precedes NDR during a cell cycle progression. However, the underlying mechanism for this coordination remains largely to be determined. Recently, we identified that a tetrapyrrole compound, Mg-Protoporphyrin IX (Mg-Proto), is a cell cycle coordinator from organelle to NDR in plant cells.1 While Mg-Proto has been suggested to be a retrograde plastid signal to modulate transcription of nuclear genes for plastid proteins, our results indicated that nuclear transcriptional regulation is not involved in the NDR induction in C. merolae. Thus, our finding for the NDR control is likely to represent a novel signaling mechanism independent of the conventional plastid signal, which we named “parasitic signal”, and suggests multiple Mg-Protoinvolved pathways for the plastid-nucleus retrograde signaling.
Key words: cell cycle, DNA replication, parasitic signal, retrograde signal, tetrapyrrole
During long history of organelles having endosymbiotic origin, gene transfer from organelle to the nucleus resulted in the organelle genome reduction. Two types of organelles in plant cells, plastids and mitochondria, have their own genome but could not transcribe or replicate them without a number of proteins encoded by the nuclear genome. Thus, it has been considered that organelles have lost their autonomy, and ODR is now under the strict control of the nucleus. On the other hand, several lines of recent evidence suggest important roles and necessity of organelles on cell proliferation and/or differentiation of eukaryotic cells. For example, decreased ATP production as well as reactive oxygen species (ROS) from mitochondria result in the G1-S cell cycle arrest through the modulation of relevant CDK activity.2,3 Also in plant cell, crumpled leaf mutant of Arabidopsis, which generates a number of leaf cells devoid of plastids, shows several abnormalities on orientation of cell division plan, cell differentiation and overall plant development.4,5
In a recent study, we have identified a critical role of plastid-derived signal for the cell cycle initiation.1 Based on detailed microscopic analyses, it was previously proposed that occurrence of mitochondria and plastids DNA replication (ODR) precedes that of nuclear DNA replication (NDR) in plant cells as C. merolae (a primitive red alga) and tobacco BY-2 cells.6,7 To understand the relationship between ODR and NDR, we specifically inhibited ODR by an inhibitor (nalidixic acid), and found that inhibition of ODR also results in the inhibition of NDR in C. merolae. This result suggested that occurrence of ODR is monitored by a checkpoint that controls the initiation of NDR. Our subsequent analyses revealed the underlying mechanism, in which ODR results in the intracellular accumulation of a tetrapyrrole compound, Mg-Proto, and this metabolite activates CDK-A by unknown mechanism to initiate NDR. Similar scheme was also demonstrated in tobacco BY-2 cells, and therefore, we postulated a novel signaling mechanism in plant cells, involvement of a plastid-derived tetrapyrrole compound in NDR initiation.1
While our finding is the tetrapyrrole signal coordinating DNA replications, the same signaling molecule, Mg-Proto, has been suggested to be the plastid signal that communicates the plastid status to the nucleus and thus regulates the transcription of nuclear genes for plastid proteins.8 Although these two schemes share a common metabolite as the key signal, it was not clear whether these are different aspects of the same pathway or represent different signal pathways. Here, we compare effects of Mg-Proto on NDR and nuclear transcriptome, and argue the independency of the two signal transduction mechanisms.
Distinct Signaling Pathways for the NDR Induction and for the Nuclear Transcriptional Regulation
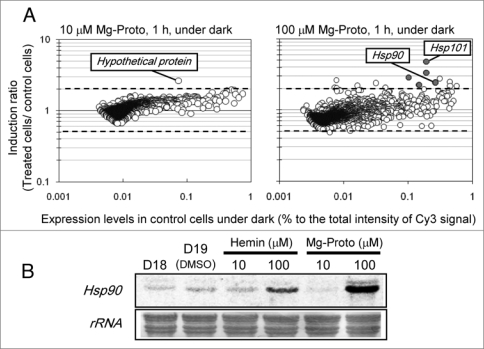
In the previous study,1 it was examined in detail that addition of Mg-Proto (10 μM) into the culture medium in the dark induced NDR of C. merolae. Since the effect of Mg-Proto as the plastid signal has been considered through the nuclear transcriptional regulation, we analyzed changes of the nuclear transcriptome in the presence or absence of Mg-Proto addition using C. merolae nuclear microarray.9 As the result, we found almost no significant change of nuclear gene expression at the Mg-Proto concentration for NDR induction (10 μM, Fig. 1A), with an exceptional induction of a hypothetical gene (CMR210C). This gene was not likely involved in NDR because it was also induced at the higher Mg-Proto condition (100 μM, data not shown and see below). When the dose effect of Mg-Proto was examined for the NDR induction, NDR was not observed at the higher concentrations (50 μM, Table 1), indicating a range of Mg-Proto concentration is critical for the replicational control. On the other hand, induction of chaperone genes, HSP90 (CMQ224C) and HSP101 (CMC125C) (Fig. 1A and B), was observed at the NDR inhibitory concentration (100 μM). Excess amount of hemin also provoked induction of the HSP90 gene (Fig. 1B). These results suggest that addition of excess amount of tetrapyrroles could be perceived as a stress by the cell to induce stress-responsive genes (Fig. 2). Tetrapyrrole molecules are well known as generator of reactive oxygen species (ROS) under light irradiations. Even in the absence of light, the present results suggest high concentration of free tetrapyrroles could be perceived as a stress for photosynthetic organisms.
Figure 1.
Effects of mg-Proto on the nuclear gene transcription. (a) effects of mg-Proto on the C. merolae nuclear transcriptome. C. merolae culture, after the second 18 hours dark period of the 18 h-dark/6 h-light cycles with air-bubbled conditions, were incubated for 1 hour in the dark in the absence or the presence of 10 μm or 100 μm mg-Proto. mg-Proto was added in DmSO, and therefore the same amount of DmSO without mg-Proto was added for the control culture. total rna was extracted from the cells and Dna microarray analysis9 was performed to reveal the effect of Mg-Proto on the nuclear gene expression. Dashed lines showed two times changes in the level of gene expression. each value was obtained by the mean of two reproductions. Gray circles in the right plot indicate the genes annotated as chaperones. (B) northern analysis of the chaperone gene, HSP90. mg-Proto or hemin solved in DmSO were added to the C. merolae culture after the second 18 hours dark period of the 18 h-dark/6 h-light cycles with air-bubbled conditions, and the cultivation was continued for 1 hour in the dark. total rnas were prepared at the same timings as (a), and 10 μg of rnas were analyzed by northern hybridization. D18, after the second 18 hours dark period; D19, incubated for 1 hour with DmSO; other lanes are samples after 1 hour incubation with tetrapyrroles as indicated. Probe for the HSP90 gene was prepared by Pcr using following primers, HSP90F, tac atG aGc Gcc aaG aaG atc a; HSP90r, act tcc tcc atG Gca ctc tcG a. Labeling of probes and signal detection were performed by alkPhos direct labeling kit (Ge Healthcare uK, Buckinghamshire, england) and the image analyzer, LaS-3000 (Fuji Film, tokyo, Japan), respectively.
Table 1.
Concentration-dependence of NDR induction by Mg-Proto in C. merolae
| Mg-Proto concentration | 0 µM | 5 µM | 10 µM | 20 µM | 50 µM | 100 µM |
| NDR | NI | I | I | I | NI | NI |
Various concentrations of mg-Proto were exogenously added to the C. merolae culture after the second 18 hours dark period of the 18 h-dark/6 h-light cycles, and the cultivation was continued for 2 hours in the dark. total Dna was extracted from the cells and qPcr analysis1 was performed to check the occurrence of NDR. Ng-Proto induced NDR in the lower concentrations (5-20 µm) while inhibited NDR in the higher concentrations (50-100 µm). NI, not induced; I, induced.
Figure 2.

Independent pathways for Mg-Proto activity. a suitable concentration of mg-Proto could induce nDr, while excess mg-Proto concentration is likely to induce cellular stress to activate stress-responsive gene expression.
Apparently, effective concentrations for the NDR induction and the chaperone gene induction are distinctive from each other in C. merolae, and thus, the underlying mechanisms are also likely to be different. Thus far, three types of responses by Mg-Proto have been proposed in plant cells: First is the plastid signal suggested based on genetic analysis of Arabidopsis,8 which is involved in transcriptional repression of nuclear genes for plastid proteins. Although Mg-Proto was proposed as the signaling molecule,8 evidence against the working model was presented recently,10,11 and the physiological relevance of Mg-Proto should be further investigated. Second is the signal to induce some nuclear genes in Chlamydomonas reinhardtii. In this case, addition of Mg-Proto as well as hemin into the cultivation medium results in the transcriptional activation of genes such as HEMA and HSP70A,12,13 and our observation for the stress gene expression in C. merolae could be categorized here. And third is the signal that we found on the NDR induction; Given that plastids are descendants of ancient cyanobacteria, DNA replication of the nucleus and the endosymbiont should have acquired a coordination mechanism to maintain the cell integrity. Especially in the absence of light, the engulfed plastid is almost a kind of parasite consuming resources and energy of the host cell, and therefore could be easily removed from the host cell. Thus, a mechanism to inhibit NDR under dark and restore NDR in the presence of light would have been critical to maintain the endosymbiotic association. Because we consider the third type of Mg-Proto signaling was evolved to stabilize the plastid- nucleus relationship in case of such parasitic dark phase, we here propose to name this signaling “parasitic signal”.
Further analysis including identification of the components of the Mg-Proto signaling pathways, such as Mg-Proto receptor(s) in the cytosol, will uncover the interrelationship between organelles and the nucleus for the cell cycle progression.
References
- 1.Kobayashi Y, Kanesaki Y, Tanaka A, Kuroiwa H, Kuroiwa T, Tanaka K. Tetrapyrrole signal as a cell cycle coordinator from organelle to nuclear DNA replication in plant cells. Proc Natl Acad Sci USA. 2009;106:803–807. doi: 10.1073/pnas.0804270105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mandel S, Guptan P, Owusu-Ansah E, Banerjee U. Mitochondrial regulation of cell cycle progression during development as revealed by the tenured mutation in Drosophila. Dev Cell. 2005;9:843–854. doi: 10.1016/j.devcel.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Owusu-Ansah E, Yavari A, Mandal S, Banerjee U. Distinct mitochondrial retrograde signals control the G1-S cell cycle checkpoint. Nat Genet. 2008;40:356–361. doi: 10.1038/ng.2007.50. [DOI] [PubMed] [Google Scholar]
- 4.Asano T, Yoshioka Y, Kurei S, Sakamoto W, Sodmergen , Machida Y. A mutation of the CRUMPLED LEAF gene that encodes a protein localized in the outer envelope membrane of plastids affects the pattern of cell division, cell differentiation and plastid division in Arabidopsis. Plant J. 2004;38:448–459. doi: 10.1111/j.1365-313X.2004.02057.x. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Asano T, Fujiwara TM, Yoshida S, Machida Y, Yoshioka Y. Plant cells without detectable plastids are generated in the crumpled leaf mutant of Arabidopsis thaliana. Plant Cell Physiol. 2009;50:956–969. doi: 10.1093/pcp/pcp047. [DOI] [PubMed] [Google Scholar]
- 6.Kuroiwa T. The primitive red algae: Cyanidium caldarium and Cyanidioschyzon merolae as model system for investigating the dividing apparatus of mitochondria and plastids. BioEssays. 1998;20:344–354. [Google Scholar]
- 7.Sakai A, Takano H, Kuroiwa T. Organelle nuclei in higher plant: structure, composition, function and evolution. Int Rev Cytol. 2004;238:59–117. doi: 10.1016/S0074-7696(04)38002-2. [DOI] [PubMed] [Google Scholar]
- 8.Strand A, Asami T, Alonso J, Ecker JR, Chory J. Chloroplast to nucleus communication triggered by accumulation of Mg-protoporphyrin IX. Nature. 2003;421:79–83. doi: 10.1038/nature01204. [DOI] [PubMed] [Google Scholar]
- 9.Fujiwara T, Misumi O, Tashiro K, Yoshida Y, Nishida K, Yagisawa F, et al. Periodic gene expression patterns during the highly synchronized cell nucleus and organelle division cycle in the unicellular red alga Cyanidioschyzon merolae. DNA Res. 2009;16:59–72. doi: 10.1093/dnares/dsn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mochizuki N, Tanaka R, Tanaka A, Masuda T, Nagatani A. The steady-state level of Mg-protoporphyrin IX is not a determinant of plastid-to-nucleus signaling in Arabidopsis. Proc Natl Acad Sci USA. 2008;105:15184–15189. doi: 10.1073/pnas.0803245105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moulin M, McCormac AC, Terry MJ, Smith AG. Tetrapyrrole profiling in Arabidopsis seedlings reveals that retrograde plastid nuclear signaling is not due to Mg-protoporphyrin IX accumulation. Proc Natl Acad Sci USA. 2008;105:15178–15183. doi: 10.1073/pnas.0803054105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasileuskaya Z, Oster U, Beck CF. Mg-protoporphyrin IX and heme control HEMA, the gene encoding the first step of tetrapyrrole biosynthesis, in Chlamydomonas reinhardtii. Eukaryot Cell. 2005;4:1620–1628. doi: 10.1128/EC.4.10.1620-1628.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Gromoff ED, Alawady A, Meinecke L, Gromm B, Beck CF. Heme, a plastid-derived regulator of nuclear gene expression in Chlamydomonas. Plant Cell. 2008;20:552–567. doi: 10.1105/tpc.107.054650. [DOI] [PMC free article] [PubMed] [Google Scholar]