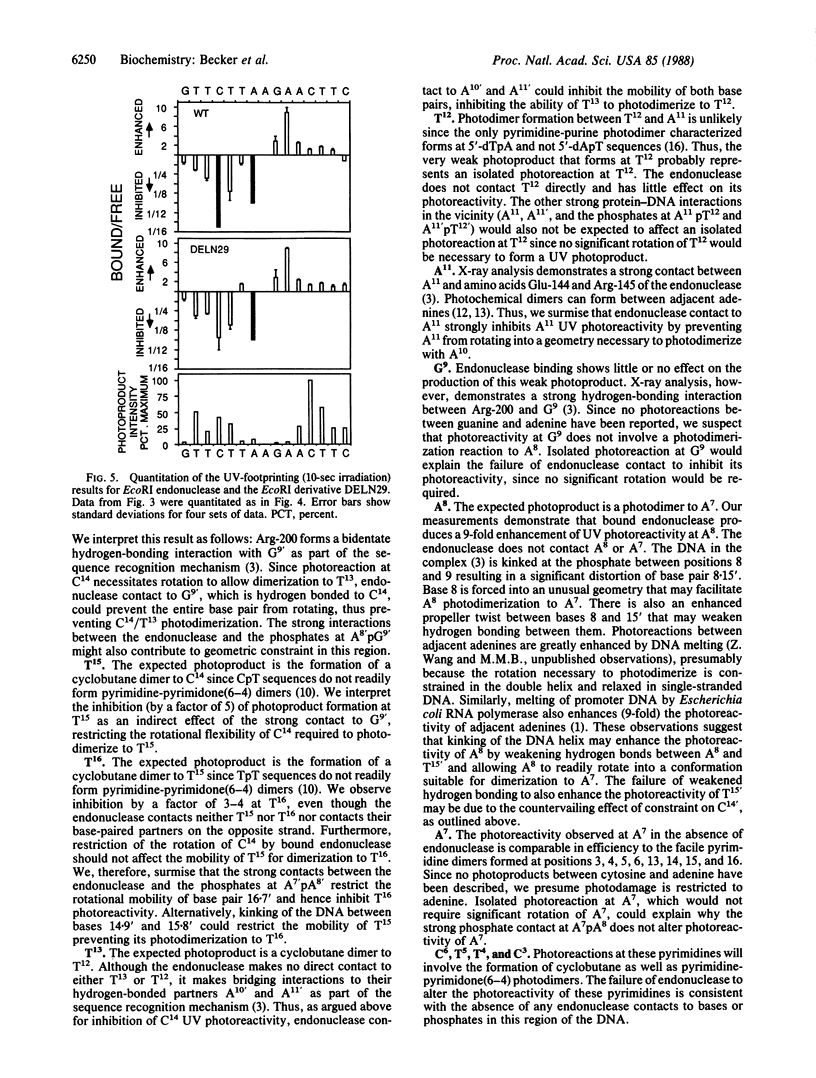
Abstract
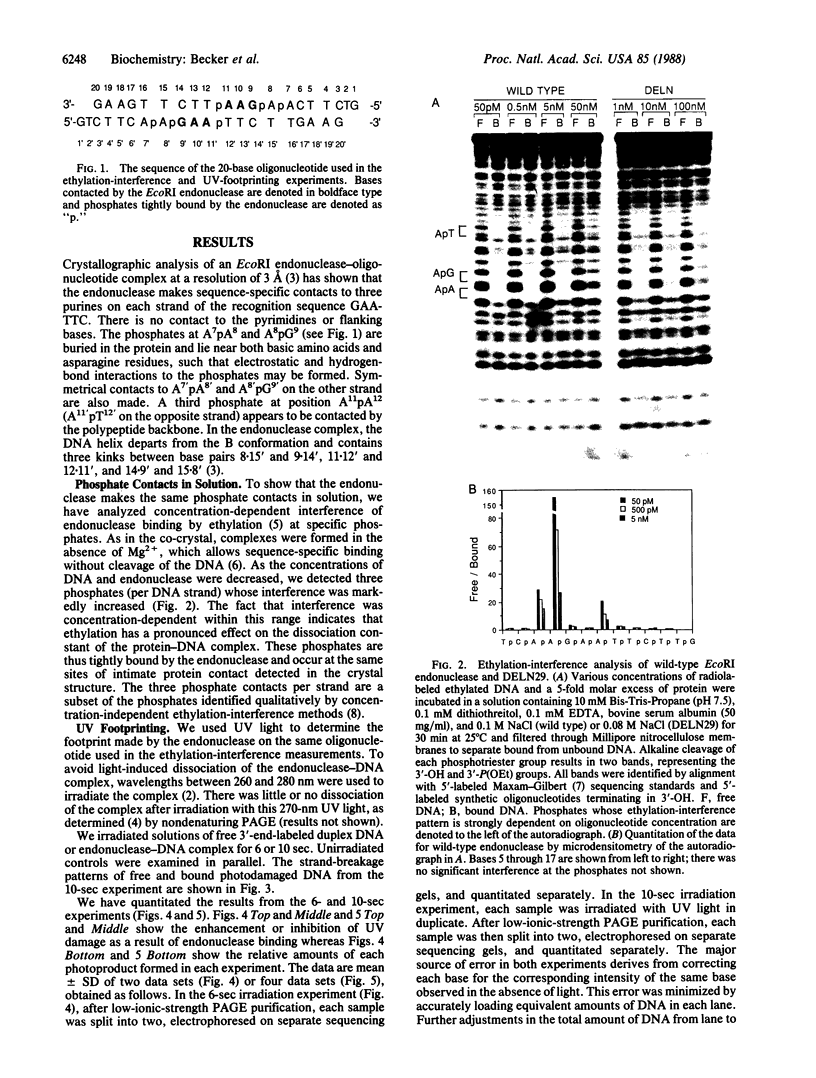
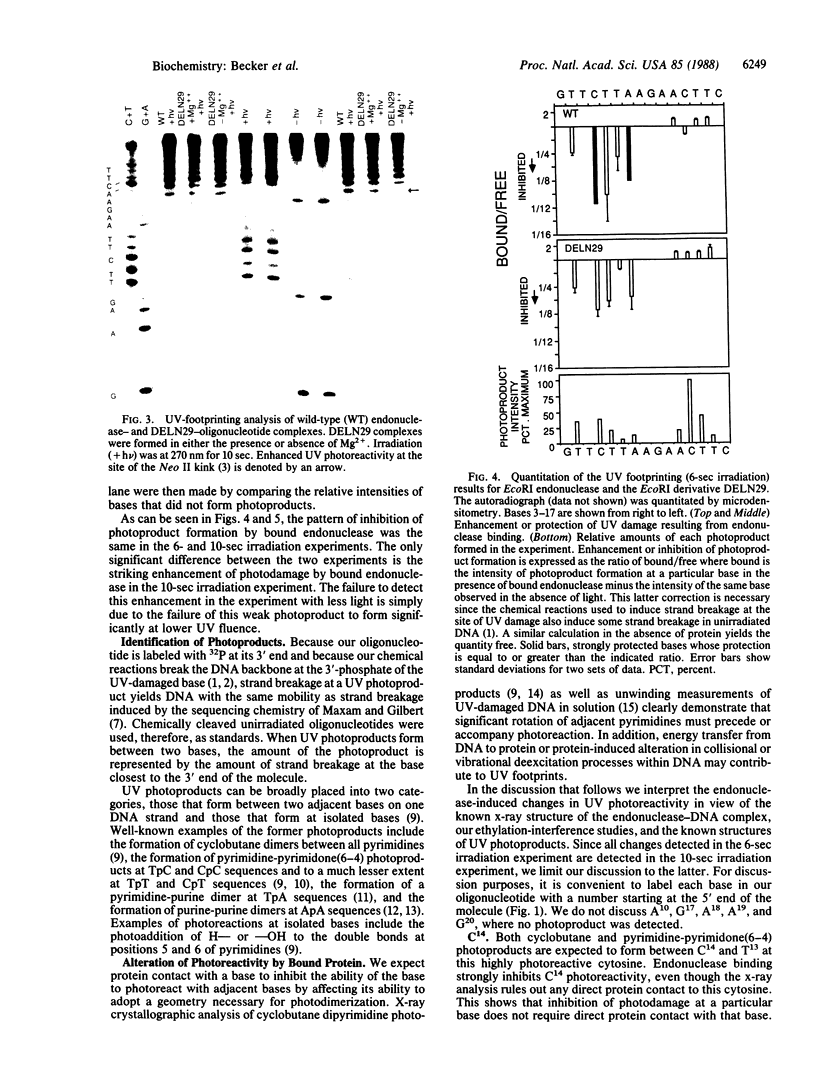
The "UV footprinting" technique has been used to detect contacts between EcoRI endonuclease and its recognition sequence at single nucleotide resolution. Comparison of the UV-footprinting results to the published crystal structure of the EcoRI endonuclease-DNA complex allows us to determine how UV light detects protein-DNA contacts. We find that kinking of the DNA helix in the complex greatly enhances the UV photoreactivity of DNA at the site of the kink. In contrast to kinking, contacts between the endonuclease and the DNA bases inhibit the UV photoreactivity of DNA. Similar analysis of a proteolytically modified endonuclease that exhibits the same sequence specificity as wild-type enzyme but that does not cleave DNA supports these conclusions. Furthermore, detection of enhanced photoreactivity at the same kink in the modified enzyme-DNA complex allows us to conclude that the loss of cleavage activity by the modified endonuclease is not due to its failure to kink DNA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker M. M., Wang J. C. Use of light for footprinting DNA in vivo. Nature. 1984 Jun 21;309(5970):682–687. doi: 10.1038/309682a0. [DOI] [PubMed] [Google Scholar]
- Bose S. N., Davies R. J. The photoreactivity of T-A sequences in oligodeoxyribonucleotides and DNA. Nucleic Acids Res. 1984 Oct 25;12(20):7903–7914. doi: 10.1093/nar/12.20.7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose S. N., Kumar S., Davies R. J., Sethi S. K., McCloskey J. A. The photochemistry of d(T-A) in aqueous solution and in ice. Nucleic Acids Res. 1984 Oct 25;12(20):7929–7947. doi: 10.1093/nar/12.20.7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brash D. E., Haseltine W. A. UV-induced mutation hotspots occur at DNA damage hotspots. Nature. 1982 Jul 8;298(5870):189–192. doi: 10.1038/298189a0. [DOI] [PubMed] [Google Scholar]
- Ciarrocchi G., Pedrini A. M. Determination of pyrimidine dimer unwinding angle by measurement of DNA electrophoretic mobility. J Mol Biol. 1982 Feb 25;155(2):177–183. doi: 10.1016/0022-2836(82)90445-4. [DOI] [PubMed] [Google Scholar]
- Gasparro F. P., Fresco J. R. Ultraviolet-induced 8,8-adenine dehydrodimers in oligo- and polynucleotides. Nucleic Acids Res. 1986 May 27;14(10):4239–4251. doi: 10.1093/nar/14.10.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jen-Jacobson L., Lesser D., Kurpiewski M. The enfolding arms of EcoRI endonuclease: role in DNA binding and cleavage. Cell. 1986 May 23;45(4):619–629. doi: 10.1016/0092-8674(86)90294-1. [DOI] [PubMed] [Google Scholar]
- Kumar S., Sharma N. D., Davies R. J., Phillipson D. W., McCloskey J. A. The isolation and characterisation of a new type of dimeric adenine photoproduct in UV-irradiated deoxyadenylates. Nucleic Acids Res. 1987 Feb 11;15(3):1199–1216. doi: 10.1093/nar/15.3.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A. L., Jack W. E., Modrich P. DNA determinants important in sequence recognition by Eco RI endonuclease. J Biol Chem. 1981 Dec 25;256(24):13200–13206. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McClarin J. A., Frederick C. A., Wang B. C., Greene P., Boyer H. W., Grable J., Rosenberg J. M. Structure of the DNA-Eco RI endonuclease recognition complex at 3 A resolution. Science. 1986 Dec 19;234(4783):1526–1541. doi: 10.1126/science.3024321. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Gilbert W. Contacts between Escherichia coli RNA polymerase and an early promoter of phage T7. Proc Natl Acad Sci U S A. 1980 Jan;77(1):122–126. doi: 10.1073/pnas.77.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry B. J., Jack W. E., Rubin R. A., Modrich P. Thermodynamic parameters governing interaction of EcoRI endonuclease with specific and nonspecific DNA sequences. J Biol Chem. 1983 Aug 25;258(16):9820–9825. [PubMed] [Google Scholar]
- Wang Z., Becker M. M. Selective visualization of gene structure with ultraviolet light. Proc Natl Acad Sci U S A. 1988 Feb;85(3):654–658. doi: 10.1073/pnas.85.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]