Why a Sjögren’s syndrome international registry?
Of the major autoimmune connective tissue diseases, Sjören’s syndrome (SS) is perhaps the least well understood. Both primary and secondary forms of SS occur, but their phenotypes are not well defined. No less than 10 sets of diagnostic/classification criteria for SS have been applied since 1965 (1–11), but none have been universally adopted or accepted by the American College of Rheumatology. These criteria have often identified patients with similar clinical features, but not necessarily with a common disease process. There is scant longitudinal data on SS. The absence of recognized classification criteria contributes to delays in diagnosis for individual patients and hampers research into SS due to small sample sizes and heterogeneously diagnosed patient populations.
To address these issues, the ongoing Sjören’s International Collaborative Clinical Alliance (SICCA) was funded under a US National Institutes of Health contract beginning in 2003. The SICCA project has the goals of 1) designing and implementing an international SS registry for collecting and storing clinical data and biospecimens; 2) developing standardized classification/diagnostic criteria for SS that are universally applicable; and 3) providing these resources for future studies of SS funded by the NIH or comparable agencies.
SICCA is an ongoing longitudinal multi-site observational study that is developing and studying a large and growing cohort of uniformly evaluated individuals from ethnically diverse populations to achieve these goals. SICCA participants must be at least 21 years of age and have: a complaint of dry eyes or dry mouth or a previous suspicion or diagnosis of SS or elevated serum ANA, RF, SS-A, or SS-B or bilateral parotid enlargement in a clinical setting of SS, or a recent increase in dental caries, or have diagnoses of rheumatoid arthritis (RA) or systemic lupus erythematosus (SLE) and thus have secondary SS. We enroll individuals using broad criteria to create a cohort reflecting a wide range of symptoms, from possible early SS to well established disease. However, to avoid circular reasoning in developing a new definition of SS, no diagnostic labels are used at study entry even for participants previously diagnosed with primary SS.
SICCA recruits participants using announcements in patient support group publications and public media, and from patients referred by intramural and extramural practices or clinics having populations meeting these enrollment criteria, Enrollment began in fall 2004 at six international SICCA Research Groups that recruit, enroll and examine participants, and collect and ship biospecimens to the central repository in San Francisco. The Groups are located at University of Buenos Aires and German Hospitals, Buenos Aires, Argentina; Peking Union Medical College Hospital, Beijing, China; Copenhagen University Hospital Glostrup, Denmark; Kanazawa Medical University, Japan; Kings College London, UK; and University of California, San Francisco, USA.
All SICCA groups use the same protocol-directed methods to provide uniform evaluations and record data from ocular, oral and rheumatologic examinations and biospecimen collections. The latter include serum, DNA, whole and parotid saliva, tears, conjunctival cells, frozen and paraffin-embedded labial salivary gland (LSG) biopsy specimens, peripheral blood mononuclear cells and plasma from all participants and DNA from blood-related controls. SICCA participants, who have any objective measures of salivary hypofunction, ocular dryness, any amount of focal lymphocytic infiltration in their LSG biopsy specimen, or positive anti SS-A/-B (95.3% of the cohort so far) at their baseline examination, are being recalled two years later to repeat all examinations and specimen collections. Participants with no positive objective tests are not recalled. This group may serve as controls in future analyses. SICCA clinical questionnaires, data collection forms, and protocols for clinical examination and specimen collection are available for review from the SICCA website <http://sicca.ucsf.edu>
Early lessons from SICCA
Preliminary cohort-wide analyses of SICCA data have already provided new information about SS. Data from 1208 baseline and 134 recall evaluations were available for these analyses. The demographic and SS-related phenotypic characteristics of the SICCA cohort at this stage are summarized in Table 1. Interestingly, positive responses to the questions “Does your mouth feel dry?” or “Do your eyes feel dry?” were not statistically associated with the presence of focal lymphocytic sialadenitis (focus score >1), serum anti-SS-A/B, or ocular staining ≥ 3 (indicating keratoconjunctivitis sicca-KCS). Responses to more specific questions such as “Do you need to sip liquids to swallow dry foods?” or “Does your mouth feel dry when eating a meal?” (13) were associated with the presence of LSG biopsy focus scores >1 (p=0.001 and p=0.007 respectively), but had weaker or no associations with serum anti-SSA/B (p=0.03 and p=0.08 respectively). Analysis of future follow-up examination data should reveal whether these symptomatic individuals develop signs of primary SS over time.
Table 1.
Demographic and SS-related phenotypic characteristics among 1208 participants enrolled in the SICCA Registry as of September 15, 2008.
| Characteristics | |||||
|---|---|---|---|---|---|
| Sources of complete baseline enrollments: | N | (%)1 | |||
| Argentina | 221 | (18) | |||
| China | 236 | (20) | |||
| Denmark | 231 | (19) | |||
| Japan | 184 | (15) | |||
| United Kingdom (since May 2007) | 55 | (5) | |||
| United States | 281 | (23) | |||
| Gender: | |||||
| Women | 1116 | (93) | |||
| SS-related characteristics: | |||||
| Symptoms of: | dry mouth | 1093 | (91) | ||
| dry eyes | 1025 | (85) | |||
| both dry mouth and eyes | 960 | (80) | |||
| Positive serum: | anti-SS-A (Ro) | 456 | (39) | ||
| anti-SS-B (La) | 289 | (25) | |||
| anti-SS-A and –B | 285 | (26) | |||
| rheumatoid factor | 468 | (40) | |||
| ANA ≥ 1:40 | 787 | (67) | |||
| Anti-Hepatitis C (repeatedly +) | 15 | (1) | |||
| Hypergammaglobulinema (IgG > 1445 mg/dL) | 484 | (41) | |||
| Unanesthetized Schirmer test ≤ 5 mm/5 min | 388 | (33) | |||
| Tear break-up time < 10 sec | 991 | (83) | |||
| Ocular staining score ≥ 3 (max of left and right2) | 869 | (72) | |||
| Unstimulated whole salivary flow < 0.5 ml/5 min | 666 | (55) | |||
| Labial salivary gland biopsy diagnosis results:3 | |||||
| Non-specific/sclerosing chronic sialadenitis | 409 | (34) | |||
| Granulomatous inflammation / within normal limits | 11 | (1) | |||
| Inadequate specimen | 26 | (2) | |||
| Focal lymphocytic sialadenitis4 (focus scores on N=740): | 762 | (63) | |||
| focus score > 1 | 500 | (68) | |||
| focus score = 1 | 27 | (4) | |||
| focus score < 1 | 213 | (29) | |||
| Continuous variables: | |||||
| Age, years (median and range) | 54 (21–90) | ||||
| Unstimulated whole salivary flow rate (UWS): (ml/5 min) (median and 25th – 75th percentile) |
0.42 (0.13 – 0.93) | ||||
| Stimulated parotid flow (PFR): (ml/min) (median and 25th – 75th percentile) |
0.12 (0.03 – 0.26) | ||||
Denominators may vary due to missing observations (< 3%) for some variables
Ocular surface staining is assessed by fluorescein staining of the cornea and lissamine green staining of the interpalpebral conjunctivae and scored by a system in which ≥3 represents the presence of keratoconjunctivitis sicca. Details and results of these examinations will be published elsewhere.
Details of the histopathological examination and further analyses of the labial salivary gland biopsy specimens will be published elsewhere.
Reference #12
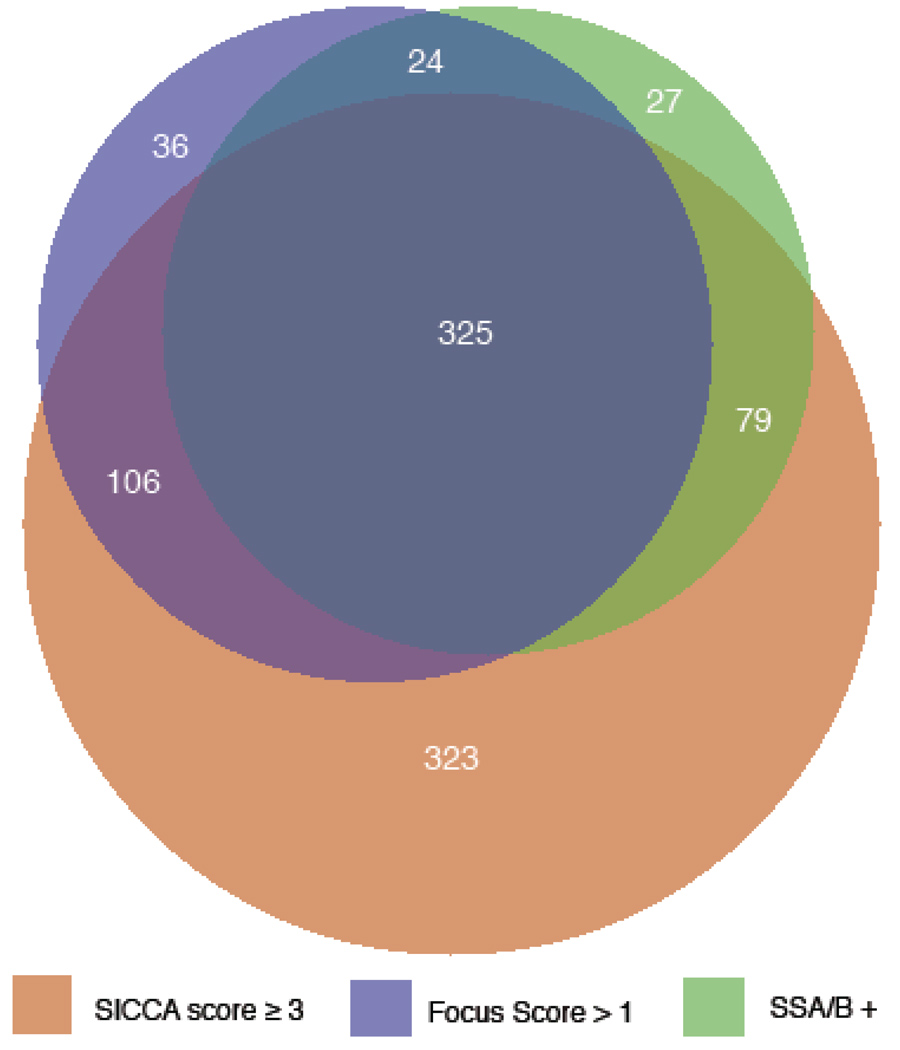
In the process of developing new classification criteria for SS, we use area-proportional Venn diagrams (14) to visualize the interrelationship between various phenotypic characteristics of SS. Figure 1 illustrates the overlapping relationships of three objective SS signs in the cohort (72% exhibit positive ocular staining for KCS, 42% positive labial salivary gland biopsy, 39% positive serum anti-SS-A and/or –B, and 20% none of these). Interestingly, 3% of the participants had only an abnormal focus score, 2% had only a positive serology to anti SS-A or B, whereas 28% had only an abnormal ocular staining score, The high proportion of participants with only abnormal ocular staining led us to compare this sub-group (termed “KCS-only”; n = 323) with those having KCS and at least one of the two other main phenotypic features of SS (focal lymphocytic sialadenitis with a focus score >1 and/or a positive serology to anti-SS-A or B antibodies) (termed “SS-KCS” n=510). KCS-only participants had significantly lower levels of serum auto antibodies (RF, ANA titer, IgG), higher ocular physiologic tests (Schirmer, tear break-up time), more frequent use of anticholinergic drugs (all at p<.0001) and were older (p=.001) than SS-KCS participants. This suggests that KCS-only cases may represent a distinct entity of sero-negative KCS that is significantly less severe than SS-KCS. Additional recall data will allow us to explore if KCS-only subjects progress into SS-KCS or whether KCS-only is a disease entity distinct from SS.
Figure 1.
An area-proportional Venn diagram of the SICCA cohort (n = 1156) illustrates the partially overlapping relationships of three objective Sjören’s syndrome signs. 920 (80%) of the cohort had one or more of the following: positive serum anti-SS-A/B (n=455, 39%); LSG biopsy with FLS and FS >1 (n=491, 42%); ocular surface stain ≥3 (keratoconjunctivitis sicca - KCS) (n=833, 72%); 236 (20%) of the cohort had none of these.
Currently, 78% of baseline participants screened for follow-up completed the follow-up examinations (21% of which declined a second biopsy), about 10% are being scheduled or waiting for follow-up examination and 12% refused further participation. In preliminary analyses of two-year follow up LSG biopsy specimens (n=108), we found marked progression in two participants. One progressed from focal lymphocytic sialadenitis (FLS) with a focus score of 4 foci/4 mm2 at baseline to confluent follicular lymphocytic proliferation two years later. In the other, the baseline biopsy had FLS with a focus score of 9 foci/4 mm2 and in two years the participant had progressed to marginal zone/MALT lymphoma with diffuse and homogenous B-cell (CD-20 positive) proliferation. The number of recall specimens will continue to increase for the duration of the registry, thus providing valuable additional data related to this important issue.
During the two-year period between baseline and follow-up examinations (n=134), the serologic, and physiologic tests (e.g. salivary flow rates, Schirmer tear test and tear break-up time) showed no significant changes. Overall, there was no measurable progression or regression in this group, but it is too soon and the sample too small to adequately assess such changes at this stage. However, there appears to be a subgroup of participants on a faster track of progression detectable through progression of their LSG biopsy.
The data from recall examinations will inform the characterization of the natural history of SS through analysis of progression of individual symptoms and signs, assessing the onset of extra glandular diseases, and supporting validation of preliminary classification criteria developed from baseline examination data. Our ultimate goal is to provide reliable data and analysis from which to describe the phenotype of primary SS, new classification criteria for identifying the disease and uniformly well-documented biospecimens for future research on SS. Additional information about the SICCA project is available from the website at http://sicca.ucsf.edu.
Acknowledgments
Supported by NIH contract NOI-DE-32636
Footnotes
Professional Collaborators: In addition to the Co-Principal Investigators and Group Directors listed as authors, professional collaborators also include:
University of California, San Francisco, USA. Coordinating Center: Rheumatology K Sack, Ophthalmology J Whitcher, Oral Medicine A Wu, D Greenspan, Pathology D Cox, R Jordan, Operations Director Y DeSouza, Project Coordinator M Rasmussen. Research Group: Ophthalmology N McNamara. Rheumatology D Lee, Clinical Coordinator / Phlebotomy D Drury, Clinical Assistant L Scott.
University of Buenos Aires and German Hospitals, Buenos Aires, Argentina: Co-Director / Ophthalmology A Heidenreich, Rheumatology C Vollenweider, Stomatology I Adler, A Smith, LSG biopsies M Gandolfo, PM Bisio, Pathology A Keszler, A Chirife, Specimen processing S Daverio, Group Coordinator V Kambo
Peking Union Medical College Hospital, Beijing, China: Co-Director / Rheumatology Zhao Y, Rheumatology Li M, Zheng W, Su J, Shi Q, Wang Y, Tong S, Ophthalmology Zhang S, Zhao J, Stomatology / Pathology Du D, Stomatology / LSG biopsies Xiao J, Wang H, Specimens / Rheumatology Wu Q, Phlebotomy Zhang C, Meng W.
Copenhagen University Hospital Glostrup, Denmark: Rheumatology P Helin, Ophthalmology S Johansen, Stomatology / LSG biopsies S Jensen, Pathology P Ibsen, Project Nurse T Schnefeldt, Specimen processing / Group Coordinator A Vang.
Kanazawa Medical University, Ishikawa, Japan: Rheumatology Y Masaki, M Tanaka, N Ogawa, K Shimoyama, Rheumatology / LSG biopsies T Sawaki, Ophthalmology K Kitagawa, K Hagiwara, Pathology T Nojima, N Kurose, Stomatology M Hondo, M Takahashi, Specimen processing T Kawanami, Group Coordinator K Fujimoto.
King’s College London, UK: Co-Director P Shirlaw, Oral Medicine B Jacobs, Rheumatology B Kirkham, Ophthalmology G Larkin, Pathology P Morgan.
REFERENCES
- 1.Bloch KJ, Buchanan WW, Wohl MJ, Bunim JJ. Sjören's syndrome. A clinical, pathological, and serological study of sixty-two cases. Medicine. 1965;44:187–231. [PubMed] [Google Scholar]
- 2.Daniels TE, Silverman S, Jr, Michalski JP, Greenspan JS, Sylvester RA, Talal N. The oral component of Sjören's syndrome. Oral Surg Oral Med Oral Pathol. 1975;39:875–885. doi: 10.1016/0030-4220(75)90108-5. [DOI] [PubMed] [Google Scholar]
- 3.Ohfuji T. Review on research reports. Japan: Annual report of the ministry of Health and Welfare: Sjören’s disease Research Committee. 1977:3–8. (published in English by Homma et al in 1986)
- 4.Manthorpe R, Frost-Larsen K, Isager H, Prause JU. Sjören's syndrome. A review with emphasis on immunological features. Allergy. 1981;36:139–153. doi: 10.1111/j.1398-9995.1981.tb01829.x. [DOI] [PubMed] [Google Scholar]
- 5.Skopouli FN, Drosos AA, Papaioannou T, Moutsopoulos HM. Preliminary diagnostic criteria for Sjören's syndrome. Scand J Rheumatol. 1986;61(Suppl):22–25. [PubMed] [Google Scholar]
- 6.Fox RI, Robinson CA, Curd JG, Kozin F, Howell FV. Sjören's syndrome. Proposed criteria for classification. Arthritis Rheum. 1986;29:577–585. doi: 10.1002/art.1780290501. [DOI] [PubMed] [Google Scholar]
- 7.Homma M, Tojo T, Akizuki M, Yamagata H. Criteria for Sjören's syndrome in Japan. Scand J Rheumatol. 1986;61(Suppl):26–27. [PubMed] [Google Scholar]
- 8.Vitali C, Bombardieri S, Moutsopoulos HM, Balestrieri G, Bencivelli W, Bernstein RM, et al. Preliminary criteria for the classification of Sjören's syndrome. Results of a prospective concerted action supported by the European Community. Arthritis Rheum. 1993;36:340–347. doi: 10.1002/art.1780360309. [DOI] [PubMed] [Google Scholar]
- 9.Vitali C, Bombardieri S, Moutsopoulos HM, Coll J, Gerli R, Hatron PY, et al. Assessment of the European classification criteria for Sjören’s syndrome in a series of clinically defined cases: results of a prospective multicentre study. Ann Rheum Dis. 1996;55:116–121. doi: 10.1136/ard.55.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujibayashi T. Revised diagnostic criteria for Sjören’s syndrome. Rheumatology. 2000;24:421–428. (in Japanese) [Google Scholar]
- 11.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjören’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniels TE, Whitcher JP. Association of patterns of labial salivary gland inflammation with keratoconjunctivitis sicca. Analysis of 618 patients with suspected Sjören’s syndrome. Arthritis Rheum. 1994;37:869–877. doi: 10.1002/art.1780370615. [DOI] [PubMed] [Google Scholar]
- 13.Fox PC, Busch KA, Baum BJ. Subjective reports of xerostomia and objective measures of salivary gland performance. JADA. 1987;115:581–584. doi: 10.1016/s0002-8177(87)54012-0. [DOI] [PubMed] [Google Scholar]
- 14.Area-proportional Venn diagrams. http://www.cs.kent.ac.uk/people/staff/pjr/EulerVennCircles/EulerVennApplet.html.