Abstract
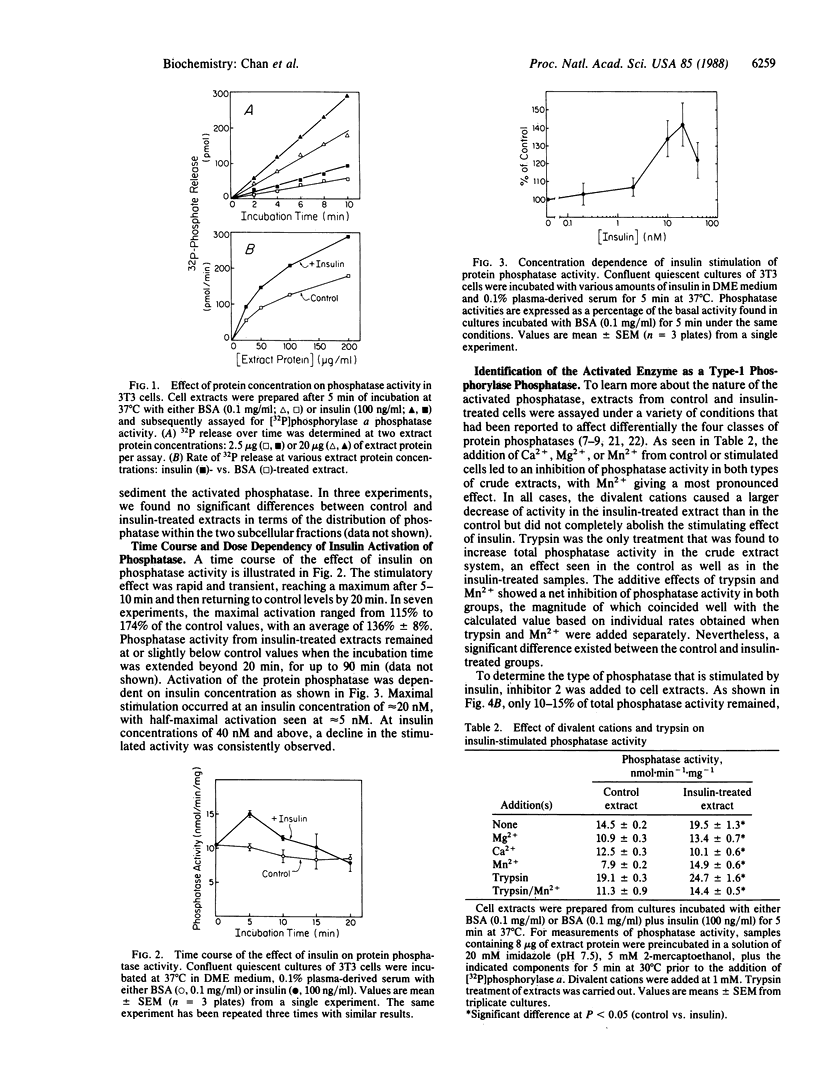
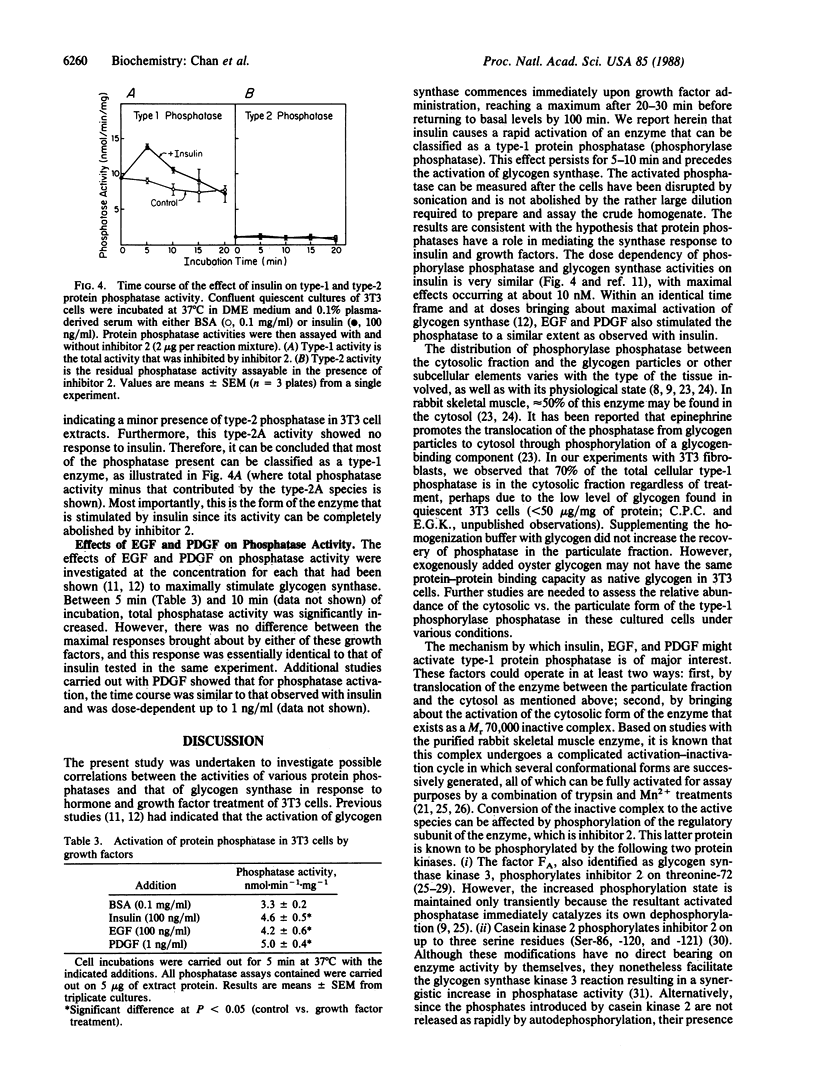
Incubation of Swiss mouse 3T3-D1 cells with physiological concentrations of insulin resulted in a rapid and transient activation of protein phosphatase activity as measured by using [32P]phosphorylase a as substrate. Activation reached a maximum level (140% of control value) within 5 min of addition and returned to control levels within 20 min. The effect of insulin was dose-dependent with half-maximal activation occurring at approximately 5 nM insulin. This activity could be completely inhibited by addition of the heat-stable protein inhibitor 2, which suggests the presence of an activated type-1 phosphatase. Similar effects on phosphatase activity were seen when epidermal growth factor and platelet-derived growth factor were tested. These results suggest that some of the intracellular effects caused by insulin and growth factors are mediated through the activation of a protein phosphatase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alemany S., Pelech S., Brierley C. H., Cohen P. The protein phosphatases involved in cellular regulation. Evidence that dephosphorylation of glycogen phosphorylase and glycogen synthase in the glycogen and microsomal fractions of rat liver are catalysed by the same enzyme: protein phosphatase-1. Eur J Biochem. 1986 Apr 1;156(1):101–110. doi: 10.1111/j.1432-1033.1986.tb09554.x. [DOI] [PubMed] [Google Scholar]
- Ballou L. M., Brautigan D. L., Fischer E. H. Subunit structure and activation of inactive phosphorylase phosphatase. Biochemistry. 1983 Jul 5;22(14):3393–3399. doi: 10.1021/bi00283a014. [DOI] [PubMed] [Google Scholar]
- Bosch F., Bouscarel B., Slaton J., Blackmore P. F., Exton J. H. Epidermal growth factor mimics insulin effects in rat hepatocytes. Biochem J. 1986 Nov 1;239(3):523–530. doi: 10.1042/bj2390523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brautigan D. L., Ballou L. M., Fischer E. H. Activation of skeletal muscle phosphorylase phosphatase. Effects of proteolysis and divalent cations. Biochemistry. 1982 Apr 27;21(9):1977–1982. doi: 10.1021/bi00538a001. [DOI] [PubMed] [Google Scholar]
- Chan C. P., Bowen-Pope D. F., Ross R., Krebs E. G. Regulation of glycogen synthase activity by growth factors. Relationship between synthase activation and receptor occupancy. J Biol Chem. 1987 Jan 5;262(1):276–281. [PubMed] [Google Scholar]
- Chan C. P., Krebs E. G. Epidermal growth factor stimulates glycogen synthase activity in cultured cells. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4563–4567. doi: 10.1073/pnas.82.14.4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C. K., Dull T. J., Russell D. S., Gherzi R., Lebwohl D., Ullrich A., Rosen O. M. Human insulin receptors mutated at the ATP-binding site lack protein tyrosine kinase activity and fail to mediate postreceptor effects of insulin. J Biol Chem. 1987 Feb 5;262(4):1842–1847. [PubMed] [Google Scholar]
- Chowdhury M. H., Agius L. Epidermal growth factor counteracts the glycogenic effect of insulin in parenchymal hepatocyte cultures. Biochem J. 1987 Oct 15;247(2):307–314. doi: 10.1042/bj2470307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePaoli-Roach A. A., Ahmad Z., Camici M., Lawrence J. C., Jr, Roach P. J. Multiple phosphorylation of rabbit skeletal muscle glycogen synthase. Evidence for interactions among phosphorylation sites and the resolution of electrophoretically distinct forms of the subunit. J Biol Chem. 1983 Sep 10;258(17):10702–10709. [PubMed] [Google Scholar]
- DePaoli-Roach A. A. Synergistic phosphorylation and activation of ATP-Mg-dependent phosphoprotein phosphatase by F A/GSK-3 and casein kinase II (PC0.7). J Biol Chem. 1984 Oct 10;259(19):12144–12152. [PubMed] [Google Scholar]
- Di Salvo J., Gifford D., Kokkinakis A. Modulation of aortic protein phosphatase activity by polylysine. Proc Soc Exp Biol Med. 1984 Oct;177(1):24–32. doi: 10.3181/00379727-177-41907. [DOI] [PubMed] [Google Scholar]
- Edelman A. M., Blumenthal D. K., Krebs E. G. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- Fiol C. J., Mahrenholz A. M., Wang Y., Roeske R. W., Roach P. J. Formation of protein kinase recognition sites by covalent modification of the substrate. Molecular mechanism for the synergistic action of casein kinase II and glycogen synthase kinase 3. J Biol Chem. 1987 Oct 15;262(29):14042–14048. [PubMed] [Google Scholar]
- Hemmings B. A., Yellowlees D., Kernohan J. C., Cohen P. Purification of glycogen synthase kinase 3 from rabbit skeletal muscle. Copurification with the activating factor (FA) of the (Mg-ATP) dependent protein phosphatase. Eur J Biochem. 1981 Oct;119(3):443–451. doi: 10.1111/j.1432-1033.1981.tb05628.x. [DOI] [PubMed] [Google Scholar]
- Hiraga A., Cohen P. Phosphorylation of the glycogen-binding subunit of protein phosphatase-1G by cyclic-AMP-dependent protein kinase promotes translocation of the phosphatase from glycogen to cytosol in rabbit skeletal muscle. Eur J Biochem. 1986 Dec 15;161(3):763–769. doi: 10.1111/j.1432-1033.1986.tb10505.x. [DOI] [PubMed] [Google Scholar]
- Holmes C. F., Campbell D. G., Caudwell F. B., Aitken A., Cohen P. The protein phosphatases involved in cellular regulation. Primary structure of inhibitor-2 from rabbit skeletal muscle. Eur J Biochem. 1986 Feb 17;155(1):173–182. doi: 10.1111/j.1432-1033.1986.tb09473.x. [DOI] [PubMed] [Google Scholar]
- Holmes C. F., Kuret J., Chisholm A. A., Cohen P. Identification of the sites on rabbit skeletal muscle protein phosphatase inhibitor-2 phosphorylated by casein kinase-II. Biochim Biophys Acta. 1986 Apr 22;870(3):408–416. doi: 10.1016/0167-4838(86)90248-7. [DOI] [PubMed] [Google Scholar]
- Ingebritsen T. S., Cohen P. Protein phosphatases: properties and role in cellular regulation. Science. 1983 Jul 22;221(4608):331–338. doi: 10.1126/science.6306765. [DOI] [PubMed] [Google Scholar]
- Ingebritsen T. S., Stewart A. A., Cohen P. The protein phosphatases involved in cellular regulation. 6. Measurement of type-1 and type-2 protein phosphatases in extracts of mammalian tissues; an assessment of their physiological roles. Eur J Biochem. 1983 May 2;132(2):297–307. doi: 10.1111/j.1432-1033.1983.tb07362.x. [DOI] [PubMed] [Google Scholar]
- Jurgensen S., Shacter E., Huang C. Y., Chock P. B., Yang S. D., Vandenheede J. R., Merlevede W. On the mechanism of activation of the ATP X Mg(II)-dependent phosphoprotein phosphatase by kinase FA. J Biol Chem. 1984 May 10;259(9):5864–5870. [PubMed] [Google Scholar]
- Lawrence J. C., Jr, Hiken J., Burnette B., DePaoli-Roach A. A. Phosphorylation of phosphoprotein phosphatase inhibitor-2 (I-2) in rat fat cells. Biochem Biophys Res Commun. 1988 Jan 15;150(1):197–203. doi: 10.1016/0006-291x(88)90505-0. [DOI] [PubMed] [Google Scholar]
- Picton C., Woodgett J., Hemmings B., Cohen P. Multisite phosphorylation of glycogen synthase from rabbit skeletal muscle. Phosphorylation of site 5 by glycogen synthase kinase-5 (casein kinase-II) is a prerequisite for phosphorylation of sites 3 by glycogen synthase kinase-3. FEBS Lett. 1982 Dec 13;150(1):191–196. doi: 10.1016/0014-5793(82)81332-x. [DOI] [PubMed] [Google Scholar]
- Raines E. W., Ross R. Platelet-derived growth factor. I. High yield purification and evidence for multiple forms. J Biol Chem. 1982 May 10;257(9):5154–5160. [PubMed] [Google Scholar]
- Roach P. J., Larner J. Rabbit skeletal muscle glycogen synthase. II. Enzyme phosphorylation state and effector concentrations as interacting control parameters. J Biol Chem. 1976 Apr 10;251(7):1920–1925. [PubMed] [Google Scholar]
- Sommercorn J., Mulligan J. A., Lozeman F. J., Krebs E. G. Activation of casein kinase II in response to insulin and to epidermal growth factor. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8834–8838. doi: 10.1073/pnas.84.24.8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonks N. K., Cohen P. The protein phosphatases involved in cellular regulation. Identification of the inhibitor-2 phosphatases in rabbit skeletal muscle. Eur J Biochem. 1984 Nov 15;145(1):65–70. doi: 10.1111/j.1432-1033.1984.tb08522.x. [DOI] [PubMed] [Google Scholar]
- Vandenheede J. R., Yang S. D., Goris J., Merlevede W. ATP x Mg-dependent protein phosphatase from rabbit skeletal muscle. II. Purification of the activating factor and its characterization as a bifunctional protein also displaying synthase kinase activity. J Biol Chem. 1980 Dec 25;255(24):11768–11774. [PubMed] [Google Scholar]
- Villa-Moruzzi E., Ballou L. M., Fischer E. H. Phosphorylase phosphatase. Interconversion of active and inactive forms. J Biol Chem. 1984 May 10;259(9):5857–5863. [PubMed] [Google Scholar]
- Villa-Moruzzi E., Heilmeyer L. M., Jr Phosphorylase phosphatase from skeletal muscle membranes. Eur J Biochem. 1987 Dec 15;169(3):659–667. doi: 10.1111/j.1432-1033.1987.tb13658.x. [DOI] [PubMed] [Google Scholar]
- Yang S. D., Chang S. Y., Soderling T. R. Characterization of an autophosphorylation-dependent multifunctional protein kinase from liver. J Biol Chem. 1987 Jul 5;262(19):9421–9427. [PubMed] [Google Scholar]



