Abstract
The importance of CD8+ T cells in the control of viral infections is well established. However, what differentiates CD8+ T cell responses in individuals who control infection and those who do not is not well understood. ‘Functional sensitivity’ describes an important quality of the T cell response and is determined in part by the affinity of the T cell receptor for antigen. A more sensitive T cell response is generally believed to be more efficient and associated with better control of viral infection, yet may also drive viral mutation and immune escape. Various in vitro techniques have been used to measure T cell sensitivity; however, rapid ex vivo analysis of this has been made possible by the application of the ‘magic’ tetramer technology. Such tools have potentially important applications in the design and evaluation of vaccines.
Keywords: CD8 T cells, T cells, viruses/viral immunity
T cells are essential for viral immunity
T cells play an important role in containment of persistent viral infections such as human immunodeficiency virus (HIV) and hepatitis C virus (HCV). For example, depletion studies in models of both HCV [1] and HIV [2] have demonstrated the importance of CD8+ cytotoxic T lymphocytes (CTL) in the control of virus replication. Additionally, immunogenetic studies reveal an important impact of human leucocyte antigen (HLA) class I and class II genes, such as HLA B27 and B57, on disease outcome [3]. There has been extensive characterization of the CD8 T cell response in acute and chronic HCV [4] and HIV [5] infections, comparing responses in those who control infection to those in whom disease progresses. However, comprehensive understanding of what determines a successful as opposed to an unsuccessful response requires more precise analysis of the mechanisms involved. This endeavour is important in the development of immunotherapy and vaccines.
T cells vary in quality
A number of factors have been suggested to play a role in determining disease outcome in viral infections; these include general features such as the magnitude of T cell response and the number of epitopes targeted, the functionality of such responses and their longevity. At a more detailed level it is likely that the exact peptides targeted, their ability to mutate and escape T cell recognition and the sensitivity of the individual T cells to peptide all play a major role. All these factors have been under intense scrutiny in HIV and, to a lesser extent, in HCV infection.
T cells that are able to recognize the same peptide bound to major histocompatibility complex (pMHC) vary in their sensitivity for antigen by several orders of magnitude [6,7] and it has been shown in both murine models and human infection that CD8+ CTLs that are able to recognize very low antigen densities are most efficient at eliminating viruses [6,8–10].
A number of factors contribute to the sensitivity of the CTL response. On the T cell side this is determined in large part by T cell receptor (TCR) affinity, but also the level of TCR expression, TCR valency CD8 expression and expression of accessory molecules on the CTL clones comprising a polyclonal response. On the antigen-presenting cell or infected target cell, a major contributor to the ability of T cells to recognize low levels of antigen is the processing and binding of peptide to MHC class I (MHCI). T cell sensitivity has been referred to in the literature as ‘functional avidity’. However, there are recent data to suggest that sensitivity is not an entirely fixed property and sensitivity can be fine-tuned in response to other factors such as cytokines and antigen level [11]. We therefore propose the use of the term ‘functional sensitivity’ in place of ‘functional avidity’, as it is usually the sensitivity (which is determined by all of the above) rather than the actual avidity of the interaction that has been measured.
In principle, increased functional sensitivity by definition allows T cells to recognize lower levels of peptide and thus target cells early in infection, or overcome immune evasion mechanisms such as down-regulation of MHCI. Because responses to different peptides, different HLA alleles or in different individuals might comprise cells bearing different T cell receptors, it is plausible that such variation may contribute to the efficacy of T cell responses.
Relating TCR affinity to T cell sensitivity
Induction of functional, long-lived CD8+ T cell responses requires interaction with a professional antigen-presenting cell, its co-stimulatory molecules and help from CD4+ T cells. Once primed, CTL effector function is activated upon engagement between the T cell receptor (TCR) and cognate pMHCI [12], expressed on the surface of almost all nucleated cells. On interaction of a TCR with its cognate pMHCI there is ultimately a formal assembly of these molecules with the formation of an immunological synapse. Thus, although it is possible to measure specific TCR/pMHCI interactions in isolation, and in a resting cell TCRs may move independently within the membrane [13], inevitably the overall binding must be viewed as a complex multimeric interaction.
The TCR interaction with pMHC is both sensitive and specific. Cognate pMHC class II complexes are able to activate CD4 T cells when as few as 0·03% of total MHC molecules present on the cell surface contain antigen [14]. T cells flux calcium ions in response to engagement of a single MHC [15] and CD8 T cell clones can be activated by as few as 1–50 pMHCI complexes [16,17]. Single amino acid substitution of presented peptides dictates strongly the ability of T cells to respond to the antigen [18]. Such sensitivity and specificity allows for appropriate responses to low levels of presentation of non-self antigen. However, as it is known that pMHCI/TCR interactions are very weak, this has led to much interest in how this sensitivity and specificity are achieved.
Kinetic models of the TCR : pMHCI interaction are popular approaches to explain this paradox. The serial engagement model proposes that a single agonist pMHCI engages multiple TCRs on a given T cell to enable sustained engagement and CTL triggering [17,19]. This is thought to explain the observation that T cell activation is possible despite low physiological levels of pMHCI on the surface of cells [16,17]. The low affinity of the TCR : pMHCI interaction enables rapid dissociation, ensuring that serial TCRs are able to engage [20]. The kinetic proof-reading model suggests that the TCR : pMHCI complex must engage for a minimum half-life (t) for completion of intracellular signalling events: if the off rate is too rapid the T cell cannot be activated [21–23]. The kinetic discrimination model expands on this to suggest that incomplete receptor activation leads to inhibition of T cell activation [23]. Combined, these models predict that there is an optimal t1/2 required for T cell activation [20,24]. Too short a t1/2 fails to activate T cells and too long a t1/2 results in too long an interaction preventing serial engagement [17,25]. These models have been supported by experimental data using TCR mutants conferring varying half-lives on the TCR : pMHCI interaction [25–29].
Thus, although the details of TCR activation still require much further work, a central role for TCR off-rate and TCR affinity in determining the threshold for triggering of a CD8+ T cell in response to peptide appears to be emerging. Many groups have hypothesized that this triggering threshold may impact to the function or ‘quality’ of T cells in vivo. In fact, surface plasmon resonance (SPR) has been used to show that the affinity of the interaction between TCR and pMHCI correlates with the ‘quality’ of the response of T cell clones [30]. How may such complex biochemical measures be translated to allow simple measurement of T cell sensitivity during responses against persistent viral infections?
Assessment of T cell sensitivity
Functional assays
In the literature, ‘functional avidity’ or ‘sensitivity’ is defined simply as the sensitizing dose of peptide epitope added exogenously to target cells yielding half-maximal CTL triggering [sensitizing dose (SD) 50] in functional assays (such as cytolysis and cytokine release). These can be performed ex-vivo in some settings, if the frequency of T cells is relatively high, or if the assay for T cell function is sensitive [such as interferon (IFN)-γ enzyme-linked immunospot assay (ELISPOT)]. However, the readout from such assays is complex, as it depends not only on the TCR affinity for MHCI (and the peptide binding to MHCI) but also the functional state of the T cells in the assay, and the exact assay conditions.
Expansion in vitro of T cells is often used to perform such analyses. However, the expansion in vitro leads to even further complexity. T cell lines of differing functional sensitivities can be generated in vitro by stimulation of peripheral blood polymorphonuclear cells (PBMCs) with distinct doses of peptide antigen. Exposure to low-dose antigen generates clones able to lyse cells more efficiently (i.e. at lower peptide concentrations) than clones generated by high-dose antigen [6,8]. This type of experiment would suggest that cells activated by lower doses of antigen are of higher sensitivity than those requiring large doses of antigen and thus the exact conditions of culture may skew the composition of the response. Therefore, although such assays have been used conventionally, more recent approaches to measurement of TCR sensitivity for peptide have been developed.
Tetramers and assessment of T cell sensitivity
Because the interaction between a single TCR and pMHCI is of low affinity, even if it is specific, staining with single pMHCI-labelled complexes does not lead to stable binding of T cells. However, multimerization of pMHCIs, described typically as ‘tetramers’ or ‘multimers’, takes advantage of the capacity for aggregation of receptors in the cell membrane and leads to high-level staining of specific cells (see Fig. 1). Such technology has transformed our ability to identify antigen-specific CD8 T cells ex vivo, and allows measurement of such populations independent of function.
Fig. 1.
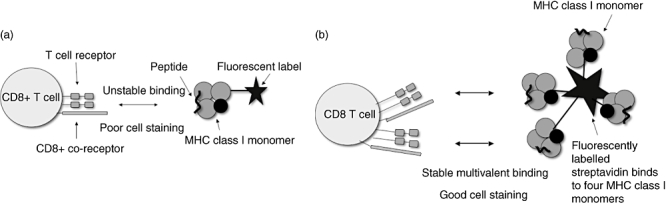
Tetramer staining of antigen-specific CD8 T cells for flow cytometry analysis. (a) Fluorescently labelled major histocompatibility complex (MHC) class I monomers bind CD8 T cells with high off rate. Binding is unstable and staining poor. (b) Fluorescently labelled MHC class I tetramers or other multimers bind CD8 T cells with low off rate. Antigen-specific CD8 T cells can be stained and studied using flow cytometry.
Measurement of the kinetic dissociation of pMHCI tetramer constructs can be used to estimate the overall sensitivity of the TCR : pMHCI interactions on a population of T cells. Although simple staining intensity of pMHCI tetramers does not correlate well with sensitivity [29,31,32], an association can be demonstrated between sensitivity and the stability of TCR : tetramer binding. Dissociation of pMHCI tetramers from CTLs specific for tumour antigen was found to correlate with the functional sensitivity of CTL clones [33] (see Fig. 2).
Fig. 2.
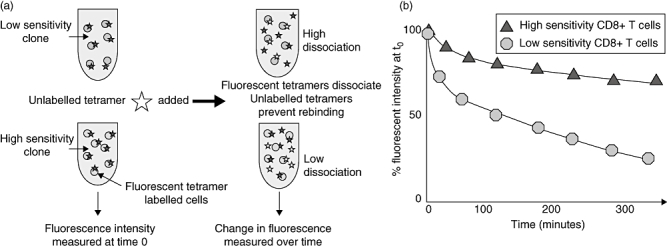
Tetramer dissociation assay. (a) Antigen-specific CD8 T cells are stained with fluorescently labelled major histocompatibility complex class I tetramers. Fluorescent intensity is measured at time 0. Unlabelled multimer is added to the tetramer-stained cells after time 0, which prevent rebinding of dissociated labelled tetramers. (b) Change in fluorescent intensity is measured over time. High-sensitivity CD8 T cells demonstrate slower dissociation than low-sensitivity CD8 T cells.
‘Magic’ tetramers with modified CD8 binding
The T cell surface glycoprotein CD8 binds independently from the TCR to an invariant region of the pMHCI complex. This interaction is of extremely low affinity, even weaker than that of TCR : pMHCI, with a KD in the order of 100 µM. CD8 is believed to have a number of roles in T cell activation: extracellularly stabilizing TCR : pMHCI interactions [34], promoting association of TCR and pMHCI [35] and participating intracellularly in signal transduction in initiating the cascade of T cell activation [36–40]. Although there is evidence for all of these, CD8 binding is not essential for all T cells, as so-called CD8 ‘independent’ epitopes exist naturally. HLA–A*68 is structurally incapable of binding CD8 yet still functions normally in antigen presentation and T cell activation [41].
CD8 co-receptor dependence varies inversely with affinity of the TCR [42–46]. CTLs bearing high-affinity TCRs may be activated independently of CD8 binding [43]. To exploit this it is possible to evaluate the affinity of TCRs on a T cell through modifications of the pMHCI : CD8 binding interaction. Because the structures of pMHCI : CD8 have been solved, it is possible to make specific mutants that reduce, abrogate or enhance this binding (see Fig. 3).
Fig. 3.
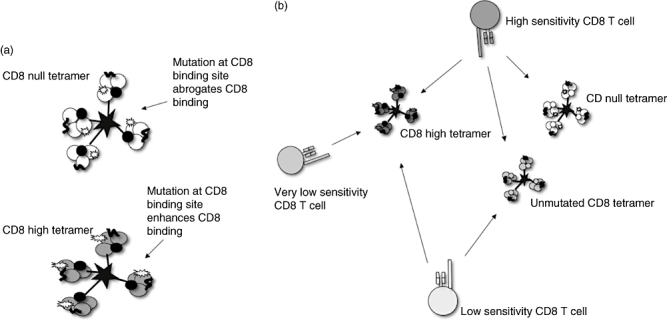
‘Magic tetramers’: tetramers with mutated CD8 binding sites enable detection of CD8 T cell populations with different sensitivities. (a) CD8 null and CD8 high tetramers. (b) CD8 null tetramer has abrogated CD8 binding (through mutation D227K/T228A in the α3 domain); only high sensitivity CD8 T cells are stained. High- and low-sensitivity CD8 T cells are able to bind unmutated tetramers. CD8 high tetramers have enhanced CD8 binding (through mutation Q115E in α2 domain) and enable staining of very low sensitivity CD8 T cells.
These tools allow an immediate ex vivo analysis of the CD8 dependence of the TCR : pMHCI interaction. T cells that bind tetramers where CD8 binding is abrogated (CD8null) are considered to be ‘high avidity’. Those which bind tetramers only in the presence of intact CD8 interactions may be considered low avidity. It is also possible to generate a set of mutants where CD8 binding is partially reduced where the spectrum of cells with intermediate affinities may be observed. CD8-enhanched tetramers have been dubbed ‘magic’ tetramers, as they allow the population of specific T cells to effectively ‘appear’ and ‘disappear’ on flow cytometric analysis [47].
Enhancement of CD8 binding may lead ultimately to a complete loss of peptide specificity for TCR : pMHCI interactions, as the tetramers will bind all CD8+ T cells. However, very small increases in CD8 binding can have surprisingly large effects functionally. TCR : pMHCI interactions which are weak, for example in the case of singly substituted peptides and where conventional tetramers will not bind, may still be visualized using pMHCIs with subtly enhanced CD8 : pMHCI binding (CD8high) [48].
pMHCI tetramers with abrogated CD8 binding (CD8null) demonstrate a correlation between affinity and efficiency of effector function [44] (see Fig. 4). These have been explored in detail using highly defined CTL clones, where the responses to wild-type and mutant peptides have been mapped tightly. However, the technology has only generated limited data so far in polyclonal responses to virus infection, especially those measured ex vivo.
Fig. 4.
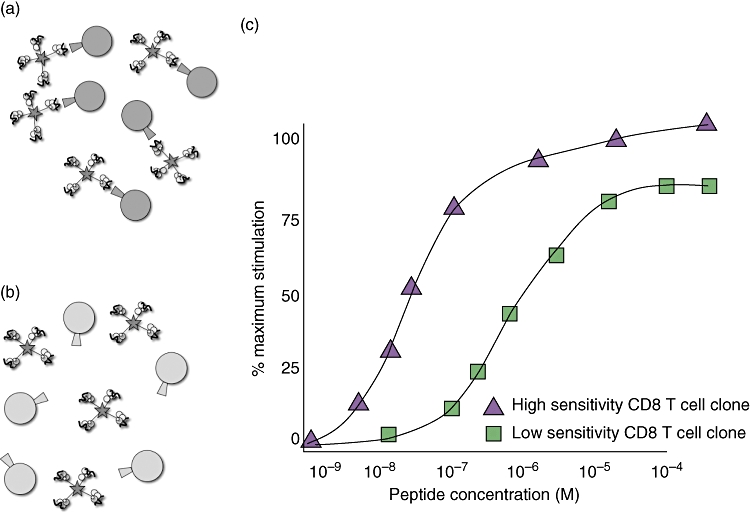
CD8 null tetramers distinguish CD8 T cell clones of high and low sensitivity. (a) CD8 T cell clones able to bind CD8 null tetramers have high functional sensitivity. (b) Low-avidity clones unable to bind CD8 null tetramers have low functional sensitivity. (c) ‘Functional avidity’ or sensitivity assays distinguish sensitivity of CD8 T cell clones that are able and unable to bind CD8 null tetramers.
Given these tools to measure T cell sensitivity in various ways, what information do we currently have that links differences in T cell sensitivity with differences in the outcome of viral infection?
The influence of T cell sensitivity on viral infection
The overall efficiency of CTL effector function may influence the outcome to viral infection through effects on acute control, induction of viral escape, CTL exhaustion and the induction of memory. We consider these in turn.
Acute control
CTLs with high functional sensitivity have been shown to be protective against viral infection in a number of settings. This has been demonstrated clearly on adoptive transfer in murine models [6,8]. In-vitro, highly sensitive CTL clones from mice vaccinated with a recombinant vaccina virus that expresses the gp160 protein from the IIIB strain of HIV-1 were able to lyse infected target cells much earlier in the course of cellular infection than the low-affinity clones. On adoptive transfer into severe combined immunodeficiency (SCID) mice inoculated simultaneously with the recombinant virus, the high-avidity CTL clones were found to be 10-fold more effective at reducing the viral burden than those of low avidity [8].
Protective immune responses against lymphocytic choriomeningitis virus (LCMV) in mice are associated with induction of CTL responses of high functional sensitivity in a comparison between vaccine strategies. More sensitive responses were induced by intraperitoneal immunization of mice with non-replicative porcine parvovirus-like particles bound to LCMV virus epitopes compared to synthetic latex microspheres carrying the same peptides. The former CTL response provided protection from subsequent challenge with lethal doses of virus [45].
A number of studies have demonstrated the importance of functional sensitivity in HIV. In vitro, the functional sensitivity of CTLs for panels of HIV-1 epitope variants were compared to the efficiency of CTL killing of cells infected with whole HIV-1 containing the same epitope variant. Efficiency of CTL killing of the HIV-1 infected target cells was found to correlate with sensitivity. A narrow threshold of functional sensitivity was demonstrated, below which there was little or no killing of the target cells [46]. Analysis of CTL responses to immunodominant HIV-1 epitopes demonstrated an inverse correlation between CTL sensitivity and cell-associated viral load. HLA B27-restricted CTLs in HIV-1 target the immunodominant epitope B27-K10, and CTL clones specific for this epitope are found to have higher functional sensitivity in comparison to other HLA-A- and HLA-B-restricted CTL responses [9]. This is clearly of interest in context of the observation that HIV progresses much more slowly in patients with HLA B27.
In HCV, in vitroanalysis of the cytotoxicity of CTL clones against target cells pulsed with exogenous peptide found there to be a significantly greater functional sensitivity in clearers compared to non-clearers [10]. This finding has been supported by a further study where patients who had cleared HCV genotype 1 were found to have higher-avidity CTL responses, with enhanced IFN-γ, tumour necrosis factor (TNF)-α and cytotoxic activity compared to chronic patients infected with the same genotype. Interestingly, the same authors also found a difference in the ability of NS31073-specific clones from clearers and chronics to bind pMHCI high-valency multimers versus lower-valency tetramers. Clones from patients who had cleared their HCV were able to bind both multimers and tetramers, whereas the clones from patients with chronic HCV were able to bind only the high-valency multimers [49]. A formal assessment of TCR affinity in such cases has not been made; however, this potentially provides further insight into the role of CTL sensitivity and in defining viral clearance.
Viral escape
Mutations within a viral genome often confer advantages in vivo, the evolution of which is driven strongly by immune selection pressures. Immune control of the virus before it is able to mutate is therefore crucial in determining long-term outcome to infection (see Fig. 5). In HIV and simian immunodeficiency virus (SIV), viral escape mutations within immunodominant epitopes play a critical role in early and late loss of immune control [50–52] and this is also shown to influence long-term outcome in acute HCV infection [53,54].
Fig. 5.
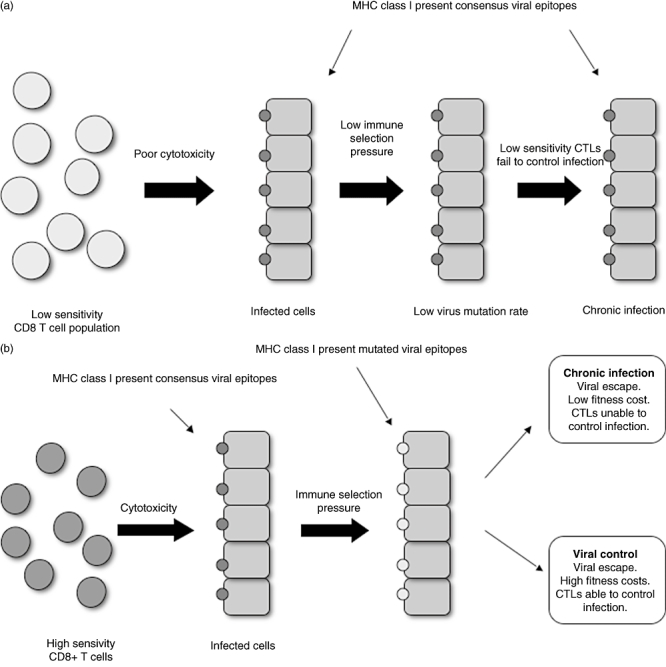
High-avidity CD8 T cells drive viral escape. (a) Low-avidity T cells exert low immune pressure on the infecting virus. This results in a low rate of virus mutation, no viral fitness cost and chronic infection. (b) High-avidity T cells exert immune pressure on the infecting virus and drive viral escape. Two outcomes can be observed: viral control, where viral escape results in mutations at a cost to viral fitness, and chronic infection, where viral escape occurs without fitness costs and the host CD8 T cells are unable to control infection. MHC, major histocompatibility complex; CTLs, cytotoxic T lymphocytes.
There is a variation in the degree of escape between different epitopes within the viral genome of such persistent viral infections, where some epitopes are observed to escape while others are often conserved. One explanation which has been proposed for this is that more sensitive T cells are associated with escape (‘driver’ responses), while less sensitive cells may be simply ‘passengers’ which have little impact on viral evolution or disease outcome [55]. More sensitive populations are observed to drive viral escape, whereas less sensitive CTLs are associated with epitope stability in both HCV [56] and SIV [57]. In HIV, CTL responses to the promiscuous epitope TL9-Gag were compared between HLA types within the B7 supertype. B*8101-restricted TL9-Gag responses were found to be of significantly higher functional sensitivity than those restricted by B*4201. Higher TL9-Gag sequence variation is observed in B*8101 compared to B*4201-positive patients [58].
There is a clear conflict of interest in the outcome of better-quality CTL responses. The immune advantages of improved clearance of the more sensitive responses would appear to be balanced against the disadvantage of driving evolution of the virus in its ability to escape the host immune response. However, viral fitness costs associated with the acquisition of escape mutations may contribute to the protective nature of some HLA class I alleles, such as B57 [3].
Deletion and anergy
CTL dysfunction is seen in a number of chronic viral infections in humans [59,60] and animal models [61,62]. The genesis of such dysfunction is not well understood, but is thought to be related to repetitive triggering through the TCR. One possible outcome is that more sensitive cells might become preferentially over-stimulated and anergic in the presence of high antigen load. This is supported by in vivo studies showing the persistence of anergic CTLs with high functional sensitivity under such conditions [63,64]. The distinct sensitivities observed in cells of the acute and chronic phase of HIV-1 appears to be a consequence of deletion of the more sensitive cells, as determined by clonotypic analysis of TCR VB chains by polymerase chain reaction (PCR). Interestingly, sensitivity and clonotype were preserved in those who controlled HIV-1 replication spontaneously to very low levels [65]. This phenomenon is also observed in the mouse model of LCMV. High-dose viral infection led to clonotypic switching in the repertoire of epitope-specific cells and emergence of dominant T cells with intermediate and low sensitivity in chronic infection [66].
The affinity of the TCR, a fixed property of the cell, plays an important role in determining CTL sensitivity. However, the overall triggering threshold of a T cell in response to peptide is determined not only by the affinity of the TCR, but seems to be regulated. Naive CTLs have inherent differences in sensitivity to peptide, pre-determining the ability of a given CTL repertoire to clear infection; interindividual difference in outcome from viral infection are thus influenced by inherent differences in the quality of the host's T cell repertoire. Differences in functional sensitivity are not seen after stimulation of naive CTLs from TCR transgenic mice with varying levels of peptide antigen. Paired daughter clones from CTLs were, however, able to give rise to populations of cells of distinct sensitivity dependent upon the level of antigen used to maintain the clones [11]. Such plasticity would enable peptide sensitivity to be tuned in response to the level of antigen presented, while at the same time provide protection against apoptosis induced by high amounts of peptide. This may explain the observation of loss of CTL function at high viral doses [67–69], suggesting that sensitivity is tuned down. Such a phenomenon may be explained by the inducible expression of the inhibitory co-stimulatory molecule programmed death-1 (PD-1) with antigen exposure. Expression is up-regulated markedly on antigen-experienced CTLs in both HIV [70] and HCV [71], as well as LCMV [72].
Heterologous immunity
Previous infection with viruses containing sequences that partially cross-react has been observed to influence the subsequent response to heterologous infection – so-called heterologous immunity. This has been observed in some murine models, and includes viruses which are quite unrelated genetically [73]. The overall impact of this process in human infection is not understood fully, and in particular the quality of such responses has not been examined in detail. It has been suggested that such responses may skew the subsequent response to a pathogen and lead to immunopathology.
We have recently examined one of the best-documented examples of this in HCV using pMHCI with modified CD8 binding (‘magic tetramers’) as described above [47]. The response concerned is specific for an immunodominant and highly conserved epitope in HCV NS3. Tetramers created using this peptide bind only in the presence of an intact CD8 recognition site, indicating that this is a low-avidity response in natural infection. Responses to the HCV-NS3 epitope have been reported to cross-react with an epitope derived from influenza virus neuraminidase protein (Flu-NA). However, in healthy donors and also in HCV+ donors we could not detect cross-reactive T cells using conventional tetramer staining, suggesting that this cross-reactivity was very weak. Interestingly, using tetramers with enhanced CD8 binding (CD8hi) revealed cross-reactivity for the Flu-NA peptide. This poor-quality response is therefore measurable, although functionally the Flu-NA peptide was unable to trigger IFN-γ release. In further experiments it was possible to enhance the sensitivity of the T cell response by using a modified peptide derived from genotype 4. Here, increased sensitivity to peptide was accompanied by loss of dependence on CD8 for binding (i.e. binding of a CD8 null tetramer).
Thus, overall, this examination in detail of a case of heterologous reactivity has revealed some of the limits of T cell cross-reactivity and its dependence on T cell sensitivity. The ability to define T cell sensitivity readily using polyclonal responses independently of function may allow further examination of the importance of heterologous immunity in man.
Conclusion
Advances in understanding of the basic biology of TCR interactions with pMHCI have led to the development of new tools and assays for determining the quality of the T cell response. Conceptually, the presence of highly sensitive T cells should be of benefit in control of viral infections, although the twin threats of immune escape and immune exhaustion act to diminish the power of anti-viral responses. However, although there are some data to support the model that TCR avidity is a key determinant of outcome, a casual link is not established fully. We suggest that there are two models which might be considered in trying confirm such a link (see Fig. 6).
Fig. 6.
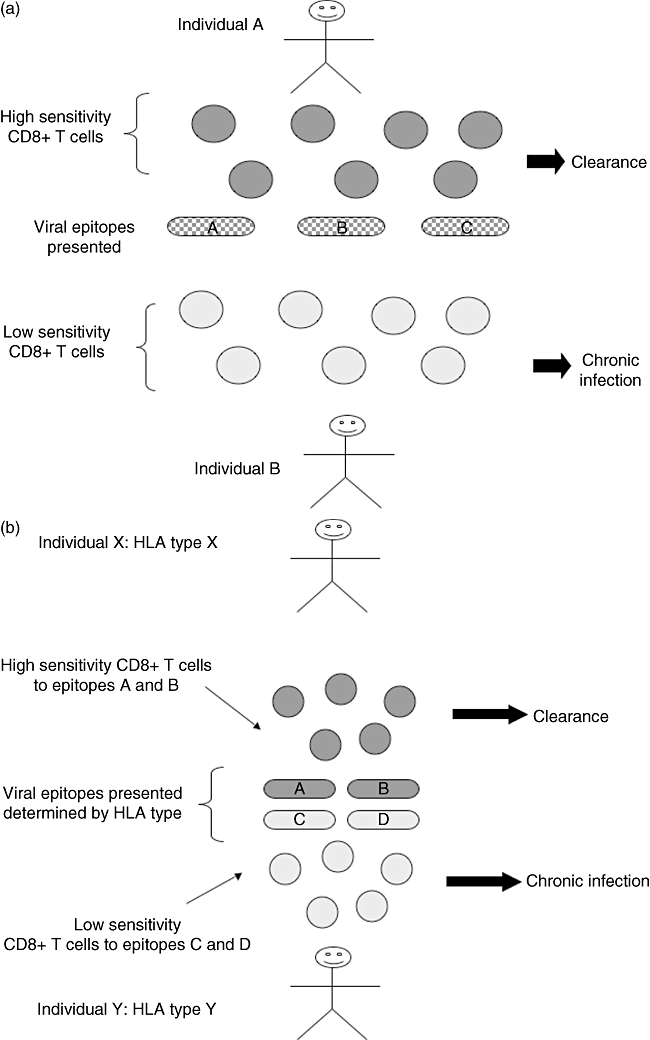
Two models of how CD8 T cell sensitivity could determine outcome to viral infection. (a) Different individuals mount responses of different sensitivity for the same epitopes. Different factors determine outcome to infection, e.g. CD8 T cell avidity, dose of antigen, site of antigen, viral fitness. (b) Outcome to infection is a fixed property of the epitope presented and the avidity of responses created, which is determined in turn by the human leucocyte antigen (HLA) type of an individual.
On one hand, different individuals may mount responses of different quality for the same epitope (depending upon a number of factors including site, duration and dose of antigen, as well as host genetics). The variation in such responses might be linked to the suppression of viraemia or the induction of immune escape (‘private avidity’). Alternatively, all individuals may make responses of similar quality against specific epitopes, i.e. the quality of the response is essentially a fixed property of the epitope (‘public avidity’). In this case, the overall picture will be determined by the choice of epitopes available to the individual, which is driven in turn largely by MHC. In this respect, the overall role of TCR avidity in determining the striking protective effect of HLA B27 and B57 in the outcome of both HIV and HCV has not yet been explained fully. However, it has been suggested that avidity plays some role [9].
Overall, we have a large number of new tools at our disposal to dissect further the impact of changes in TCR avidity or quality on the outcome of virus infection. Further work is required in man, using carefully defined clinical cohorts studied ideally from acute infection onwards. If we can exploit these tools to strengthen a link between T cell sensitivity and clinical outcome, this will be of substantial benefit in both designing and analysing vaccines.
Acknowledgments
We acknowledge the Wellcome Trust, NIHR Biomedical Research Centre Programme (Oxford) and the MRC.
Disclosure
None.
References
- 1.Shoukry NH, Grakoui A, Houghton M, et al. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J Exp Med. 2003;197:1645–55. doi: 10.1084/jem.20030239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmitz JE, Kuroda MJ, Santra S, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–60. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 3.Scherer A, Frater J, Oxenius A, et al. Quantifiable cytotoxic T lymphocyte responses and HLA-related risk of progression to AIDS. Proc Natl Acad Sci USA. 2004;101:12266–70. doi: 10.1073/pnas.0404091101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dustin LB, Rice CM. Flying under the radar: the immunobiology of hepatitis C. Annu Rev Immunol. 2007;25:71–99. doi: 10.1146/annurev.immunol.25.022106.141602. [DOI] [PubMed] [Google Scholar]
- 5.Goulder PJ, Watkins DI. Impact of MHC class I diversity on immune control of immunodeficiency virus replication. Nat Rev Immunol. 2008;8:619–30. doi: 10.1038/nri2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexander-Miller MA, Leggatt GR, Berzofsky JA. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc Natl Acad Sci USA. 1996;93:4102–7. doi: 10.1073/pnas.93.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander-Miller MA. Differential expansion and survival of high and low avidity cytotoxic T cell populations during the immune response to a viral infection. Cell Immunol. 2000;201:58–62. doi: 10.1006/cimm.1999.1632. [DOI] [PubMed] [Google Scholar]
- 8.Derby M, Alexander-Miller M, Tse R, Berzofsky J. High-avidity CTL exploit two complementary mechanisms to provide better protection against viral infection than low-avidity CTL. J Immunol. 2001;166:1690–7. doi: 10.4049/jimmunol.166.3.1690. [DOI] [PubMed] [Google Scholar]
- 9.Almeida J, Price D, Papagno L, et al. Superior control of HIV-1 replication by CD8(+) T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J Exp Med. 2007;204:2473–85. doi: 10.1084/jem.20070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yerly D, Heckerman D, Allen TM, et al. Increased cytotoxic T-lymphocyte epitope variant cross-recognition and functional avidity are associated with hepatitis C virus clearance. J Virol. 2008;82:3147–53. doi: 10.1128/JVI.02252-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kroger CJ, Alexander-Miller MA. Cutting edge: CD8+ T cell clones possess the potential to differentiate into both high- and low-avidity effector cells. J Immunol. 2007;179:748–51. doi: 10.4049/jimmunol.179.2.748. [DOI] [PubMed] [Google Scholar]
- 12.Henrickson SE, von Andrian UH. Single-cell dynamics of T-cell priming. Curr Opin Immunol. 2007;19:249–58. doi: 10.1016/j.coi.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 13.James JR, White SS, Clarke RW, et al. Single-molecule level analysis of the subunit composition of the T cell receptor on live T cells. Proc Natl Acad Sci USA. 2007;104:17662–7. doi: 10.1073/pnas.0700411104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demotz S, Grey HM, Sette A. The minimal number of class II MHC-antigen complexes needed for T cell activation. Science. 1990;249:1028–30. doi: 10.1126/science.2118680. [DOI] [PubMed] [Google Scholar]
- 15.Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM. Direct observation of ligand recognition by T cells. Nature. 2002;419:845–9. doi: 10.1038/nature01076. [DOI] [PubMed] [Google Scholar]
- 16.Sykulev Y, Joo M, Vturina I, Tsomides TJ, Eisen HN. Evidence that a single peptide–MHC complex on a target cell can elicit a cytolytic T cell response. Immunity. 1996;4:565–71. doi: 10.1016/s1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- 17.Valitutti S, Lanzavecchia A. Serial triggering of TCRs: a basis for the sensitivity and specificity of antigen recognition. Immunol Today. 1997;18:299–304. [PubMed] [Google Scholar]
- 18.Sloan-Lancaster J, Evavold BD, Allen PM. Induction of T-cell anergy by altered T-cell-receptor ligand on live antigen-presenting cells. Nature. 1993;363:156–9. doi: 10.1038/363156a0. [DOI] [PubMed] [Google Scholar]
- 19.Lanzavecchia A, Lezzi G, Viola A. From TCR engagement to T cell activation: a kinetic view of T cell behavior. Cell. 1999;96:1–4. doi: 10.1016/s0092-8674(00)80952-6. [DOI] [PubMed] [Google Scholar]
- 20.Davis MM, Boniface JJ, Reich Z, et al. Ligand recognition by alpha beta T cell receptors. Annu Rev Immunol. 1998;16:523–44. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- 21.McKeithan TW. Kinetic proofreading in T-cell receptor signal transduction. Proc Natl Acad Sci USA. 1995;92:5042–6. doi: 10.1073/pnas.92.11.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wulfing C, Rabinowitz JD, Beeson C, Sjaastad MD, McConnell HM, Davis MM. Kinetics and extent of T cell activation as measured with the calcium signal. J Exp Med. 1997;185:1815–25. doi: 10.1084/jem.185.10.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rabinowitz JD, Beeson C, Lyons DS, Davis MM, McConnell HM. Kinetic discrimination in T-cell activation. Proc Natl Acad Sci USA. 1996;93:1401–5. doi: 10.1073/pnas.93.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Merwe PA, Davis SJ. Molecular interactions mediating T cell antigen recognition. Annu Rev Immunol. 2003;21:659–84. doi: 10.1146/annurev.immunol.21.120601.141036. [DOI] [PubMed] [Google Scholar]
- 25.Coombs D, Kalergis AM, Nathenson SG, Wofsy C, Goldstein B. Activated TCRs remain marked for internalization after dissociation from pMHC. Nat Immunol. 2002;3:926–31. doi: 10.1038/ni838. [DOI] [PubMed] [Google Scholar]
- 26.Degano M, Garcia KC, Apostolopoulos V, Rudolph MG, Teyton L, Wilson IA. A functional hot spot for antigen recognition in a superagonist TCR/MHC complex. Immunity. 2000;12:251–61. doi: 10.1016/s1074-7613(00)80178-8. [DOI] [PubMed] [Google Scholar]
- 27.Hudrisier D, Kessler B, Valitutti S, Horvath C, Cerottini JC, Luescher IF. The efficiency of antigen recognition by CD8+ CTL clones is determined by the frequency of serial TCR engagement. J Immunol. 1998;161:553–62. [PubMed] [Google Scholar]
- 28.Kessler B, Hudrisier D, Cerottini JC, Luescher IF. Role of CD8 in aberrant function of cytotoxic T lymphocytes. J Exp Med. 1997;186:2033–8. doi: 10.1084/jem.186.12.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalergis AM, Boucheron N, Doucey MA, et al. Efficient T cell activation requires an optimal dwell-time of interaction between the TCR and the pMHC complex. Nat Immunol. 2001;2:229–34. doi: 10.1038/85286. [DOI] [PubMed] [Google Scholar]
- 30.McMahan RH, McWilliams JA, Jordan KR, Dow SW, Wilson DB, Slansky JE. Relating TCR-peptide-MHC affinity to immunogenicity for the design of tumor vaccines. J Clin Invest. 2006;116:2543–51. doi: 10.1172/JCI26936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubio-Godoy V, Dutoit V, Rimoldi D, et al. Discrepancy between ELISPOT IFN-gamma secretion and binding of A2/peptide multimers to TCR reveals interclonal dissociation of CTL effector function from TCR-peptide/MHC complexes half-life. Proc Natl Acad Sci USA. 2001;98:10302–7. doi: 10.1073/pnas.181348898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Derby MA, Wang J, Margulies DH, Berzofsky JA. Two intermediate-avidity cytotoxic T lymphocyte clones with a disparity between functional avidity and MHC tetramer staining. Int Immunol. 2001;13:817–24. doi: 10.1093/intimm/13.6.817. [DOI] [PubMed] [Google Scholar]
- 33.Dutoit V, Rubio-Godoy V, Doucey MA, et al. Functional avidity of tumor antigen-specific CTL recognition directly correlates with the stability of MHC/peptide multimer binding to TCR. J Immunol. 2002;168:1167–71. doi: 10.4049/jimmunol.168.3.1167. [DOI] [PubMed] [Google Scholar]
- 34.Wooldridge L, van den Berg HA, Glick M, et al. Interaction between the CD8 coreceptor and major histocompatibility complex class I stabilizes T cell receptor-antigen complexes at the cell surface. J Biol Chem. 2005;280:27491–501. doi: 10.1074/jbc.M500555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gakamsky DM, Luescher IF, Pramanik A, et al. CD8 kinetically promotes ligand binding to the T-cell antigen receptor. Biophys J. 2005;89:2121–33. doi: 10.1529/biophysj.105.061671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Purbhoo MA, Boulter JM, Price DA, et al. The human CD8 coreceptor effects cytotoxic T cell activation and antigen sensitivity primarily by mediating complete phosphorylation of the T cell receptor zeta chain. J Biol Chem. 2001;276:32786–92. doi: 10.1074/jbc.M102498200. [DOI] [PubMed] [Google Scholar]
- 37.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fahmy TM, Bieler JG, Edidin M, Schneck JP. Increased TCR avidity after T cell activation: a mechanism for sensing low-density antigen. Immunity. 2001;14:135–43. [PubMed] [Google Scholar]
- 39.Alam SM, Davies GM, Lin CM, et al. Qualitative and quantitative differences in T cell receptor binding of agonist and antagonist ligands. Immunity. 1999;10:227–37. doi: 10.1016/s1074-7613(00)80023-0. [DOI] [PubMed] [Google Scholar]
- 40.Gascoigne NR, Alam SM, Lin CM, et al. T cell receptor binding kinetics and special role of Valpha in T cell development and activation. Immunol Res. 2000;21:225–31. doi: 10.1385/IR:21:2-3:225. [DOI] [PubMed] [Google Scholar]
- 41.Hutchinson SL, Wooldridge L, Tafuro S, et al. The CD8 T cell coreceptor exhibits disproportionate biological activity at extremely low binding affinities. J Biol Chem. 2003;278:24285–93. doi: 10.1074/jbc.M300633200. [DOI] [PubMed] [Google Scholar]
- 42.Holler PD, Kranz DM. Quantitative analysis of the contribution of TCR/pepMHC affinity and CD8 to T cell activation. Immunity. 2003;18:255–64. doi: 10.1016/s1074-7613(03)00019-0. [DOI] [PubMed] [Google Scholar]
- 43.Kerry SE, Buslepp J, Cramer LA, et al. Interplay between TCR affinity and necessity of coreceptor ligation: high-affinity peptide-MHC/TCR interaction overcomes lack of CD8 engagement. J Immunol. 2003;171:4493–503. doi: 10.4049/jimmunol.171.9.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laugel B, van den Berg HA, Gostick E, et al. Different T cell receptor affinity thresholds and CD8 coreceptor dependence govern cytotoxic T lymphocyte activation and tetramer binding properties. J Biol Chem. 2007;282:23799–810. doi: 10.1074/jbc.M700976200. [DOI] [PubMed] [Google Scholar]
- 45.Sedlik C, Dadaglio G, Saron MF, et al. In vivo induction of a high-avidity, high-frequency cytotoxic T-lymphocyte response is associated with antiviral protective immunity. J Virol. 2000;74:5769–75. doi: 10.1128/jvi.74.13.5769-5775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bennett MS, Ng HL, Dagarag M, Ali A, Yang OO. Epitope-dependent avidity thresholds for cytotoxic T-lymphocyte clearance of virus-infected cells. J Virol. 2007;81:4973–80. doi: 10.1128/JVI.02362-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kasprowicz V, Ward SM, Turner A, et al. Defining the directionality and quality of influenza virus-specific CD8+ T cell cross-reactivity in individuals infected with hepatitis C virus. J Clin Invest. 2008;118:1143–53. doi: 10.1172/JCI33082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Melenhorst JJ, Scheinberg P, Chattopadhyay PK, et al. Detection of low avidity CD8(+) T cell populations with coreceptor-enhanced peptide-major histocompatibility complex class I tetramers. J Immunol Methods. 2008;338:31–9. doi: 10.1016/j.jim.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neveu B, Debeaupuis E, Echasserieau K, et al. Selection of high-avidity CD8 T cells correlates with control of hepatitis C virus infection. Hepatology. 2008;48:713–22. doi: 10.1002/hep.22379. [DOI] [PubMed] [Google Scholar]
- 50.Allen TM, O'Connor DH, Jing P, et al. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature. 2000;407:386–90. doi: 10.1038/35030124. [DOI] [PubMed] [Google Scholar]
- 51.Barouch DH, Kunstman J, Kuroda MJ, et al. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature. 2002;415:335–9. doi: 10.1038/415335a. [DOI] [PubMed] [Google Scholar]
- 52.Goulder PJ, Phillips RE, Colbert RA, et al. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat Med. 1997;3:212–17. doi: 10.1038/nm0297-212. [DOI] [PubMed] [Google Scholar]
- 53.Cox AL, Mosbruger T, Mao Q, et al. Cellular immune selection with hepatitis C virus persistence in humans. J Exp Med. 2005;201:1741–52. doi: 10.1084/jem.20050121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Erickson AL, Kimura Y, Igarashi S, et al. The outcome of hepatitis C virus infection is predicted by escape mutations in epitopes targeted by cytotoxic T lymphocytes. Immunity. 2001;15:883–95. doi: 10.1016/s1074-7613(01)00245-x. [DOI] [PubMed] [Google Scholar]
- 55.Zafiropoulos A, Barnes E, Piggott C, Klenerman P. Analysis of ‘driver’ and ‘passenger’ CD8+ T-cell responses against variable viruses. Proc Biol Sci. 2004;271(Suppl 3):S53–6. doi: 10.1098/rsbl.2003.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Urbani S, Amadei B, Cariani E, et al. The impairment of CD8 responses limits the selection of escape mutations in acute hepatitis C virus infection. J Immunol. 2005;175:7519–29. doi: 10.4049/jimmunol.175.11.7519. [DOI] [PubMed] [Google Scholar]
- 57.O'Connor DH, Allen TM, Vogel TU, et al. Acute phase cytotoxic T lymphocyte escape is a hallmark of simian immunodeficiency virus infection. Nat Med. 2002;8:493–9. doi: 10.1038/nm0502-493. [DOI] [PubMed] [Google Scholar]
- 58.Leslie A, Price DA, Mkhize P, et al. Differential selection pressure exerted on HIV by CTL targeting identical epitopes but restricted by distinct HLA alleles from the same HLA supertype. J Immunol. 2006;177:4699–708. doi: 10.4049/jimmunol.177.7.4699. [DOI] [PubMed] [Google Scholar]
- 59.Lechner F, Gruener NH, Urbani S, et al. CD8+ T lymphocyte responses are induced during acute hepatitis C virus infection but are not sustained. Eur J Immunol. 2000;30:2479–87. doi: 10.1002/1521-4141(200009)30:9<2479::AID-IMMU2479>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 60.Appay V, Nixon DF, Donahoe SM, et al. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J Exp Med. 2000;192:63–75. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vogel TU, Allen TM, Altman JD, Watkins DI. Functional impairment of simian immunodeficiency virus-specific CD8+ T cells during the chronic phase of infection. J Virol. 2001;75:2458–61. doi: 10.1128/JVI.75.5.2458-2461.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gallimore A, Glithero A, Godkin A, et al. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med. 1998;187:1383–93. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ueno T, Tomiyama H, Fujiwara M, Oka S, Takiguchi M. Functionally impaired HIV-specific CD8 T cells show high affinity TCR–ligand interactions. J Immunol. 2004;173:5451–7. doi: 10.4049/jimmunol.173.9.5451. [DOI] [PubMed] [Google Scholar]
- 64.Probst HC, Tschannen K, Gallimore A, et al. Immunodominance of an antiviral cytotoxic T cell response is shaped by the kinetics of viral protein expression. J Immunol. 2003;171:5415–22. doi: 10.4049/jimmunol.171.10.5415. [DOI] [PubMed] [Google Scholar]
- 65.Lichterfeld M, Yu XG, Mui SK, et al. Selective depletion of high-avidity human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T cells after early HIV-1 infection. J Virol. 2007;81:4199–214. doi: 10.1128/JVI.01388-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin MY, Welsh RM. Stability and diversity of T cell receptor repertoire usage during lymphocytic choriomeningitis virus infection of mice. J Exp Med. 1998;188:1993–2005. doi: 10.1084/jem.188.11.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wherry EJ, Puorro KA, Porgador A, Eisenlohr LC. The induction of virus-specific CTL as a function of increasing epitope expression: responses rise steadily until excessively high levels of epitope are attained. J Immunol. 1999;163:3735–45. [PubMed] [Google Scholar]
- 68.Zajac AJ, Blattman JN, Murali-Krishna K, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–13. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gallimore A, Dumrese T, Hengartner H, Zinkernagel RM, Rammensee HG. Protective immunity does not correlate with the hierarchy of virus-specific cytotoxic T cell responses to naturally processed peptides. J Exp Med. 1998;187:1647–57. doi: 10.1084/jem.187.10.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 71.Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol. 2007;81:9249–58. doi: 10.1128/JVI.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wherry EJ, Ha SJ, Kaech SM, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–84. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 73.Selin LK, Brehm MA, Naumov YN, et al. Memory of mice and men: CD8+ T-cell cross-reactivity and heterologous immunity. Immunol Rev. 2006;211:164–81. doi: 10.1111/j.0105-2896.2006.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


