Abstract
We have demonstrated previously that, in primary Sjögren's syndrome (SS), immature myeloid dendritic cells (DCs) are decreased in blood and mature myeloid DCs are accumulated in salivary glands, suggesting recruitment of the myeloid DCs from blood to salivary glands. To verify whether this finding is universal in patients of not only primary SS but also secondary SS, in this study we analysed the blood DCs of secondary SS patients. We examined 24 secondary SS and 29 primary SS patients. A direct correlation between the decreased number of myeloid DCs and the duration of Sicca syndrome in primary and secondary SS was observed; namely, the reduction of myeloid DCs in blood was restored spontaneously with duration time of Sicca syndrome. We also examined the immunohistochemical staining of salivary glands of SS patients with monoclonal antibodies against fascin, CD11c and human leucocyte antigen DR (HLA-DR). Fascin+ or CD11c+/HLA-DR+ mononuclear cells were present in the salivary glands of secondary SS patients, as in primary SS. However, fascin+ mononuclear cells were barely detected in the salivary glands of a chronic phase of SS patients. We also found a negative correlation between the frequency of blood myeloid DCs and salivary gland-infiltrating DCs in secondary SS patients, as well as primary SS. Our results suggest that the reduction of blood myeloid DCs and preferential trafficking of myeloid DCs into salivary glands is a common event in the early stage of SS. Myeloid DCs may play essential roles in the pathogenesis of Sicca syndrome of SS by initiating T helper cell immune responses.
Keywords: cytokines, dendritic cells, Sjögren's syndrome
Introduction
Sjögren's syndrome (SS) is an autoimmune disease that affects primarily the salivary and lachrymal glands, causing xerostomia and so-called ‘Sicca syndrome’, and is categorized thus as an organ-specific autoimmune disease. The pathogenetic mechanisms consist of an autoimmune process leading to the progressive destruction of salivary and lachrymal glands. Therefore, symptoms of SS are chronic and sometimes irreversible. It is well known that autoimmune diseases often overlap with other collagen diseases, and this is also the case for SS. Without overlapping with any other autoimmune diseases, SS is called primary SS, while SS that overlaps with other autoimmune diseases is termed secondary SS. It has been reported that approximately half of SS cases are secondary SS [1]. SS can be seen alone (primary SS) or in association with other autoimmune rheumatic disease, especially rheumatoid arthritis (RA), systemic sclerosis (SSc) and systemic lupus erythematosus (SLE) (secondary SS). It has not been explained clearly why SS is prone to merge with these autoimmune diseases.
Although the essential mechanism of autoimmune diseases is still largely unknown, various immune cells are suggested to be involved in their genesis. Among those immune cells, dendritic cells (DCs) have emerged recently as candidates for the master cells that elicit aberrant immune reactions in autoimmune diseases [2–7]. DCs are professional antigen-presenting cells (APCs) that have a unique capacity to prime naive T cells and induce them to develop into effector T cells. Thus, DCs are regarded as being the master regulators of adaptive immune responses. Furthermore, recent progress of DC biology has highlighted the functional plasticity of DCs; DCs can induce not only inflammatory immune responses but also peripheral tolerance, depending upon their subsets, the maturation stage of DCs and microenvironments such as cytokine milieu or stimuli [8,9]. These biological properties of DCs may lead to a hypothesis that functional alteration of the DC system causes development of autoimmune diseases. Human peripheral blood contains two major subsets of DCs: CD11c+ myeloid DCs and plasmacytoid DCs [10,11]. Blood myeloid DCs are in the immature stage and seem to be en route to peripheral and lymphoid tissues; they may contribute mainly to T helper type 1 (Th1)-mediated adaptive immune responses by producing interleukin (IL)-12 in response to microbial pathogens. On the other hand, blood plasmacytoid DCs are identical to circulating natural type 1 interferon (IFN)-producing cells, which may contribute to anti-viral innate immunity. The analysis of blood DCs may provide a novel and unique perspective in dissecting the pathogenesis of autoimmune diseases.
There is evidence that mature DCs infiltrated into the RA joint mediate immunopathology in RA [3,4]. Recent evidence also indicates the pathogenic role of type 1 IFN produced by plasmacytoid DCs in SLE [5,6]. However, it remains to be clarified whether DCs may participate in the pathogenesis of other autoimmune diseases.
Previously we have demonstrated that, in primary SS, blood immature myeloid DCs are decreased and mature myeloid DCs are accumulated in salivary glands, suggesting the recruitment of myeloid DCs from blood to inflamed salivary glands. In addition, we demonstrated that numerous IFN-γ-producing CD4+ T cells are also infiltrated into the salivary glands from primary SS patients [2]. Based upon these findings, we proposed a hypothesis that myeloid DCs play a role in pathogenesis of primary SS by initiating Th1 immune response. In this study, we report that the decrease of blood myeloid DCs and accumulation of salivary gland-infiltrating DCs is universal in the early phase of not only primary SS but also secondary SS, and this alteration was restored spontaneously during the natural clinical course.
Materials and methods
Patients
As shown in Table 1, patients enrolled into this study comprised 24 patients with secondary SS (two men and 22 women, mean age 55·5 years), 29 with primary SS (two men and 27 women, mean age 58·6 years), 11 with SLE (two men and nine women, mean age 25·3 years), 14 with SSc (one man and 13 women, mean age 54·9 years) and 12 with RA (three men and nine women, mean age 55·9 years). In addition, 32 healthy volunteers (12 men and 20 women, mean age 48·0 years) were also enrolled into this study as normal controls. All patients presented to our hospital between May 1999 and June 2003 and were diagnosed freshly as having autoimmune diseases. No patients or volunteers had evidence of infections at the time of this study. All patients underwent routine laboratory examinations and were also examined for a variety of autoantibodies. Informed consent was obtained for this study in accordance with the provisions of the Declaration of Helsinki. All SS patients met the criteria of the Research Committee on SS of the Ministry of Health and Welfare of Japan [12], as well as the European Community criteria [13]. Patients with SLE or SLE-merged secondary SS fulfilled the diagnostic criteria for SLE of the American College of Rheumatology (ACR) [14,15]. Patients with RA or RA-merged secondary SS fulfilled the diagnostic criteria for RA of the ACR [16]. Patients with SSc or SSc-merged secondary SS fulfilled the diagnostic criteria for SSc of the ACR [17]. We determined the onset of SS by a patient complaint about Sicca syndrome in a medical interview (Table 1).
Table 1.
Summary of clinical and serological findings for each autoimmune disease.
| Age (years) |
Total PBDCs/ml |
Myeloid DCs/ml |
Plasmacytoid DCs/ml |
WBCs/µl |
ANA log titre |
RF IU/ml |
Anti-SSA |
Anti-SSB |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Sex (M/F) | Mean range | Mean range | Mean range | Mean range | Mean range | Positive/n range | Positive/n range | Positive/n | Positive/n | |
| Normal | 32 | 12/20 | 48·0 | 19 380 | 12 356 | 7 105 | 4 822 | 0/32 | 0/32 | 0/32 | 0/32 |
| 25–72 | 12 009–32 708 | 7 090–21 760 | 3 356–12 270 | 3 800–10 200 | – | – | |||||
| SLE | 11 | 2/9 | 25·3 | 9 749 | 4 876 | 4 873 | 3 864 | 11/11 | n.d. | n.d. | n.d. |
| 14–66 | 3 179–22 464 | 563–15 298 | 2 313–8 240 | 1 900–8 400 | 20–2,560 | ||||||
| SSc | 14 | 1/13 | 54·9 | 17 738 | 10 655 | 7 083 | 5 371 | 11/12 | n.d. | n.d. | n.d. |
| 41–67 | 7 399–23 855 | 2 862–15 345 | 2 216–13 676 | 3 400–8 300 | < 20–1,280 | ||||||
| RA | 12 | 3/9 | 55·9 | 19 437 | 11 738 | 7 699 | 8 650 | n.d. | 8/12 | n.d. | n.d. |
| 30–79 | 10 987–32 019 | 4 776–26 655 | 2 673–16 505 | 4 500–12 600 | < 20–828 | ||||||
| Primary SS | 29 | 2/27 | 58·6 | 11 719 | 5 265 | 6 460 | 5 224 | 29/29 | 15/29 | 27/29 | 11/29 |
| 29–76 | 4 213–24 786 | 1 441–15 900 | 2 416–13 271 | 2 900–9 100 | 20–1280 | < 20–244 | |||||
| Secondary SS | 24 | 2/22 | 55·5 | 14 548 | 7 312 | 7 236 | 5 704 | 21/24 | 12/24 | 21/24 | 5/24 |
| 28–72 | 1 243–25 982 | 286–15 081 | 957–15 427 | 2 800–11 300 | < 20–1280 | < 20–760 | |||||
| Secondary SS with SLE | 5 | 1/4 | 39·0 | 6 358 | 2 863 | 3 495 | 3 900 | 5/5 | 1/5 | 5/5 | 2/5 |
| 30–50 | 1 243–13 665 | 286–7 599 | 957–6 066 | 2 900–5 300 | 160–1,280 | < 20–39 | |||||
| Secondary SS with SSc | 8 | 0/8 | 63·1 | 17 855 | 8 959 | 8 897 | 5 550 | 7/8 | 1/8 | 5/8 | 1/8 |
| 50–72 | 13 003–24 256 | 4 876–14 135 | 4 821–15 427 | 3 100–8 500 | < 20–320 | < 20–65 | |||||
| Secondary with RA | 11 | 1/10 | 57·5 | 15 866 | 8 137 | 7 729 | 6 636 | 9/11 | 10/11 | 10/11 | 2/11 |
| 28–69 | 8 526–25 982 | 2 065–15 081 | 4 220–10 998 | 2 800–11 300 | < 20–640 | < 20–760 |
Normal values are as follows: white blood cells (WBCs) 3000–8500, anti-nuclear antibodies (ANA) < 20, rheumatoid factor (RF) < 20, and anti-Sjögren's syndrome antigen A (SSA) and anti-SSB negative. PBDCs: peripheral blood dendritic cells. Absolute numbers (per ml) of PBDCs were calculated by multiplying the percentage contributed by the lineage marker-negative, human leucocyte antigen D-related (HLA-DR+) fraction to the total number of flow cytometry events by count (per ml) of peripheral blood mononuclear cells (PBMCs) after negative selection; M/F: male/female; n.d.: not done.
In order to assess whether the number of peripheral blood DCs (PBDCs) changes during the natural course of primary SS, six primary SS patients with long-term follow-up were examined sequentially. All the six primary SS patients' PBDCs were examined in the chronic phase of the disease, 24 months or after the onset of Sicca syndrome [all women, mean age 56·5 years (range 51–71 years)].
Media
RPMI-1640 medium supplemented with 2 mM l-glutamine, 100 units/ml penicillin, 100 ng/ml streptomycin and heat-inactivated 10% fetal bovine serum (Irvine Scientific, Santa Ana, CA, USA) was used throughout the experiments.
Enrichment and analyses of the PBDCs
PBDCs were enriched using a previously described protocol [2]. Briefly, peripheral blood mononuclear cells (PBMCs) were isolated freshly by Lymphoprep (Nycomed Pharma, Oslo, Norway) gradient centrifugation of heparinized blood. PBMCs were incubated with anti-CD3 (HIT3a), anti-CD14 (M5E2) and anti-CD19 (B43) mAbs (Pharmingen, San Diego, CA, USA), and cells binding these mAbs were removed using sheep anti-mouse Ig-coated magnetic beads (M-450; Dynal, Oslo, Norway). The resultant DC-enriched population (CD3-/CD14-/CD19- cells) was stained with phycoerythrin (PE)-labelled anti-CD11c (Leu-M5; Becton Dickinson, San Jose, CA, USA), fluorescein isothiocyanate (FITC)-labelled mixture against lineage markers (lin), CD3 (M2AB; Exalpha, Boston, MA, USA), CD14 (M5E2; BD Biosciences, San Jose, CA, USA), CD15 (M5E2; BD Biosciences), CD16 (J5511; Exalpha), CD19 (HIB19; BD Biosciences) and CD56 (NCAM16·2; BD Biosciences) and peridinin chlorophyll protein (PerCP)-labelled HLA-DR (L-243; Becton Dickinson). Consequently, two phenotypically distinct fractions of DCs were found: (1: myeloid DCs) CD11c+/lin-/HLA-DR+ (2: plasmacytoid DCs) CD11c-/lin-/HLA-DR+ cells. Absolute numbers of DCs (/ml) were calculated by multiplying the percentage of lineage-/HLA-DR+ fraction within total events on flow cytometry by PBMC count after negative selection (/ml) (designated R1 in Fig. 1a). The absolute number of each fractions of DC (/ml) was calculated by multiplying the percentage of each region by the total number of DCs (designated R2 and R3 in Fig. 1b).
Fig. 1.
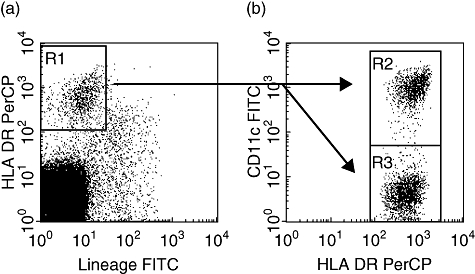
Detection of fractions of peripheral blood dendritic cells (PBDCs). The DC-enriched population was stained with fluorescein isothiocyanate (FITC)-labelled anti-CD11c monoclonal antibodies (mAbs), phycoerythrin (PE)-labelled mAbs against lineage markers (CD3, CD14, CD15, CD16 and CD19) (lin) and peridinin chlorophyll (PerCP)-labelled anti-human leucocyte antigen D-related (HLA-DR) mAbs. (a) DCs were detected as lin-/HLA-DR+ fraction [designated as region 1 (R1)]. (b) Two fractions of DCs were identified, with phenotypes of CD11c+/lin-/HLA-DR+ (myeloid DC; R2), CD11c-/lin-/HLA-DR+ (plasmacytoid DC; R2). Absolute numbers of PBDCs (/ml) were calculated by multiplying the percentage of lin-/HLA-DR+ fraction (designated R1) within total flow cytometry events by the peripheral blood mononuclear cell (PBMC) count after negative selection (/ml).
Immunohistochemistry
We performed immunohistochemical staining against labial salivary glands from 16 of 24 secondary SS patients who agreed to biopsy. Formalin-fixed, paraffin-embedded sections and frozen sections, which were stored in liquid nitrogen, were prepared from biopsied specimens of labial salivary glands of SS patients and normal volunteers. Hematoxylin and eosin (H&E) staining was performed and immunohistochemical staining was performed with several monoclonal antibodies known to react with DCs or lymphocytes, using the avidin–biotin–peroxidase complex method with the Dako LSAB® (labelled streptavidin–biotin) kit plus haematoxylin and diaminobenzidine. Monoclonal antibodies against fascin (55K-2; Dako, Carpinteria, CA, USA) [18], HLA-DR (TAL.1B5; Dako) and CD11c (3·9; Anaspec, Fremont, CA, USA) were used for staining of DCs. Monoclonal antibodies against CD4 (MT310; Dako) and CD8 (DK25; Dako) were used for staining of T cells. Formalin-fixed, paraffin-embedded sections were stained with anti-fascin and anti-HLA-DR. Anti-CD11c, CD4 and CD8 staining were performed in cryosections.
Statistical analysis
In the numbers of PBDCs of normal control subjects, differences by aging were calculated by Pearson's correlation coefficient, and differences by sex were calculated by F-test. The Mann–Whitney U-test was used for statistical analysis of differences in PBDC counts. Differences of PBDC and tissue-infiltrated DC counts by duration time of the clinical course in patients with Sicca syndrome were calculated by Pearson's correlation coefficient. These tests were used for statistical analysis using a Statview statistical program (Abacus Concepts, Berkeley, CA, USA). Differences were considered significant when P-values were less than 0·05.
Results
Clinical characteristics
The clinical characteristics of the patients are shown in Tables 1 and 2. All but seven patients with secondary SS (three overlapping with SLE and four overlapping with RA) and none of the normal volunteers received medication of corticosteroids and immunosuppressants during the study (Table 2). The clinical characteristics of secondary SS and primary SS patients are shown in Table 1. Five of the 24 secondary SS patients had an overlapping SLE. The SLE disease activity index (SLEDAI) [19] in these patients was 6, 12, 13, 22 and 26, respectively, at the time of the examination. In two patients, the symptoms of SLE and those of SS developed almost simultaneously. In the remaining three patients, SLE symptoms preceded those of SS. These three patients were receiving 5 mg/day of prednisolone at the time of the examination. Barnett classification is an evaluation system for the severity of SSc determined by the extent of skin sclerotization caused by this disease. When skin sclerotization is localized only at the fingers and hands, the case is classified as class I (B-I). Conversely, when skin sclerotization is extended to the face or further to the trunk the classification of B-II or B-III is made, respectively. According to the Barnett classification, the eight secondary SS patients who had an overlapping SSc were classified into four B-I, three B-II and one B-III. The onset profile of the symptoms was variable among patients with SSc-merged secondary SS. The symptoms of SSc and those of SS almost appeared simultaneously in three patients. In two patients the symptoms of SSc preceded those of SS, while in the remaining three patients Sicca syndrome appeared first and skin sclerotization developed several years later. Eleven secondary SS patients had an overlapping RA. Two of the 11 patients were diagnosed as having RA-merged secondary SS at the initial presentation. On the other hand, five of the 11 patients were diagnosed originally as primary SS and subsequently as RA-merged secondary SS when the RA symptoms developed later. By contrast, in the remaining four patients, Sicca syndrome appeared after the diagnosis of RA was established. Disease modified anti-rheumatic drugs (methotrexate 6 mg/week, 8 mg/week, bucillamine 50 mg/day and salazosulphapyridine 1000 mg/day, respectively) had been administered to four patients whose RA preceded SS. SLE patients showed low white blood cell (WBC) numbers (normal control: mean 4822/µl, range 3800–10 200; SLE: mean 3864, range 1900–8400) (Table 1).
Table 2.
Clinical and serological findings for patients with secondary Sjögren's syndrome (SS).
| Patient/age/sex | Total PBDCs/ml | Myeloid DCs/ml | Plasmacytoid DCs/ml | Marged autoimmune disease | Contraction of disease period of Sicca syndrome (month) | Onset pattern | Medication | WBCs/µl | ANA, log titre | RF, IU/ml | Anti-SSA | Anti-SSB |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/36/F | 4 617 | 2 116 | 2 501 | SLE/26 (SLEDAI) | 4 | The same time | – | 2 900 | 320 | < 20 | + | + |
| 2/43/F | 6 533 | 2 326 | 4 207 | SLE/22 | 4 | The same time | – | 4 200 | 160 | < 20 | + | − |
| 3/50/F | 5 730 | 1 988 | 3 742 | SLE/6 | 2 | SLE → SS | PSL = 5 mg/day | 5 300 | 1280 | < 20 | + | − |
| 4/36/F | 1 243 | 286 | 957 | SLE/13 | 10 | SLE → SS | PSL = 5 mg/day | 3 500 | 160 | < 20 | + | − |
| 5/30/M | 13 665 | 7 599 | 6 066 | SLE/12 | 3 | SLE → SS | PSL = 5 mg/day | 3 600 | 640 | 39 | + | + |
| 6/50/F | 21 740 | 14 135 | 7 605 | SSc/B-I (Barnett classification) | 8 | The same time | – | 3 400 | 320 | < 20 | + | − |
| 7/58/F | 15 831 | 11 010 | 4 821 | SSc/B-I | 18 | SS → SSc | – | 8 500 | 40 | 65 | + | + |
| 8/59/F | 15 220 | 10 395 | 4 825 | SSc/B-I | 3 | SSc → SS | – | 4 400 | < 20 | < 20 | + | − |
| 9/65/F | 16 174 | 7 489 | 8 685 | SSc/B-II | 24 | SS → SSc | – | 3 100 | 320 | < 20 | + | − |
| 10/65/F | 24 256 | 8 829 | 15 427 | SSc/B-II | 20 | The same time | – | 6 500 | 160 | < 20 | − | − |
| 11/67/F | 23 092 | 9 699 | 11 393 | SSc/B-III | 30 | SS → SSc | – | 6 200 | 80 | < 20 | − | − |
| 12/69/F | 13 003 | 4 876 | 8 127 | SSc/B-II | 12 | SSc → SS | – | 5 900 | 20 | < 20 | + | − |
| 13/72/F | 13 522 | 5 233 | 8 289 | SSc/B-I | 24 | The same time | – | 6 400 | 320 | < 20 | − | − |
| 14/28/F | 22 620 | 12 321 | 10 299 | RA | 48 | SS → RA | – | 5 700 | 40 | 567 | + | − |
| 15/53/F | 17 917 | 11 197 | 6 720 | RA | 54 | SS → RA | – | 2 800 | 320 | 86 | + | + |
| 16/53/F | 13 395 | 2 183 | 11 212 | RA | 2 | RA → SS | SASP = 1000 mg/day | 4 900 | < 20 | 29 | + | − |
| 17/54/F | 25 982 | 15 081 | 10 901 | RA | 24 | SS → RA | – | 7 700 | 160 | 22 | − | − |
| 18/56/F | 8 526 | 4 306 | 4 220 | RA | 12 | The same time | – | 5 900 | 320 | 143 | + | − |
| 19/60/F | 20 504 | 11 784 | 8 720 | RA | 20 | The same time | – | 6 800 | 640 | 760 | + | − |
| 20/62/F | 12 690 | 1 692 | 10 998 | RA | 1 | RA → SS | MTX = 8 mg/week | 11 300 | 20 | < 20 | + | − |
| 21/65/F | 12 856 | 8 003 | 4 853 | RA | 6 | RA → SS | BU = 50/day | 8 200 | < 20 | 670 | + | − |
| 22/66/F | 8 603 | 2 065 | 6 538 | RA | 2 | RA → SS | MTX = 6 mg/week | 3 200 | 40 | 65 | + | + |
| 23/66/M | 11 035 | 5 884 | 5 151 | RA | 12 | SS → RA | – | 6 400 | 80 | 39 | + | − |
| 24/69/F | 20 398 | 14 989 | 5 409 | RA | 60 | SS → RA | – | 10 100 | 40 | 375 | + | − |
SLE: systemic lupus erythematosus; SSc: systemic sclerosis; RA: rheumatoid arthritis; SLEDAI: SLE disease activity index; PSL: prednisolone; SASP: salazosulphapyridine; MTX: methotrexate; BU: bucillamine; M: male; F: female.
In this study, we compared the number of PBDCs in each autoimmune disease with that in normal controls. In the analysis of the number of PBDC in autoimmune diseases, however, age or sex may possibly affect the results. Therefore, we first investigated whether the number of PBDCs is affected by ageing in normal control subjects. There was no alteration in the total number of PBDCs by ageing (correlation 0·01, P = 0·96). Furthermore, the number of myeloid DCs (correlation 0·13, P = 0·50) and plasmacytoid DCs (correlation 0·21, P = 0·26) did not show a significant difference by ageing (data not shown). We investigated whether a sex difference was observed in the number of PBDCs in normal control subjects. No sex difference was observed in the total number of PBDCs (male: mean 19 099/ml, range 12 009–32 708; female: mean 19 549, range 13 566–31 672), myeloid DCs (male: mean 12 076, range 7090–21 760; female: mean 12 525, range 7293–20 595) or plasmacytoid DCs (male: mean 7023, range 3356–10 948; female: mean 7153, range 3292–12 270) (data not shown). These findings indicate that age or sex does not affect the number of PBDCs.
The number of PBDCs in primary and secondary SS
Figure 2 shows the number of PBDCs in various autoimmune diseases. We have reported previously that the number of myeloid DCs is decreased in peripheral blood in patients with primary SS [2]; the data are included in Fig. 2. Similarly to patients with primary SS (mean 11 719/ml), those with secondary SS (mean 14 584) also had a significantly lower number of PBDCs compared with normal controls (mean 19 380, tied P < 0·01) (Fig. 2a). In addition, the number of myeloid DCs was significantly lower in both primary SS patients (mean 5265, tied P < 0·01) and secondary SS patients (mean 7312, tied P < 0·01) than in normal controls (mean 12 356) (Fig. 2b). Conversely, the number of plasmacytoid DCs was similar among primary SS (mean 6460), secondary SS (mean 7236) and normal controls (mean 7105) (Fig. 2c).
Fig. 2.
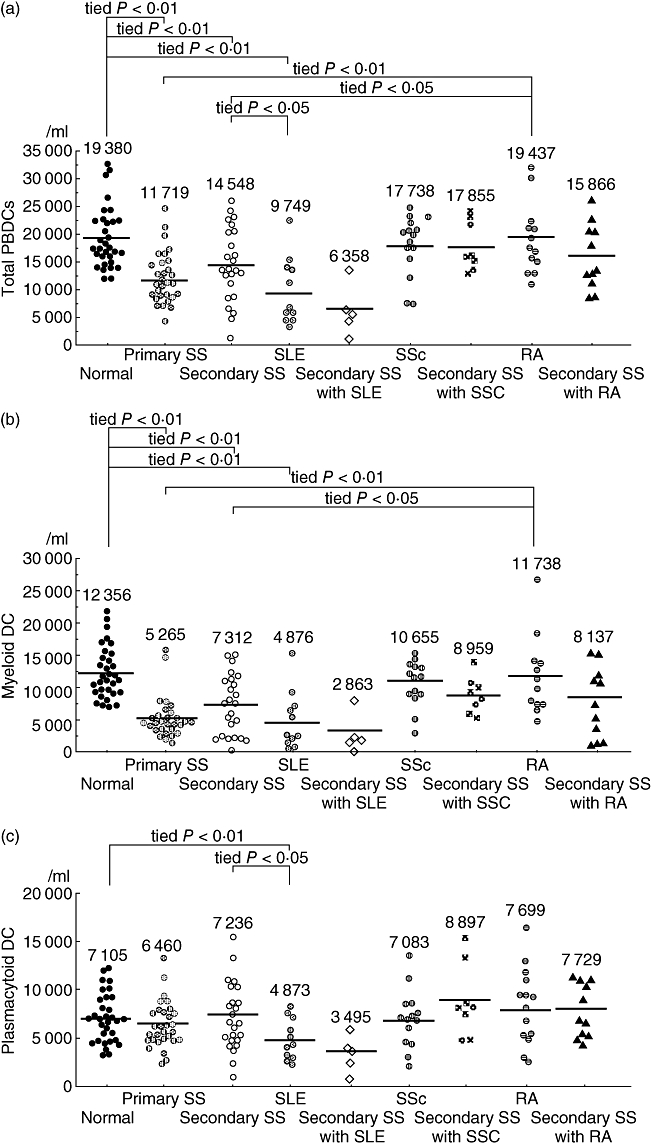
Absolute numbers of peripheral blood dendritic cells (PBDCs) in each autoimmune disease patients. (a) The mean numbers of PBDCs in primary Sjögren's syndrome (SS) (mean 11 719/ml), secondary SS (mean 14 584) and systemic lupus erythematosus (SLE) (mean 9749/ml) were significantly lower than those in 32 normal control subjects (mean 19 380) (tied P < 0·01). However, in systemic sclerosis (SSc) (mean 17 738) and rheumatoid arthritis (RA) (mean 19 437), mean numbers of PBDCs were not decreased. (b) Similarly, the mean numbers of myeloid DCs in patients with primary SS (mean 5265), secondary SS (mean 7312) and SLE (mean 4876) were significantly lower than those in controls (mean 12 356) (tied P < 0·01). (c) On the other hand, the number of plasmacytoid DCs only in patients with SLE (mean 4873) was significantly lower than in normal controls (mean 7105) (tied P < 0·05).
The number of PBDCs in primary autoimmune diseases
There is a possibility that the decrease in the number of PBDCs in secondary SS could be related to the individual autoimmune disease (SLE, SSc and RA) that merges in secondary SS. Therefore, we investigated the number of PBDCs in patients with SLE, SSc and RA. As shown in Fig. 2a, the total number of PBDCs was decreased significantly in SLE patients (mean 9749/ml, tied P < 0·01) compared with normal controls. Meanwhile, the number of PBDCs was not altered significantly in SSc (mean 17 738) and RA patients (mean 19 437). The number of myeloid and plasmacytoid DCs in each autoimmune disease is shown in Fig. 2b,c. The number of myeloid DCs in SLE patients (mean 4876, tied P < 0·01) was significantly lower than that in normal controls. By contrast, no significant alteration in the number of myeloid DCs was observed in SSc patients (mean 10 655) and RA patients (mean 11 738). The decrease in the number of plasmacytoid DCs was observed only in SLE patients (mean 4873, tied P = 0·0154) but not in SSc (mean 7083) and RA (mean 7699) patients.
The number of PBDCs in secondary SS is affected by overlapping autoimmune diseases
As described above, each autoimmune disease showed a different pattern of the decrease in the number of PBDCs. In addition, although the number of total PBDCs and myeloid DCs was decreased significantly in secondary SS patients, the number was distributed more widely than that in primary SS patients (Fig. 2a,b). Based upon these findings, we hypothesized that the number of PBDCs in secondary SS might be influenced or determined by the autoimmune diseases that overlap with SS. Therefore, we compared the number of total PBDCs, myeloid DCs and plasmacytoid DCs in each subgroup of secondary SS (five SLE-merged secondary SS, 11 RA-merged secondary SS and eight SSc-merged secondary SS) with that in each corresponding primary autoimmune disease and in normal controls. There was no significant difference in the number of total PBDCs, myeloid DCs and plasmacytoid DCs among SSc-merged secondary SS (total PBDCs: mean 17 855/ml; myeloid DCs: mean 8959; plasmacytoid DCs: mean 8897), RA-merged secondary SS (total PBDCs: mean 15 866; myeloid DCs: mean 8137; plasmacytoid DCs: mean 7729) and normal controls. PBDCs, myeloid DCs and plasmacytoid DCs were all decreased significantly in SLE-merged secondary SS (total PBDCs: mean 6358; myeloid DCs: mean 2863; plasmacytoid DCs: mean 3495) (Table 1). The number of total PBDCs, myeloid DCs and plasmacytoid DCs in each subgroup of secondary SS was similar to that in the corresponding primary autoimmune disease that overlaps in each subgroup of secondary SS.
Furthermore, we analysed the PBDC numbers of primary SS and secondary SS which were compared with RA and SLE. The total numbers of PBDC and myeloid DC were decreased significantly in primary and secondary SS patients in comparison with RA, which was similar to healthy donors, but not with SLE (Fig. 2a,b). Meanwhile, the numbers of total PBDCs and plasmacytoid DCs in secondary SS were significantly larger than those in SLE. These results might be due to the decreased plasmacytoid DCs in SLE.
The decreased number of PBDCs in primary SS is restored naturally during the clinical course.
In our previous report, we put forward a hypothesis that the decrease of PBDCs might be a critical event in the pathogenesis of primary SS [2]. Thus, in this study we examined whether the decrease of PBDCs continues during the natural course of primary SS.
As shown in Fig. 3a–c, a direct correlation was observed between the number of PBDCs and the time from onset of Sicca syndrome in primary SS. None of the 29 patients received therapeutic agents, including corticosteroids. In addition, six of the 29 patients with primary SS were examined twice sequentially for PBDC numbers (Fig. 3g–i). Four of the six patients and all six patients showed an increase in the number of total PBDCs and myeloid DCs, respectively, after an average of 43 months from the initial examination. However, plasmacytoid DC numbers did not show a distinct alteration in all the six patients. The increased level of IgG is observed generally to rise in SS patients. We examined the titre of IgG of these six patients. Their serum levels of IgG were not altered markedly (Fig. 3j).
Fig. 3.
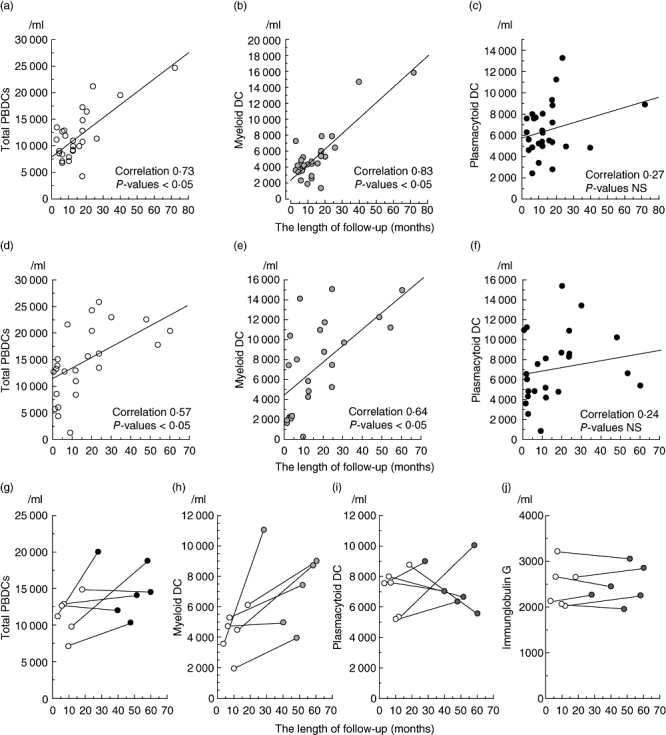
The number of peripheral blood dendritic cells (PBDCs) in Sjögren's syndrome (SS) is restored during the clinical course. We plotted the number of PBDCs, myeloid DCs or plasmacytoid DCs in 29 primary SS and 19 secondary SS patients except those with systemic lupus erythematosus – merged secondary SS against the x-axis that shows the time after the onset of Sicca syndrome. A direct correlation was observed between the number of total PBDCs (a, correlation 0·73, P < 0·01) and myeloid DCs (b, correlation 0·83, P < 0·01) and the time from onset of Sicca syndrome in primary SS. A direct correlation was observed between the number of PBDCs (d, total PBDCs; correlation 0·53, P = 0·0189; and e, myeloid DCs; correlation 0·60, P = 0·0053) and the time from the onset of Sicca syndrome in secondary SS, the same as primary SS. On the other hand, the number of plasmacytoid DCs was not correlated significantly with the length of follow-up period (c,f). PBDC number and serum immunoglobulin (Ig)G levels of six (of the 29) patients with primary SS were monitored. Although four of the six patients (g) and all six patients (h) showed an increase in the number of total PBDCs after an average of 43 months from initial examination, their serum IgG levels were not altered markedly (j). Plasmacytoid DC numbers did not show distinct alteration in all six patients (i).
The decreased number of PBDCs is associated with duration time of Sicca syndrome in secondary SS
Next, we investigated the relationship between the number of PBDCs and duration time of Sicca syndrome in secondary SS.
As shown in Fig. 3d–f, a direct correlation was observed between the number of PBDCs and the time from the onset of Sicca syndrome in secondary SS, as in primary SS.
Immunohistochemical analysis of labial salivary glands from SS patients
We have demonstrated previously that, in primary SS, a number of mature myeloid DCs as well as numerous IFN-γ-producing T cells are infiltrated in the interstitial areas of labial salivary glands [2]. In this study, we also carried out similar histological examinations on the labial salivary glands biopsied from secondary SS patients by staining with DC markers CD11c, HLA-DR and fascin. We found infiltration of a number of mononuclear cells (MNCs) around the glandular structures by H&E staining of the labial salivary gland from 16 of 24 secondary SS patients who agreed to undergo biopsy (Fig. 4a, patient 22 in Table 2; Sicca syndrome onset, 2 months). Similar to primary SS [2], many fascin-positive MNCs were detected among numerous fascin-negative MNCs in the areas surrounding the tubular ducts in secondary SS (Fig. 4b). In addition, immunohistochemical double-staining of CD11c and HLA-DR demonstrated that the CD11c/HLA-DR double-positive cells with DC morphology had infiltrated the MNC area at the same frequency as the fascin-positive cells (Fig. 4c), suggesting that these cells are myeloid DCs.
Fig. 4.
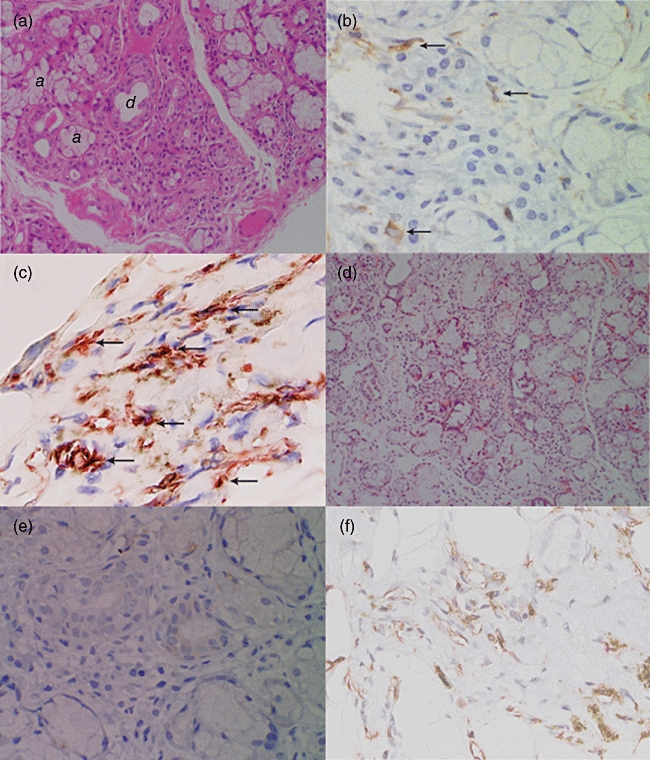
Histological findings for dendritic cells (DCs) of labial salivary glands from a patient with Sjögren's syndrome (SS). Immunohistochemical staining of the same biopsied specimens [from early phase of secondary SS (a–c) and primary SS (d–f)] performed on formalin-fixed paraffin-embedded sections demonstrated infiltration of mononuclear cells (MNCs) within the aggregates of lymphoid cells around the tubular ducts of secretary glands. (a) Haematoxylin and eosin (H&E) staining (from early phase of secondary SS patient 22 in Table 2; Sicca syndrome onset, 2 months); a and d in the picture indicate acini and tubular ducts, respectively. (b) Staining with anti-fascin monoclonal antibody (mAb) (arrows) (from patient 22). (c) Double staining with anti-CD11c mAb (red) and anti-human leucocyte antigen D-related (HLA-DR) mAb (brown) showed that both CD11c/HLA-DR-positive cells with dendritic cell morphology had infiltrated into the lymphocyte-infiltrated layer (from patient 22). (d) H&E staining (late phase of primary SS patient; 60 months from the Sicca syndrome onset). (e) Staining with anti-fascin mAb (late phase of primary SS patient). (f) Double staining with anti-CD11c mAb and anti-HLA-DR mAb (brown) (late phase of primary SS patient) showed numerous HLA-DR+ MNCs were detected in labial salivary glands. However, these cells were negative for anti-CD11c mAb [original magnification ×10 in (a) and (e); ×50 in (b–d), and (f)]. Fascin+ MNCs also infiltrated the inflammatory cells (b) at a frequency similar to that of CD11c+/HLA-DR+ MNCs in secondary SS (c). Although similar to the early phase of SS, numerous HLA-DR+ MNCs (f) were detected in the interstitial areas in labial salivary glands in the later phase of primary SS (60 months from the Sicca syndrome onset); fascin+ MNCs were barely detected among the MNCs (e).
As described above, patients in the early phase of primary SS showed a significant decrease of total PBDCs and myeloid DCs, whereas patients in the chronic phase of primary SS showed a lesser extent of decrease of PBDCs and myeloid DCs (Fig. 3). These findings suggest that the decreased levels of PBDCs and myeloid DCs restore gradually to normal levels during the natural course of the disease. This prompted us to examine how infiltration of mature myeloid DCs in labial salivary glands in primary SS is altered as the clinical course proceeds. Thus, we examined the immunohistochemical staining of labial salivary glands of primary SS patients who passed through a long period of time after the onset of Sicca syndrome (60 months from the Sicca syndrome onset) and calculated the percentage of fascin-positive cells to the total infiltrating MNCs in salivary glands. Similar to the early phase of primary SS [2], numerous MNCs were detected in the interstitial areas around the tubular ducts in labial salivary glands in the later phase of primary SS (Fig. 4d). However, in contrast to the early phase of primary SS, fascin-positive MNCs were barely detected in the later phase of primary SS (Fig. 4e). We confirmed that the percentage of fascin-positive cells to infiltrated MNCs was decreased statistically in salivary gland sections during the natural course of primary SS (Fig. 5).
Fig. 5.
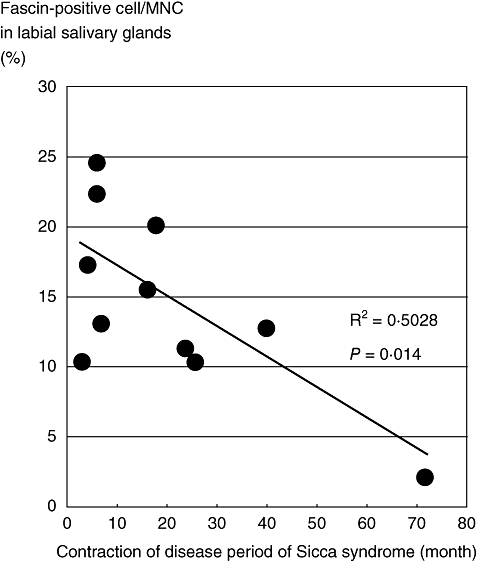
Frequency of dendritic cells (DCs) in the labial salivary glands from a patient with primary Sjögren's syndrome (SS) during the disease time–course. We examined immunohistochemical staining of biopsied specimens from 11 cases of primary SS with anti-fascin monoclonal antibody (mAb) and counted both fascin-positive cells and total mononuclear cells (MNCs) per 100 × 100 µm at least five fields of view (magnification × 100) per each sample. The y-axis indicates mean percentage of fascin-positive cells to total MNCs in labial salivary glands.
We finally investigated the possible relation between the decrease of blood myeloid DCs and accumulated tissue-infiltrated DCs in patients with primary and secondary SS. We found that the numbers of myeloid DCs in the peripheral blood were correlated negatively with the frequency of infiltrated fascin-positive mononuclear cells in salivary glands in not only primary SS (Fig. 6a), but also secondary SS (Fig. 6b). This finding supports the hypothesis that blood DCs recruit to inflamed salivary glands in Sicca syndrome in both primary and secondary SS.
Fig. 6.
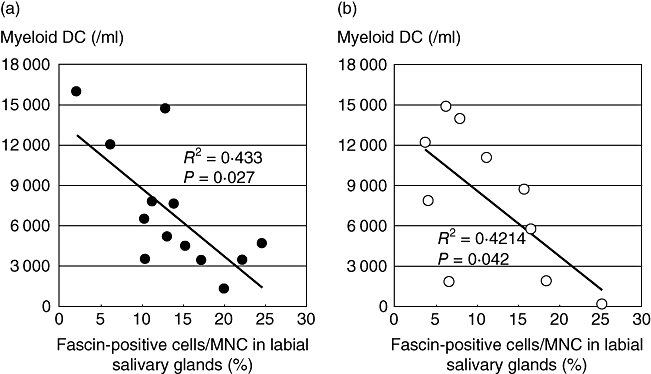
The number of peripheral blood dendritic cells (PBDCs) and tissue dendritic cells (DCs) is related negatively in both primary and secondary SS during clinical time–course. We analysed the number of blood myeloid DCs and examined immunohistochemical staining of biopsied specimens from the 11 patients with primary SS (a) and 10 patients with secondary SS (b). We counted both fascin-positive cells and total MNCs per 100 ×100 µm at least five fields of view (magnification × 100) per each sample. The x-axis indicates mean percentage of fascin-positive cells to total MNCs in labial salivary glands and the y-axis indicates the number of myeloid DCs in blood.
Discussion
It is believed that the various DCs encountered in the different organs are interconnected by defined pathways of migration [20]. DCs are not a single cell type, but a system of cells that arise from both the myeloid and lymphoid haemopoietic lineages [10,11]. Various DC subtypes are thought to differ in their capacity to either stimulate or inhibit the immune response [8,9,21]. The factors that influence the ability of DCs to instruct the naive CD4+ T cells to differentiate into a Th1 or Th2 cell phenotype are becoming clear. The environment in which the DCs have been stimulated, the type of stimulus and the origin of the DCs play a part in the fate of the T cell response. These biological properties of DCs may lead to the hypothesis that alteration of the DC system causes autoimmune diseases.
One of the major immunopathological events in SS is epithelial cell destruction by infiltrating lymphocytes, leading to subsequent replacement of the salivary gland tissue by mononuclear cells. As is well documented, the majority of the infiltrating cells within the salivary glands of early phase SS patients are T lymphocytes of the helper/inducer (CD4) phenotypes, with a relative paucity of the suppressor/cytotoxic (CD8) phenotypes. This predominance of CD4+ T cell infiltration suggests the presentation of antigen in association with class II by APCs to helper T cells. Although little is known about the antigens that trigger the onset of SS directly, many reports of evidence from human studies have suggested that a Th1-mediated process might contribute mainly to the local immune responses in SS [22,23]. Therefore, APCs such as DCs may play an important role in triggering CD4+ T cell-mediated immune responses in the salivary gland tissue by inducing Th1 cells. Indeed, in the non-obese diabetic (NOD) mouse models for SS, it has been observed that DCs infiltrated into the parotid glands early phase of the clinical course, preceding T cells [7]. Consistent with this finding we found previously that, in primary SS, myeloid DCs were decreased selectively in peripheral blood, and that this was associated with infiltration of myeloid DCs in minor salivary glands. We also found that the numbers of IFN-γ-producing Th1 cells were increased in peripheral blood as well as in the minor salivary glands of patients, and that this appeared to be generated by interaction with myeloid DCs [2].
Recent evidence also indicates that SLE may be caused by alterations in the functions of plasmacytoid DCs [5,6]. In our study, we have shown that the numbers of myeloid and plasmacytoid DCs in patients with SLE are the same as in previous reports. Furthermore, the same decrease of myeloid and plasmacytoid DCs were observed in patients with SLE-merged secondary SS. Meanwhile, there were no significant differences in the number of myeloid and plasmacytoid DCs among SSc-merged secondary SS patients and RA-merged secondary SS patients, as well as SSc and RA patients. However, we found a direct correlation between the number of myeloid DCs and the time from the onset of Sicca syndrome in patients of secondary SS. A similar correlation was also observed in patients with primary SS. We also found a negative correlation between the number of blood myeloid DCs and the frequency of tissue-infiltrated DCs in both primary and secondary SS. Furthermore, in contrast to the early phase of primary SS, in the minor salivary glands of primary later-phase SS patients the mature DCs disappeared. These findings suggest that the reduction of myeloid DCs is a common finding in the early stage of Sicca syndrome and that myeloid DCs contribute to the critical and pathogenic roles of Sicca syndrome of SS. In this study we hypothesized that preferential trafficking of myeloid DCs into salivary or lachrymal glands play essential roles in the pathogenesis of Sicca syndrome of primary and secondary SS by initiating Th1 immune responses.
It has been reported that in patients in the later phase of SS, the percentage of infiltrating B cells within the salivary glands is increasing [24–26], suggesting that cell interaction between DCs and helper T cells is no longer required. Further detailed studies will be required to determine which antigens trigger DC-mediated immune responses in the salivary glands of SS patients. Our data raise the possibility that the infiltration of myeloid DCs within salivary glands has been caused by the early onset of SS; meanwhile, retaining inflammation may require another mechanism in the later phase of SS.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (C) (subject 11670466) from the Japan Society for the Promotion of Science.
Disclosure
None of the authors have any conflict of interest with the subject matter or materials discussed in the manuscript.
References
- 1.Moutsopoulos HM. Sjogren's syndrome: autoimmune epithelitis. Clin Immunol Immunopathol. 1994;72:162–5. doi: 10.1006/clin.1994.1123. [DOI] [PubMed] [Google Scholar]
- 2.Ozaki Y, Amakawa R, Ito T, et al. Alteration of peripheral blood dendritic cells in patients with primary Sjogren's syndrome. Arthritis Rheum. 2001;44:419–31. doi: 10.1002/1529-0131(200102)44:2<419::AID-ANR61>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 3.Page G, Lebecque S, Miossec P. Anatomic localization of immature and mature dendritic cells in an ectopic lymphoid organ: correlation with selective chemokine expression in rheumatoid synovium. J Immunol. 2002;168:5333–41. doi: 10.4049/jimmunol.168.10.5333. [DOI] [PubMed] [Google Scholar]
- 4.Santiago-Schwarz F, Anand P, Liu S, Carsons SE. Dendritic cells (DCs) in rheumatoid arthritis (RA): progenitor cells and soluble factors contained in RA synovial fluid yield a subset of myeloid DCs that preferentially activate Th1 inflammatory-type responses. J Immunol. 2001;167:1758–68. doi: 10.4049/jimmunol.167.3.1758. [DOI] [PubMed] [Google Scholar]
- 5.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–3. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 6.Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25:383–92. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 7.van Blokland SC, van Helden-Meeuwsen CG, Wierenga-Wolf AF, et al. Two different types of sialoadenitis in the NOD- and MRL/lpr mouse models for Sjogren's syndrome: a differential role for dendritic cells in the initiation of sialoadenitis? Lab Invest. 2000;80:575–85. doi: 10.1038/labinvest.3780062. [DOI] [PubMed] [Google Scholar]
- 8.Reid SD, Penna G, Adorini L. The control of T cell responses by dendritic cell subsets. Curr Opin Immunol. 2000;12:114–21. doi: 10.1016/s0952-7915(99)00059-x. [DOI] [PubMed] [Google Scholar]
- 9.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 10.Rissoan MC, Soumelis V, Kadowaki N, et al. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–6. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 11.Ito T, Inaba M, Inaba K, et al. A CD1a+/CD11c+ subset of human blood dendritic cells is a direct precursor of Langerhans cells. J Immunol. 163:1409–19. [PubMed] [Google Scholar]
- 12.Homma M, Tojo T, Akizuki M, Yamagata H. Criteria for Sjogren's syndrome in Japan. Scand J Rheumatol. 1986;61:26–7. [PubMed] [Google Scholar]
- 13.Vitali C, Bombardieri S, Moutsopoulos HM, et al. Preliminary criteria for the classification of Sjogren's syndrome. Results of a prospective concerted action supported by the European Community. Arthritis Rheum. 1993;36:340–7. doi: 10.1002/art.1780360309. [DOI] [PubMed] [Google Scholar]
- 14.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 15.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 16.Hochberg MC, Chang RW, Dwosh I, Lindsey S, Pincus T, Wolfe F. The American College of Rheumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritis. Arthritis Rheum. 1992;35:498–502. doi: 10.1002/art.1780350502. [DOI] [PubMed] [Google Scholar]
- 17.Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23:581–90. doi: 10.1002/art.1780230510. No authors listed. [DOI] [PubMed] [Google Scholar]
- 18.Ross R, Ross XL, Schwing J, Langin T, Reske-Kunz AB. The actin-bundling protein fascin is involved in the formation of dendritic processes in maturing epidermal Langerhans cells. J Immunol. 1998;160:3776–82. [PubMed] [Google Scholar]
- 19.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI: a disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630–40. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 20.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 21.Caux C, Massacrier C, Vanbervliet B, et al. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte–macrophage colony-stimulating factor plus tumor necrosis factor alpha: II. Functional analysis. Blood. 1997;90:1458–70. [PubMed] [Google Scholar]
- 22.Fox RI, Kang HI, Ando D, Abrams J, Pisa E. Cytokine mRNA expression in salivary gland biopsies of Sjogren's syndrome. J Immunol. 1994;152:5532–9. [PubMed] [Google Scholar]
- 23.Kolkowski EC, Reth P, Pelusa F, et al. Th1 predominance and perforin expression in minor salivary glands from patients with primary Sjogren's syndrome. J Autoimmun. 1999;13:155–62. doi: 10.1006/jaut.1999.0289. [DOI] [PubMed] [Google Scholar]
- 24.Isenberg DA, Rowe D, Tookman A, et al. An immunohistological study of secondary Sjogren's syndrome. Ann Rheum Dis. 1984;43:470–6. doi: 10.1136/ard.43.3.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moutsopoulos HM, Hooks JJ, Chan CC, Dalavanga YA, Skopouli FN, Detrick B. HLA-DR expression by labial minor salivary gland tissues in Sjogren's syndrome. Ann Rheum Dis. 1986;45:677–83. doi: 10.1136/ard.45.8.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiroki A, Nakamura S, Shinohara M, Oka M. Significance of oral examination in chronic graft-versus-host disease. J Oral Pathol Med. 1994;23:209–15. doi: 10.1111/j.1600-0714.1994.tb01115.x. [DOI] [PubMed] [Google Scholar]


