Abstract
Selective immunoglobulin (Ig)G3 subclass deficiency in adults, especially its immunological profile, has not been described previously in detail. Therefore, a retrospective chart review was conducted to characterize the immune profile and clinical manifestations in adult patients with selective IgG3 deficiency. We reviewed the charts of 17 adult patients attending our subspeciality immunology clinic with a diagnosis of selective IgG3 deficiency. The following immunological test results were recorded: lymphocyte subsets, proliferative response to mitogens (phytohaemagglutinin, concanavalin A, pokeweed mitogen) and soluble antigens (mumps, Candida albicans, tetanus toxoid), specific antibody response to tetanus toxoid and pneumococcal antigens, neutrophil oxidative burst and natural killer cell cytotoxicity. In addition, we recorded information about the types of infections and other associated diseases, and response to intravenous immunoglobulin therapy (IVIG). In the majority of patients, lymphocyte subsets were normal. Proliferative responses to mitogens and antigens were decreased in 33% and 40% of patients, respectively. Specific antibody responses to tetanus were normal; however, responses to various pneumococcal serotypes were impaired in a subset of patients. Patients suffered from recurrent upper respiratory tract infections, which usually decreased in frequency and severity following treatment with IVIG. The majority of these patients also had concurrent atopic diseases in the form of allergic rhinitis or asthma. Selective IgG3 subclass deficiency should be considered in adults with recurrent upper respiratory tract infections with or without allergic rhinitis or asthma, who may have normal levels of total IgG. IVIG appears to be an effective therapy.
Keywords: IgG3, immunodeficiency, infections, intravenous immunoglobulin, subclass
Introduction
The four subclasses of immunoglobulin G (IgG) have structural differences which confer different biological properties. IgG3, comprising 4–8% of total serum IgG, is the most susceptible to proteolytic digestion; its half-life is much shorter (7–9 days) in comparison to the half-lives of the other IgG subclasses (21–23 days), it is an excellent activator of the complement system and it is directed predominantly towards protein antigens [1]. Furthermore, IgG3 binds with high affinity to Fc receptors on macrophages, and thus may be important in antibody-mediated phagocytosis [2]. These factors may explain why patients with isolated IgG3 deficiency present with recurrent upper respiratory tract infections. However, the propensity for infections in these patients may not be attributed solely to IgG3 deficiency. There have been reports of patients with complete absence of IgG3 due to gene deletion in the heavy chain constant regions, but these patients have had no infectious complications [3]. Therefore, other immune dysfunctions might exist in those patients with isolated IgG3 deficiency and recurrent infections. A more detailed analysis of immune function in IgG3-deficient patients is needed.
The majority of reported studies for IgG subclass deficiency have been in children [4–6], and very few studies have reported detailed clinical and immunological features of adult patients with IgG3 deficiency [7–8]. In some of these reports, IgG3 subclass deficiency was associated with either IgA deficiency or another subclass deficiency, and therefore may not be considered selective IgG3 deficiency. Moreover, none of these studies reported immunological data. Finally, there is a lack of information about the use of intravenous immunoglobulin for treatment of IgG3 subclass deficiency. In this study, we present detailed information regarding immune functions of patients with recurrent infections and isolated IgG3 deficiency, and their response to intravenous Ig therapy (IVIG).
Materials and methods
Patients
We reviewed the charts of patients with recurrent infections referred to one of us (S. G.) at Immunology Clinic, University of California, Irvine (UCI) from 1998 to 2007. We identified 17 adult patients with a diagnosis of selective IgG3 deficiency. The diagnosis was made according to published guidelines [9]. Patients were 16 years of age or older at the time of diagnosis, suffered from recurrent infections, had an IgG3 level that was greater than 2 standard deviations below the mean on at least two separate occasions and had normal levels of IgA, IgM, IgG, IgG1, IgG2 and IgG4. The charts of these 17 patients were reviewed for immunological data, the type and frequency of infections and response to IVIG treatment. This study was approved by the UCI Institutional Review Board, and the patients signed informed consent.
Reagents
Fluorescein isothiocyanate (FITC)- and phycoerythrin (PE)-conjugated monoclonal antibodies to CD3, CD4, CD8, CD19, CD16, CD56, CD14, Toll-like receptor-4 (TLR-4) and isotype controls were obtained from Becton Dickinson (San Jose, CA, USA). Tritiated thymidine [3H] for lymphocyte transformation assays was obtained from New England Nuclear (Boston, MA, USA). Mitogens phytohaemagglutinin (PHA), concavalin A (ConA) and pokeweed mitogen (PWM) were obtained from Sigma Chemicals (St Louis, MO, USA). Antigens consisted of mumps virus (Whittaker Bioproducts, Walkersville, MD, USA), Candida albicans (Greer Laboratories, Lenoir, NC, USA) and tetanus toxoid (Connaught Laboratories Ltd, Swiftwater, PA, USA).
Methods
Serum immunoglobulin levels and IgG subclasses were measured by rate nephelometry. Pneumococcal and tetanus antibody titres were measured by multi-analyte fluorescence detection (Arup Laboratories, Salt Lake City, UT, USA). Pneumococcal antibody titres against 14 serotypes (1, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 12F, 14, 18C, 19F, 23F) were obtained prior to and 4 weeks after administration of the 23-valent polysaccharide Pneumovax-23 vaccine (Merck, Whitehouse Station, NJ, USA). Protective pneumococcal antibody titres were defined as IgG > 1 µg/ml, or a greater than fourfold increase of titres after vaccination with Pneumovax-23. Protective antibody titres to tetanus were defined as anti-tetanus toxoid IgG > 0·10 IU/ml.
Lymphocyte subsets were measured in whole blood. One hundred µl blood was mixed with 25 µl of fluorochrome-conjugated antibodies and isotype controls for 30 min at room temperature followed by lysis by lysing buffer (Becton Dickinson). Cells were centrifuged and then washed 1× with phosphate-buffered saline (PBS), acquired by fluorescence activated cell sorter (FACS)Calibur and analysed by Simultest (Becton Dickinson). Lymphocyte subsets and TLR-4 expression on CD14+ macrophages were determined by multi-colour flow cytometry (FACScalibur) with FITC- and PE-conjugated monoclonal antibodies and isotype controls, using Simulset software (Becton Dickinson). Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque density gradient centrifugation, and lymphocyte proliferation in response to mitogens (PHA, ConA, PWM) and antigens (mumps, C. albicans, tetanus toxoid) were measured by [3H]-thymidine incorporation. Data were analysed as net counts/min after subtracting background counts.
Natural killer (NK) cell-mediated cytotoxicity was determined by a non-radioactive cytotoxicity assay kit (ACT1; Cell Technology Inc., Mountain View, CA, USA), using flow cytometry according to the manufacturer's instructions. Briefly, human erythroleukaemic tumour cells K562 (target cells) were labelled with the cell-tracking dye carboxyfluorescein diacetate succinimidyl ester (CFSE) and cultured with PBMCs (2·5 × 105 cells) at effector : target ratios of 12·5:1, 25:1, 50:1 and 100:1. After 6-h incubation at 37°C, 7-amino-actinomycin D (7AAD) stain was added to measure cell death. Data from 1 × 104 cells were collected and analysed by FACScalibur flow cytometer.
To measure neutrophil oxidative burst, 1 µl of 5 mM dihydrorhodamine and 1 µl of dimethyl sulphoxide were added to 100 µl of heparinized blood. After 15 min at 37°C, the cells were activated with 10 µg/ml phorbol myristate acetate and FACS lysing solution was added. Cells were washed, resuspended and analysed by FACSCalibur (Becton Dickinson).
For cytokine studies, PBMCs (1 × 106 /ml) were activated with anti-CD3 (100 ng/ml) plus anti-CD28 (200 ng/ml) for 48 h, and supernatants were collected for the analysis of cytokines [interferon (IFN)-γ and interleukin (IL)-5] by enzyme-linked immunosorbent assay (ELISA) (BD Pharmingen, San Diego, CA, USA).
Most of the data, including total IgG, IgG subclasses, lymphocyte subsets, lymphocyte proliferation assays and specific antibody responses, were obtained at the time of diagnosis, prior to the start of IVIG. Studies of NK cytotoxicity, neutrophil oxidative burst and cytokine levels were measured later while patients were receiving IVIG; however, blood samples were drawn immediately prior to receiving the next scheduled IVIG dose (at trough level). All laboratory tests listed above were performed by a California State and CLIA (Clinical Laboratory Improvement Amendments)-certified laboratory, which requires validation and reproducibility of data.
Results
Clinical characteristics
Demographic and clinical features of 17 adult patients with selective IgG3 deficiency are listed in Table 1. There was a significant female predominance (female : male, 3:1), and the mean age at diagnosis was 47 years. The majority of patients presented with recurrent upper respiratory infection, sinusitis and pneumonia. In addition, 10 of 17 patients had concurrent allergic rhinitis and/or asthma. This was based upon patients' history and statement that radioallergosorbent tests (RAST) and skin tests were performed by the referring allergists.
Table 1.
Patient characteristics.
| Patient no. | Sex | Age at dx: years | Total IgG: mg/dl* | IgG3: mg/dl (normal range) | Clinical presentation | Other diagnoses |
|---|---|---|---|---|---|---|
| 1 | F | 41 | 794 | 11 (41–129) | URIs, 4 PNs as a child, Lyme disease | AR, asthma |
| 2 | M | 24 | 801 | 23 (41–129) | Recurrent URIs since childhood | |
| 3 | F | 47 | 1005 | 10 (41–129) | URIs, sinusitis, 2 PNs | AR, sister with low IgG3 |
| 4 | F | 71 | 803 | 34 (41–129) | URIs, PNs | AR, rheumatoid arthritis |
| 5 | M | 55 | 968 | 19 (21–114) | Rash on legs (biopsy: many eosinophils) | High IgE |
| 6 | F | 62 | 1070 | 17 (21–114) | Recurrent URIs | |
| 7 | F | 63 | 876 | 20 (21–114) | Recurrent URIs | Asthma, multiple drug allergies |
| 8 | F | 56 | 760 | 27 (41–129) | URIs, sinusitis, 2 PNs, hospitalizations | AR, asthma, Grave's disease, temporal arteritis |
| 9 | F | 57 | 1120 | 20 (21–114) | Recurrent URIs, recurrent anaphylactoid urticaria | AR, asthma |
| 10 | F | 45 | 966 | 13 (41–129) | Recurrent URIs, recurrent herpes infections, interstitial cystitis | AR, psoriasis |
| 11 | M | 32 | 1120 | 23 (41–129) | Recurrent sinusitis, chronic fungal infection of skin and prostate | |
| 12 | F | 25 | 808 | 20 (41–129) | Recurrent URIs and sinusitis | AR |
| 13 | M | 17 | 747 | 28 (41–129) | Childhood infections, 2 PNs | AR |
| 14 | F | 61 | 1300 | 17 (21–114) | Multiple sinus infections and surgeries (Pseudomonas on culture), bronchitis, hospitalizations | Non-Hodgkin's lymphoma (diagnosed 3 years after IgG3 deficiency diagnosis) |
| 15 | F | 36 | 818 | 19 (21–114) | Recurrent URIs, sinusitis | AR, polyneuritis |
| 16 | F | 53 | 1190 | 16 (21–114) | Chronic fatigue | |
| 17 | F | 41 | 1481 | 14 (21–114) | Recurrent URIs and sinusitis, severe CMV and EBV infections |
Normal range for total immunoglobulin G (IgG): 700–1600 mg/dl. Description of each patient's age, sex, total IgG, IgG3 subclass, presenting complaints and other associated diagnoses; dx, diagnosis; M: male; F: female; URI: upper respiratory infection; PN: pneumonia; AR: allergic rhinitis; CMV: cytomegalovirus; EBV: Epstein–Bar virus.
Adaptive immune responses
Lymphocyte subpopulations
Figure 1 show proportions of CD3+ T cells, CD3+CD4+ helper/inducer T cells, CD3+CD8+ cytotoxic T cells, CD3–CD19+ B cells and CD3–CD16+CD56+ NK cells. The majority of patients had percentages of subsets within the range of age- and sex-matched controls (Fig. 1, top panel). When data were analysed for absolute numbers, two patients each had low CD8+ T cells and low B cells (Fig. 1, bottom panel).
Fig. 1.
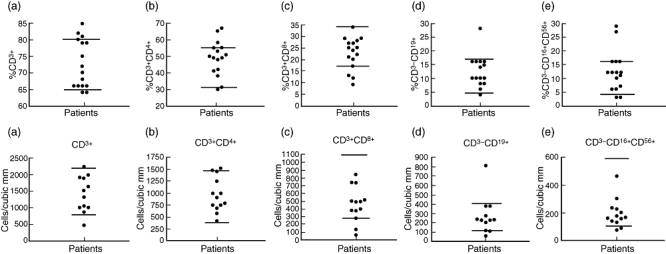
Top panel: each point represents percentage of total lymphocytes for an individual patient. Bottom panel: each point represents the absolute lymphocyte count for an individual patient. Horizontal lines indicate upper and lower limits of normal for each lymphocyte subset. (a) CD3+ T cells; (b) CD3+CD4+ helper T cells; (c) CD3+CD8+ T cells; (d) CD3–CD19+ B cells; (e) CD3–CD16+CD56+ natural killer (NK) cells.
DNA synthesis in lymphocytes
Data for lymphocyte proliferation are shown in Fig. 2. Low response to at least two of three mitogens or two of three antigens was considered abnormal. Four of 12 patients (33%) on whom mitogen studies were performed had low mitogen responses, and four of 10 patients (40%) had low antigen responses.
Fig. 2.
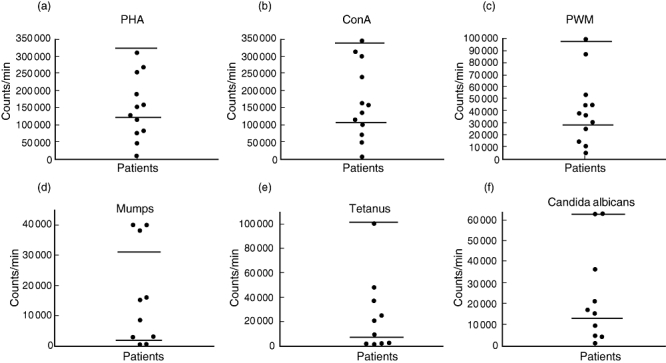
Each point represents counts for an individual patient. Horizontal lines indicate upper and lower limits of normal for each mitogen or antigen tested. (a) Response to mitogen phytohaemagglutinin (PHA); (b) response to mitogen concavalin A (ConA); (c) response to pokeweed mitogen (PWM); (d) response to antigen mumps virus; (e) response to antigen Candida albicans; (f) response to antigen tetanus toxoid.
Specific antibody responses
The pneumococcal antibody responses were recorded in 11 patients, five of whom had protective prevaccination titres greater than 1·0 IU/ml for at least half of the 14 serotypes. Of the six patients who had low prevaccination titres, two patients had no response to vaccination with Pneumovax-23. The most common unprotective antibody levels were observed against serotypes 3, 8, 9N and 12F, and the least common impairment was observed against serotypes 4, 5, 7F, 18C and 23F. Specific antibody responses to tetanus toxoid were recorded in 10 of 17 patients. Nine patients had protective titres of greater than 0·10 IU/ml at baseline, and the one who had low titres at baseline developed protective levels after immunization with tetanus toxoid vaccine.
Cytokine production
Cytokines were measured in seven patients using ELISA assay. Production of IFN-γ was used to assess T helper type 1 (Th1) function, whereas production of IL-5 was used to assess Th2 function. One patient (#9) had decreased IFN-γ production, whereas two patients (#2 and #12) had decreased production of IL-5.
Innate immune responses
Natural killer cells and activity
CD3–CD16+CD56+ NK cells were analysed by multi-colour flow cytometry, whereas NK cytotoxicity was measured by lysis of labelled target K562 cells. Proportions of NK cells were increased in two subjects and decreased in another two subjects (Fig. 1, top panel), but absolute numbers were normal in all (Fig. 1, bottom panel). NK cytotoxicity was reduced in only one of eight patients tested (patient #12).
Neutrophil function
Oxidative burst was tested in eight patients; two patients (#2 and #9) showed a modest decrease in neutrophil oxidative burst.
Complement components
Six patients had data on levels of 50% haemolytic complement (CH50) assay, C3 and C4. All were normal.
TLRs
Two of the five patients who were tested had low proportions of TLR-4+CD14+ cells (#2 and #7), and one patient had high proportions of TLR-4+CD14+ cells (#4).
Clinical response to intravenous immunoglobulin
Four of the 17 patients had mild symptoms that could be managed with antibiotic therapy, and therefore IVIG was not administered to them. Thirteen of 17 patients received IVIG treatment. They received IVIG at standard doses of 300–400 mg/kg body weight every 2 weeks (because IgG3 half-life is only 7 days). Initially, patients were started on 300 mg/kg body weight every 2 weeks and IgG3 levels and clinical status were determined. In those patients whose IgG3 levels were not normalized, dose was increased to 400 mg/kg body weight. All patients had normal IgG3 levels while on IVIG treatment. Two of the patients (#5 and #13) did not show any clinical improvement, and therefore their IVIG was discontinued. Patient 3 had a history of five episodes of sinusitis per year and two pneumonias requiring hospitalization. After receiving IVIG, the frequency and severity of her infections decreased. She had no further episodes of pneumonia, and only two sinus infections per year. Patient 4 reported recurrent episodes of bronchitis and history of pneumonia. While on IVIG, she had no pneumonias and only one URI per year. Patient 7 complained of recurrent sinusitis and bronchitis. While on IVIG she continued to have frequent sinusitis and bronchitis, but subjectively she felt better overall and had lessened severity of infections. Patient 8 had a history of two pneumonias and hospitalizations with recurrent pulmonary and sinus infections (and recovery of multiple organisms from sputum cultures). While on IVIG, her infections decreased in frequency from once a month to once every 3 months, and she had no further pneumonia or hospitalization. Patient 9 experienced fewer episodes of URIs while on IVIG. Patient 10 had recurrent URIs, recurrent herpes infections, ongoing interstitial cystitis and severe psoriatic plaques, all of which improved dramatically with IVIG treatment. Patient 11 had a history of recurrent sinus infections resistant to multiple antibiotics and chronic fungal infection of the skin and prostate. While on IVIG he felt better subjectively and had decreased URIs and sinusitis, but his chronic fungal infections persisted. Patient 12 improved from multiple URIs per year to only one URI per year on IVIG. Patient 14 presented with multiple sinus infections, sinus surgeries (Pseudomonas on culture) and recurrent URIs. While on IVIG she had less severe sinus infections, and the number of URIs decreased from once a month to once a year. While on IVIG patient 15 noted less frequent and less severe URIs. Prior to treatment, patient 17 suffered from recurrent URIs and sinus infections, as well as severe CMV and EBV infections requiring hospitalization. She had dramatic improvement on IVIG with no further hospitalizations, and fewer than one URI per year. IVIG was generally well tolerated and brand of product did not make any difference in clinical response. No patient had to discontinue IVIG due to adverse reactions. The side effects occurred during the first infusion and included rigours/chills (two patients), aseptic meningitis (two patients) and shortness of breath (one). These effects were ameliorated by decreasing the infusion rate, and did not occur in subsequent IVIG infusions. One patient had an urticarial reaction on one IVIG preparation, which did not occur when the patient was switched to another IVIG preparation.
Discussion
In the present study we have reported the immunological and clinical findings of 17 adult patients with recurrent infections and isolated IgG3 subclass deficiency. All patients have normal levels of total IgG (Table 1). Therefore, their deficiency may have been missed if IgG subclasses were not analysed. Our data show a female predilection with a female : male ratio of 3:1. Bjorkander et al.[10] observed a similar female : male ratio. The majority of our patients presented with recurrent episodes of sinusitis, bronchitis and/or pneumonia. In addition, commonly associated diseases included allergic rhinitis and/or asthma. Oxelius et al.[3] also found a high prevalence of asthma (more than 20%) in adults and children with isolated IgG3 deficiency and recurrent upper respiratory tract infections.
To the best of our knowledge, this is the first study that has analysed immunological functions in detail in adult patients with selective IgG3 subclass deficiency. In our study, the majority of patients had normal lymphocyte subsets, which is similar to those reported by Soderstrom et al.[11]. Furthermore, we observed that almost all our patients were able to make protective levels of anti-tetanus IgG. IgG3 responds predominantly to protein antigens. Tetanus toxoid is a protein antigen and elicits a strong specific antibody response. In our experience, impaired response to tetanus toxoid is observed only in severe immune deficiency; even patients with common variable immunodeficiency who have impaired specific antibody response to pneumococci do not display impaired specific antibody response to tetanus toxoid. Only two patients in this study had impaired protective levels to most of the 14 polysaccharide antigens; the majority of patients had impaired responses to serotypes 3, 8, 9N and 12F. Oxelius et al.[3] reported normal responses to polysaccharide antigens in their mixed sample of 10 adults and children (although they had data only for pneumococcal serotypes 3, 6A, 19F and 23F). This is in contrast to a report by Popa et al.[8], who observed decreased response to tetanus and Haemophilus influenza vaccines in IgG3-deficient adults. Soderstrom et al.[11] reported that 75% of adults with selective IgG3 deficiency had low B cell function, as defined by EBV- or PWM-stimulated protein A plaque-forming cells lower than 50% of healthy controls.
Data on T cell function in selective IgG3 deficiency are limited. We observed that 30–40% of patients display impaired T cell proliferative response to mitogens and recall antigens. Soderstrom et al.[11] reported decreased T cell function (defined as PHA or ConA stimulation indices of <0·8) in 40% of IgG3-deficient adult subjects. In their study, data were presented as stimulation index, which may be skewed due to differences in background counts. In our study, we analysed data as net counts per minute after subtracting the background.
T helper-1 (IFN-γ) and T helper-2 (IL-5) cytokine production was analysed in seven subjects; abnormal IFN-γ production was observed in one patient and abnormal IL-5 production in two patients. It is not possible to suggest the significance of these cytokine results in IgG3 subclass deficiency, as the number of samples tested is small. Finally, NK cell cytotoxicity and neutrophil oxidative burst (reactive oxygen species generation) were relatively normal. In two patients oxidative burst was modestly reduced; however, it was not to a level observed in chronic granulomatous disease. Furthermore, patients did not have diabetes mellitus.
In general, IgG1 or IgG2 deficiencies are reported to cause more severe infections, and there is greater acceptance of the use of immunoglobulin prophylaxis in such cases [7]. In our study, clinical response to IVIG was observed in the majority of patients with IgG3 deficiency. Six of 13 patients who received IVIG had dramatic relief from their recurrent infections, five patients experienced moderate clinical improvement and two patients had no response. We did not observe any correlation between response to IVIG and immunological parameters. However, our sample size is too small to reach a definitive conclusion.
Olinder-Nielsen et al.[7] recently examined the effect of subcutaneous immunoglobulin on a group of adults with different IgG subclass deficiencies and greater than four antibiotic-requiring respiratory tract infections per year. In their study, the number of respiratory tract infections prior to immunoglobulin treatment was significantly higher in the selective IgG3 deficiency group than in the group with selective IgG1 deficiency, but comparable to the number of infections in IgG2-deficient patients. Moreover, patients with IgG3 deficiency responded to treatment just as well as did patients with deficiency of IgG1, IgG2 or combinations of subclasses. The researchers found that subcutaneous immunoglobulin prophylaxis reduced the frequency of respiratory tract infections from 6·045 episodes per year to only 2·258 episodes per year in patients with selective IgG3 deficiency [7].
The mechanism by which IVIG reduces infections in IgG3-deficient patients is due probably to passive transfer of specific antibodies against multiple pathogens, rather than simple replacement of IgG3. Barlan et al.[5] reported clinical improvement after administration of IVIG devoid of IgG3. This would suggest that the normalization of IgG3 should not be the aim of IVIG therapy or for modifying the dosage of IVIG in patients with selective IgG3 deficiency. The effectiveness of IVIG therapy should be judged by clinical response.
Popa et al.[12] suggested that the clinical effects of IVIG were due to its anti-inflammatory properties. This possibility was based upon their observation that a subgroup of patients who had recurrent respiratory infections, interstitial lung disease and isolated or combined deficiencies of IgG1, IgG2, IgG3 or IgG4 demonstrated improvement in symptoms, spirometry, and in radiological and histological findings after treatment with IVIG. However, the majority of anti-inflammatory effects of IVIG are observed generally with higher immunomodulatory doses of IVIG rather than with replacement dosage.
In summary, our retrospective study of patients with selective IgG3 deficiency shows that selective IgG3 subclass deficiency should be considered in adults with recurrent upper respiratory tract infections with or without allergic rhinitis and asthma, and therefore IgG subclasses should be analysed even when total IgG levels are normal. Furthermore, this study suggests that a subset of patients with selective IgG3 deficiency have combined T and B cell defects. Patients with selective IgG3 deficiency respond clinically to IVIG treatment, and it should be incorporated as a standard of care therapy. A detailed study of cytokine and other components of the innate immune system is needed in a large cohort of patients with IgG3 subclass deficiency.
Acknowledgments
We would like to thank our patients for their participation. The study was supported by the University of California, Irvine Division of Basic and Clinical Immunology.
Disclosure
None.
References
- 1.Buckley RH. Immunoglobulin G subclass deficiency: fact or fancy? Curr Allergy Asthma Rep. 2002;2:356–60. doi: 10.1007/s11882-002-0067-1. [DOI] [PubMed] [Google Scholar]
- 2.Shackelford PG. IgG subclasses: importance in pediatric practice. Pediatr Rev. 1993;14:291–6. doi: 10.1542/pir.14-8-291. [DOI] [PubMed] [Google Scholar]
- 3.Oxelius VA, Hanson LA, Bjorkander J, et al. IgG3 deficiency: common in obstructive lung disease. Hereditary in families with immunodeficiency and autoimmune disease. Monogr Allergy. 1986;20:106–15. [PubMed] [Google Scholar]
- 4.Meyts I, Bossuyt X, Proesmans M, De B. Isolated IgG3 deficiency in children: to treat or not to treat? Case presentation and review of the literature. Pediatr Allergy Immunol. 2006;17:544–50. doi: 10.1111/j.1399-3038.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- 5.Barlan IB, Geha RS, Schneider LC. Therapy for patients with recurrent infections and low serum IgG3 levels. J Allergy Clin Immunol. 1993;92:353–5. doi: 10.1016/0091-6749(93)90179-j. [DOI] [PubMed] [Google Scholar]
- 6.Umetsu DT, Ambrosino DM, Quinti I, et al. Recurrent sinopulmonary infection and impaired antibody response to bacterial capsular polysaccharide antigen in children with selective IgG-subclass deficiency. N Engl J Med. 1985;313:1247–51. doi: 10.1056/NEJM198511143132002. [DOI] [PubMed] [Google Scholar]
- 7.Olinder-Nielsen AM, Granert C, Forsberg P, et al. Immunoglobulin prophylaxis in 350 adults with IgG subclass deficiency and recurrent respiratory tract infections: a long-term follow-up. Scand J Infect Dis. 2007;39:44–50. doi: 10.1080/00365540600951192. [DOI] [PubMed] [Google Scholar]
- 8.Popa V, Kim K, Heiner DC. IgG deficiency in adults with recurrent respiratory infections. Ann Allergy. 1992;70:418–24. [PubMed] [Google Scholar]
- 9.Bonilla FA, Bernstein L, Khan DA, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. Ann Allergy Asthma Immunol. 2005;94S:S1–60. doi: 10.1016/s1081-1206(10)61142-8. [DOI] [PubMed] [Google Scholar]
- 10.Bjorkander J, Bengtsson U, Oxelius VA, Hanson LA. Symptoms in patients with lowered levels of IgG subclasses, with or without IgA deficiency, and effects of immunoglobulin prophylaxis. Monogr Allergy. 1986;20:157–63. [PubMed] [Google Scholar]
- 11.Soderstrom T, Soderstrom R, Avanzini A, et al. Immunoglobulin G subclass deficiencies. Int Arch Allergy Appl Immunol. 1987;82:476–80. doi: 10.1159/000234258. [DOI] [PubMed] [Google Scholar]
- 12.Popa V, Colby TV, Reich SB. Pulmonary interstitial disease in Ig deficiency. Chest. 2002;122:1594–603. doi: 10.1378/chest.122.5.1594. [DOI] [PubMed] [Google Scholar]


